Abstract
Cysteinyl leukotrienes (CysLTs) have been implicated as inflammatory mediators of cardiovascular disease. Three distinct CysLT receptor subtypes transduce the actions of CysLTs but the role of the endothelial CysLT2 receptor (CysLT2R) in cardiac function is unknown. Here, we investigated the role of CysLT2R in myocardial ischemia-reperfusion (I/R) injury using transgenic (tg) mice overexpressing human CysLT2R in vascular endothelium and nontransgenic (ntg) littermates. Infarction size in tg mice increased 114% compared with ntg mice 48 hours after I/R; this increase was blocked by the CysLT receptor antagonist BAY-u9773. Injection of 125I-albumin into the systemic circulation revealed significantly enhanced extravasation of the label in tg mice, indicating increased leakage of the coronary endothelium, combined with increased incidence of hemorrhage and cardiomyocyte apoptosis. Expression of proinflammatory genes such as Egr-1, VCAM-1, and ICAM was significantly increased in tg mice relative to ntg controls. Echocardiographic assessment 2 weeks after I/R revealed decreased anterior wall thickness in tg mice. Furthermore, the postreperfusion time constant τ of isovolumic relaxation was significantly increased in tg animals, indicating diastolic dysfunction. These results reveal that endothelium-targeted overexpression of CysLT2R aggravates myocardial I/R injury by increasing endothelial permeability and exacerbating inflammatory gene expression, leading to accelerated left ventricular remodeling, induction of peri-infarct zone cellular apoptosis, and impaired cardiac performance.
Myocardial infarction results from severe impairment of the coronary blood supply usually provoked by thrombotic or other acute alterations of coronary atherosclerotic plaque.1 It remains the chief cause of death in North America and Europe.2 With loss of oxygen supply, apoptosis and necrosis of cardiac myocytes in the ischemic area ensues leading to decreased cardiac performance.1 Rapid reperfusion is essential to limit the extent of myocardial necrosis.3 However, the consequences of reperfusion are complex and include various deleterious effects collectively referred to as ischemia-reperfusion (I/R) injury.1 The intense inflammatory response after reperfusion plays a central role not only in promoting tissue injury, but also in repair after infarction.4 The inflammatory process characterizing early and late reperfusion is an important aspect of the changes leading to tissue damage.4 Increased vascular permeability and expression of adhesion molecules initiates the inflammatory reaction, and alterations of endothelial function are pivotal in the development of reperfusion damage.4,5
Cysteinyl leukotrienes (CysLTs), leukotriene C4 (LTC4), leukotriene D4 (LTD4), and leukotriene E4 (LTE4), are well established inflammatory agents that mediate bronchial and vascular smooth muscle constriction and enhance vascular permeability.6 CysLTs are implicated in inflammatory conditions such as asthma and more recently in cardiovascular disease.7,8,9 CysLTs mediate their actions via G protein-coupled receptor (GPCR) proteins, cysteinyl leukotriene 1 receptor (CysLT1R), cysteinyl leukotriene 2 receptor (CysLT2R), and a recently deorphanized GPCR known as GPR17.8,10 The CysLT2R gene is expressed in human heart and coronary vessels, also within the cardiac Purkinje system, as well as in human coronary smooth muscle cells and umbilical vein endothelial cells.8,11,12,13,14 CysLT2R expression in mouse heart appears to be more restricted with diffuse expression within endothelial cells.15 We generated previously transgenic (tg) mice overexpressing the human CysLT2R in vascular endothelium to characterize the role of this receptor in vascular function.16
The involvement of CysLTs and their receptors in inflammation and fibrosis has been confirmed in various animal and human studies.17 Several studies reported enhanced edema and neutrophil infiltration after myocardial I/R concomitant with elevation of CysLTs.18,19 These eicosanoids are detected as increased urinary LTE4 levels in patients after admission for suspected acute myocardial infarction and unstable angina.20 Moreover, the expression of CysLT1R and CysLT2R is increased in organs that are prone to ischemic damage and CysLT1R antagonism exerts anti-inflammatory effects on cerebral and renal I/R injury.21,22,23 Few studies have investigated CysLTs and their receptors in acute myocardial infarction and specifically the role of CysLT2R in myocardial I/R injury has not been established. Here, we report that endothelium- targeted overexpression of CysLT2R aggravates myocardial I/R injury by increasing endothelial permeability and exacerbating inflammatory gene expression, leading to accelerated left ventricular (LV) remodeling and impaired cardiac performance.
Materials and Methods
Animals
The generation of EC-CysLT2R transgenic mice has been described previously.16 These mice express seven copies of the human CysLT2R gene under control of the Tie2 promoter/enhancer, integrated in a gene-sparse region of chromosome 6. Hemizygous mice were continuously backcrossed with C57BL/6 mice to obtain equal numbers of transgenic and wild-type littermates. 5-Lipoxygenase-deficient (5LO−/−) mice, developed in our laboratory previously,24 were obtained from The Jackson Laboratory (Bar Harbor, ME). The mice were backcrossed for more than nine generations to the C57BL/6 background. The 5LO−/− mice show absence of 5-lipoxygenase mRNA, protein, and leukotriene synthesis in inflammatory cells. CysLT2R-deficient LacZ mice were generated by standard gene targeting procedures using C57BL/6 embryonic stem cells (S. Ishii, unpublished data) and embryos heterozygous for the genetic modification were transferred from Japan, revived at Queen’s University, and littermates of heterozygous offspring (all on a C57BL/6 genetic background) were used in these studies.
Mouse Model of Myocardial I/R and Drug Treatment
Mice (8 to 12 weeks) underwent coronary artery occlusion or sham surgery as previously described.25 Briefly, mice were anesthetized with sodium pentobarbital (45 mg/kg) intraperitoneally, intubated, and ventilated with a rodent ventilator (Harvard Apparatus, St. Laurent, Canada). A midsternal thoracotomy was performed at the fourth intercostal space to expose the anterior surface of the heart. The proximal left anterior descending artery (LAD) was identified and a 6-0 silk Ethilon suture was placed around the artery and surrounding myocardium just below the atrioventricular border. Regional ischemia was induced for 30 minutes by tightening the suture against a small piece of PE-10 tubing placed on top of the LAD. Ischemia was confirmed by the discoloration of the myocardium. Sham-operated animals served as surgical controls and were subjected to the same surgical procedures as the experimental animals, with the exception that the LAD was not ligated. At the end of ischemia, the ligature was loosened and reperfusion was achieved. The lungs were reinflated and the muscle and skin layers were closed separately. The animals were weaned from the ventilator, extubated, and allowed to recover under a heat lamp before being returned to their cages. For animals receiving drug treatment, Bay-u9773 (0.25 mg/kg; Biomol Research Products, Plymouth Meeting, PA) was diluted in 1× phosphate-buffered saline (PBS) and injected intraperitoneally 4 hours before surgery, and 2, 8, and 16 hours after reperfusion. Surgical procedures and treatment regimens were approved by the University Animal Care Committee at Queen’s University and adhered to the guidelines of the Canadian Council of Animal Care and the Guiding Principles in the Care and Use of Animals of the American Physiological Society.
