Abstract
The adenovirus E1A 243R oncoprotein encodes a potent transcription-repression function within the N-terminal 80 amino acids. Our proposed model of E1A repression predicts that E1A interacts with important cellular proteins on chromatin. Consistent with this idea, we report here that E1A proteins from in vivo formaldehyde cross-linked 293 cells are closely associated with chromatin even after several stringent purification steps including double isopycnic CsCl density gradient centrifugation and size exclusion chromatography. Likewise, E1A proteins expressed from virus during productive infection of HeLa cells are closely associated with chromatin starting at early times after infection. No other adenoviral proteins are necessary for E1A 243R protein to associate with chromatin. Analyses of chromatin from HeLa cells infected with adenovirus vectors expressing E1A 243R protein with deletions in different E1A functional domains indicate that sequences within the E1A N-terminal repression domain are needed for the majority of E1A’s interactions with chromatin.
Keywords: Adenovirus, Ad E1A, chromatin, N-terminal transcription-repression domain
Introduction
The E1A oncogene is the first viral gene expressed during productive infection of cells with human adenoviruses (Ad) and is essential for virus replication. There are two major isoforms of group C Ad E1A proteins of 243 and 289 amino acid residues (E1A 243R and E1A 289R) that are synthesized from alternatively spliced RNA transcripts (reviewed in Shenk, 2001). The multifunctional E1A 243R protein encodes a potent transcription repression function within the N-terminal 80 amino acids (Song et al., 1995a; Song et al., 1995b; Song et al., 1995c; Song et al., 1997; Boyd et al., 2002; Loewenstein et al., 2006; Green, Panesar, and Green, submitted for publication). We have proposed a two step molecular model for E1A repression (Loewenstein et al., 2006; Green, Panesar, and Loewenstein, submitted). E1A gains access to repressible promoters through interaction with cellular partners, that include p300, as molecular scaffolds. E1A can then interact with the basal transcription machinery where it binds TBP and disrupts TBP-TATA interaction thus aborting transcription-initiation. We have previously identified the E1A N-terminal sequences essential for E1A repression that interact with p300 and TBP and those that are needed to disrupt TBP-TATA complex formation. One consequence of the proposed model of E1A repression is that E1A must associate at one time or another with chromatin in living cells. But E1A is not a DNA binding protein in vivo (Chatterjee et al., 1988; Avvakumov et al., 2002), therefore, any interaction with chromatin must be through E1A’s N-terminal associated cellular protein partners.
In vivo formaldehyde cross-linking is a valuable tool to investigated the interaction been E1A proteins and chromatin since formaldehyde is capable of creating protein-protein and protein-DNA cross-links. It is a particularly facile reagent because it can be used to rapidly fix intact cells and its cross-links can be reversed under relatively mild conditions. Importantly, formaldehyde cross-links occur between appropriate biological molecules when they are in a molecular proximity of 2 angstroms or less (Orlando et al., 1997; Orlando, 2000). Close proximity implies a functional relationship. To investigate the association of E1A with chromatin we have used formaldehyde cross-linking of living cells followed by CsCl density gradient equilibrium centrifugation to purify chromatin bound to its closely associated proteins. Using these stringent conditions, we demonstrate here that E1A 243R associates closely with chromatin and that sequences within the N-terminal repression domain are important for this interaction.
Results
E1A proteins are closely associated with chromatin in Ad transformed human cells
The HEK 293 cell line was generated by transformation of a normal human embryonic kidney cell culture with sheared Ad5 DNA (Graham, 1977) which involved integration of ∼4.5 kb of DNA from the left arm of Ad DNA into the host genome (Louis, 1997). 293 cells constitutively express high levels of both E1A 243R and E1A 289R. Formaldehyde was used to cross-link closely associated intracellular proteins to chromatin in cultured 293 cells. Chromatin was isolated from nuclei and sonicated to produce soluble fragments of ∼500-2000 bp as described in Materials and methods. Chromatin fragments were purified and separated from free nuclear proteins by isopycnic centrifugation for 72 h in CsCl density gradients. Gradient fractions were collected and chromatin DNA visualized by agarose gel electrophoresis followed by staining with ethidium bromide (EtBr). Fractions containing E1A proteins were identified by immunoblot analysis using E1A specific antibody that detects both E1A 243R and E1A 289R proteins.
