Abstract
Intimal hyperplasia of autologous vein grafts is a critical problem affecting the long-term patency of many types of vascular reconstruction. Within intimal hyperplasia lesions, smooth muscle cells are a major component, playing an essential role in the pathological process. Given that bone marrow-derived cells may differentiate into smooth muscle cells in the neointima of injured arteries, we hypothesized that the bone marrow may serve as a source for some of the smooth muscle cells within intimal hyperplasia lesions of vein grafts. To test this hypothesis, we used an established mouse model for intimal hyperplasia in wild-type mice that had been transplanted with bone marrow from a green fluorescent protein (GFP+/+) transgenic mouse. High-resolution confocal microscopy analysis performed 2 and 8 weeks after grafting demonstrated expression of GFP in 5.4 ± 0.8% and 11.9 ± 2.3%, respectively, of smooth muscle cells within intimal hyperplasia lesions. By 16 weeks, GFP expression in smooth muscle cells was not detected by immunohistochemistry; however, real-time PCR revealed that 20.2 ± 1.7% of the smooth muscle cells captured from the neointima lesion by laser capture microdissection at 16 weeks contained GFP DNA. Our results suggest that bone marrow-derived cells differentiated into smooth muscle cells within the intimal lesion and may provide a novel clinical approach for decreasing intimal hyperplasia in vein grafts.
Use of vein grafts as bypass conduits is one of most effective treatments for ischemic hearts and limbs. In the United States alone, nearly 600,000 patients undergo coronary artery bypass surgery and/or peripheral revascularization annually.1,2 Unfortunately, the lifespan of vein grafts is limited most commonly by a decrease in the area of the vessel lumen due to intimal hyperplasia, the pathological process of thickening of the vessel wall. Intimal hyperplasia leads to recurrent chest pain when it occurs in vein grafts used in coronary bypass graft surgery, to lower limb ischemia when it occurs in vein grafts used in peripheral revascularization, and to loss of access for hemodialysis when it occurs in arteriovenous fistulas. Hemodialysis access dysfunction costs more than 1 billion dollars per year, and access-related admissions account for 25% of all hospitalizations in the United States.3,4 Currently, there is no effective therapy for intimal hyperplasia.
The predominant cellular component within the intimal hyperplasia lesion is smooth muscle cells, and smooth muscle cell proliferation is believed to play a key role in the pathogenesis of the process. Since several studies have demonstrated that bone marrow-derived cells differentiate into smooth muscle cells within the neointima of the injured artery,5,6,7 the assumption was that bone marrow-derived cells would play a similar role in the vein graft. However, recent studies have not found bone marrow-derived smooth muscle cells within the neointima of an allogeneic/isograft vein graft and arteriovenous fistula model,8,9,10 and thus, the role of bone marrow-derived smooth muscle cells in veins has been questioned. To definitively examine whether cells of bone marrow origin have a role in venous intimal hyperplasia, we used an established model of venous intimal hyperplasia in which the autologous external jugular vein is grafted into the abdominal aorta. This model was performed in wild-type C57BL/6J mice that have undergone transplantation with bone marrow from mice that express green fluorescent protein (GFP) in every cell. The pathology of the intimal hyperplasia lesion from 2 to 16 weeks after grafting of these chimeric mice was analyzed using immunohistochemistry as well as laser capture microscopy followed by real-time polymerase chain reaction (PCR).
In this study, we determined the level of proliferation of the cellular components of the intimal hyperplasia lesion and provided evidence that smooth muscle cells are derived from the bone marrow. By immunohistochemistry, smooth muscle cells of bone marrow origin can be detected at 2 and 8 weeks, but not at 16 weeks, after grafting. It is interesting that smooth muscle cells captured from the neointima lesion at 16 weeks still contained GFP DNA, indicating a bone marrow origin. Our data show strong evidence that bone marrow cells that have differentiated into smooth muscle cells are “permanent residents” of the intimal hyperplasia lesion and may provide a novel clinical avenue for decreasing intimal hyperplasia.
Materials and Methods
All animal procedures were performed according to protocols approved by the Institutional Animal Care and Use Committee and complied with the Guide for the Care and Use of Laboratory Animals.
Chimeric GFP mice
Chimeric GFP mice were generated as described previously.11 In brief, GFP transgenic mice (C57BL/6J background) obtained from The Jackson Laboratory (Bar Harbor, ME) carry the GFP driven by chicken β-actin promoter and cytomegalovirus intermediate-early enhancer leading to expression of GFP in every cell. Wild-type C57BL/6J mice were irradiated by 950 rads and injected with bone marrow cells (1 × 106) from GFP mice into the retro-orbital sinus.
Mouse Venous Intimal Hyperplasia Model
Similar to our previous report, a novel mouse intimal hyperplasia model was used in which the autologous external jugular vein was grafted into the infrarenal abdominal aorta by end-to-end anastomosis.12 In brief, mice were anesthetized using a portable anesthesia machine (Summit Medical, Bend, OR) with isoflurane. The left external jugular vein was grafted into the infrarenal abdominal aorta with 11/0 Ethilon suture (Ethicon Inc. Somerville, NJ) in a nonreversed configuration. Six or seven interrupted sutures were required per end. The infrarenal abdominal aorta was clamped for an average of 68 minutes. The operative field was irrigated thoroughly with a penicillin saline before closing the muscle and skin.
Tissue Harvest and Histology
Vein grafts were harvested from wild-type and chimeric mice at 2, 8, and 16 weeks after surgery. Before mice were sacrificed, with an overdose of isoflurane, peripheral blood was collected from the right atrium for analysis by flow cytometry to determine the degree of transplantation. Frozen sections were obtained and processed as described previously.12 Four adjacent sections were cut every 100 μm at three different points and stained with: 1) hematoxylin and eosin stain; 2) Verhoeff elastin stain [used to determine the area (micrometer squared) of the lumen and neointima to calculate the degree of lumen narrowing13]; 3) antiproliferation cell nuclear antigen (PCNA) antibody; or 4) control antibody. An additional 16 6-μm slices were obtained between each point (16 6-μm slices × 2 intervals = 32 sections per mouse) for dual and triple immunohistochemistry (8 to 12 sections per mouse) and laser capture microdissection (20+ sections per mouse) analysis. Quantitative evaluation was performed at ×400 magnification using a Zeiss Axiovert Microscope (Carl Zeiss MicroImaging, Thornwood, NY); the images were analyzed with Axiovision 4.1 software (Carl Zeiss MicroImaging).
