Abstract
We hypothesized that human ocular surface squamous neoplasia (OSSN) may result from the continuous growth stimulation of corneal epithelial progenitor cells. In the present study, we analyzed the effects of excess fibroblast growth factor-7 (FGF-7) on both the proliferation and differentiation of corneal epithelium in a novel Krt12-rtTA/tet-O-FGF-7 double transgenic mouse model in which cornea-specific FGF-7 overexpression is achieved by doxycycline (Dox) treatment. When such adult mice were exposed to Dox, they exhibited epithelial hyperplasia with increases in phospho-extracellular signal-regulated kinase 1/2-, nuclear β-catenin-, and 5-bromo-2′-deoxyuridine-labeled cells and altered keratin (K) 14 (K14) expression pattern, a normal K12 expression pattern, and the normal absence of K10. Hyperplasia of the adult cornea was fully reversible 2 weeks after the removal of Dox from chow. In contrast, double transgenic embryos that were exposed to Dox from embryonic day 0.5 to postnatal day 21 developed papillomatous tumors in the cornea, resembling human OSSN, and ectopic gland-like structures in the limbus, accompanied by the down-regulation of K12 and the up-regulation of K14, Pax6, and p63. These epithelial anomalies observed in young experimental mice were not fully resolved after the termination of Dox induction. Taken together, Krt12-rtTA/tet-O-FGF-7 mice may be a suitable animal model for the study of the molecular and cellular mechanisms of human OSSN.
Ocular surface squamous neoplasia (OSSN) is rare, but it is the most common ocular surface precancerous and cancerous lesion previously known by various names, such as conjunctival intraepithelial neoplasia, corneal intraepithelial neoplasia, or both together.1 Clinically, OSSN manifests in different grades ranging from simple dysplasia to squamous cell carcinoma.1 Because of the high incidence of OSSN in the limbal area, where the corneal epithelial stem cells are located, the limbal transition zone/stem cell theory has been proposed for the development of corneal intraepithelial neoplasia by Lee and Hirst.2 Tseng et al3 have suggested that the slow cycling limbal stem cells may become hyperproliferative by stimulations, such as alterations in this anatomical site influenced by other factors, which can cause abnormal maturation of the conjunctival and corneal epithelium and lead to the formation of corneal intraepithelial neoplasia. Nevertheless, the etiology and pathogenesis of corneal intraepithelial neoplasia and ocular surface carcinoma remain elusive, because there is no appropriate animal model available to study the molecular and cellular mechanisms of this disease.
Fibroblast growth factor-7 (FGF-7), a potent mitogen, enhances epithelial cell proliferation in various organs.4,5,6 The precise spatio-temporal expression of FGF-7 and the related polypeptide FGF-10 is important for governing ocular surface morphogenesis. FGF-7 and FGF-10 are secreted by mesenchymal cells, which bind with high affinity to the same FGF receptor 2 (FGFR2-IIIb) isoform expressed mainly by the epithelium.7 In the cornea, expression of FGF-7 and its cognate receptor FGFR2-IIIb is higher in limbal stroma and epithelium, respectively, than in the central cornea, implicating that FGF-7 may promote limbal stem cell proliferation and participate in modulation of corneal epithelium renewal and homeostasis.8,9 It has been reported that overexpression of FGF-7 or FGF-10 driven by αA-crystalline promoter, which is activated at mouse E11.5 before corneal epithelial differentiation, resulted in the suppression of cornea-type epithelial differentiation and the induction of ectopic lacrimal gland formation in the corneas of the transgenic mice.10,11,12 These results indicate that excess FGF-7 and FGF-10 are capable of altering epithelial fate decision during embryonic development. However, it remains unclear whether they are capable of exerting such an influence at a later stage when epithelial cells have committed to corneal epithelial differentiation. To answer this question, we developed a Krt12-rtTA/tet-O-FGF-7 double transgenic mouse line in which overexpression of FGF-7 in cornea is achieved by administering experimental mice doxycycline (Dox).
In the present study, we show that excess FGF-7 induced by Dox in the Krt12-rtTA/tet-O-FGF-7 double transgenic mice beginning from embryonic day 0.5 to postnatal day 21 and beyond results in squamous cell carcinoma of the cornea, resembling OSSN in human. However, excess FGF-7 induced by Dox for up to 2 months in adult Krt12-rtTA/tet-O-FGF-7 double transgenic mice exhibits only corneal epithelial hyperplasia without tumor formation. The clinical manifestations of excess FGF-7 showed age-dependent lesions in the experimental mice.
Materials and Methods
Animals
Experimental animals were housed under pathogen-free conditions in accordance with institutional guidelines. Animal care and use conformed to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. All animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Cincinnati.
Generation and Genotyping for Krt12-rtTA/tet-O-FGF-7 Double Transgenic Mice
Krt12-rtTA knock-in mice13 were crossed to tet-O-FGF-7 mice14 for the preparation of Krt12-rtTA/tetO-FGF7 double transgenic mice, which overexpress FGF-7 by corneal epithelium on induction of Dox.
Transgenic mice were identified by genotyping of PCR using oligonucleotide primers specific for each transgene as follows. Krt12-rtTA knock-in allele was identified by PCR with the following oligonucleotide primers: forward Krt12-1 (primer 1), 5′-GTGTGTGCCTGCCATCCCATC-3′; Neo 781-803 (primer 2), 5′-CGCCTTCTTGACGAGTTCTTCTG-3′ for knock-in allele; and Krt12-1 primer and reverse Krt12-2 (primer 3), 5′-GAT CTG GGG TTG CAA TGA AGA C-3′ for wild-type allele. The primers for detecting tet-O-FGF-7 transgene in the transgenic mice were as follows: forward primer in CMV minimum promoter, 5′-GTCAGATCGCCTGGAGACGCC-3′; reverse primer in hFGF-7, 5′-AATTAGTTCTTTGAAGTTACAATCT-3′. PCR for Krt12-rtTA was performed by denaturation at 94°C for 5 minutes, 35 cycles of amplification (30 seconds at 94°C, 30 seconds at 64°C, and 45 seconds at 72°C), followed by a 5-minute final extension step at 72°C. PCR products were analyzed by agarose gel electrophoresis. Detection of tet-O-FGF-7 was identical except the annealing temperature was 54°C.
Administration of Dox
To induce FGF-7 expression, mice were injected once intraperitoneally with Dox (80 μg/g body weight; Clontech Laboratories, Mountain View, CA) dissolved in PBS (pH 7.4) at a concentration of 10 mg/ml15 and fed Dox-chow (1 g/kg chow; Bioserv, Frenchtown, NJ). Control animals were fed regular chow.
Immunohistological and Western Blotting Analysis
The following antibodies were used: rabbit anti-K12 antibody16; rabbit anti-keratocan antibody17; rabbit anti-VP-16 antibodies to detect rtTA (Clontech Laboratories); rabbit anti-hFGF7 (GeneTex, Inc., San Antonio, TX); rabbit anti-K14 and rabbit anti-Pax6 (Covance, Princeton, NJ); rabbit anti-phosphoERK1/2 (Neuromics, Inc., Edina, MN), monoclonal antibody 4A4 anti-p63 and monoclonal antibody anti-β-catenin (BD Biosciences); anti-E-cadherin (Chemicon, Inc., Temecula, CA) and 5-bromo-2′-deoxyuridine (BrdU) monoclonal antibody (Lab Vision, Corp., Fremont, CA). Secondary Alexa488, Alexa555, labeled antibodies were obtained from Molecular Probes, Inc. (Eugene, OR). Secondary horseradish peroxidase-conjugated antibodies were obtained from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA).