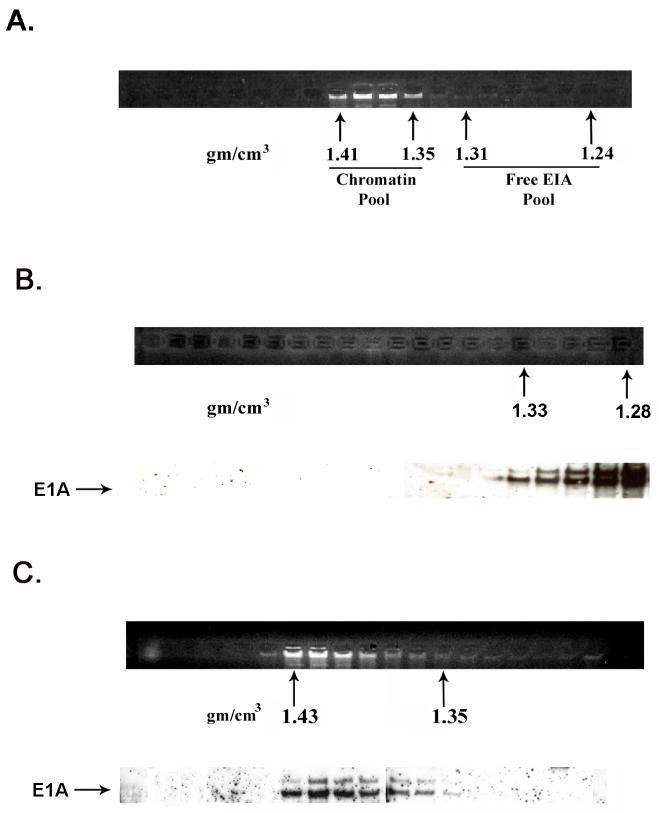
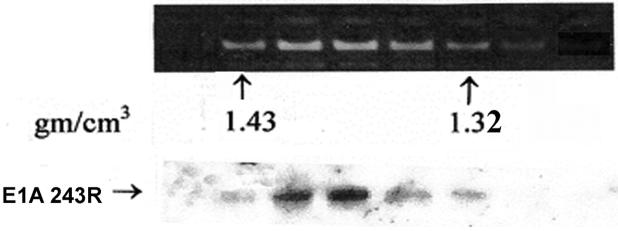
Chromatin containing fractions which equilibrate near the middle of the CsCl gradient at a buoyant density of ∼1.35-1.43 g/cm3 contain appreciable amounts of E1A protein, whereas fractions at the top of the gradient at a density of ∼1.24-1.31 (see Fig. 1A) contain the majority of E1A as free protein. To determine whether E1A protein associated with chromatin represent contamination with free E1A protein, fractions containing chromatin and fractions containing free E1A protein were separately pooled and subjected to a second round of CsCl density gradient centrifugation. E1A protein in the second gradient of the free protein pool equilibrated at the top of the gradient as expected (Fig. 1B). Significantly, E1A protein in the double purified chromatin-containing fractions remained associated with chromatin (Fig. 1C). These results demonstrate that E1A proteins are closely associated with chromatin and consequently are cross-linked to chromatin in 293 cells.
Fig. 1.