Dual and Triple Immunohistochemistry and Confocal Laser Scanning Microscopy
Frozen sections were rehydrated, blocked, and incubated with biotin-conjugated monoclonal anti-PCNA (BD PharMingen, San Diego, CA) followed by streptavidin-peroxidase polymer (Sigma, St. Louis, MO) and developed using a diaminobenzidine hydrochloride. After diaminobenzidine hydrochloride deposits were observed on the sections, each section was stained with an antibody for a different cell type. For smooth muscle and endothelial cells, the sections were incubated overnight with either a rabbit anti-smooth muscle-myosin heavy chain (Biomedical Technologies Inc. Stoughton, MA) or a rabbit anti-von Willebrand factor (vWF; DAKO, Carpinteria, CA) antibody, respectively, followed by staining with Alexa 594-conjugated donkey anti-rabbit secondary antibody (Molecular Probes, Eugene, OR). To stain for leukocytes, the sections were incubated with a rat anti-CD-45 antibody (BD Biosciences, San Jose, CA) followed by a biotinylated goat anti-rat Fab secondary antibody (Jackson ImmunoResearch, West Grove, PA) and then Alexa 594-conjugated streptavidin.
To evaluate the role of bone marrow-derived cells within the intimal hyperplasia lesion at 2 weeks after grafting, sections obtained from the chimeric mice were stained by the dual-immunohistochemistry technique. To enhance the GFP signal, an anti-GFP antibody (Abcam, Cambridge, MA) was used. In sections in which smooth muscle or endothelial cells were examined, the sections were stained with rabbit anti-GFP, followed by donkey anti-rabbit biotin-conjugated Fab and then streptavidin-conjugated fluorescein isothiocyanate (Jackson ImmunoResearch). Each section was then incubated with rabbit anti-smooth muscle-myosin heavy chain or rabbit anti-vWF antibodies followed by Alexa 594-conjugated donkey anti-rabbit antibody. To stain for leukocytes, sections were incubated simultaneously with rat anti-CD45 and rabbit anti-GFP, followed by simultaneous staining with biotinylated goat anti-rat Fab and fluorescein isothiocyanate-conjugated donkey anti-rabbit Fab secondary antibodies, and then the sections were stained with streptavidin-conjugated Alexa 594. Sections from an aorta artery isolated from a GFP+/+ mouse served as positive controls. Two different negative controls were used: sections from chimeric mice incubated with isotype-matched IgG in lieu of anti-GFP primary antibody and sections from wild-type mice at 2 weeks after grafting were incubated with anti-GFP primary antibody. A similar procedure was performed on sections of intimal hyperplasia lesions from chimeric mice obtained at 8 and 16 weeks after grafting.
To assess whether smooth muscle cells derived from the bone marrow were actively proliferating, sections obtained 2 and 8 weeks after grafting from the chimeric mice were stained by the triple-immunohistochemistry technique similar to above. First, sections were stained in sequence for PCNA, rabbit anti-GFP, donkey anti-rabbit biotin-conjugated Fab, and streptavidin-conjugated fluorescein isothiocyanate. Second, sections were incubated with rabbit anti-smooth muscle-myosin heavy chain followed by Alexa 594-conjugated donkey anti-rabbit antibody. Third, sections were counterstained with 4,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA). Some sections were pretreated with a high-temperature antigen-unmasking technique before being stained.
Sections were examined under a confocal laser scanning microscope (LSM, 510 UV; Carl Zeiss). Images were acquired with a Plan-Neofluor 40× (numerical aperture = 0.75) and Plan-Fluor 100× oil (numerical aperture = 1.4) objectives and evaluated with Zeiss LSM 510 imaging software including three-dimensional reconstruction.
Laser Capture Microdissection and DNA Isolation
Smooth muscle cells in 6-μm frozen sections from the 16-week intimal hyperplasia lesion of chimeric mice were stained for smooth muscle heavy chain by immunohistochemistry and processed for microdissection using a PixCell II laser capture microscope with a diode laser (Arcturus Bioscience, Inc. Mountain View, CA). In brief, the dehydrated sections were overlaid with a thermoplastic membrane mounted on optically transparent caps (CapSure Macro LCM Caps 0211; Arcturus Bioscience, Inc.), and smooth muscle cells were captured with focal melting of the membrane through laser activation. Cells that did not stain positive for myosin heavy chain were manually removed with a CapSure Pad (CP-100; Arcturus Bioscience, Inc.), according to manufacturer’s instructions. The total number of smooth muscle cell nuclei on the membrane was determined by DAPI staining and by counting the nuclei before immersing in DNA isolation solution (Pinpoint Slide DNA Isolation System; ZYMO Research, Orange, CA). Smooth muscle cells captured from wild-type vein graft lesions, 16 weeks postsurgery, and from a GFP abdominal aorta were used as negative and positive controls, respectively. All DNA were transferred into PCR reaction tubes containing 10 μl of UltraPure water (Molecular Biology Grade; Invitrogen Corp, Grand Island, NY) and stored at −20°C until PCR analysis.
Real-Time Quantitative PCR
Real-time PCR was performed in duplicate using SYBR Green Core Reagent Kit (Applied Biosystems, Foster City, CA) with an Opticon II thermocycler (Bio-Rad, Hercules, CA). Amplification conditions were as follows: 95°C at 10 minutes, followed by 45 cycles of 94°C for 15 seconds, 60°C for 25 seconds, and 72°C for 25 seconds. After the final extension period (72°C for 10 minutes), a melting-curve analysis was performed to ensure specificity of the products. Expression of each gene was normalized to β-actin, and fold expression values (relative to the sample with the lowest expression) were calculated using the 2-ΔΔct method. Primers used in this study were as follows: β-actin (forward 5′-TGGCGCTTTTGACTCAGGATT-3′, reverse 5′-GACGCGACCATCCTCCTCTTAG-3′), and GFP (forward 5′-ACCCTCGTGACCACCCTGACCT-3′, reverse 5′-TCGGCGCGGGTCTTGTAGTT-3′).
Statistics
Quantitative morphological data were presented as mean ± SE and analyzed by the unpaired Student’s t-test. The analyses were performed with SigmaStat 2.0 and SigmaPlot 8.0 software, and significance was defined by P < 0.05.