For Western blotting analysis, adult Krt12-rtTA/tet-O-FGF-7 mice (2 months old) were administrated with or without Dox for 2 weeks, and the neonatal Krt12-rtTA/tet-O-FGF-7 mice were administrated with or without Dox from embryonic day (E) 0.5 to postnatal day (P) 3. Eyes were then enucleated and incubated with Dispase II (0.5%) in PBS at 37°C for 2 hours to isolate corneal epithelial sheets. The corneal epithelial sheets were pooled and incubated with 1× cell lysis buffer containing 50 mmol/L Tris-HCl, pH 7.4, 1% Nonidet P-40, 0.25% sodium deoxycholate, 1 mmol/L EDTA, 1 mmol/L phenylmethylsulfonyl fluoride, and protease inhibitor cocktail (Sigma, St. Louis, MO). Twenty micrograms of the total protein extract were separated on a SDS-PAGE and Western blotting analysis with rabbit anti-VP-16 antibodies and rabbit anti-hFGF7 followed by a goat anti-rabbit IgG (H+L)-alkaline phosphatase. Immune reactivity was visualized with Western blue (Promega, Madison, WI).
Immunohistological analysis was performed to determine the consequences of excess FGF-7 in corneas of Krt12-rtTA/tet-O-FGF-7 mice and control single transgenic littermates. Excised eyes were fixed in 4% paraformaldehyde in 0.1 mol/L phosphate buffer (pH 7.4) at 4°C overnight and paraffin embedded. Five-micrometer sections were then mounted on Super Frost slides (Fisher Scientific, Pittsburgh, PA). The sections were de-paraffinized and hydrated in a graded ethanol series (95% and 75% ethanol and PBS for 3 minutes each). All incubations were performed at room temperature. Sections for immunofluorescence analysis were mounted (SlowFade Light Antifade kit; Molecular Probes) in the presence of 4-,6-diamidino-2-phenylindole, observed with an epifluorescence microscope (Axioscop2; Carl Zeiss, München-Hallbergmoos, Germany) and were photographed with a digital camera system (Axiocam, Carl Zeiss). The same fields were photographed under Nomarski optics, and the Nomarski and immunofluorescence images were overlaid.
Results
Induction of Adult Krt12-rtTA/tet-O-FGF-7 Double Transgenic Mice by Dox
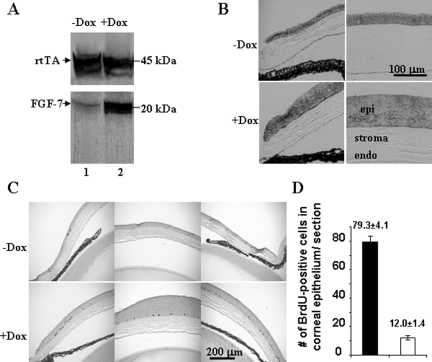
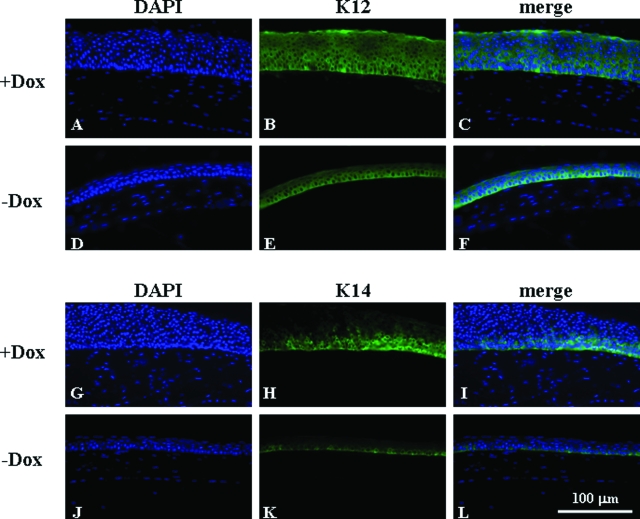
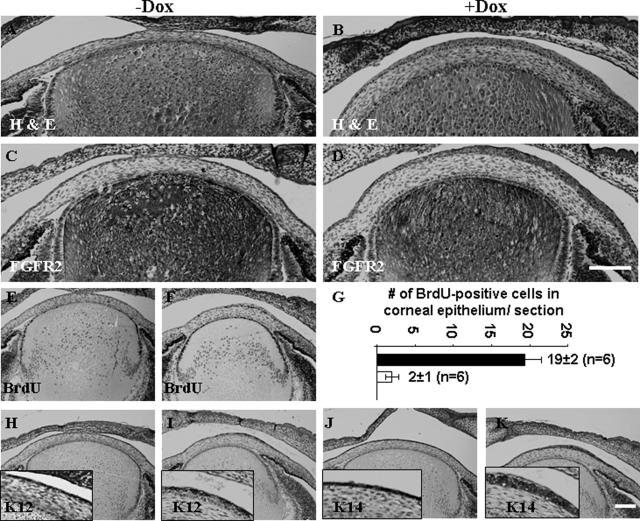
Transgenic mice bearing a single transgene, either Krt12-rtTA or tet-O-FGF-7, and noninduced Krt12-rtTA/tet-O-FGF-7 double transgenic mice had no anomaly in cornea or other tissues and survived normally in the vivarium.13,14 Adult Krt12-rtTA/tet-O-FGF-7 double transgenic and tet-O-FGF-7 single transgenic mice were treated with or without Dox for 2 weeks. Experimental animals were then sacrificed, and enucleated eyes were subjected to biochemical and histological analyses. Western blotting analysis of corneal epithelial extract showed compatible rtTA expression levels regardless of Dox treatment, but a large increase in FGF-7 only in Dox-treated Krt12-rtTA/tet-O-FGF-7 double transgenic mice (Figure 1A). None of Krt12-rtTA and tet-O-FGF-7 single transgenic mice fed Dox, showed elevated FGF-7 in the cornea (data not shown). The expression of FGFR2 receptor, which binds FGF-7 and FGF-10, was detected in corneal epithelium but did not seem to increase in response to FGF-7 overexpression (Figure 1B). The corneas remain transparent in all of the adult Krt12-rtTA/tet-O-FGF-7 mice treated with Dox (data not shown), however, histological examination of the enucleated eyes exhibited marked epithelial hyperplasia in the cornea (Figure 1, B and C). Corneal epithelium contained five to seven cell layers in untreated mouse but this increased to 15 to 18 cell layers in those treated with Dox (Figures 1B and 2). No corneal abnormality was observed in Krt12-rtTA or tet-O-FGF-7 single transgenic mice treated with Dox (data not shown). Incorporation of BrdU into DNA was used to estimate cell proliferation in the transgenic mice. As shown in Figure 1, C and D, Dox treatment caused an approximately 6.5-fold increase in BrdU incorporation by basal epithelial cells in the cornea and by some suprabasal cells in the peripheral cornea of Krt12-rtTA/tet-O-FGF-7 double transgenic mice. Because FGF/FGFR signaling transduction can phosphorylate and activate cytoplasmic ERK, we therefore examined the phospo-ERK by immunohistochmistry. As expected, nuclear localization of phospho-pERK1/2 was more prominent in Dox-induced compared with noninduced Krt12-rtTA/tet-O-FGF-7 double transgenic mice (Supplemental Figure 1A, see http://ajp.amjpathol.org). Likewise, β-catenin expression level was increased and was found in both membrane and nucleus (Supplemental Figure 1B, see http://ajp.amjpathol.org). In comparison, β-catenin was mostly found in the cytoplasm of control un-induced double transgenic Krt12-rtTA/tet-O-FGF-7 mice. The K12 expression pattern remained unchanged in all cell layers (basal through superficial layer) whether treated (Figure 2, A–C) or not treated by Dox (Figure 2, D–F). In contrast, K14 expression, normally restricted to the basal cell layer of stratified epithelium, was altered and detectable in nearly all cell layers in Dox-treated Krt12-rtTA/tet-O-FGF-7 double transgenic mice (Figure 2, compare G–I with J–L). Moreover, use of anti-K10 antibodies failed to detect the presence of this epidermal epithelium marker in the hyperplastic corneal epithelium, suggesting that no epithelial metaplasia was found in excess of FGF-7 (data not shown).