A portion of E1A proteins in HEK 293 cells is closely associated with chromatin. Chromatin from formaldehyde cross-linked 293 cells was subjected to isopycnic centrifugation on CsCl gradients. (A) Fractions containing chromatin and fractions at the top of the gradient containing free proteins were separately pooled and subjected to a second CsCl centrifugation. (B) EtBr staining and E1A immunoblot analysis of the second CsCl gradient of the “free E1A pool” showing that free E1A proteins band reproducibly at the top of the gradient. (C) EtBr staining and E1A immunoblot analysis of the second CsCl gradient of the “chromatin pool” showing that E1A protein associated with chromatin faithfully co-equilibrates with chromatin after a second CsCl gradient. (D) E1A proteins in HEK 293 cells not cross-linked with formaldehyde dissociate from chromatin DNA in the high salt conditions of a CsCl gradient. EtBr staining and E1A immunoblot shows that all E1A proteins equilibrate at the top of the gradient and are not artificially associated with DNA at the bottom of the gradient. (E) E1A proteins in formaldehyde cross-linked 293 cells remain closely associated with chromatin after stringent purification. (Upper panel) Chromatin from cross-linked 293 cells was subjected to isopycnic centrifugation on a CsCl gradient. (Lower panel) Chromatin containing fractions from the CsCl gradient were pooled and subjected to Superose 12 size exclusion chromatography. E1A remained closely associated with chromatin in the excluded fractions (fractions 8 and 9) as indicated by immunoblot analysis. Y-axis values are absorbance at A280.
When chromatin from non cross-linked 293 cells was examined by CsCl centrifugation, all E1A protein equilibrated at the top of the CsCl gradient at the position of free protein and is clearly not associated with cell DNA (Fig. 1D). This is due to the fact that the high salt environment of a CsCl gradient dissociates non covalently-linked proteins from DNA in non cross-linked cells (Orlando et al., 1997).
To provide further evidence that free E1A protein does not contaminate chromatin fractions, chromatin from cross-linked 293 cells after purification by CsCl density gradient centrifugation was subjected to size exclusion chromatography on Superose 12. As shown in Fig. 1E, E1A protein co-purified with the excluded chromatin peak. Since E1A proteins are not sequence-specific DNA binding proteins (Chatterjee et al., 1988; Avvakumov et al., 2002), we conclude that a portion of the E1A proteins in the HEK 293 transformed cell line closely associates (within 2 angstroms) with chromatin because of specific interactions with promoter-bound transcription regulatory proteins (see Discussion).
E1A proteins expressed in Ad infected HeLa cells are bound to chromatin
Because E1A proteins are expressed constitutively in 293 cells, association with chromatin may not represent a natural function of E1A during virus replication. To explore this possibility, HeLa cells at 24 h after productive infection with Ad2 were cross-linked and chromatin isolated and subjected to two rounds of CsCl centrifugation. As with 293 cells, a portion of E1A protein expressed from adenovirus is closely associated with chromatin (Fig. 2A). In other experiments, semi-quantitative immunoblot analysis by ECF (GE Healthcare) showed that from 8 to 12% of E1A proteins in HeLa cells at 24 h after infection are closely associated with chromatin (data not shown).
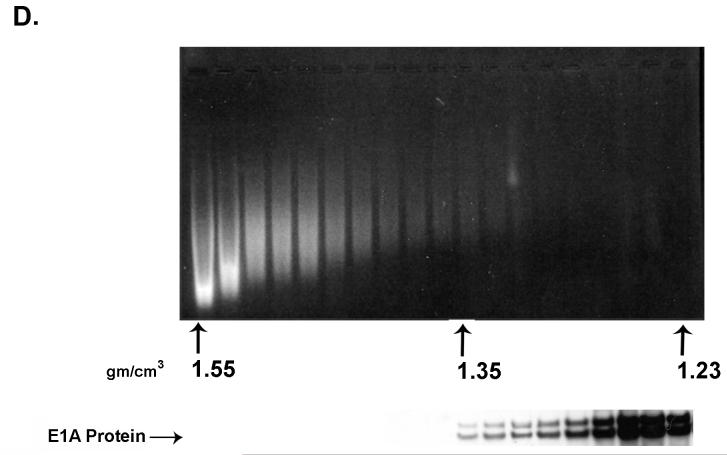
Fig. 2.