Morphometric Evaluation of Risk Area and Infarction Size
Forty-eight hours after reperfusion, mice were euthanized by an intraperitoneal pentobarbital overdose. The 48-hour time point was selected because it is commonly used to assess early inflammatory events (eg, leukocyte infiltration, vascular leakage). The heart was exposed and the original suture was religated. The heart was then perfused retrogradely with 100 to 200 μl of 2% Evans blue dye in PBS (pH 7.4) to delineate the nonischemic area. The heart was excised and rinsed in ice-cold PBS and the LV, including the interventricular septum, was sectioned into four or five slices of similar thickness perpendicular to the long axis of the heart. The slices were incubated in 1% 2,3,5-triphenyltetrazolium chloride (TTC; Sigma Chemicals, St. Louis, MO) at 37°C for 15 minutes to demarcate viable and necrotic tissue. The thickness of each slice was measured using calipers. The slices were photographed on both sides with a digital camera (Canon Corp., Tokyo, Japan). The infarct area (pale white), the area at risk (area excluding Evans Blue), and the total left ventricular area were traced and calculated for both sides of each slice using Image software (National Institutes of Health, Bethesda, MD). The areas for each slice were multiplied by the thickness of the slice to obtain a measure of volume. The cumulative volume for all sections for each heart was used for comparisons. The size of LV at risk was calculated as the ratio of the LV volume excluding Evans blue dye to the total LV volume. Infarct size was calculated as the ratio of the infarct volume to the volume of the risk area as previously described.26 Animals with infarct volume in the 35 to 70% range of total LV volume were used as inclusion criteria in the study.27 Only one mouse was excluded based on these criteria.
Lactate Dehydrogenase (LDH) and Creatine Kinase (CK) Activity in Plasma
Biochemical analysis of myocardial injury was performed in heparinized arterial blood collected at termination of the experiment. Plasma LDH and CK were measured using an automated clinical analyzer at the Kingston General Hospital using clinical grade reagents.
Vascular Permeability Assay in Cardiac Tissue
Forty-eight hours after reperfusion, mice (8 to 12 weeks) were anesthetized by an intraperitoneal injection of pentobarbital (45 mg/kg). 125I-albumin (106 cpm, 1.44 mCi/mg; MP Biomedicals, Inc., Mississauga, Canada) was injected into the right external jugular vein via a PE-10 catheter. Twenty minutes after injection, the mice were euthanized, and blood was obtained as above and weighed. Exsanguination and removal of excess 125I-albumin proceeded via the right atrium. A 23-gauge needle was inserted into the apex of the left ventricle and the mouse was perfused retrogradely at 40 mmHg with 5.85 ml/100 g of 0.9% NaCl containing 100 U/ml heparin as described previously.28 The LAD was then religated and Evans blue dye was perfused as above to delineate the risk area, which was then dissected from the remaining myocardial tissue, weighed, and placed in individual tubes. The radioactivity in the blood, nonrisk area, and risk area were counted separately using a gamma counter (Beckman Instruments, Irvine, CA). The permeability index of the different regions was calculated as the radioactivity per g of wet tissue divided by the radioactivity in 1 g of blood.29 Sham-surgery controls were subjected to the same manipulations, with the exception that the ligature was not tied.
RNA Extraction and Real-Time Polymerase Chain Reaction (PCR)
Total RNA was isolated from the risk area of the left ventricle 3 hours after reperfusion using Trizol reagent (Sigma). Total RNA was reverse-transcribed to cDNA using the Synthesis System for RT-PCR kit (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. For detection of mouse gene expression, quantitative real-time PCR was performed using a 7500 thermal cycler with TaqMan Universal PCR master mix and TaqMan gene expression assays (Egr-1, VCAM-1, and ICAM-1; Applied Biosystems, Foster City, CA) or with SYBR Green PCR master mix (CysLT1R, and CysLT2R) as described.16 GAPDH was used as a control housekeeping gene. Data are calculated by the 2−ΔΔCT method and are presented as fold induction of transcripts for target genes normalized to GAPDH, with respect to the sham controls.30
Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick End-Labeling (TUNEL) Staining
TUNEL assays were performed on LV samples with the CardioTACS in situ apoptosis detection kit (Trevigen, Gaithersburg, MD) as described by Takahashi and colleagues31 with some modification. The hearts were arrested in diastole with 0.2 N KCl 48 hours after reperfusion and perfused with 3.7% neutralized formaldehyde solution. The heart was then excised, postfixed in the same fixative for another 12 hours, then cut into three sections corresponding approximately to the apex, mid-papillary, and base. The slices were embedded in paraffin, cut into 5-μm sections, and transferred to silicon-coated slides. High-power fields (12 to 20 at ×400 magnification) were obtained at the different levels to measure the number of TUNEL-positive cardiomyocyte nuclei in the peri-infarct border and uninfarcted remote zones, respectively. Only nuclei that were clearly located in cardiomyocytes were scored. The number of TUNEL-positive cardiomyocyte nuclei was divided by the total number of nuclei to determine the ratio of TUNEL-positive nuclei.
Immunohistochemical Staining
To determine the numbers of infiltrating leukocytes, formalin-fixed, paraffin-embedded 4-μm sections were mounted on silicon-coated slides and treated with 3% H2O2 to block endogenous peroxidase. The sections were incubated for 1 hour at room temperature with rat polyclonal anti-mouse CD45 antibody (PharMingen, San Diego, CA) at a dilution of 1:50. The sections were then incubated with biotinylated rabbit IgG (Vector Laboratories, Burlingame, CA), and CD45 immunoreactivity was visualized using diaminobenzidine substrate. The number of leukocytes in the boundary area was counted in 10 random high-power fields, and the average number in each group was calculated.32 X-gal staining to determine endogenous CysLT2R expression based on the LacZ reporter gene was performed essentially as described.33
Echocardiography
Mice (8 to 12 weeks) underwent transthoracic echocardiography 1 day before and 2 weeks after acute I/R using a Phillips (Andover, MA) Sonos 5500 equipped with a 15-6L (15-6 MHz) intraoperative linear array transducer essentially as previously described.34 The 2-week time point was chosen as one of the earliest time points to clearly define remodeling responses in rodents.34 Briefly, in preparation for echocardiography, animals were lightly anesthetized by halothane using a nose cone, shaved, and positioned on a heated pad in a recumbent position. Measurements were performed at the midpapillary level from well aligned M-mode images from the parasternal short-axis view. LVd (LV diastolic diameter), PWd (end-diastolic posterior wall thickness), and IVSd (interventricular septum thickness) were determined. The relative wall thickness for each level of the LV was calculated as (PWd + IVSd)/LVd. For each parameter, an average of five cardiac cycles was used for calculations.
Hemodynamic Measurements
Two weeks after acute I/R injury, mice were anesthetized with isoflurane (2%) in medical grade oxygen. The animals were then intubated and ventilated using a pressure controlled respirator (Kent Scientific Corp., Litchfield, CN) at a tidal volume of 200 μl and a frequency of 130 strokes/minute. Body temperature was monitored with a rectal thermometer and maintained at 37°C with the aid of a heat lamp. A midsternal thoracotomy was performed as above to expose the heart. The right jugular vein was cannulated for drug administration. A 1.4F ultra-miniature Millar catheter (SPR 839; Millar Instruments, Houston, TX) was placed into the left ventricle through the apex to record LV pressure. After recording steady-state LV pressures, mice were given an intravenous administration of the synthetic catecholamine dobutamine (10 ng/g body weight) to investigate the functional integrity of adrenergic signaling in the heart. The peak hemodynamic response was recorded using a data acquisition system (PVAN, Millar Instruments). The PVAN software was used for off-line calculation of LV peak systolic pressure, LV end-diastolic pressure, LV peak-positive developed pressure (dP/dtmax), LV peak-negative developed pressure (dP/dtmin), LV pressure at peak positive developed pressure (P@dP/dtmax), heart rate, and tau (τ) as described.35 For calculation of hemodynamic parameters, a minimum of 50 consecutive cardiac cycles were used.
Statistical Analysis
All data are expressed as mean ± SEM. One-way analysis of variance followed by Student-Neuman-Keuls t-test were used to compare differences in risk area, infarct size, myocardial enzyme activities, and endothelial permeability, as well as differences in inflammatory gene expression and cardiomyocyte apoptosis. Unpaired t-test was used to compare differences in neutrophil infiltration and echocardiographic and functional parameters between tg and ntg mice. Paired t-test was used to compare before and after I/R changes in echocardiographic parameters and LV functional responses to dobutamine in the same animals. A P value <0.05 was considered to indicate statistical significance.