Results
Surgical and Bone Marrow Transplantation Success Rate
We performed the intimal hyperplasia model in 39 mice, and three mice died secondary to a prolonged clamp time (>90 minutes), which lead to thrombosis (n = 1), hemorrhage (n = 1), and postoperative intestinal obstruction (n = 1). The overall surgical success rate was 92% (36/39). Vein grafts were harvested and analyzed at 2 weeks (n = 5, wild-type mice; n = 5 chimeric mice), 8 weeks (n = 10, wild-type mice; n = 6 chimeric mice), and 16 weeks (n = 5, wild-type mice; n = 5 chimeric mice). All vein grafts were patent when harvested. Flow-cytometric analysis of circulating mononuclear cells from blood in bone marrow transplantation mice at 2, 8, and 16 weeks after grafting indicated an average chimerism rate of 70 ± 7%.
Proliferating Cells within the Vein Graft
There was no significant difference in the lumen-narrowing rate between wild-type and chimeric mice at 2 (16.4 ± 0.87 versus 14.9 ± 0.75%, P = 0.21), 8 (22.3 ± 0.79 versus 24.6 ± 1.29%, P = 0.12), or 16 weeks (23.9 ± 1.6 versus 24.9 ± 1.1%, P = 0.58) after grafting. Similar results were obtained for cell density and PCNA-positive cell percentage between the two groups (data not shown).
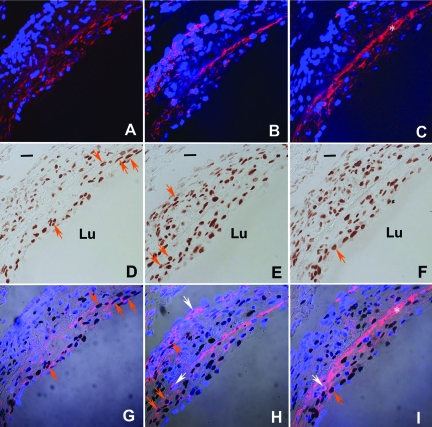
Previous studies have demonstrated that PCNA-positive cells are present early after grafting a vein into an artery.12,13 In the present study, we determined the percentage of proliferation for each cell type within the neointima in wild-type mice. Two weeks after grafting, the majority (52 ± 5%) of the PCNA-positive cells expressed smooth muscle-myosin heavy chain and were identified as smooth muscle cells; 25 ± 4% stained for CD45, identifying them as leukocytes, and only 18 ± 1% stained positive for vWF, indicating an endothelial cell phenotype (Figure 1). Conversely, 57 ± 5% of all smooth muscle myosin heavy chain cells, 52 ± 6% of all CD45-positive cells, and only 17 ± 2% of all vWF-positive cells were PCNA-positive.
Figure 1.
Analysis of 2 week neointima lesion:proliferating cell type. A–C: Confocal image of smooth muscle myosin heavy chain (A, red) and CD45-positive cells (B, red) and DAPI nuclear staining (blue). C: vWF (red) and DAPI nuclear staining (blue). Because of injury to the endothelium, platelets (which are vWF-positive) accumulate along the lining of the vessel. This is reflected as a bright, red, anuclear, fluorescent band indicated by the *. Given that platelets do not have a nucleus, their positive fluorescence is not an indication of an endothelial cell. D–F: Bright-field image of PCNA-positive cells (brown). G–I: Merged image: orange arrows indicate example of PCNA-positive smooth muscle cells (G), leukocytes (H), or endothelial cells (I). The identical cells in D, E, and F are indicated by orange arrows. Example of nonproliferating leukocytes (H) and endothelial cells (I) are indicated by white arrows. Note: in the adventitia there is a predominance of CD45-positive cells (H) with a paucity of smooth muscle cells (G) and endothelial cells (I). Bar = 20 μm, Lu, lumen.
Bone Marrow-Derived Cells in the Intimal Hyperplasia Lesion
To assess the involvement of bone marrow-derived cells during the intimal hyperplasia process, the expression of GFP was determined in endothelial cells, leukocytes, and smooth muscle cells in the vein graft performed in chimeric mice. At two, 8, and 16 weeks after grafting, the percentage of GFP-positive cells that colocalized with vWF antibody remained constant. Breaks in the endothelium could be observed up to 8 weeks, but the endothelium was intact by 16 weeks. GFP-positive cells that co-localized with CD45 antibody were observed only at 2 and 8 weeks after grafting.
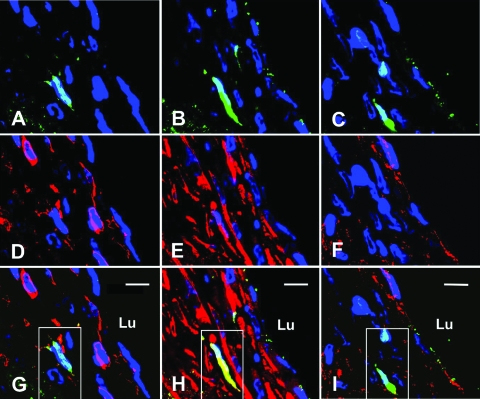
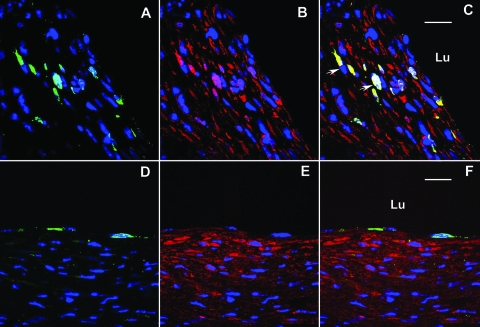
With regard to smooth muscle cells, two weeks after grafting, 5.4 ± 0.8% of cells identified as smooth muscle cells expressed GFP. To confirm that the GFP-positive cells were indeed smooth muscle cells, two adjacent sections to the GFP and smooth muscle myosin heavy chain slide were stained with vWF and CD45, respectively. Cells that expressed GFP and smooth muscle myosin heavy chain did not express vWF or CD45 (Figure 2). By 8 weeks after vein grafting, 11.9 ± 2.3% of the smooth muscle myosin heavy chain expressing cells also expressed GFP (Figure 3, A–C). The specificity of our GFP detection was confirmed by the inability to detect any GFP-positive cells in two different negative controls: sections from bone marrow transplantation mice incubated with isotype-matched IgG in lieu of anti-GFP primary antibody and sections from wild-type mice at 2 and 8 weeks after grafting incubated with anti-GFP primary antibody (data not shown).