Figure 1.
Dox-induced FGF-7 overexpression results in hyperplasia and increased BrdU uptake in corneal epithelium of Krt12-rtTA/tet-O-FGF-7 mice. A: Western blotting analyses of 20 μg of total protein extract isolated from corneal epithelial sheets showed that rtTA expression was comparable regardless of Dox treatment, but hFGF-7 was dramatically up-regulated in the corneal epithelium of Dox-treated (lane 2) compared with nontreated mice (lane 1). Immunohistochemical staining of corneal sections of the Krt12-rtTA/tet-O-FGF-7 mice treated with or without Dox with anti-FGFR2 (B) and anti-BrdU (C), respectively. FGFR2 was expressed mainly in the corneal epithelium but not in stroma. Hyperplastic epithelium retained a high level of FGFR2 expression (B), suggesting excess FGF-7 continuously triggered an autocrine/paracrine action via FGFR2 signaling within Dox-treated Krt12-rtTA/tet-O-FGF-7 mice. As a result, BrdU uptake in central cornea, and more prominently, at the limbus was significantly increased in the Dox-treated compared with the nontreated Krt12-rtTA/tet-O-FGF-7 mice (C). A histogram shows that there is a 6.5-fold increase of BrdU uptake per corneal sections in the Dox-treated compared with the nontreated Krt12-rtTA/tet-O-FGF-7 mice (D).
Figure 2.
Dox-induced FGF-7 overexpression changes K14 but not K12 expression pattern. Immunohistofluorescent staining of corneas of the Krt12-rtTA/tet-O-FGF-7 mice administered with Dox chow (A–C and G–I) or Dox-free chow (D–F and J–L) with anti-K12 (A–F) or anti-K14 (G–L). K12 expression was detected in the full thickness of hyperplastic corneal epithelium in Dox-treated and of normal corneal epithelium in nontreated Krt12-rtTA/tet-O-FGF-7 mice. In contrast, K14 expression, which was only detected in the basal epithelium of the nontreated mice, was detected not only in the basal epithelium but also in suprabasal and superficial layers of the Dox-treated Krt12-rtTA/tet-O-FGF-7 mice.
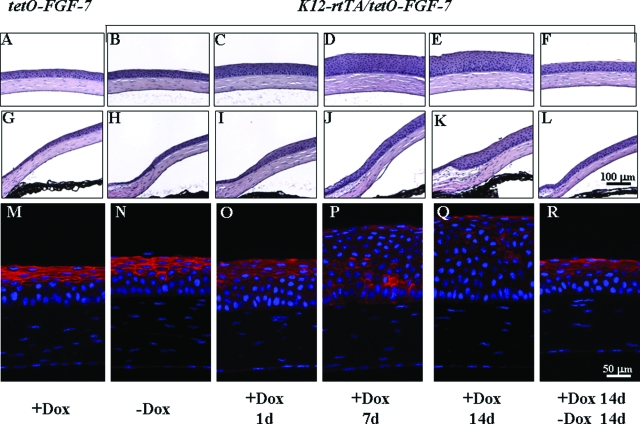
Kinetics of Hyperplasia and Phenotype Reversion
The effects of excess FGF-7 on corneal morphology in adult (older than 2 months) Krt12-rtTA/tet-O-FGF-7 double transgenic mice fed with Dox for various periods of time was assessed by histology and immunofluorescence staining (Figure 3). Without Dox induction (Figure 3, B, H, and N), corneal epithelial morphology and E-cadherin expression pattern of double transgenic mice were normal and indistinguishable from that of control tet-O-FGF-7 single transgenic mouse fed Dox for 14 days (Figure 3, A, G, and M). In contrast, corneal epithelium became hyperplasic after 24 hours Dox administration (Figure 3, C, I, and O) and increased to 15 to 18 cell layers at 7 days (Figure 3, D, J, and P) and 14 days (Figure 3, E, K, and Q), with altered E-cadherin expression pattern. Interestingly, 14 days after the termination of a 14-day Dox induction, the corneas of Krt12-rtTA/tet-O-FGF-7 double transgenic mice regained nearly normal corneal epithelial morphology and E-cadherin expression pattern (Figure 3, F, L, and R).
Figure 3.
Reversibility of epithelial hyperplasia induced by Dox in adult Krt12-rtTA/tet-O-FGF-7 mice. Histological H&E staining of central cornea (A–F) and limbal area (G–L) of tet-O-FGF-7 (A and G) mice and Krt12-rtTA/tet-O-FGF-7 (B–F and H–L) mice. Corneal epithelium of Krt12-rtTA/tet-O-FGF-7 without Dox consists of five to seven cell layers (B and H). However, Dox caused hyperplasia with 10 cell layers at day 1 (C and I) and 15 cell layers at days 7 to 14 (D, J, E, and K) exposure. Mice treated with Dox for 14 days followed by Dox-free diet for 14 days exhibited complete reversal of corneal hyperplasia (F and L). Note that Dox-treated tet-O-FGF-7 mice exhibited normal cornea morphology (A and G), suggesting that Dox had no adverse effect by itself. Immunofluorescent staining of E-cadherin expression pattern (red) in corneal epithelium (M–R). Sections were counterstained with 4-,6-diamidino-2-phenylindole.