Results
CysLT Receptor Expression in Mouse Hearts
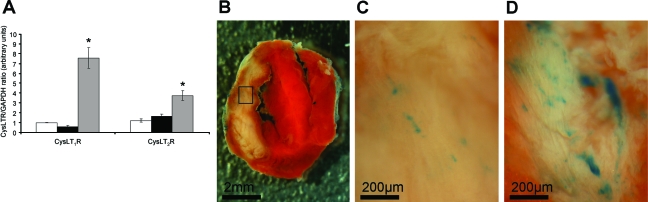
The expression of both native murine CysLT1R and CysLT2R was examined in hearts by real-time quantitative PCR as previously done in mouse ear tissue.16 Gene expression for both CysLT receptors was low in noninfarcted ntg hearts and in infarcted hearts 3 hours after I/R injury (Figure 1). However, 48 hours after I/R injury CysLT1R expression had increased 7.5-fold whereas CysLT2R expression increased 3.5-fold. The human CysLT2R transgene, using specific primers that can distinguish between species, could only be detected in tg mice. Using a second independent technique, we were also able to document elevation of CysLT2R expression after 48 hours of I/R. Thus, using a novel mouse strain in which the Cysltr2 gene is deleted and replaced with a LacZ reporter gene under control of the Cysltr2 gene regulatory elements (S. Ishii et al, unpublished data) we were able to demonstrate sparse blue X-gal staining in noninfarcted ventricular tissue and 3 hours after I/R injury (Figure 1, B and C), consistent with the pattern observed previously by in situ hybridization in normal mouse heart.15 After 48 hours of I/R injury, staining intensity increased in the infarct and peri-infarct zones (Figure 1D), which was in harmony with the PCR data.
Figure 1.
Expression of CysLT1R and CysLT2R in mouse hearts. A: Quantitative real-time PCR was used to assess gene expression for the two CysLT receptors relative to GAPDH expression in ntg mouse hearts (n = 3) as described in the Materials and Methods section. Open bars, sham-operated mice; black bars, hearts 3 hours after I/R; gray bars, hearts 48 hours after I/R. *P < 0.05 compared to sham-operated controls. B: TTC-stained heart slice from a CysLT2R-deficient LacZ mouse 3 hours after I/R injury. C: Representative CysLT2R expression in boxed region of the slice shown in B detected via the reporter gene LacZ with blue X-gal staining. D: CysLT2R expression (via reporter LacZ/X-gal staining) in a heart slice from a CysLT2R-deficient LacZ mouse having undergone 48 hours of I/R. Similar patterns of expression were observed in two additional mice at 3 and 48 hours after I/R.
Endothelial CysLT2R Overexpression Increases Myocardial Infarct Size after LAD Occlusion and Reperfusion
The effect of endothelial overexpression of CysLT2R on myocardial I/R injury is shown in Figure 2. Gross histological analysis of TTC-stained sections 48 hours after reperfusion showed a larger necrotic area in tg animals compared to ntg littermates and 5-lipoxygenase-null 5LO−/− mice (Figure 2A). Histomorphometric analysis revealed that infarct size in CysLT2R tg mice was increased by 114% relative to ntg mice (56 ± 15% versus 26 ± 9%, n = 8, P < 0.01) (Figure 2C), despite comparable risk area in all groups (Figure 2B). Infarct size in the 5LO−/− null mice was comparable to ntg mice (21 ± 9% versus 26 ± 9%). Treatment of tg mice with the nonselective dual CysLT1R/CysLT2R antagonist Bay-u9773, at a dose tested empirically to evoke CysLT2R antagonism, markedly reduced infarct size by nearly 60% (56% versus 23%, n = 8, P < 0.05) to levels comparable to ntg and 5LO−/− mice (Figure 2, A and C). The antagonist had no additional effect on infarct size in ntg mice.
Figure 2.
Effect of endothelial CysLT2R overexpression on LV infarct size after acute I/R injury. A: Representative TTC-stained ventricular sections from ntg, tg, and 5LO−/− mice at 48 hours after I/R. Representative sections of ntg and tg mice treated with the nonselective dual CysLT1R/CysLT2R receptor antagonist Bay-u9773 are also shown. B: Morphometric analysis of LV area at risk (B) and infarct size (C) in the five groups mentioned above. D and E: Serum levels of CK (D) and LDH (E) in sham and infarcted ntg, tg, and 5-LO−/− mice at 48 hours after reperfusion. *P < 0.05; **P < 0.01; n = 8 for groups in A–C; n = 6 for groups in D and E.
We measured serum levels of CK and LDH 48 hours after reperfusion. CK (Figure 2D) and LDH (Figure 2E) activities in infarcted ntg mice were increased by ∼26% compared to the baseline levels in sham-operated controls. In contrast, CK and LDH levels were markedly elevated by 357% and 123%, respectively, in tg mice subjected to I/R compared to tg sham controls (Figure 2, D and E). Compared to ntg I/R mice, the levels of CK and LDH were elevated by ∼230% and 100%, respectively, in tg mice. In concordance with the histopathological findings, treatment with Bay-u9773 reduced levels of CK and LDH after reperfusion in the tg animals (Figure 2, D and E), while having no significant effect on these markers in ntg mice. I/R increased the levels of CK and LDH in 5LO−/− mice but this was significantly smaller than in tg mice (Figure 2, D and E).
Endothelial CysLT2R Overexpression Increases Permeability in the Infarcted Region of Transgenic Mouse Hearts
Previously, we detected enhanced vascular permeability responses to leukotriene challenge and passive cutaneous anaphylaxis in mouse ear vasculature of tg mice.16 To examine if similar vascular responses occur in the coronary endothelium after myocardial I/R, we assessed the histopathology of the infarct. In addition, we measured extravasation of 125I-BSA in the ischemic and remote areas of the left ventricle at 48 hours after reperfusion. Microscopic examination of the infarct in hematoxylin and eosin (H&E)-stained sections showed minimal accumulation of erythrocytes in the infarcted region of ntg mice (Figure 3A). In contrast, tg mice presented significant accumulation of red cells in the interstitium, resulting in hemorrhage of the infarcted area (Figure 3B). Basal coronary endothelial permeability to 125I-BSA did not differ significantly between ntg and tg mice (Figure 3C). I/R injury led to significant interstitial accumulation of 125I-BSA in both ntg and tg mice. However, the increase in coronary circulation permeability was more pronounced in tg versus ntg mice (202% versus 93%, Figure 3C). No differences in permeability were seen in the nonischemic region of the myocardium.
Figure 3.
Effect of endothelial CysLT2R overexpression on myocardial histopathology and coronary endothelial permeability after acute I/R injury. A and B: Microscopic appearance of infarcted left ventricle in H&E-stained paraffin sections from ntg (A) and tg (B) mice 48 hours after acute I/R injury. C: Permeability of coronary endothelium to 125I-BSA in the at-risk and nonrisk regions of the left ventricle of ntg and tg mice. **P < 0.01; n = 6. Scale bars = 50 μm.
Endothelial CysLT2R Overexpression Increases CD45+ Leukocyte Infiltration after I/R in Transgenic Mouse Hearts
We used immunostaining of the pan leukocyte cell surface marker CD45 to determine whether the enhanced permeability of coronary endothelium leads to increased leukocyte infiltration of the infarcted region after I/R injury. Figure 4A shows representative cross-sections from the peri-infarct region in ntg and tg mice. The tg mice showed greater density of CD45-positive cells than ntg mice. Morphometric analysis showed >100% increase in the number of infiltrating leukocytes in tg compared to ntg mice (1289 ± 113/mm2 versus 528 ± 131/mm2) (Figure 4).
Figure 4.