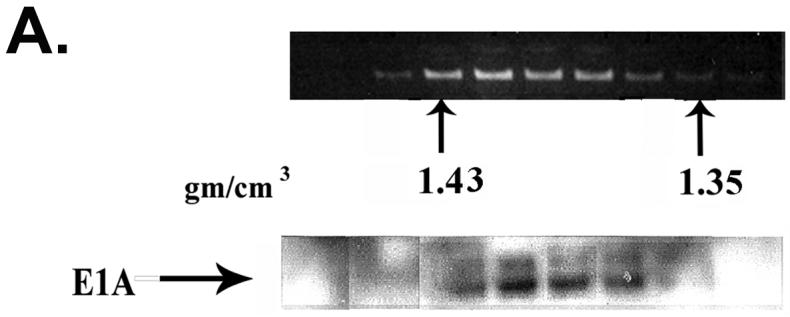
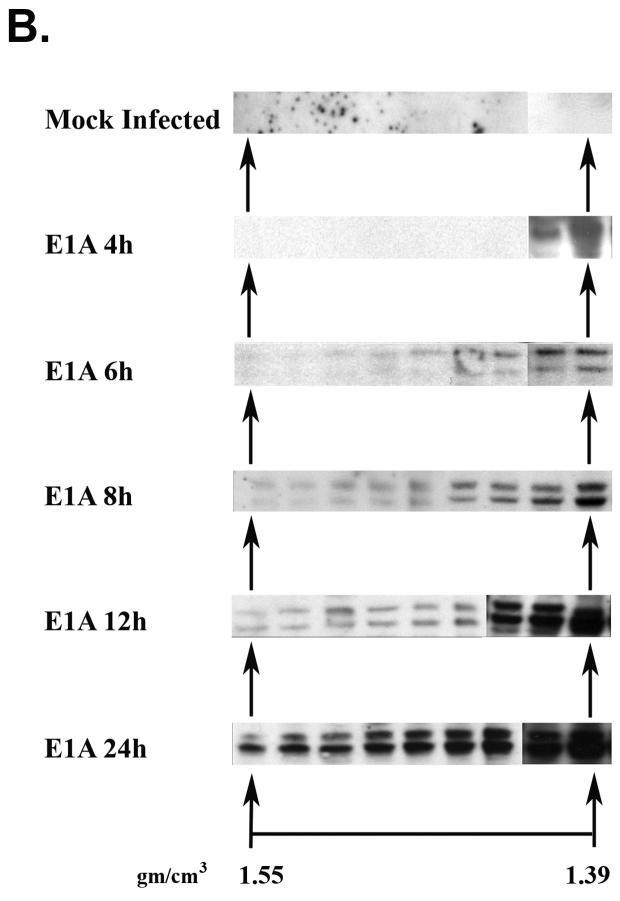
(A) A portion of E1A proteins expressed in Ad2 infected HeLa cells is bound to chromatin. EtBr staining and E1A immunoblot analysis of chromatin-containing fractions from formaldehyde cross-linked Ad2 infected (30 moi) HeLa cells (24 h post-infection) after two rounds of isopycnic CsCl centrifugation. (B) E1A proteins associate with chromatin beginning at early times after Ad2 infection. Replicate flasks of HeLa cells were infected with 30 moi of Ad2. Cells were cross-linked with formaldehyde at the indicated times post-infection, chromatin isolated, and subjected to two rounds of isopycnic centrifugation on CsCl density gradients. Shown are E1A immunoblots of the density gradient fractions at different times after infection. Approximately equal amounts of chromatin from each time point were analyzed.
The association of E1A protein with chromatin occurs at early times during productive infection
The interaction of E1A proteins with chromatin implies functional significance. Another indication of functional significance would be the association of E1A with chromatin at early times after productive infection, as opposed to E1A association with chromatin only at late times which could reflect pathological aftermaths of virus replication and cell lyses. To explore this possibility, HeLa cells were infected with Ad2 and chromatin cross-linked, isolated and purified by double CsCl centrifugation at 4 h, 6 h, 8 h, 12 h, and 24 h after infection. Shown in Fig. 2B are the chromatin-containing fractions from the second gradient probed for E1A protein by immunoblot analysis. E1A is first detected at 4 h at a density of 1.39. By 6 h, E1A is found in all chromatin-containing fractions. As might be expected, as the concentration of E1A within the cell increases over time, the amount associated with chromatin also increases. These findings imply that E1A associates with chromatin in a functional manner early after infection.