Detrimental Consequences of Excess FGF-7 after Dox Induction in Utero
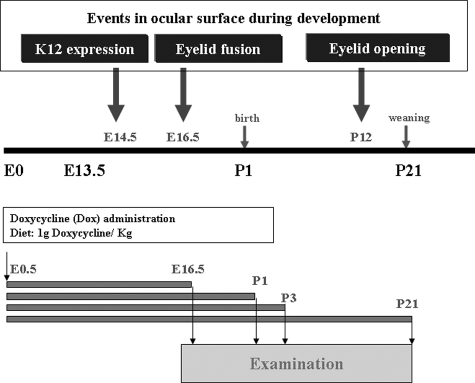
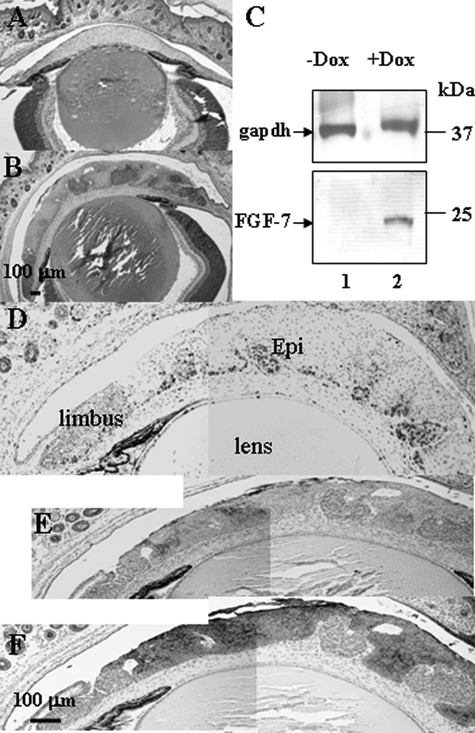
Because K12 gene expression commences at E14.5,18,19 systematic administration of Dox to pregnant Krt12-rtTA/tet-O-FGF-7 double transgenic females should lead to FGF-7 overexpression in the fetal corneal epithelium. To determine the effects of excess FGF-7 on corneas in utero, female Krt12-rtTA/tet-O-FGF-7 double transgenic mice were fed Dox from the time of conception (E0.5), and at least five embryos or pups were examined at various developmental stages: E16.5, P1, P3, and P21 as shown in Figure 4. Histological examination of the developing eyes from nontreated Krt12-rtTA/tet-O-FGF-7 double transgenic embryos at E16.5 showed that corneal epithelium consisted of two cell layers (Figure 5A), whereas Dox-treated embryos revealed marked hyperplasia (five to six layers) (Figure 5B). Immunohistochemical staining with anti-FGFR2 showed that nearly the entire corneal epithelium was FGFR2 positive (Figure 5C), and Dox-induced hyperplastic epithelium retains high expression of FGFR2 (Figure 5D), suggesting it continues to respond to the excess FGF-7. When BrdU labeling was compared between corneal sections in the Dox-treated (Figure 5F) and nontreated Krt12-rtTA/tet-O-FGF-7 mice (Figure 5E), quantitation of cell proliferation revealed that there was an approximately 9-fold increase of BrdU uptake (Figure 5G) in the Dox-treated mice. K14 keratin, which is normally expressed only in the basal epithelium, was detected in the entire hyperproliferative epithelium (Figure 5, J and K), but the corneal differentiation marker K12 was found only in the superficial layer (Figure 5, H and I).
Figure 4.
Diagram showing Dox induction in utero. Top panel shows the ocular surface developmental events, including K12 expression (at E14.5), eyelid fusion (E16.5), and eyelid reopening (P12). Bottom panel shows the Dox administration protocol.
Figure 5.
Dox-induced epithelial hyperplasia in the embryonic cornea. Histological examination of Krt12-rtTA/tet-O-FGF-7 embryos (E16.5) with (B, D, F, I, and K) or without (A, C, E, H, and J) exposure to Dox through pregnant mothers from E0.5. Dox treatment induced corneal epithelial hyperplasia (B). Immunohistochemical staining with anti-FGFR2 (C and D) showed that nearly the entire epithelium is FGFR-positive (C); likewise, hyperplastic epithelium retains high expression of FGFR2 (D). Hyperplastic epithelium responds to FGF-7 with increased BrdU uptake (F) compared with noninduced embryos (E). A histogram shows that there is a 9.1-fold increase of BrdU uptake per corneal sections in the Dox-treated (filled bar) compared with the nontreated (open bar) Krt12-rtTA/tet-O-FGF-7 mice (G). Immunohistochemical stainings with anti-K12 (H and I) and anti-K14 (J and K) were used to characterize the epithelial differentiation status. Nearly the entire hyperplastic epithelium is K14-positive (K), but only the superficial layer exhibits K12-positive staining (I). Scale bars = 100 μm.
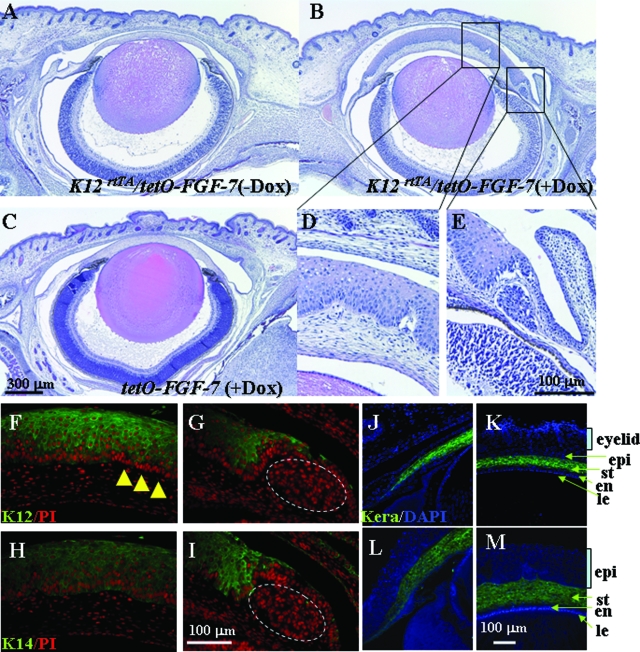
At P1, corneal epithelium of Krt12-rtTA/tet-O-FGF-7 double transgenic pups that had been exposed to Dox from E0.5 had around 20 cell layers, 10 times more than that of single tet-O-FGF-7 transgenic littermates and of age-matched noninduced Krt12-rtTA/tet-O-FGF-7 double transgenic pups (Figure 6). K12 was detected in the superficial layer but absent in the basal and suprabasal layers (Figure 6, F and G). However, K14 was expressed throughout the entire epithelial layer (Figure 6, H and I). It is of interest to note that K12- and K14-negative hyperproliferative nodules existed in the limbal basal region of the Dox-treated Krt12-rtTA/tet-O-FGF-7 double transgenic eyes as shown in Figure 6, G and I. This observation was extended in the postnatal day 3, Krt12-rtTA/tet-O-FGF-7 double transgenic pups exposed to Dox from E0.5 (Figure 7), in that K12-negative (Figure 7E) and K14-negative (Figure 7F) hyperproliferative nodules labeled by BrdU were present across the entire corneal epithelium (Figure 7D). Although hyperplasia is prominent in the epithelium, corneal and limbal stroma remained relatively unaffected by excess FGF-7 present in the epithelium, because the expression pattern of keratocan, a keratocyte-specific marker, was not altered (Figure 6, J–M).
Figure 6.