Effect of endothelial CysLT2R overexpression on leukocyte infiltration after I/R. A: Representative photomicrographs showing immunohistochemical detection of pan-leukocyte cell surface marker CD45 in the peri-infarct region of the LV in ntg and tg mice at 48 hours after reperfusion. Arrows indicate CD45-positive cells. B: Quantitative morphometric analysis of leukocyte infiltration. **P < 0.01; n = 4. Scale bars = 50 μm. Original magnifications, ×400.
Endothelial CysLT2R Overexpression Increases Egr-1, ICAM, and VCAM-1 Gene Expression in Transgenic Mouse Hearts
To examine potential molecular correlates for the I/R-induced histopathological and permeability alterations seen in tg mice, we determined myocardial mRNA expression of adhesion molecules ICAM and VCAM-1, as well as Egr-1 transcription factor (Figure 5). These genes have been implicated in the myocardial inflammatory response to I/R injury. No significant genotype-related differences were seen in basal expression of these genes. Myocardial expression of ICAM (Figure 5A), VCAM-1 (Figure 5B), and Egr-1 (Figure 5C) were increased significantly in both ntg and tg mice 3 hours after reperfusion. However, the I/R-induced increase in expression of these genes was greater in the tg mice (Figure 5).
Figure 5.
Effect of endothelial CysLT2R overexpression on proinflammatory gene expression. A–C: Quantitative real-time PCR evaluation of ICAM (A), VCAM-1 (B), and Egr-1 (C) gene expression in total RNA extracted from the ischemic area of mouse hearts at 3 hours after reperfusion. *P < 0.05; n = 3.
Endothelial CysLT2R Overexpression Increases Cardiomyocyte Apoptosis in Transgenic Mouse Hearts
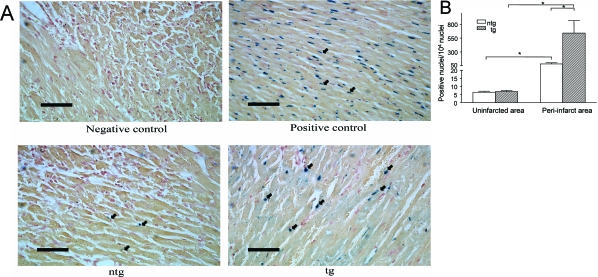
Because apoptosis plays a central role in myocardial cell loss after I/R, we determined whether endothelial overexpression of CysLT2R influences the number of apoptotic nuclei in cardiomyocytes in the peri-infarct region of tg and ntg mice after I/R. We found increased apoptosis of cells with cardiomyocyte morphology in both groups at 48 hours after reperfusion (Figure 6, A and B). However, the number of apoptotic nuclei in the peri-infarct region of tg animals was significantly greater than in ntg animals (641 ± 222 TUNEL-positive myocytes/104 nuclei versus 84 ± 21/104 nuclei) (Figure 6B). At 48 hours after reperfusion, cardiomyocyte apoptosis was confined primarily to the peri-infarct region, although at earlier time points (ie, 6 to 24 hours after reperfusion), apoptosis is typically elevated in the infarct core. The number of apoptotic nuclei in the noninfarcted region was markedly lower than in the peri-infarct region and did not differ between ntg and tg mice (Figure 6B). It should be noted that apoptotic nuclei in noncardiomyocytes were observed; however, the precise cell types were not identified nor were they quantified in the present studies.
Figure 6.
Effect of endothelial CysLT2R overexpression on myocardial apoptosis after I/R injury. A: Immunohistochemical detection of apoptotic nuclei in the peri-infarct region using TUNEL assay in ntg and tg mice (bottom) at 48 hours after reperfusion. Negative and positive reagent controls are shown in the top panels. Arrows indicate some blue-stained TUNEL-positive nuclei. B: Apoptotic index (no. positive nuclei/104 nuclei) in the peri-infarct and noninfarct regions of the left ventricle in ntg and tg mice. *P < 0.05, n = 4. Scale bars = 50 μm. Original magnifications, ×400.
Endothelial CysLT2R Overexpression Accelerates Left Ventricular Remodeling after I/R
We used two-dimensional echocardiography to examine early (2 week) changes in LV wall and chamber dimensions after I/R. We chose the I/R model of myocardial infarction because it recapitulates some of the features of pathology after infarction seen in humans with reperfused MI, namely slow-developing LV remodeling that is generally complete by 3 to 6 weeks in rodents.36,37 Representative M-mode frames taken before and 2 weeks after acute I/R injury are shown in Figure 7, and echocardiographic data are summarized in Table 1. Pre-I/R wall and chamber dimensions did not differ significantly between ntg and tg mice, with the exception of left ventricular diastolic dimension (LVDd) that was found to be slightly increased in tg mice (Figure 7, A and C; Table 1). Two weeks after reperfusion the tg mice presented significant thinning of the anterior wall/interventricular septum, whereas the anterior wall remained relatively unchanged in ntg mice (Figure 7, B and D; Table 1). Systolic and diastolic LV chamber dimensions after infarction remained relatively unchanged from preinfarction values in ntg mice (Figure 7, C and D; Table 1). However, tg mice showed a trend toward greater LV systolic dimension after infarction than ntg mice (21% versus 12% increase with respect to preinfarction values; Figure 7, A and B, and Table 1).
Figure 7.
Effect of endothelial CysLT2R overexpression on left ventricular wall and chamber dimensions. A–D: Representative M-mode frames from the mid-papillary region of tg (A, B) and ntg (C, D) before (A, C) and 2 weeks after (B, D) acute I/R injury. Arrow indicates width of the LV chamber.
Table 1.
Two-Dimensional Echocardiographic Analysis of Left Ventricular Wall and Chamber Dimension before and 2 Weeks after Acute Myocardial I/R in CysLT2R Transgenic and Nontransgenic Mice
| Pre-ischemia/reperfusion
|
Post-ischemia/reperfusion
|
% change from pre I/R
|
||||
|---|---|---|---|---|---|---|
| ntg | tg | ntg | tg | ntg | tg | |
| LVDd (mm) | 0.350 ± 0.010 | 0.389 ± 0.0120* | 0.378 ± 0.0087 | 0.424 ± 0.0300 | 8.6 ± 4.4 | 8.8 ± 6.5 |
| LVDs (mm) | 0.200 ± 0.0090 | 0.229 ± 0.0173 | 0.220 ± 0.0185 | 0.27 ± 0.0342 | 11.5 ± 10.2 | 20.9 ± 15.9 |
| IVSd (mm) | 0.0751 ± 0.0030 | 0.0707 ± 0.0022 | 0.0727 ± 0.0025 | 0.0712 ± 0.0008 | −2.6 ± 4.0 | 1.3 ± 3.8 |
| IVSs (mm) | 0.127 ± 0.0047 | 0.125 ± 0.0083 | 0.118 ± 0.0085 | 0.103 ± 0.0067† | −6.1 ± 8.8 | −16.9 ± 4.3 |
| PWd (mm) | 0.0719 ± 0.0017 | 0.0763 ± 0.0044 | 0.0811 ± 0.0045 | 0.0867 ± 0.0041 | 13.9 ± 8.4 | 16.7 ± 11.2 |
| PWs (mm) | 0.124 ± 0.0052 | 0.125 ± 0.0044 | 0.143 ± 0.0148 | 0.148 ± 0.0060† | 15.9 ± 11.4 | 19.6 ± 7.6 |
| HR (bpm) | 472 ± 17 | 479 ± 30 | 476 ± 21 | 481 ± 40 | 1.2 ± 4.9 | 0.30 ± 4.6 |
LVDd, left ventricular chamber diameter at diastole; LVDs, left ventricular chamber diameter at systole; IVSd, interventricular septum thickness at diastole; IVSs, interventricular septum thickness at systole; PWd, posterior wall thickness at diastole; PWs, posterior wall thickness at systole; HR, heart rate.