Expression of E1A 243R alone is sufficient for association of E1A protein with chromatin
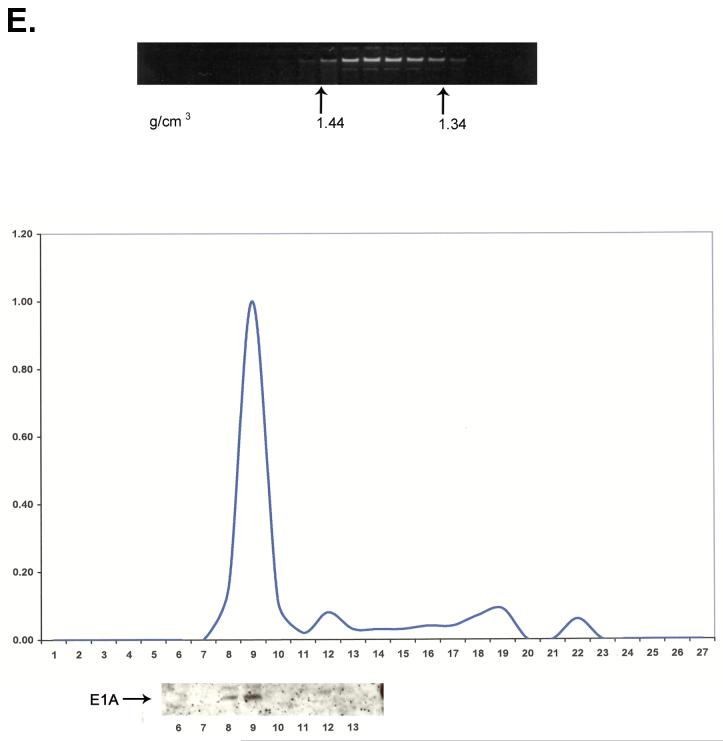
The interaction of E1A proteins with chromatin during productive infection with Ad2 may reflect a unique property of E1A or may require the functions of other Ad proteins. Additionally, experiments described thus far do not determine whether E1A 243R, E1A 289R, or both E1A proteins associate with chromatin. To determine whether E1A 243R can by itself associate with chromatin, HeLa cells were infected with an Ad vector expressing only the E1A 243R protein. Cells were cross-linked with formaldehyde at 36 h after infection and cross-linked chromatin was subjected to two CsCl density gradient centrifugations. Immunoblot analysis showed that double CsCl gradient purified chromatin contains closely associated E1A 243R protein (Fig. 3). These results lead to the conclusion that association with chromatin is a distinct property of the E1A 243R oncoprotein that requires no other viral proteins.
Fig. 3.
E1A 243R expressed in HeLa cells by infection with Ad/CMV-E1A 243R closely associates with chromatin. Shown are E1A immunoblots of the chromatin-containing fractions from the second CsCl gradient.
When chromatin from cross-linked Ad2 infected HeLa cells was analyzed by immunoblot analysis using antibody directed against E1A conserved domain 3 which is specific for E1A 289R, E1A protein was also found associated with chromatin (data not shown). Together, these findings suggest that protein domains common to both E1A 243R and E1A 289R proteins are important for interaction with chromatin.