Dox-induced epithelial hyperplasia and ectopic gland in the neonatal cornea. Krt12-rtTA/tet-O-FGF-7 mouse exposed to Dox through pregnant mothers from E0.5 showed hyperproliferative epithelium (B) compared with age-matched noninduced Krt12-rtTA/tet-O-FGF-7 (A) or Dox-induced tet-O-FGF-7 mice (C). Interestingly, Dox-treated cKrt12-rtTA/tet-O-FGF-7 mouse exhibit ectopic gland induction in the limbal basal area (B, D, and E). Immunofluorescent staining (green color) was performed to detect K12 (F and G) and K14 expression (H and I) and counterstained with propidium iodide (PI, red). All layers of epithelium are K14 positive (H and I), which is a marker of basal cells. Although almost all basal cells express K14, many basal cells are K12 negative (F and G) in this cornea. Immunofluorescent staining was performed to detect keratocan (green) and counterstained with 4-,6-diamidino-2-phenylindole (blue) (J–M). The keratocan expression pattern did not change regardless of Dox treatment. epi, corneal epithelium; St, stroma; en, corneal endothelium; le, lens.
Figure 7.
Dox-induced epithelial hyperplasia and ectopic gland invasion in the neonatal cornea. Krt12-rtTA/tet-O-FGF-7 mouse exposed to Dox through pregnant mothers from E0.5 showed hyperproliferative epithelium at postnatal day 3 (B) compared with age-matched noninduced Krt12-rtTA/tet-O-FGF-7 mouse (A). C: Western blotting analyses of 20 μg of total protein extract isolated from corneal epithelial sheets showed that hFGF-7 was dramatically up-regulated in the corneal epithelium of Dox-treated (lane 2) compared with nontreated age-matched mice (lane 1). gapdh served as a reference of protein loading. At this stage, it was clear that the stromal-epithelial boundary was not preserved due to the corneal invasion by the ectopic glandular cell mass (see Figure 6), which was extremely hyperproliferative as revealed by strong BrdU immunoreactivity (D) and K12 (E) and K14 (F) negativity.
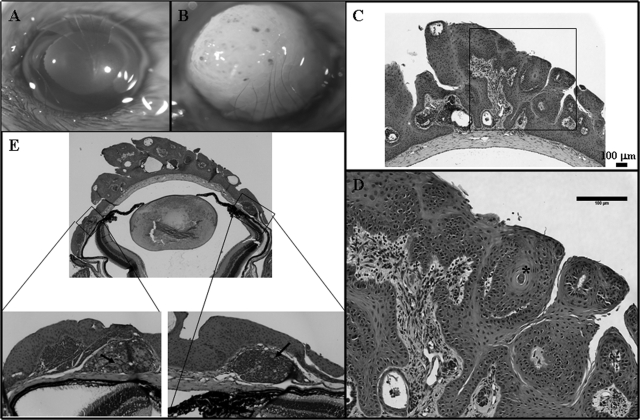
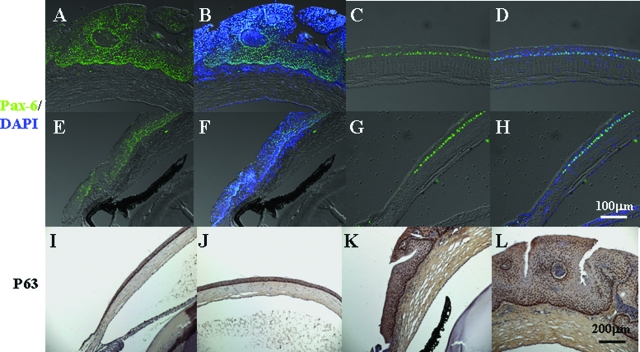
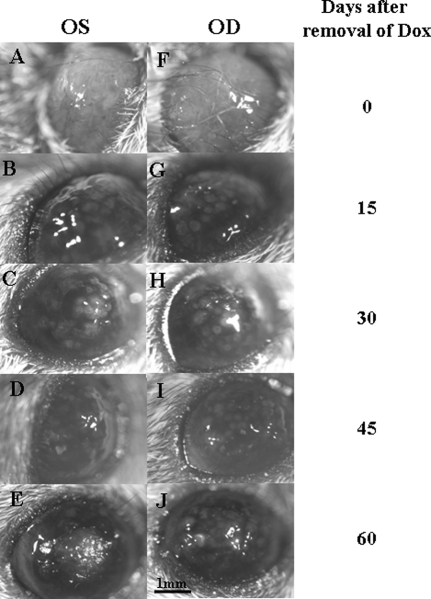
Krt12-rtTA/tet-O-FGF-7 double transgenic mice under continued Dox induction from E0.5 to P21 manifested thickened mulberry-like gelatinous lesions with gray intraepithelial plaques and tufts of vessels in the corneas, resembling human corneal intraepithelial neoplasia (Figure 8B). Histological examination revealed that excess FGF-7 induced a papilloma-like tumor with neovascularization and stromal invasions into the corneal epithelium (Figure 8, C and D). A differentiated glandular cell mass was apparent in the limbal basal region (Figure 8E, inset). Immunohistochemical analyses showed that the hyperplastic epithelial cells stained heavily for Pax-6 (Figure 9, A–D) in comparison with a single basal layer staining in noninduced corneas (Figure 9, E–H). Similarly, overexpression of ΔNp63 was observed in almost all epithelial cells (Figure 9, K and L), whereas in control un-induced double transgenic mice, ΔNp63 was observed only in the basal corneal epithelial cells (Figure 9, I and J). Unlike Dox-treated adult Krt12-rtTA/tet-O-FGF-7 mice in which the epithelial hyperplasia is reversible when Dox is removed, corneal tumors in Krt12-rtTA/tet-O-FGF-7 mice induced from E0.5 to P21 did not completely regress, and normal corneal morphology did not return even 60 days after Dox withdrawal (Figure 10).
Figure 8.
Dox-induced corneal intraepithelial and papillomatous neoplasia in young animals. Krt12-rtTA/tet-O-FGF-7 mice exposed to Dox through pregnant mothers from E0.5 exhibited corneal intraepithelial neoplasia at postnatal day 21 (B) compared with age-matched noninduced Krt12-rtTA/tet-O-FGF-7 mice (A). Papilloma-like epithelial lesion with mesenchymal invasion was found in the central cornea (C; magnified in D). In the limbal region, ectopic glandular cell mass (see Figure 6) becomes more differentiated with acinar-like morphology (E; arrows in inset).
Figure 9.
Corneal intraepithelial neoplasia induced by Dox expressed both Pax-6 and p63 progenitor markers. Krt12-rtTA/tet-O-FGF-7 mice exposed to Dox through pregnant mothers from E0.5 exhibited corneal intraepithelial neoplasia at postnatal day 21 (A, B, C, D, K, and L) compared with age-matched noninduced Krt12-rtTA/tet-O-FGF-7 (E–J). Immunofluorescent staining was performed to detect Pax-6 (green) and counterstained with 4-,6-diamidino-2-phenylindole (blue) (A–H). Immunohistochemical staining was performed to detect p63 (I–L). Both Pax-6 and p63 were detected throughout the entire hyperplastic epithelium in the Dox-induced Krt12-rtTA/tet-O-FGF-7 mice (A, B, C, D, K, and L), whereas they were only detected in the basal epithelial layer in the noninduced Krt12-rtTA/tet-O-FGF-7 (E–J).