P < 0.05, tg versus ntg by unpaired t-test;
P < 0.05, pre-I/R versus post-I/R by paired t-test.
Endothelial CysLT2R Overexpression Impairs Left Ventricular Function after I/R
We also assessed the effect of endothelial CysLT2R overexpression on LV function using a microtip pressure catheter (Table 2). Basal LV function did not differ significantly between ntg and tg mice. Furthermore, both types of mice responded comparably to an acute bolus injection of dobutamine with increases in heart rate, LV pressures, and maximal and minimal values of the first derivative of LV pressure (Table 2). Two weeks after I/R, function remained relatively unchanged in ntg mice. In contrast, tg animals showed a trend toward decreased LV +dP/dt and LV −dP/dt and a significant increase in the time constant of isovolumic relaxation (τ), indicating the presence of both systolic and diastolic dysfunction (Table 2). Interestingly, both genotypes showed refractoriness of heart rate and LV pressures to dobutamine after infarction.
Table 2.
Left Ventricular Function in Control CysLT2R-Transgenic and Nontransgenic Mice and 2 Weeks after Acute Myocardial I/R Injury
|
ntg
|
tg
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Control (n = 6)
|
I/R (n = 5)
|
Control (n = 5)
|
I/R (n = 5)
|
|||||
| Before DB | After DB | Before DB | After DB | Before DB | After DB | Before DB | After DB | |
| Heart rate, beats/minute | 633 ± 14 | 709 ± 17* | 616 ± 26 | 664 ± 44 | 575 ± 13‡ | 642 ± 11*‡ | 519 ± 32‡ | 522 ± 39†‡ |
| LV function | ||||||||
| LV peak pressure, mmHg | 71 ± 2 | 138 ± 13* | 69 ± 4 | 72 ± 3 | 74 ± 4 | 130 ± 14* | 70 ± 4 | 83 ± 8 |
| LVESP, mmHg | 70 ± 2 | 138 ± 13* | 68 ± 4 | 71 ± 3 | 72 ± 4 | 130 ± 14* | 70 ± 4* | 83 ± 8† |
| LVEDP, mmHg | 3. ± 0 | 5 ± 1 | 3 ± 0.3 | 4 ± 0.4 | 5 ± 1 | 7 ± 1* | 4 ± 0.6 | 4 ± 0.2 |
| LV +dP/dt, mmHg/second | 5636 ± 320 | 16,046 ± 1281* | 6409 ± 425 | 9952 ± 1557*† | 6571 ± 604 | 13,921 ± 1867* | 4896 ± 569 | 7412 ± 1728† |
| LV −dP/dt, mm Hg/second | −6058 ± 423 | −10,377 ± 648* | −6624 ± 489 | −6454 ± 397† | −6267 ± 621 | −9144 ± 680* | −4865 ± 662 | −5370 ± 735† |
| τ, ms | 6.37 ± 0.44 | 5.10 ± 0.19* | 5.91 ± 0.33 | 4.94 ± 0.17* | 5.76 ± 0.22 | 5.77 ± 0.20‡ | 7.91 ± 0.82† | 7.17 ± 0.77‡ |
DB, dobutamine; LV, left ventricle; LVESP, left ventricular end-systolic pressure; LVEDP, left ventricular end-diastolic pressure; LV +dP/dt, maximal value of the first derivative of LV pressure; LV −dP/dt, minimal value of the first derivative of LV pressure; τ, time constant for isovolumic relaxation.
P < 0.05 after DB versus before DB;
P < 0.05, control versus I/R;
P < 0.05 tg versus ntg.
Discussion
The endothelium plays a pivotal role in maintaining vessel homeostasis by elaborating a variety of vasoactive, anti-inflammatory and antithrombotic factors that help maintain coronary vessel tone and protect the vessel wall against inflammatory cell and platelet adhesion.38 Endothelial dysfunction plays a central role in the pathogenesis of myocardial I/R injury1,5,18 and is characterized by impaired vessel relaxation, and enhanced expression of inflammatory and adhesion molecules, leading to increased vascular permeability, inflammatory cell infiltration, and platelet adhesion and thrombus formation.5 CysLTs are major inflammatory mediators and activation of endothelial CysLT2R markedly increases vascular permeability in transgenic mice.16 We now report that myocardial injury in response to acute I/R is exacerbated in endothelium-targeted CysLT2R transgenic mice, in association with increased coronary vascular permeability, inflammatory cell infiltration, and heightened myocyte loss through apoptosis.
CysLT synthesis increases in humans20 with myocardial infarction. Furthermore, pretreatment with leukotriene biosynthesis inhibitors, AA-861 or Bay X1005, reduces neutrophil influx and infarct size after I/R injury in rats39 and rabbits,40 respectively, and intravenous infusion of a CysLT receptor antagonist LY-171883 at reperfusion decreases infarct size and improves LV functional recovery after myocardial infarction in cats.41 In addition, recent linkage analysis studies revealed increased risk of stroke and myocardial infarction in some ethnic groups harboring a distinct haplotype in the ALOX5AP gene encoding 5-lipoxygenase-activating protein.42 Thus, it is plausible in the current study that CysLTs released from resident and/or circulating inflammatory cells or synthesized by transcellular pathways between interacting neutrophils and endothelial cells40,43 may activate CysLT2Rs in vascular endothelium to promote endothelial leakage and subsequent myocardial damage. CysLTs influence the adhesion of neutrophils to endothelium by up-regulating adhesion molecules44,45 and may also act as chemotactic factors in recruitment of leukocytes to the infarcted myocardium. There is compelling evidence that activation of CysLT2R in cultured human endothelial cells leads to activation of a distinct set of immediate-early gene signatures46 including the transcription factor Egr-1 and a variety of signaling and adhesion molecules that have been shown to participate in ischemic stress and reperfusion injury in mice.47 In agreement with these findings, the expression of Egr-1, as well as ICAM and VCAM-1 genes, were elevated in the present study after activation of the human transgene in vascular endothelium, suggesting that they may contribute to the enhanced endothelial permeability and neutrophil infiltration in the infarcted myocardium of tg mice.
To examine the relative impact of transgenic endothelial overexpression of CysLT2 receptor vis-à-vis native CysLT2 expression after I/R, a number of tests were performed including analysis of the expression level of CysLT1 and CysLT2 receptors before and after injury (Figure 1), as well as comparative analysis of treatment with the dual CysLT1/CysLT2 receptor antagonist Bay-u9773, combined with infarction analysis of 5LO/leukotriene-deficient mice (Figure 2). Real-time PCR analysis indicated low levels of native murine CysLT1 and CysLT2 receptors in sham-operated mouse hearts and early after I/R (3 hours) with significantly higher levels of both receptors 48 hours after I/R. We speculate that the increased CysLT1R levels at 48 hours of I/R are attributable to infiltrating mononuclear leukocytes, a predominant cell type at this time point32 and cells known to express this receptor subtype.48 Based on the blue X-gal/LacZ staining in heart tissue as a surrogate for CysLT2R expression, enhanced expression of this receptor subtype after 48 hours of I/R in the infarct and peri-infarct zones was observed (Figure 1). Although the specific cell types expressing the induced receptor were not positively identified, several cell types including vascular smooth muscle12 and endothelial cells,15 Purkinje conducting cells,11 mesenchymal stem cells, and perhaps some leukocytes are possible candidates.