Figure 2.
Identification of bone marrow-derived cells within the neointima 2 weeks after grafting. A–C: Three adjacent sections imaged by confocal microscopy after immunofluorescent detection of GFP-positive cells (green) and stained with DAPI nuclear stain (blue). D–F: Red fluorescence indicates CD45 (D), smooth muscle myosin heavy chain (E), or vWF (F) of same sections pictured in A to C, respectively. G–I: Merged image of GFP fluorescence (A–C) and red fluorescence images (D–F). Note the GFP-positive cell expressing smooth muscle myosin heavy chain appears yellow (outlined by a white box) in H. This same cell, since it does not express CD45 or vWF, appears green in G and I, respectively. Bar = 10 μm, Lu, lumen.
Figure 3.
Identification of bone marrow-derived cell at 8 weeks (A–C) and 16 weeks (D–F) after grafting. A,D: Confocal image of GFP-positive cells (green) and DAPI nuclear stain (blue). B,E: smooth muscle myosin heavy chain-positive cells (red) and DAPI nuclear stain (blue). C: Merged image of 8-week lesion demonstrating that GFP-positive cells also express smooth muscle myosin heavy chain (C, white arrow). F: Merged image of 16-week lesion demonstrating GFP-positive cells outline the lumen (and stain positive for vWF (data not shown)), but no smooth muscle myosin heavy chain expressing cells expresses GFP. Bar = 20 μm, Lu, lumen.
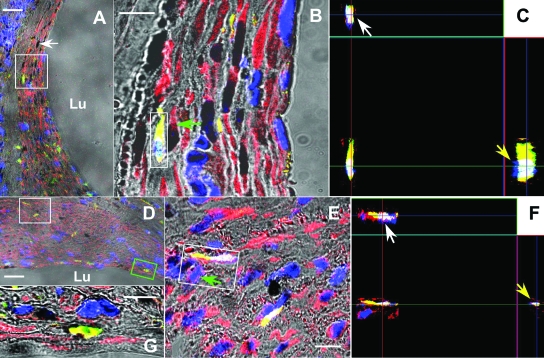
Although we optimized the pinhole size of the confocal microscope during imaging, collection and the thickness of the scanning section in Figures 2 and 3 is less than 0.9 μm; to further reduce the possibility of false-positive signal derived from neighboring cells or overlapping cells, we performed imaging stacks. Orthogonal sections of cells staining positive for GFP and smooth muscle myosin heavy chain (ie, X-Z or Y-Z imaging) were analyzed by three-dimensional analysis of imaging sequences of single cells (ie, three-dimensional reconstruction). The dual positivity of cytoplasm around a single nucleus for GFP and smooth muscle myosin heavy chain was confirmed (Figure 4 and Supplemental Figure 1, see http://ajp.amjpathol.org). More interestingly, even at 8 weeks, cells positive for GFP, smooth muscle myosin heavy chain, and PCNA were observed (Figure 4G), albeit at a lower frequency. Unexpectedly, using immunohistochemistry, at 16 weeks after grafting, we did not detect any GFP-positive cells that expressed smooth muscle myosin heavy chain (Figure 3, D–F).
Figure 4.
Orthogonal analysis of bone marrow-derived cells in the neointima 2 (A–C) and 8 weeks (D–G) after grafting. A,D: Typical triple immunofluorescent sections imaged by low resolution (object: ×20) confocal microscopy demonstrating of bone marrow-derived smooth muscle cells. The yellow represents the cytoplasm of the cell staining both with anti-GFP (fluorescein isothiocyanate) and anti-smooth muscle heavy chain (Alexa 594). The black stain (white arrow) represents nuclei staining PCNA (black), non-PCNA-staining nuclei appear blue (DAPI). Bar = 40 μm, Lu, lumen. B,E: High-resolution pictures (object: ×100) from area within the white box in A and D, respectively. Bar = 10 μm. C,F: X-Z (white arrows) and Y-Z (yellow arrows) imaging from the area within the white box in B and E, respectively, demonstrating that the yellow fluorescent signal is not derived from neighboring smooth muscle cells, green arrows in B and E, or overlapping cells. G: A cell that stains both with smooth muscle myosin heavy chain and GFP (yellow, outlined by a green box in D) has a PCNA positive-nucleus (black area).
Laser Capture Microdissection and Real-Time PCR
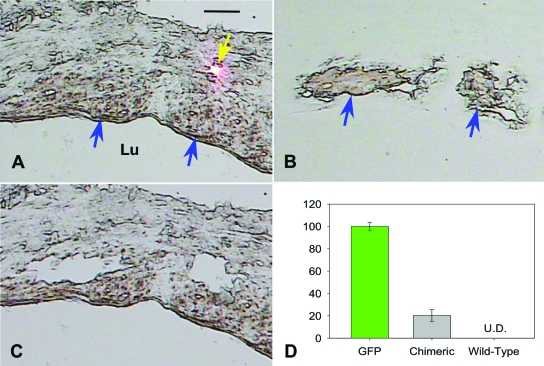
The lack of smooth muscle cells expressing GFP at 16 weeks could be explained by apoptosis of bone marrow-derived smooth muscle cells or lack of robust GFP expression at latter time points, making it difficult to detect the GFP-positive smooth muscle cells. To differentiate between these alternatives, we processed smooth muscle cells captured by laser capture microdissection and analyzed them by real-time PCR. We successfully isolated 1757, 2131, 700, and 1780 smooth muscle cells from the intimal hyperplasia lesion of four chimeric mice. Real-time PCR demonstrated that, on average, 20.2 ± 1.7% of the smooth muscle cells contained the DNA for GFP at 16 weeks after grafting (Figure 5).
Figure 5.
DNA determination of origin of smooth muscle cells at 16 weeks after grafting. A: Bright-field image of section before capture. Smooth muscle cells were identified with immunohistochemistry stain (brown, blue arrow) and captured by laser (pink dot indicates laser (yellow arrow)). B: Captured smooth muscle cells on a transparent cap. C: Void within neointima lesion left by captured smooth muscle cells. Bar = 20 μm, Lu, lumen. D: Real-time PCR determination of percentage of GFP-positive cells, as determined by copy number of GFP vs. β-actin, among captured smooth muscle cells isolated from chimeric mice. Shown as controls are results of real-time PCR of smooth muscle cells isolated from a GFP and wild-type mice (U.D., undetectable).