Figure 10.
Reversibility of corneal intraepithelial neoplasia. Photographs of both left (OS) and right (OD) eyes were taken from Krt12-rtTA/tet-O-FGF-7 mice exposed to Dox from E0.5-P21 followed by Dox-free diet for 0 (A and F), 15 (B and G), 30 (C and H), 45 (D and I), and 60 (E and J) days.
Discussion
To examine the hypothesis that continuous growth stimulation may result in the alteration of corneal epithelial homeostasis that can lead to corneal pathology, such as OSSN, we have established cornea-specific and Dox-inducible overexpression of FGF-7 in transgenic mouse lines, namely, Kerapr-rtTA/tet-O-FGF-720 and Krt12-rtTA/tet-O-FGF-7 (this study). Unlike Dox-treated Krt12-rtTA/tet-O-FGF-7 mice, which exhibited severe corneal phenotypes, Kerapr-rtTA/tet-O-FGF-7 double transgenic mice exposure to Dox exhibited a subtle phenotypic change of epithelial hyperproliferation without significant corneal pathology.20 The distinct phenotype is most likely due to the different anatomical sites of FGF-7 overexpression in these two double transgenic mouse lines. Given that keratocan promoter/enhancer activates rtTA in keratocytes but not in epithelium, the subsequent Dox-induced FGF-7 overexpression is primarily present in the corneal stroma. However, the presence of extracellular matrix in stroma, and the basement membrane may preclude sufficient amounts of FGF-7 from reaching the epithelium. Alternatively, due to the avascular characteristic of the cornea, the concentration of doxycycline in corneal stroma may be insufficient to induce tet-O-FGF-7 transgene, and doxycycline derived from tears may be readily accessible to epithelial cells but not to stromal keratocytes. In contrast, Dox-induced overexpression of FGF-7 can quickly execute an autocrine/paracirne loop within corneal epithelium and trigger corneal epithelial hyperplasia and tumor formation in double transgenic Krt12-rtTA/tet-O-FGF-7 mice.
Our data indicate that FGF-7 overexpression by the corneal epithelium in Krt12-rtTA/tet-O-FGF-7 double transgenic mice does not produce adverse effects to other ocular tissues, such as conjunctiva, eyelid, lens, and retina, but only affects corneal epithelium. Using this model, we demonstrated that excess FGF-7 in corneal epithelium perturbed tissue homeostasis. This was manifested as corneal epithelium hyperplasia leading to increased epithelial thickness from 6 to 7 to more than 20 cell layers and increased number of cells labeled by BrdU incorporation. Furthermore, K14 expression was no longer limited to basal cell layer and extended to suprabasal and superficial cells (Figure 2), consistent with the altered K14 expression pattern in the pathogenesis of hyperproliferative stratified epithelium.21 In contrast, no perturbation of normal corneal type epithelial differentiation of K12 expression was noted in that full-thickness expression pattern of K12 was detected (Figure 2). There was no evidence of keratinization, eg, expression of epidermal epithelium K10 keratin, fillagrin, and formation of envelope (data not shown). Therefore, FGF-7 overexpression in the adult mature corneal epithelium only caused enhanced cell proliferation without perturbing cornea-type epithelium differentiation of keratin K12 expression. This result is in agreement with the data reported by Wilson et al22 that KGF (FGF-7) stimulates proliferation of cultured corneal epithelial cells at low density and in confluent islands of cells and does not affect expression of keratin K3 and migration of epithelial cells. Collectively, these results were consistent with the notion that FGF-7 is a mesenchyme-derived mitogen for epithelial cells,16,17 including the corneal epithelial cells. It has previously been reported by Rosner et al23 that the canonical Wnt pathway was involved in mammary tumorigenesis in transgenic mouse overexpressing FGF-7 driven by MMTV promoter. We observed that corneal epithelial hyperplasia was associated with up-regulation and nuclear translocation of β-catenin (Supplemental Figure 1, see http://ajp.amjpathol.org), suggesting that FGF-7 may trigger a mitogenic pathway mediated though β-catenin besides the well known MAP kinase/ERK pathway (Figure 1D).24 Because such corneal epithelial hyperplasia was not associated with any notable alteration of the underlying corneal stroma and could be thoroughly reversed in 14 days after the removal of Dox from the diet in adult Krt12-rtTA/tet-O-FGF-7 mice (Figure 3), we concluded that FGF-7 did not alter the plasticity of differentiated corneal transient amplifying cells nor indirectly affect the underlying corneal stroma.
In contrast, administration of Dox beginning at E0.5 and continued up to P21 produced a much more severe epithelial phenotype manifesting epithelial hyperplasia (Figures 4 and 5), epithelial intrastromal invasion (Figure 6), and formation of papillomatous tumor-like tissues in the cornea at P21 and beyond (Figure 7). Such altered epithelial and stromal phenotypes resembled the precancerous lesion known as corneal squamous papilloma that is thought to be derived from untreated OSSN. Human OSSN usually initiates from the limbus, where the corneal epithelium stem cells reside and then expands to the corneal and/or conjunctival surface.25 Interestingly, we also observed that Krt12-rtTA/tet-O-FGF-7 mice exposed to Dox from P2, P3, and P7 to P60 all developed corneal tumor at the peripheral corneal/limbal region (J.T.A. Meij et al, unpublished data), implicating that the Dox-inducible corneal epithelial tumor in Krt12-rtTA/tet-O-FGF-7 mice may be initiated from the limbus.