Bay-u9773 is a nonspecific antagonist of CysLT1R and CysLT2R, rendering it difficult to determine the precise contributions of each receptor subtype to ischemic injury. This is further complicated by the recent discovery of a third CysLT receptor subtype termed GPR17, that can bind CysLT1R antagonists and was found to participate in focal rat brain ischemic injury.10 Therefore, depending on the organ and tissue-specific vascular beds, various CysLT receptor subtypes might contribute to inflammatory vascular permeability changes in ischemic injury. In our studies, absence of leukotriene ligand to CysLT2R, as represented in 5LO-deficient mice, and in preliminary studies with the recently acquired CysLT2R-deficient LacZ mice (n = 3, data not shown), lack of ligand/receptor did not significantly influence myocardial injury compared to ntg mice. These data are consistent with those in a recent study showing that I/R injury did not differentially affect infarct size in 5LO-deficient mice compared to wild-type controls49 but apparently not in agreement with the studies mentioned above with leukotriene biosynthesis inhibitors.39,40 Moreover, the finding that Bay-u9773 did not reduce infarct size below baseline levels in ntg mice suggests that endogenous CysLT2R does not play a significant role in I/R injury in contrast to the transgenic overexpression of the receptor. However, the observation that I/R induces murine CysLT2R in our study at 48 hours, along with a recent report examining CysLT2R expression in human brain tissue finding increased expression in microvascular endothelium after traumatic injury,50 warrants further study to examine the pathophysiological sequelae of induction of CysLT2R.
The mechanism by which endothelial CysLT2R overexpression leads to increased myocyte apoptosis is not known. To our knowledge no direct role of CysLT2R in cardiomyocyte apoptosis has been established. The cascade of events leading to myocyte apoptosis during I/R involves the activation of both the intrinsic mitochondrial proapoptotic pathway as well as the extrinsic pathway mediated by cytokine activation of death receptors.51 Myocytes are particularly prone to apoptosis during reperfusion,52 where up to 30% in the risk area may undergo apoptosis in the first few hours after reperfusion. The events of reperfusion that lead to cardiomyocyte apoptosis have not been fully elucidated. However, reactive oxygen species and cytokines produced by infiltrating inflammatory cells appear to play a central role in activating apoptotic pathways in myocytes.52,53 For example, genetic mouse models harboring deletions of tumor necrosis factor-α and CD18 genes show reduced infarct size in response to I/R in association with decreased neutrophil infiltration, whereas null mice for the anti-oxidant gene heme oxygenase-1 have increased infarct size and reduced LV recovery in parallel with a decrease in antioxidant load.52 In the current study, the enhanced influx of CD45+ leukocytes, presumably mostly neutrophils, after reperfusion in CysLT2R tg mice could potentially contribute to the enhanced myocyte apoptosis seen in these animals by a similar mechanism; however, additional mechanisms may also be at play. Regardless of mechanism, the increased apoptosis in tg mice would predictably lead to greater long-term loss of myocardial contractile mass, resulting in LV chamber remodeling and impairment of contractile function. Indeed, in the current study, CysLT2R mice show accelerated LV remodeling, highlighted by decreased anterior wall thickness and increased LV systolic dimensions 2 weeks after reperfusion. Typically, LV remodeling in mice with reperfused myocardium is slow and often absent,37 unless a significant amount of the LV (>40% of the area at risk) is infarcted. Our results indicate that tg CysLT2R mice had significantly larger infarcts than the ntg counterparts. We presume that the heightened apoptosis is, at least partially, responsible for the larger infarct sizes and subsequent LV remodeling in these mice.
As expected, LV remodeling in tg mice was accompanied by impaired LV function after reperfusion. We believe that this is directly attributable to the greater myocyte loss in tg mice, because basal LV function and responsiveness to β-adrenergic stimulation did not differ significantly between the two groups of animals. In contrast, ntg mice were able to preserve LV function after reperfusion because of the significantly smaller infarcts and absence of negative remodeling. Interestingly, both genotypes showed marked refractoriness to dobutamine after reperfusion. The mechanism underlying this lack of response appears to be unrelated to CysLT2R overexpression, because it is also present in ntg controls. β-Adrenergic receptor desensitization usually occurs after myocardial infarction as the sympathetic nervous system attempts to maintain hemodynamic homeostasis. However, desensitization usually occurs throughout a longer time course than in the current studies, and is unlikely to be the explanation for the postreperfusion refractoriness to dobutamine.
In summary, the results of the current study indicate that endothelial-targeted overexpression of CysLT2R exacerbates myocardial injury after ischemia reperfusion in association with increased inflammatory cell infiltration and cardiomyocyte apoptosis. Inhibition of endothelial CysLT2R activity should be explored further as a potential strategy for myocardial protection.
Acknowledgments
We thank Karoline Machado and the staff at the Queen’s University Transgenic Animal Facility for performing the embryo transfer procedures to obtain CysLT2R-deficient LacZ mice. Yiqun Hui is kindly acknowledged for supplying the CysLT2R transgenic mice.
Footnotes
Address reprint requests to Colin D. Funk, Department of Physiology, 433 Botterell Hall, Stuart St., Queen’s University, Kingston, ON K7L 3N6 Canada. E-mail: funkc@queensu.ca.
Supported by the Canadian Institutes of Health Research (grants MOP 68930 to C.D.F. and MOP 79506 to L.G.M.), the Heart and Stroke Foundation of Ontario (grant NA 5779 to L.G.M.), and the Pharmacological Society of Canada (Merck Frosst postdoctoral fellowship to S.R.H.).
This publication is dedicated to the honor of Luis G. Melo who passed away suddenly on September 26, 2007 after a brief and courageous battle with pancreatic cancer.
C.D.F. and L.G.M. hold Canada Research Chairs. C.D.F. is a career investigator of the Heart and Stroke Foundation of Ontario.
References
- Buja LM. Myocardial ischemia and reperfusion injury. Cardiovasc Pathol. 2005;14:170–175. doi: 10.1016/j.carpath.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Moens AL, Claeys MJ, Timmermans JP, Vrints CJ. Myocardial ischemia/reperfusion-injury, a clinical view on a complex pathophysiological process. Int J Cardiol. 2005;100:179–190. doi: 10.1016/j.ijcard.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Park JL, Lucchesi BR. Mechanisms of myocardial reperfusion injury. Ann Thorac Surg. 1999;68:1905–1912. doi: 10.1016/s0003-4975(99)01073-5. [DOI] [PubMed] [Google Scholar]