Discussion
After implantation into the arterial circulation, a vein graft undergoes three distinct phases: thrombosis, intimal hyperplasia/remodeling, and accelerated atherosclerosis.13 Intimal hyperplasia, defined as the proliferation of smooth muscle cell and the accumulation of extracellular matrix, occurs between 1 month and 5 years after surgery.14 Our mouse model of intimal hyperplasia, followed for 16 weeks, recapitulated most of the mid-to-late phase of human vein grafts in an accelerated time frame. In addition, the results from the chimeric mice appear to recapitulate the results from the wild-type mouse, suggesting that the intimal hyperplasia lesion was not affected by either the radiation necessary to prepare the mice for transplantation or the bone marrow transplantation procedure itself.
Smooth muscle cells play an essential role in the pathological process of intimal hyperplasia. To accurately and definitively identify smooth muscle cells, we used the smooth muscle myosin heavy chain marker, which has been used as a marker for smooth muscle cells in a number of studies,15,16 and the antibody clone used in this study has previously been published as a specific marker of smooth muscle cells.17,18,19 Whereas smooth muscle α-actin has been used as a marker of smooth muscle cells, due to the availability of high affinity antibodies, numerous studies indicated that smooth muscle α-actin is expressed by myofibroblasts,20 endothelial cells,21 and even in tumor cells.22
In the current study, we have demonstrated that approximately half of PCNA-positive cells are smooth muscle cells. However, inflammatory cells proliferate at a rate equal to that of smooth muscle cells. Interestingly, endothelial cells, thought to play a major protective role in the pathological process, have a significantly lower proliferation rate. This may explain the dependence on bone marrow progenitor cells for protection against intimal hyperplasia.23 Although the role of bone marrow-derived cells in endothelial repair in transplant arteriopathy has been controversial,24,25,26 our findings support the ability of bone marrow-derived cells to differentiate into endothelial cells (Supplemental Figure 2, see http://ajp.amjpathol.org).
More importantly, at 2 and 8 weeks after grafting, a significant fraction of smooth muscle cells within the intimal hyperplasia lesion are derived from the bone marrow and have a capacity for proliferation, as determined by immunohistochemistry. Thus, cells derived from the bone marrow appear to have considerable plasticity differentiating into smooth muscle cells as well as endothelial cells.
Previously, our preliminary data suggested that smooth muscle cells originating from the bone marrow could be found within the venous intimal hyperplasia at early time points after grafting;27 however, we were not able to detect smooth muscle cells that express GFP at 16 weeks after grafting. A possible explanation for this inability to detect GFP by immunohistochemistry at 16 weeks is a change in the expression of various genes, including GFP. It is known that compared with native medial smooth muscle cells, neointimal smooth muscle cells in the injured artery have a different phenotype and gene expression profile.28,29 However, further studies will be needed to address the mechanism responsible for the diminished GFP expression or detection at 16 weeks in vein grafts.
Whereas in situ PCR of a single smooth muscle cell may be the preferred method to detect GFP DNA within the neointima lesion, we were unable to successfully carry out this procedure due to technical difficulties. With methodological modification, we performed laser capture and real-time PCR to identify the origin of the smooth muscle cells. The ability to accurately capture only smooth muscle cells from the neointima lesion was aided by the fact that, at 16 weeks after grafting, up to 70% of the cells within the neointima were smooth muscle cells.30 In addition, chromogenic immunohistochemical staining for smooth muscle cells further enhanced our ability to precisely isolate only smooth muscle cells. Although the capture of other cell types from the neointima lesion cannot be formally excluded, if it had occurred, it would be limited to a very small percentage. Previously, two groups8,9,10 have suggested that bone marrow-derived smooth muscle cells are absent in the neointima of an allogeneic/isograft vein graft and arteriovenous fistula model. Our difficulty in detecting GFP may carry over to the ability to detect β-galactosidase within smooth muscle cells used in those studies. Unfortunately, we do not know the sensitivity of the previous methods. In our hands, when immunohistochemistry was performed on the aorta of a GFP+/+ mouse in which every cell expresses GFP, GFP could be detected in only ∼50% of smooth muscle cells, whereas GFP DNA was detected in every cell (data not shown). In addition, the previous studies relied on the expression of β-galactosidase under the smooth muscle cell-specific SM22 promoter. Although the SM22 promoter is active in bone marrow-derived smooth muscle cells in vitro,8 it is unknown whether comparable activation occurs in vivo within a grafted vein. Nevertheless, both the studies provide important evidence that smooth muscle cells at least partly originate from the local vessel.
More recently, Bentzon et al31 reported that they could not replicate the results of Sata et al6 and provided strong evidence that hematopoietic stem cells did not contribute to smooth muscle cells in the fibrous cap of atherosclerotic/arterial transplant atherosclerosis lesions in a chronic (more than 20 weeks) mouse model. This is consistent with our current study that GFP could not be detected in smooth muscle cells at longer time points (16 weeks) by immunohistochemistry. Nevertheless, fibrous caps are absent or poorly developed in vein grafts,14,32,33,34 and thus, the origin of smooth muscle cells may be different in vein grafts.
Currently, the origin of smooth muscle cells in neointima in injury artery is highly controversial. However, it is reasonable that bone marrow-derived cells may differentiate into smooth muscle cells only in severe artery injury.35,36 Injury to a vein may be similar to that of a severe injured artery in that the vessel wall of intact veins has only one or two layers of smooth muscle cells12,30 and early after grafting a high apoptosis rate is observed within veins37; there have even been reports of the vein consisting of only an acellular band surrounding the lumen.37,38 By 16 weeks, the wall of the vein graft had increased up to 18-fold, with the majority of cells being smooth muscle cells.30 This appears to necessitate a vein graft-extrinsic origin of smooth muscle cells within the neointima.9 In the few reports that refute a bone marrow origin of smooth muscle cells into vein, the models used are ones that induce a less severe mechanical injury such as the “cuff” model30 or arteriovenous fistula model.10 Consistent with the conflicting artery injury results,39 perhaps the degree of venous injury explains the differing results.