Up-regulation of FGF-7 has been reported to be associated with many human neoplastic tumors of epithelial cell origin26,27,28,29,30,31 and during multistage epidermal carcinogenesis in K14-human papilloma virus 16 transgenic mice.32 HPV is also one of the risk factors for corneal intraepithelial neoplasia.33 We, thus, suggest that up-regulation of FGF-7 signaling pathway(s) may be common in tumorigenesis derived from limbal stem cells that undergo oncogenic transformation by insults, such as long-term UVB exposure, infection of human papilloma virus and human immunodeficiency virus, and cigarette smoking, etc, which often exhibit the characteristic phenotypes of OSSN.34,35,36
The notion that limbal stem cells might be involved in the aforementioned Krt12-rtTA/tet-O-FGF-7 double transgenic mice was further supported by the findings in that the lesion of corneal epithelial neoplasia in the double transgenic mice fed Dox beginning at E0.5 was first noted in the limbus at P1∼P3 (Figures 4 and 5). The lesion then expanded into the corneal surface and beyond (Figures 5 and 6), and after removal of Dox from the diet, the diseased tissue failed to revert to a normal corneal morphology (Figure 9). Furthermore, the notion of limbal stem cell involvement in the pathogenic effects of excess FGF-7 on corneal epithelium was strengthened by the observations that such epithelial hyperplasia was characterized by an increased number of K12-negative cells in basal and suprabasal corneal epithelium (Figures 5 and 6). Thus it is very likely that excess FGF-7 led to the expansion of the K12-negative corneal epithelial progenitor cells residing in limbus, eg, limbal stem cells, and alteration of such progenitor cells may account for the papilloma-like tissue.37,38
It remains unknown whether such expansion of K12-negative cells resulted from de-differentiation of K12-positive cells (via an autocrine mechanism), activation of limbal stem cells (via a paracrine mechanism), or both. Because such an expansion of progenitor cells was not observed in corneas of adult mice even under a prolonged induction of Dox for more than 4 months (data not shown), it is very likely that K12-negative epithelial progenitor cells in embryonic corneas are more susceptible or responsive to stimulation than their adult counterparts and undergo oncogenic transformation by excess FGF-7. Further studies are needed to determine the intrinsic difference between embryonic progenitors and adult progenitors. For ectoderm-derived ocular surface progenitors, overexpression of FGF-7 and FGF-10 driven by lens-specific αA crystalline promoter began at E11.5 and led to ectopic lacrimal gland formation in the cornea after birth.10,11,12 In contrast, we noted that at P21 ectopic gland-like tissues formed in limbal region where stem cells reside (Figure 7) when overexpression of FGF-7 began at E14.5 in our Krt12-rtTA/tet-O-FGF-7 double transgenic mice fed Dox. These gland-like tissues maintained high expression levels of Pax6 and p63 (Figure 8), suggesting preservation of ocular surface lineage commitment under the influence of excess FGF-7. These findings also supported multipotent plasticity of ectodermal progenitor cells to different lineages at various stages of embryonic development. The presence of excess FGF-7 in the corneas of Krt12-rtTA/tet-O-FGF-7 mice by Dox induction begins at E14.5 at a time that the fates of cells derived from ocular surface ectoderm may have been determined. It is possible that FGF-7 triggers different signaling pathways in corneal epithelial cells of different ages (E16.5 or P60). Alternately, the differences in tissue environment between embryonic and adult corneas may account for the variations in FGF-7 effects on corneal morphogenesis. For example, Alvarado et al39 have previously demonstrated that the composition of the corneal epithelial basement membrane changed as the mouse matured and that this may contribute to altered functions of corneal epithelium basement membrane in an age-dependent manner. Nevertheless, it remains to be shown whether such an age-related change in the basement membrane of the corneal epithelium contributes to sieving of factors, such as growth factors and cytokines, and modulates epithelium/mesenchyme interactions.
Taken together, Krt12-rtTA/tet-O-FGF-7 mice may be an animal model for human OSSN. In addition, they can potentially be used for treatment trials. Moreover, the Krt12-rtTA knock-in mice will likely be useful in generating mouse models in which diverse biologically active and/or dominant-negative molecules can be expressed through administration of Dox in developing or mature cornea.
Acknowledgments
We thank Dr. Scheffer C.G. Tseng (Ocular Surface Research and Education Foundation, Miami, FL) for his critical reading and instructive suggestions.
Footnotes
Address reprint requests to Chia-Yang Liu and Winston Kao, Department of Ophthalmology, University of Cincinnati Medical Center, 3223 Eden Ave., Suite 350, Cincinnati, OH 45267-0527. E-mail: liucg@uc.edu and winston.kao@uc.edu.
Supported by National Institutes of Health/National Eye Institute grants RO1-EY10556 (to W.W.Y.K.) and RO1-EY12486 (to C.Y.L.), by RPB, Inc., and by Ohio Lion Eye Research Foundation.
References
- Grossniklaus HE, Green WR, Lukenback M, Chan CC. Conjunctival lesions in adults: a clinical and histopathologic review. Cornea. 1987;6:78–116. doi: 10.1097/00003226-198706020-00002. [DOI] [PubMed] [Google Scholar]
- Lee GA, Hirst LW. Ocular surface squamous neoplasia. Surv Ophthalmol. 1995;39:429–450. doi: 10.1016/s0039-6257(05)80054-2. [DOI] [PubMed] [Google Scholar]
- Tseng SCG. Concept and application of limbal stem cells. Eye. 1989;3:141–157. doi: 10.1038/eye.1989.22. [DOI] [PubMed] [Google Scholar]
- Finch PW, Rubin JS, Miki T, Ron D, Aaronson SA. Human KGF is FGF-related with properties of a paracrine effector of epithelial cell growth. Science. 1989;245:752–755. doi: 10.1126/science.2475908. [DOI] [PubMed] [Google Scholar]
- Rubin JS, Osada H, Finch PW, Taylor WG, Rudikoff S, Aaronson SA. Purification and characterization of a newly identified growth factor specific for epithelial cells. Proc Nat Acad Sci USA. 1989;86:802–806. doi: 10.1073/pnas.86.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin JS, Bottaro DP, Chedid M, Miki T, Ron D, Cheon G, Taylor WG, Fortney E, Sakata H, Finch PW, LaRochelle WJ. Keratinocyte growth factor. Cell Biol Intern. 1995;19:399–412. doi: 10.1006/cbir.1995.1085. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Finch PW, Aaronson SA. Characterization of recombinant human fibroblast growth factor (FGF)-10 reveals functional similarities with keratinocyte growth factor (FGF-7). J Biol Chem. 1998;273:13230–13235. doi: 10.1074/jbc.273.21.13230. [DOI] [PubMed] [Google Scholar]
- Li DQ, Tseng SC. Differential regulation of cytokine and receptor transcript expression in human corneal and limbal fibroblasts by epidermal growth factor, transforming growth factor-alpha, platelet-derived growth factor B, and interleukin-1 beta. Invest Ophthalmol Vis Sci. 1996;37:2068–2080. [PubMed] [Google Scholar]