- Di Napoli P, Taccardi AA, De Caterina R, Barsotti A. Pathophysiology of ischemia-reperfusion injury: experimental data. Ital Heart J. 2002;3(Suppl 4):24S–28S. [PubMed] [Google Scholar]
- Lefer AM, Tsao PS, Lefer DJ, Ma XL. Role of endothelial dysfunction in the pathogenesis of reperfusion injury after myocardial ischemia. FASEB J. 1991;5:2029–2034. doi: 10.1096/fasebj.5.7.2010056. [DOI] [PubMed] [Google Scholar]
- Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- Funk CD. Leukotriene modifiers as potential therapeutics for cardiovascular disease. Nat Rev Drug Disc. 2005;4:664–672. doi: 10.1038/nrd1796. [DOI] [PubMed] [Google Scholar]
- Hui Y, Funk CD. Cysteinyl leukotriene receptors. Biochem Pharmacol. 2002;64:1549–1557. doi: 10.1016/s0006-2952(02)01357-6. [DOI] [PubMed] [Google Scholar]
- Folco G, Rossoni G, Buccellati C, Berti F, Maclouf J, Sala A. Leukotrienes in cardiovascular diseases. Am J Respir Crit Care Med. 2000;161:S112–S116. doi: 10.1164/ajrccm.161.supplement_1.ltta-22. [DOI] [PubMed] [Google Scholar]
- Ciana P, Fumagalli M, Trincavelli ML, Verderio C, Rosa P, Lecca D, Ferrario S, Parravicini C, Capra V, Gelosa P, Guerrini U, Belcredito S, Cimino M, Sironi L, Tremoli E, Rovati GE, Martini C, Abbracchio MP. The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. EMBO J. 2006;25:4615–4627. doi: 10.1038/sj.emboj.7601341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise CE, O'Dowd BF, Figueroa DJ, Sawyer N, Nguyen T, Im DS, Stocco R, Bellefeuille JN, Abramovitz M, Cheng R, Williams DL, Jr, Zeng Z, Liu Q, Ma L, Clements MK, Coulombe N, Liu Y, Austin CP, George SR, O'Neill GP, Metters KM, Lynch KR, Evans JF. Characterization of the human cysteinyl leukotriene 2 receptor. J Biol Chem. 2000;275:30531–30536. doi: 10.1074/jbc.M003490200. [DOI] [PubMed] [Google Scholar]
- Takasaki J, Kamohara M, Matsumoto M, Saito T, Sugimoto T, Ohishi T, Ishii H, Ota T, Nishikawa T, Kawai Y, Masuho Y, Isogai T, Suzuki Y, Sugano S, Furuichi K. The molecular characterization and tissue distribution of the human cysteinyl leukotriene CysLT(2) receptor. Biochem Biophys Res Commun. 2000;274:316–322. doi: 10.1006/bbrc.2000.3140. [DOI] [PubMed] [Google Scholar]
- Lötzer K, Spanbroek R, Hildner M, Urbach A, Heller R, Bretschneider E, Galczenski H, Evans JF, Habenicht AJ. Differential leukotriene receptor expression and calcium responses in endothelial cells and macrophages indicate 5-lipoxygenase-dependent circuits of inflammation and atherogenesis. Arterioscler Thromb Vasc Biol. 2003;23:e32–e36. doi: 10.1161/01.ATV.0000082690.23131.CB. [DOI] [PubMed] [Google Scholar]
- Kamohara M, Takasaki J, Matsumoto M, Matsumoto Si, Saito T, Soga T, Matsushime H, Furuichi K. Functional characterization of cysteinyl leukotriene CysLT(2) receptor on human coronary artery smooth muscle cells. Biochem Biophys Res Commun. 2001;287:1088–1092. doi: 10.1006/bbrc.2001.5695. [DOI] [PubMed] [Google Scholar]
- Hui Y, Yang G, Galczenski H, Figueroa DJ, Austin CP, Copeland NG, Gilbert DJ, Jenkins NA, Funk CD. The murine cysteinyl leukotriene 2 (CysLT2) receptor. cDNA and genomic cloning, alternative splicing, and in vitro characterization. J Biol Chem. 2001;276:47489–47495. doi: 10.1074/jbc.M107556200. [DOI] [PubMed] [Google Scholar]
- Hui Y, Cheng Y, Smalera I, Jian W, Goldhahn L, Fitzgerald GA, Funk CD. Directed vascular expression of human cysteinyl leukotriene 2 receptor modulates endothelial permeability and systemic blood pressure. Circulation. 2004;110:3360–3366. doi: 10.1161/01.CIR.0000147775.50954.AA. [DOI] [PubMed] [Google Scholar]
- Kanaoka Y, Boyce JA. Cysteinyl leukotrienes and their receptors: cellular distribution and function in immune and inflammatory responses. J Immunol. 2004;173:1503–1510. doi: 10.4049/jimmunol.173.3.1503. [DOI] [PubMed] [Google Scholar]
- Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- Barst S, Mullane K. The release of a leukotriene D4-like substance following myocardial infarction in rabbits. Eur J Pharmacol. 1985;114:383–387. doi: 10.1016/0014-2999(85)90384-x. [DOI] [PubMed] [Google Scholar]
- Carry M, Korley V, Willerson JT, Weigelt L, Ford-Hutchinson AW, Tagari P. Increased urinary leukotriene excretion in patients with cardiac ischemia. In vivo evidence for 5-lipoxygenase activation. Circulation. 1992;85:230–236. doi: 10.1161/01.cir.85.1.230. [DOI] [PubMed] [Google Scholar]
- Yu GL, Wei EQ, Zhang SH, Xu HM, Chu LS, Zhang WP, Zhang Q, Chen Z, Mei RH, Zhao MH. Montelukast, a cysteinyl leukotriene receptor-1 antagonist, dose- and time-dependently protects against focal cerebral ischemia in mice. Pharmacology. 2005;73:31–40. doi: 10.1159/000081072. [DOI] [PubMed] [Google Scholar]
- Fang SH, Zhou Y, Chu LS, Zhang WP, Wang ML, Yu GL, Peng F, Wei EQ. Spatio-temporal expression of cysteinyl leukotriene receptor-2 mRNA in rat brain after focal cerebral ischemia. Neurosci Lett. 2007;412:78–83. doi: 10.1016/j.neulet.2006.10.065. [DOI] [PubMed] [Google Scholar]
- Sener G, Sehirli O, Velioglu-Ogunc A, Cetinel S, Gedik N, Caner M, Sakarcan A, Yegen BC. Montelukast protects against renal ischemia/reperfusion injury in rats. Pharmacol Res. 2006;54:65–71. doi: 10.1016/j.phrs.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Chen XS, Sheller JR, Johnson EN, Funk CD. Role of leukotrienes revealed by targeted disruption of the 5-lipoxygenase gene. Nature. 1994;372:179–182. doi: 10.1038/372179a0. [DOI] [PubMed] [Google Scholar]
- Tarnavski O, McMullen JR, Schinke M, Nie Q, Kong S, Izumo S. Mouse cardiac surgery: comprehensive techniques for the generation of mouse models of human diseases and their application for genomic studies. Physiol Genom. 2004;16:349–360. doi: 10.1152/physiolgenomics.00041.2003. [DOI] [PubMed] [Google Scholar]
- Liu X, Wei J, Peng DH, Layne MD, Yet SF. Absence of heme oxygenase-1 exacerbates myocardial ischemia/reperfusion injury in diabetic mice. Diabetes. 2005;54:778–784. doi: 10.2337/diabetes.54.3.778. [DOI] [PubMed] [Google Scholar]
- Petzelbauer P, Zacharowski PA, Miyazaki Y, Friedl P, Wickenhauser G, Castellino FJ, Groger M, Wolff K, Zacharowski K. The fibrin-derived peptide Bbeta15-42 protects the myocardium against ischemia-reperfusion injury. Nat Med. 2005;11:298–304. doi: 10.1038/nm1198. [DOI] [PubMed] [Google Scholar]
- Schumacher J, Binkowski K, Dendorfer A, Klotz KF. Organ-specific extravasation of albumin-bound Evans blue during nonresuscitated hemorrhagic shock in rats. Shock. 2003;20:565–568. doi: 10.1097/01.shk.0000093540.78705.71. [DOI] [PubMed] [Google Scholar]
- Younger JG, Sasaki N, Delgado J, Ko AC, Nghiem TX, Waite MD, Till GO, Ward PA. Systemic and lung physiological changes in rats after intravascular activation of complement. J Appl Physiol. 2001;90:2289–2295. doi: 10.1152/jappl.2001.90.6.2289. [DOI] [PubMed] [Google Scholar]