E1A N-terminal sequences required for the E1A transcription repression function are important for association of the E1A 243R oncoprotein with cellular chromatin
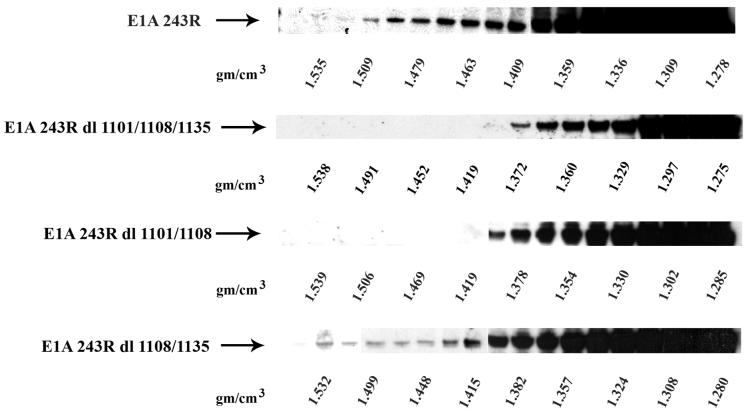
Because E1A 243R is a multifunctional transcription regulatory protein whose multiple domains interact with a number of key cellular proteins, it was of interest to determine which domains are important for interaction of E1A 243R with chromatin. HeLa cells were infected with Ad vectors expressing: (i) full length E1A 243R; (ii) an E1A 243R triple mutant with deletions in the binding sites for TBP and p300 within the N-terminal domain, the Rb binding site domain, and the CtBP binding site domain (dl1101/1108/1135); (iii) an E1A 243R mutant with deletions in the N-terminal domain and the Rb binding site (dl1101/1108); and (iv) an E1A 243R mutant with deletions of both the Rb and CtBP binding sites (dl1108/1135). Cross-linked chromatin was isolated at 36 h after infection, subjected to two CsCl density gradient fractionations, and CsCl fractions analyzed by immunoblot for E1A protein. As seen in Fig. 4, chromatin fractions from both wild type E1A 243R and dl1108/1135, both of which have an intact N-terminal domain, contained E1A protein cross-linked to chromatin. In contrast, chromatin fractions from the triple mutant and dl1101/1108/1135 did not contain E1A detectable by immunoblot analysis. These results show that sequences within the E1A N-terminus are needed for the majority of E1A’s interaction with chromatin.
Fig. 4.
Sequences within the E1A N-terminus are needed for the majority of E1A’s interaction with chromatin. Monolayer cultures of HeLa cells (T150) were infected with Ad/CMV/V5-DEST vectors (30 moi) expressing (i) full length E1A 243R, (ii) E1A 243R dl1101/1108/1135 containing deletions in the N-terminus, Rb binding site, and CtBP binding site, (iii) E1A 243R dl1101/1108 containing deletions in the N-terminus and the Rb binding site, and (iv) E1A 243R dl1108/1135 containing deletions in the Rb and CtBP binding sites. Cells were cross-linked with formaldehyde at 36 h post-infection, chromatin isolated, and subjected to CsCl density gradient centrifugation. Shown are E1A immunoblot analysis of the fractions from the various density gradients.
Discussion
Our working model of E1A transcription-repression (Lowenstein et al., 2005) and the results of transient expression analysis (Green, Panesar, and Loewenstein, submitted), predict that a fraction of E1A protein molecules within infected and transformed cells should interact with chromatin through binding one or more of E1A’s cellular partners. To test this prediction experimentally, formaldehyde was used to cross-link proteins to chromatin in living cells. As reported here, E1A proteins are found closely associated with chromatin in 293 cells which constitutively express E1A (Fig. 1). Additionally, E1A protein from productively infected HeLa cells is closely associated with chromatin (Fig. 2A). Of additional significance, E1A associates with chromatin at early times after infection when the functions of E1A are critical for virus replication (Fig. 2B). These findings taken together strongly argue for the functional interaction of E1A protein with chromatin.
E1A interacts with multiple cellular partners that are involved in functions including transcription repression, transcription activation, histone acetylation, chromatin remodeling and cell cycle regulation. For example, E1A C-terminal conserved region 4 (CR4) interacts with CtBP corepressor molecules (Chinnadurai, 2006), CR3 interacts with the mediator complex (Berk, 2005), CR1 and CR2 interact with Rb protein family members (Berk, 2005), and the N-terminal repression domain which includes the non-conserved N-terminus and CR1 interact with p300/CBP, TBP, TRRAP, GCN5, PCAF, as well as p400 (reviewed in Frisch and Mymryk, 2002). Any of these proteins could logically be involved in the functional association of E1A with chromatin. Interestingly, it has been reported that E1A can alter chromatin structure through interaction with p130 on some E2F site containing promoters thus activating transcription (Ghosh and Harter, 2003).