Although the percentage of GFP-positive smooth muscle cells detected at 2 and 8 weeks after grafting was only in the 5 to 12% range, it is important to take into account our ability to detect bone marrow-derived cells. As discussed above, immunohistochemistry only detects ∼50% of smooth muscle cells. Thus, detection of 12% of bone marrow-derived smooth muscle cells by immunohistochemistry at 8 weeks is comparable to the 20% of bone marrow-derived smooth muscle cells detected by laser capture at 16 weeks. In addition, the bone marrow transplantation efficiency was not complete. When flow cytometry was performed on the peripheral blood of the chimeric animals, at the time of sacrifice, a mean of 70% of the cells expressed GFP. Lastly, smooth muscle myosin heavy may not be present in less mature smooth muscle cells.15 Thus, the actual percentage of bone marrow-derived smooth muscle cells is substantially higher than that detected by immunohistochemistry. This suggests that bone marrow-derived smooth muscle cells may play a major role in the formation of neointima.
There are a number of possible mechanisms by which bone marrow-derived cells contribute to intimal hyperplasia. 1) Bone marrow cells can adhere to the venous wall without much infiltration/penetration. This would imply that the neointima is being built up “layer-by-layer.” 2) Bone marrow cells can enter the venous wall via the perivascular vasa vasorum. Within the wall, the bone marrow-derived cells would differentiate into smooth muscle cells and migrate to the neointima from the “outside-in.” Interestingly, we (unpublished data) and others40 found clustered bone marrow-derived cells not only in the intima but also in and around adventitial microvessels. 3) Bone marrow-derived progenitor cells may infiltrate/penetrate into the adventitia from the lumen similar to white blood cells. After differentiation into smooth muscle cells in the appropriate microenvironment, they may make a “u-turn” toward the neointima. Further experiments to identify the precise mechanisms will provide an increased understanding of the intimal hyperplasia process.
The phenotype of bone marrow-derived cells recruited to the neointima is still controversial. In an injury artery model, Wang et al41 reported that there was an increase in c-kit+ cells in the injured artery wall that assisted in the homing of stem cell factor-positive cells. However, whether these c-kit+ cells are derived from the circulation or the local vessel is still unknown. Zernecke et al42 demonstrated that bone marrow-derived c-kit-negative/Sac-1+ cells were preferentially recruited to the neointimal and differentiated into smooth muscle cells. The development of definitive stem cell markers is necessary to determine the exact phenotype of cells within the intimal hyperplasia lesion.
Excluding the bone marrow-derived cells, there are three theoretical sources of smooth muscle cells within the venous intimal hyperplasia lesion: the vein graft, the adjacent artery, and the adventitia. Using a β-galactosidase reporter model, Hu et al8 demonstrated that 60% of the smooth muscle cells within neointima are derived from the donor vein. More recently, cells derived from the adventitia have been shown to be present in the neointima lesion of veins.43 The relative contribution and role of each source of smooth muscle cells remains to be determined.
These data for the first time demonstrate that bone marrow-derived cells differentiate into smooth muscle cells as well as endothelial cells within the venous intimal hyperplasia lesion. We purposely provided high-resolution confocal pictures since previous studies, performed in the injured artery model,5,6 have been criticized because of lack of high-resolution confocal analysis.15 Our finding that bone marrow-derived cells are able to differentiate into both smooth muscle cells and endothelial cells in the venous intimal hyperplasia lesion may explain some important clinical and therapeutic findings. The failure rate of vein grafts is known to be increased in patients with renal failure.44,45 Interestingly, Westerweel46 reported that there is an imbalance between progenitor endothelial cell and smooth muscle cells in end-stage renal disease patients, perhaps explaining the low 2-year patency rate of 25% in hemodialysis accesses.4 Rotmans et al47 demonstrated that cell seeding with anti-CD34 stimulated intimal hyperplasia in a porcine arteriovenous model, perhaps due to increasing the rate of CD34 differentiation into smooth muscle cells.
One limitation of this study is that it does not rule out the possibility of cell fusion. However, from a therapeutic standpoint, it may not make a difference whether the genetic material is delivered to the venous neointima by fusion or by cell differentiation. Importantly, bone marrow-derived cells are available with a minimally invasive procedure, cell apheresis, and may be an effective vehicle to deliver a gene product without the use of genetic vectors. An alternative may be a pretreatment of progenitor cells to force differentiation along the endothelial pathway.
In summary, we demonstrate that smooth muscle cells and CD45-positive cells are the predominant proliferating cells at early time points after surgery and are also the cell types with the highest proliferating activity. More interestingly, we used dual immunohistochemistry stain and laser capture microdissection and conclusively demonstrated that smooth muscle cells (and endothelial cells), at least partly, originate from bone marrow-derived cells at up to 16 weeks after grafting in the venous intimal hyperplasia lesion. This suggests that bone marrow-derived cells maybe the critical proliferating cell in the neointima and skewing the differentiation toward the endothelial lineage or using them to deliver a gene product may provide a novel strategy for the treatment of intimal hyperplasia.
Acknowledgments
We thank Marda Jorgensen for the technical assistance with immunohistochemistry and in situ PCR. We thank the Imaging Core at the North Florida/South Georgia Veterans Administration Health System for providing confocal microscopy and related facility.
Footnotes
Address reprint requests to Dr. Mark S. Segal, P. O. Box 100224, Gainesville, FL 32610. E-mail: segalms@medicine.ufl.edu.