- Harja E, Bucciarelli LG, Lu Y, Stern DM, Zou YS, Schmidt AM, Yan SF. Early growth response-1 promotes atherogenesis: mice deficient in early growth response-1 and apolipoprotein E display decreased atherosclerosis and vascular inflammation. Circ Res. 2004;94:333–339. doi: 10.1161/01.RES.0000112405.61577.95. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Tang T, Lai NC, Roth DM, Rebolledo B, Saito M, Lew WY, Clopton P, Hammond HK. Increased cardiac adenylyl cyclase expression is associated with increased survival after myocardial infarction. Circulation. 2006;114:388–396. doi: 10.1161/CIRCULATIONAHA.106.632513. [DOI] [PubMed] [Google Scholar]
- Dewald O, Ren G, Duerr GD, Zoerlein M, Klemm C, Gersch C, Tincey S, Michael LH, Entman ML, Frangogiannis NG. Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am J Pathol. 2004;164:665–677. doi: 10.1016/S0002-9440(10)63154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Scott G, Ray MK, Mishina Y, Zhang Y. Histone demethylase JHDM2A is critical for Tnp1 and Prm1 transcription and spermatogenesis. Nature. 2007;450:119–123. doi: 10.1038/nature06236. [DOI] [PubMed] [Google Scholar]
- Liu X, Simpson JA, Brunt KR, Ward CA, Hall SR, Kinobe RT, Barrette V, Tse MY, Pang SC, Pachori AS, Dzau VJ, Ogunyankin K, Melo LG. Pre-emptive heme oxygenase-1 gene delivery reveals reduced mortality and preservation of left ventricular function one year after acute myocardial infarction. Am J Physiol. 2007;293:H48–H59. doi: 10.1152/ajpheart.00741.2006. [DOI] [PubMed] [Google Scholar]
- Hall SR, Wang L, Milne B, Hong M. Left ventricular dysfunction after acute intracranial hypertension is associated with increased hydroxyl free radical production, cardiac ryanodine hyperphosphorylation, and troponin I degradation. J Heart Lung Transplant. 2005;24:1639–1649. doi: 10.1016/j.healun.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Anversa P, Beghi C, Kikkawa Y, Olivetti G. Myocardial infarction in rats. Infarct size, myocyte hypertrophy and capillary growth. Circ Res. 1986;58:26–37. doi: 10.1161/01.res.58.1.26. [DOI] [PubMed] [Google Scholar]
- De Celle T, Cleutjens JP, Balnkesteijn WM, Debets JJ, Smits JF, Janssen BJ. Long-term structural and functional consequences of cardiac ischemia-reperfusion injury in vivo in mice. Exp Physiol. 2004;89:605–615. doi: 10.1113/expphysiol.2004.027649. [DOI] [PubMed] [Google Scholar]
- Rubanyi GM. The role of endothelium in cardiovascular homeostasis and disease. J Cardiovasc Pharmacol. 1993;22:S1–S4. doi: 10.1097/00005344-199322004-00002. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Ueno A, Kswamura M, Katori M, Shigehiro S, Kikawada R. Reduction of myocardial infarct size in rats by selective 5-lipooxygenase inhibitor (AA-861). Adv Prostglandin Thromboxane Leukot Res. 1987;17A:381–383. [PubMed] [Google Scholar]
- Rossoni G, Sala A, Berti F, Testa T, Buccellati C, Molta C, Muller-Peddinghaus R, Maclouf J, Folco GC. Myocardial protection by the leukotriene synthesis inhibitor BAY X1005: importance of transcellular biosynthesis of cysteinyl-leukotrienes. J Pharmacol Exp Ther. 1996;276:335–341. doi: 10.1163/2211730x96x00180. [DOI] [PubMed] [Google Scholar]
- Hock CE, Beck LD, Papa LA. Peptide leukotriene antagonism in myocardial ischemia and reperfusion. Cardiovasc Res. 1992;26:1206–1211. doi: 10.1093/cvr/26.12.1206. [DOI] [PubMed] [Google Scholar]
- Helgadottir A, Manolescu A, Thorleifsson G, Gretarsdottir S, Jonsdottir H, Thorsteinsdottir U, Samani NJ, Gudmundsson G, Grant SF, Thorgeirsson G, Sveinbjornsdottir S, Valdimarsson EM, Matthiasson SE, Johannsson H, Gudmundsdottir O, Gurney ME, Sainz J, Thorhallsdottir M, Andresdottir M, Frigge ML, Topol EJ, Kong A, Gudnason V, Hakonarson H, Gulcher JR, Stefansson K. The gene encoding 5-lipoxygenase activating protein confers risk of myocardial infarction and stroke. Nat Genet. 2004;36:233–239. doi: 10.1038/ng1311. [DOI] [PubMed] [Google Scholar]
- Sala A, Folco G. Neutrophils, endothelial cells, and cysteinyl leukotrienes: a new approach to neutrophil-dependent inflammation? Biochem Biophys Res Commun. 2001;283:1003–1006. doi: 10.1006/bbrc.2001.4865. [DOI] [PubMed] [Google Scholar]
- Pedersen KE, Bochner BS, Undem BJ. Cysteinyl leukotrienes induce P-selectin expression in human endothelial cells via a non-CysLT1 receptor-mediated mechanism. J Pharmacol Exp Ther. 1997;281:655–662. [PubMed] [Google Scholar]
- Di Gennaro A, Carnini C, Buccellati C, Ballerio R, Zarini S, Fumagalli F, Viappiani S, Librizzi L, Hernandez A, Murphy RC, Constantin G, De Curtis M, Folco G, Sala A. Cysteinyl-leukotrienes receptor activation in brain inflammatory reactions and cerebral edema formation: a role for transcellular biosynthesis of cysteinyl-leukotrienes. FASEB J. 2004;18:842–844. doi: 10.1096/fj.03-0599fje. [DOI] [PubMed] [Google Scholar]
- Uzonyi B, Lotzer K, Jahn S, Kramer C, Hildner M, Bretschneider E, Radke D, Beer M, Vollandt R, Evans JF, Funk CD, Habenicht AJ. Cysteinyl leukotriene 2 receptor and protease-activated receptor 1 activate strongly correlated early genes in human endothelial cells. Proc Natl Acad Sci USA. 2006;103:6326–6331. doi: 10.1073/pnas.0601223103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan SF, Fujita T, Lu J, Okada K, Shan Zou Y, Mackman N, Pinsky DJ, Stern DM. Egr-1, a master switch coordinating upregulation of divergent gene families underlying ischemic stress. (Erratum in: Nat Med 2001, 7:509). Nat Med. 2000;6:1355–1361. doi: 10.1038/82168. [DOI] [PubMed] [Google Scholar]
- Figueroa DJ, Breyer RM, Defoe SK, Kargman S, Daugherty BL, Waldburger K, Liu Q, Clements M, Zeng Z, O'Neill GP, Jones TR, Lynch KR, Austin CP, Evans JF. Expression of the cysteinyl leukotriene 1 receptor in normal human lung and peripheral blood leukocytes. Am J Respir Crit Care Med. 2001;163:226–233. doi: 10.1164/ajrccm.163.1.2003101. [DOI] [PubMed] [Google Scholar]
- Adamek A, Jung S, Dienesch C, Laser M, Ertl G, Bauersachs J, Frantz S. Role of 5-lipoxygenase in myocardial ischemia-reperfusion injury in mice. Eur J Pharmacol. 2007;57:51–54. doi: 10.1016/j.ejphar.2007.05.040. [DOI] [PubMed] [Google Scholar]
- Hu H, Chen G, Zhang JM, Zhang WP, Zhang L, Ge QF, Yao HT, Ding W, Chen Z, Wei EQ. Distribution of cysteinyl leukotriene receptor 2 in human traumatic brain injury and brain tumors. Acta Pharmacol Sin. 2005;26:685–690. doi: 10.1111/j.1745-7254.2005.00092.x. [DOI] [PubMed] [Google Scholar]
- Crow MT, Mani K, Nam Y-J, Kitsis RN. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ Res. 2004;95:957–970. doi: 10.1161/01.RES.0000148632.35500.d9. [DOI] [PubMed] [Google Scholar]
- Eefting F, Rensing B, Wigman J, Pannekoek WJ, Liu WM, Cramer MJ, Lips DJ, Doevendans PA. Role of apoptosis in reperfusion injury. Cardiovasc Res. 2004;61:414–426. doi: 10.1016/j.cardiores.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Duilio C, Ambrosio G, Kuppusamy P, DiPaula A, Becker LC, Zweier JL. Neutrophils are primary source of O2 radicals during reperfusion after prolonged myocardial ischemia. Am J Physiol. 2001;280:H2649–H2657. doi: 10.1152/ajpheart.2001.280.6.H2649. [DOI] [PubMed] [Google Scholar]