- Li DQ, Tseng SC. Differential regulation of keratinocyte growth factor and hepatocyte growth factor/scatter factor by different cytokines in human corneal and limbal fibroblasts. J Cell Physiol. 1997;172:361–372. doi: 10.1002/(SICI)1097-4652(199709)172:3<361::AID-JCP10>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Makarenkova HP, Ito M, Govindarajan V, Faber SC, Sun L, McMahon G, Overbeek PA, Lang RA. FGF10 is an inducer and Pax6 a competence factor for lacrimal gland development. Development. 2000;127:2563–2572. doi: 10.1242/dev.127.12.2563. [DOI] [PubMed] [Google Scholar]
- Govindarajan V, Ito M, Makarenkova HP, Lang RA, Overbeek PA. Endogenous and ectopic gland induction by FGF-10. Dev Biol. 2000;225:188–200. doi: 10.1006/dbio.2000.9812. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, Kao WW, Overbeek PA. Ectopic gland induction by lens-specific expression of keratinocyte growth factor (FGF-7) in transgenic mice. Mech Dev. 1999;88:43–53. doi: 10.1016/s0925-4773(99)00169-0. [DOI] [PubMed] [Google Scholar]
- Chikama T, Hayashi Y, Liu CY, Terai N, Terai K, Kao CW, Wang L, Hayashi M, Nishida T, Sanford P, Doestchman T, Kao WW. Characterization of tetracycline-inducible double transgenic Krt12rtTA/+/tet-O-LacZ mice. Invest Ophthalmol Vis Sci. 2005;46:1966–1972. doi: 10.1167/iovs.04-1464. [DOI] [PubMed] [Google Scholar]
- Tichelaar JW, Lu W, Whitsett JA. Conditional expression of fibroblast growth factor-7 in the developing and mature lung. J Biol Chem. 2000;275:11858–11864. doi: 10.1074/jbc.275.16.11858. [DOI] [PubMed] [Google Scholar]
- Utomo AR, Nikitin AY, Lee WH. Temporal, spatial, and cell type-specific control of Cre-mediated DNA recombination in transgenic mice. Nat Biotechnol. 1999;17:1091–1096. doi: 10.1038/15073. [DOI] [PubMed] [Google Scholar]
- Liu CY, Zhu G, Converse R, Kao CW, Nakamura H, Tseng SC, Mui MM, Seyer J, Justice MJ, Stech ME, Hansen GM, Kao WW. Characterization and chromosomal localization of the cornea-specific murine keratin gene Krt1.12. J Biol Chem. 1994;269:24627–24636. [PubMed] [Google Scholar]
- Liu CY, Birk DE, Hassell JR, Kane B, Kao WW. Keratocan-deficient mice display alterations in corneal structure. J Biol Chem. 2003;278:21672–21677. doi: 10.1074/jbc.M301169200. [DOI] [PubMed] [Google Scholar]
- Liu CY, Zhu G, Westerhausen-Larson A, Converse RL, Kao CW, Sun TT, Kao WW. Cornea-specific expression of K12 keratin during mouse development. Curr Eye Res. 1993;12:963–974. doi: 10.3109/02713689309029222. [DOI] [PubMed] [Google Scholar]
- Tanifuji-Terai N, Terai K, Hayashi Y, Chikama T, Kao WW. Expression of keratin 12 and maturation of corneal epithelium during development and postnatal growth. Invest Ophthalmol Vis Sci. 2006;47:545–551. doi: 10.1167/iovs.05-1182. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Hayashi Y, Liu CY, Tichelaar JW, Kao WW. Over expression of FGF7 enhances cell proliferation but fails to cause pathology in corneal epithelium of Kerapr-rtTA/FGF7 bitransgenic mice. Mol Vis. 2005;11:201–207. [PubMed] [Google Scholar]
- Coulombe PA, Kopan R, Fuchs E. Expression of keratin K14 in the epidermis and hair follicle: insights into complex programs of differentiation. J Cell Biol. 1989;109:2295–2312. doi: 10.1083/jcb.109.5.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, He YG, Weng J, Zieske JD, Jester JV, Schultz GS. Effect of epidermal growth factor, hepatocyte growth factor, keratinocyte growth factor on the motility, differentiation of human corneal epithelial cells. Exp Eye Res. 1994;59:665–678. doi: 10.1006/exer.1994.1152. [DOI] [PubMed] [Google Scholar]
- Rosner A, Miyoshi K, Landesman-Bollag E, Xu X, Seldin DC, Moser AR, MacLeod CL, Shyamala G, Gillgrass AE, Cardiff RD. Pathway pathology: histological differences between ErbB/Ras and Wnt pathway transgenic mammary tumors. Am J Pathol. 2002;161:1087–1097. doi: 10.1016/S0002-9440(10)64269-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holnthoner W, Pillinger M, Groger M, Wolff K, Ashton AW, Albanese C, Neumeister P, Pestell RG, Petzelbauer P. Fibroblast growth factor-2 induces Lef/Tcf-dependent transcription in human endothelial cells. J Biol Chem. 2002;277:45847–45853. doi: 10.1074/jbc.M209354200. [DOI] [PubMed] [Google Scholar]
- Shields CL, Shields JA. Tumors of the conjunctiva and cornea. Surv Ophthalmol. 2004;49:3–24. doi: 10.1016/j.survophthal.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Cho K, Ishiwata T, Uchida E, Nakazawa N, Korc M, Naito Z, Tajiri T. Enhanced expression of keratinocyte growth factor and its receptor correlates with venous invasion in pancreatic cancer. Am J Pathol. 2007;170:1964–1974. doi: 10.2353/ajpath.2007.060935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manavi M, Hudelist G, Fink-Retter A, Gschwandtler-Kaulich D, Pischinger K, Czerwenka K. Gene profiling in Pap-cell smears of high-risk human papillomavirus-positive squamous cervical carcinoma. Gynecol Oncol. 2007;105:418–426. doi: 10.1016/j.ygyno.2006.12.032. [DOI] [PubMed] [Google Scholar]
- Hishikawa Y, Tamaru N, Ejima K, Hayashi T, Koji T. Expression of keratinocyte growth factor and its receptor in human breast cancer: its inhibitory role in the induction of apoptosis possibly through the over-expression of Bcl-2. Arch Histol Cytol. 2004;67:455–464. doi: 10.1679/aohc.67.455. [DOI] [PubMed] [Google Scholar]
- Mehta PB, Robson CN, Neal DE, Leung HY. Serum keratinocyte growth factor measurement in patients with prostate cancer. J Urol. 2000;164:2151–2155. [PubMed] [Google Scholar]
- Kovacs D, Cota C, Cardinali G, Aspite N, Bolasco G, Amantea A, Torrisi MR, Picardo M. Expression of keratinocyte growth factor and its receptor in clear cell acanthoma. Exp Dermatol. 2006;15:762–768. doi: 10.1111/j.1600-0625.2006.00459.x. [DOI] [PubMed] [Google Scholar]
- Niu J, Chang Z, Peng B, Xia Q, Lu W, Huang P, Tsao MS, Chiao PJ. Keratinocyte growth factor/fibroblast growth factor-7-regulated cell migration and invasion through activation of NF-kappaB transcription factors. J Biol Chem. 2007;282:6001–6011. doi: 10.1074/jbc.M606878200. [DOI] [PubMed] [Google Scholar]
- Arbeit JM, Olson DC, Hanahan D. Upregulation of fibroblast growth factors and their receptors during multi-stage epidermal carcinogenesis in K14-HPV16 transgenic mice. Oncogene. 1996;13:1847–1857. [PubMed] [Google Scholar]
- Napora C, Cohen EJ, Genvert GI, Presson AC, Arentsen JJ, Eagle RC, Laibson PR. Factors associated with conjunctival intraepithelial neoplasia: a case control study. Ophthalmic Surg. 1990;21:27–30. [PubMed] [Google Scholar]
- Scott IU, Karp CL, Nuovo GJ. Human papillomavirus 16 and 18 expression in conjunctival intraepithelial neoplasia. Ophthalmology. 2002;109:542–547. doi: 10.1016/s0161-6420(01)00991-5. [DOI] [PubMed] [Google Scholar]
- Karp CL, Scott IU, Chang TS, Pflugfelder SC. Conjunctival intraepithelial neoplasia: a possible marker for human immunodeficiency virus infection? Arch Ophthalmol. 1996;114:257–261. doi: 10.1001/archopht.1996.01100130253003. [DOI] [PubMed] [Google Scholar]
- Kiire CA, Dhillon B. The aetiology and associations of conjunctival intraepithelial neoplasia. Br J Ophthalmol. 2006;90:109–113. doi: 10.1136/bjo.2005.077305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103:49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsarelis SZ, Cheng G, Dong G, Sun TT, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- Alvarado J, Murphy C, Juster R. Age-related changes in the basement membrane of the human corneal epithelium. Invest Ophthalmol Vis Sci. 1983;24:1015–1028. [PubMed] [Google Scholar]