See related Commentary on page 566
Supported by Grant-in-aid from the American Heart Association 0355334B (to M.S.S.) and Gatorade Research funds.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- American Heart Association Dallas, Texas: Heart Disease and Stroke Statistics-2006 Update. 2006 [Google Scholar]
- Feinglass J, Morasch M, McCarthy WJ. Measures of success and health-related quality of life in lower-extremity vascular surgery. Annu Rev Med. 2000;51:101–113. doi: 10.1146/annurev.med.51.1.101. [DOI] [PubMed] [Google Scholar]
- Hakim R, Himmelfarb J. Hemodialysis access failure: a call to action. Kidney Int. 1998;54:1029–1040. doi: 10.1046/j.1523-1755.1998.00122.x. [DOI] [PubMed] [Google Scholar]
- Roy-Chaudhury P, Kelly BS, Miller MA, Reaves A, Armstrong J, Nanayakkara N, Heffelfinger SC. Venous neointimal hyperplasia in polytetrafluoroethylene dialysis grafts. Kidney Int. 2001;59:2325–2334. doi: 10.1046/j.1523-1755.2001.00750.x. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Sugiyama S, Aikawa M, Fukumoto Y, Rabkin E, Libby P, Mitchell RN. Host bone-marrow cells are a source of donor intimal smooth-muscle-like cells in murine aortic transplant arteriopathy. Nat Med. 2001;7:738–741. doi: 10.1038/89121. [DOI] [PubMed] [Google Scholar]
- Sata M, Saiura A, Kunisato A, Tojo A, Okada S, Tokuhisa T, Hirai H, Makuuchi M, Hirata Y, Nagai R. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med. 2002;8:403–409. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- Sahara M, Sata M, Matsuzaki Y, Tanaka K, Morita T, Hirata Y, Okano H, Nagai R. Comparison of various bone marrow fractions in the ability to participate in vascular remodeling after mechanical injury. Stem Cells. 2005;23:874–878. doi: 10.1634/stemcells.2005-0012. [DOI] [PubMed] [Google Scholar]
- Hu Y, Mayr M, Metzler B, Erdel M, Davison F, Xu Q. Both donor and recipient origins of smooth muscle cells in vein graft atherosclerotic lesions. Circ Res. 2002;91:e13–20. doi: 10.1161/01.res.0000037090.34760.ee. [DOI] [PubMed] [Google Scholar]
- Zhang L, Freedman NJ, Brian L, Peppel K. Graft-extrinsic cells predominate in vein graft arterialization. Arterioscler Thromb Vasc Biol. 2004;24:470–476. doi: 10.1161/01.ATV.0000116865.98067.31. [DOI] [PubMed] [Google Scholar]
- Castier Y, Lehoux S, Hu Y, Foteinos G, Tedgui A, Xu Q. Characterization of neointima lesions associated with arteriovenous fistulas in a mouse model. Kidney Int. 2006;70:315–320. doi: 10.1038/sj.ki.5001569. [DOI] [PubMed] [Google Scholar]
- Grant MB, May WS, Caballero S, Brown GA, Guthrie SM, Mames RN, Byrne BJ, Vaught T, Spoerri PE, Peck AB, Scott EW. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med. 2002;8:607–612. doi: 10.1038/nm0602-607. [DOI] [PubMed] [Google Scholar]
- Diao Y, Xue J, Segal MS. A novel mouse model of autologous venous graft intimal hyperplasia. J Surg Res. 2005;126:106–113. doi: 10.1016/j.jss.2005.01.025. [DOI] [PubMed] [Google Scholar]
- Shi C, Patel A, Zhang D, Wang H, Carmeliet P, Reed GL, Lee ME, Haber E, Sibinga NE. Plasminogen is not required for neointima formation in a mouse model of vein graft stenosis. Circ Res. 1999;84:883–890. doi: 10.1161/01.res.84.8.883. [DOI] [PubMed] [Google Scholar]
- Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation. 1998;97:916–931. doi: 10.1161/01.cir.97.9.916. [DOI] [PubMed] [Google Scholar]
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- Miano JM, Cserjesi P, Ligon KL, Periasamy M, Olson EN. Smooth muscle myosin heavy chain exclusively marks the smooth muscle lineage during mouse embryogenesis. Circ Res. 1994;75:803–812. doi: 10.1161/01.res.75.5.803. [DOI] [PubMed] [Google Scholar]
- Kolega J. Cytoplasmic dynamics of myosin IIA and IIB: spatial ‘sorting’ of isoforms in locomoting cells. J Cell Sci. 1998;111:2085–2095. doi: 10.1242/jcs.111.15.2085. [DOI] [PubMed] [Google Scholar]
- Cornacchia F, Fornoni A, Plati AR, Thomas A, Wang Y, Inverardi L, Striker LJ, Striker GE. Glomerulosclerosis is transmitted by bone marrow-derived mesangial cell progenitors. J Clin Invest. 2001;108:1649–1656. doi: 10.1172/JCI12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol. 2001;159:1009–1020. doi: 10.1016/S0002-9440(10)61776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartore S, Chiavegato A, Faggin E, Franch R, Puato M, Ausoni S, Pauletto P. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: from innocent bystander to active participant. Circ Res. 2001;89:1111–1121. doi: 10.1161/hh2401.100844. [DOI] [PubMed] [Google Scholar]
- Basson CT, Kocher O, Basson MD, Asis A, Madri JA. Differential modulation of vascular cell integrin and extracellular matrix expression in vitro by TGF-beta 1 correlates with reciprocal effects on cell migration. J Cell Physiol. 1992;153:118–128. doi: 10.1002/jcp.1041530116. [DOI] [PubMed] [Google Scholar]
- Cintorino M, Bellizzi de Marco E, Leoncini P, Tripodi SA, Xu LJ, Sappino AP, Schmitt-Graff A, Gabbiani G. Expression of alpha-smooth-muscle actin in stromal cells of the uterine cervix during epithelial neoplastic changes. Int J Cancer. 1991;47:843–846. doi: 10.1002/ijc.2910470609. [DOI] [PubMed] [Google Scholar]
- Takamiya M, Okigaki M, Jin D, Takai S, Nozawa Y, Adachi Y, Urao N, Tateishi K, Nomura T, Zen K, Ashihara E, Miyazaki M, Tatsumi T, Takahashi T, Matsubara H. Granulocyte colony-stimulating factor-mobilized circulating c-Kit+/Flk-1+ progenitor cells regenerate endothelium and inhibit neointimal hyperplasia after vascular injury. Arterioscler Thromb Vasc Biol. 2006;26:751–757. doi: 10.1161/01.ATV.0000205607.98538.9a. [DOI] [PubMed] [Google Scholar]
- Hillebrands JL, Klatter FA, van Dijk WD, Rozing J. Bone marrow does not contribute substantially to endothelial-cell replacement in transplant arteriosclerosis. Nat Med. 2002;8:194–195. doi: 10.1038/nm0302-194. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao Yanpeng, Grant Maria, Guthrie Steve, Scott Edward, Segal Mark S. Role of circulating progenitor in the development of venous intimal hyperplasia (abstract). J Am Soc Nephrol. 2002;13:58A–59A. [Google Scholar]
- Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Zohlnhofer D, Klein CA, Richter T, Brandl R, Murr A, Nuhrenberg T, Schomig A, Baeuerle PA, Neumann FJ. Gene expression profiling of human stent-induced neointima by cDNA array analysis of microscopic specimens retrieved by helix cutter atherectomy: detection of FK506-binding protein 12 upregulation. Circulation. 2001;103:1396–1402. doi: 10.1161/01.cir.103.10.1396. [DOI] [PubMed] [Google Scholar]
- Zou Y, Dietrich H, Hu Y, Metzler B, Wick G, Xu Q. Mouse model of venous bypass graft arteriosclerosis. Am J Pathol. 1998;153:1301–1310. doi: 10.1016/S0002-9440(10)65675-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzon JF, Weile C, Sondergaard CS, Hindkjaer J, Kassem M, Falk E. Smooth muscle cells in atherosclerosis originate from the local vessel wall and not circulating progenitor cells in ApoE knockout mice. Arterioscler Thromb Vasc Biol. 2006;26:2696–2702. doi: 10.1161/01.ATV.0000247243.48542.9d. [DOI] [PubMed] [Google Scholar]
- Lie JT, Lawrie GM, Morris GC. Aortocoronary bypass saphenous vein graft atherosclerosis anatomic study of 99 vein grafts from normal and hyperlipoproteinemic patients up to 75 months postoperatively. Am J Cardiol. 1977;40:906–914. doi: 10.1016/0002-9149(77)90041-8. [DOI] [PubMed] [Google Scholar]
- Neitzel GF, Barboriak JJ, Pintar K, Qureshi I. Atherosclerosis in aortocoronary bypass grafts morphologic study and risk factor analysis 6 to 12 years after surgery. Arteriosclerosis. 1986;6:594–600. doi: 10.1161/01.atv.6.6.594. [DOI] [PubMed] [Google Scholar]
- Kalan JM, Roberts WC. Morphologic findings in saphenous veins used as coronary arterial bypass conduits for longer than 1 year necropsy analyses of 53 patients, 123 saphenous veins, and 1865 five-millimeter segments of veins. Am Heart J. 1990;119:1164–1184. doi: 10.1016/s0002-8703(05)80249-2. [DOI] [PubMed] [Google Scholar]
- Han CL, Campbell GR, Campbell JH. Circulating bone marrow cells can contribute to neointimal formation. J Vasc Res. 2001;38:113–119. doi: 10.1159/000051038. [DOI] [PubMed] [Google Scholar]
- Hoofnagle MH, Thomas JA, Wamhoff BR, Owens GK. Origin of neointimal smooth muscle: we’ve come full circle. Arterioscler Thromb Vasc Biol. 2006;26:2579–2581. doi: 10.1161/01.ATV.0000249623.79871.bc. [DOI] [PubMed] [Google Scholar]
- Mayr M, Li C, Zou Y, Huemer U, Hu Y, Xu Q. Biomechanical stress-induced apoptosis in vein grafts involves p38 mitogen-activated protein kinases. FASEB J. 2000;14:261–270. doi: 10.1096/fasebj.14.2.261. [DOI] [PubMed] [Google Scholar]
- Kwei S, Stavrakis G, Takahas M, Taylor G, Folkman MJ, Gimbrone MA, Jr, Garcia-Cardena G. Early adaptive responses of the vascular wall during venous arterialization in mice. Am J Pathol. 2004;164:81–89. doi: 10.1016/S0002-9440(10)63099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Sata M, Hirata Y, Nagai R. Diverse contribution of bone marrow cells to neointimal hyperplasia after mechanical vascular injuries. Circ Res. 2003;93:783–790. doi: 10.1161/01.RES.0000096651.13001.B4. [DOI] [PubMed] [Google Scholar]
- Caplice NM, Bunch TJ, Stalboerger PG, Wang S, Simper D, Miller DV, Russell SJ, Litzow MR, Edwards WD. Smooth muscle cells in human coronary atherosclerosis can originate from cells administered at marrow transplantation. Proc Natl Acad Sci USA. 2003;100:4754–4759. doi: 10.1073/pnas.0730743100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CH, Verma S, Hsieh IC, Hung A, Cheng TT, Wang SY, Liu YC, Stanford WL, Weisel RD, Li RK, Cherng WJ. Stem cell factor attenuates vascular smooth muscle apoptosis and increases intimal hyperplasia after vascular injury. Arterioscler Thromb Vasc Biol. 2007;27:540–547. doi: 10.1161/01.ATV.0000257148.01384.7d. [DOI] [PubMed] [Google Scholar]
- Zernecke A, Schober A, Bot I, von Hundelshausen P, Liehn EA, Möpps B, Mericskay M, Gierschik P, Biessen EA, Weber C. SDF-1alpha/CXCR4 axis is instrumental in neointimal hyperplasia and recruitment of smooth muscle progenitor cells. Circ Res. 2005;96:784–791. doi: 10.1161/01.RES.0000162100.52009.38. [DOI] [PubMed] [Google Scholar]
- Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, Metzler B, Xu Q. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest. 2004;113:1258–1265. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellenius GA, Mukamal KJ, Winkelmayer WC, Mittleman MA. Renal dysfunction increases the risk of saphenous vein graft occlusion: Results from the Post-CABG trial. Atherosclerosis. 2006;193:414–420. doi: 10.1016/j.atherosclerosis.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Albers M, Romiti M, De Luccia N, Brochado-Neto FC, Nishimoto I, Pereira CA. An updated meta-analysis of infrainguinal arterial reconstruction in patients with end-stage renal disease. J Vasc Surg. 2007;45:536–542. doi: 10.1016/j.jvs.2006.11.036. [DOI] [PubMed] [Google Scholar]
- Westerweel PE, Hoefer IE, Blankestijn PJ, de Bree P, Groeneveld D, van Oostrom O, Braam B, Koomans HA, Verhaar MC. End-stage renal disease causes an imbalance between endothelial and smooth muscle progenitor cells. Am J Physiol Renal Physiol. 2007;292:F1132–F1140. doi: 10.1152/ajprenal.00163.2006. [DOI] [PubMed] [Google Scholar]
- Rotmans JI, Heyligers JM, Verhagen HJ, Velema E, Nagtegaal MM, de Kleijn DP, de Groot FG, Stroes ES, Pasterkamp G. In vivo cell seeding with anti-CD34 antibodies successfully accelerates endothelialization but stimulates intimal hyperplasia in porcine arteriovenous expanded polytetrafluoroethylene grafts. Circulation. 2005;112:12–18. doi: 10.1161/CIRCULATIONAHA.104.504407. [DOI] [PubMed] [Google Scholar]