In terms of the E1A transcription-repression function, it is of interest that E1A 243R in the absence of other viral proteins is capable of interacting with chromatin (Fig. 3). Analyses using Ad vectors expressing E1A 243R mutants with deletions in functional domains show that the N-terminal repression domain is needed for the majority of E1A’s association with chromatin (Fig. 4) as predicted by our model for E1A repression. It is intriguing that the regions within the N-terminus that are important for chromatin association and for the transcriptional repression function are essential for the immortalization and cell transformation functions of E1A. The downstream genes that are the ultimate targets of the E1A functions remain largely unknown. Much more work is needed to identify these genes and to understand the E1A functions that modulate their expression.
Materials and methods
Cell culture
A clonal line of HEK 293 cells (Invitrogen) and HeLa cells (ATTC#CCL-2) were grown in monolayer culture in DME/10% fetal bovine serum. Infection with Ad2 and Ad vectors was carried out as described (Green and Loewenstein, 2005).
Construction of Ad vectors expressing E1A 243R with deletions in functional protein domains
Plasmids expressing Ad5 E1A 243R deletion mutants were kindly provided by Stan Bayley (Howe et al., 1990). E1A sequences were transferred in two steps into the replication-deficient Ad vector pAd/CMV/V5-DEST (Invitrogen Gateway system; deleted in E1A, E1B, and E3). E1A 243R from pLE2/520 and E1A 243R Δ2-25/124-127 from pLE2 dl1101/1108/520 were directionally cloned into the topoisomerase-bound cloning site which lies between two lambda aatL recombination sites in the entry vector pENTR/SD/D-TOPO (see Green and Loewenstein, 2005 for description). Clones were sequenced to assure fidelity. The E1A 243R and E1A 243R dl1101/1108 sequences located between the two aatL sites of the entry vector were exchanged with the DNA fragment located between the two aatR sites in the destination vector pAd/CMV/V5 DEST using the LR clonase reaction (Invitrogen). The resulting Ad E1A expression vectors were linearized by digestion with Pac I, transfected and amplified in HEK 293 cells, purified by CsCl gradient centrifugation, and titered in HEK 293 cells as described (Green and Loewenstein, 2005).
The Ad vector expressing E1A 243R dl1011/1108/1135 was constructed as follows. First, pUC 243R dl1135 (expressing E1A 243R Δ225-237), kindly provided by G. Chinnadurai (Chinnadurai, 2006), was used as template to clone E1A 243R dl1135 into pENTR/SD/D-TOPO by the method described above. Second, pENTR 243R dl1101/1108 and pENTR 243R dl1135 were digested with NcoI (immediately upstream of the N-terminal E1A 243R cloning site) and XbaI (internal to E1A 243R). The NcoI→XbaI fragment from pENTR 243R 1101/1108 (contains the E1A 2-25 and 124-127 deletions and E1A sequences up to the XbaI site) were purified with a gel purification kit (Qiagen). Likewise, the large XbaI→NcoI fragment from pENTR 243R dl1135 (contains E1A C-terminal sequences including the E1A 225-237 deletion and most of the pENTR vector) was purified. These two fragments were ligated to create the vector pENTR E1A 243R dl1101/1108/1135. In a similar manner, the NcoI→XbaI fragment from pENTR 243R dl1108 was isolated and ligated with the Xba→NcoI fragment from pENTR 243R dl1135 to generate pENTR E1A 243R dl1108/1135. The E1A 243R constructs were transferred to pAd/CMV/V5-DEST and the adenovirus expression vectors amplified and purified as described above.
Purification of chromatin from formaldehyde cross-linked cells
T150 flasks of Ad infected HeLa cells or 293 cells were cross-linked with formaldehyde and nuclei isolated as described in Takahashi et al. (2000). Nuclei suspended in 3 ml of sonication buffer (10 mM Tris-HCl, pH 8.0, 1 mM EDTA, 0.5 mM EGTA) were sonicated in 15 ml conical centrifuge tubes on ice under conditions that yield chromatin fragments of 200 to 2000 bp. Three ml of additional buffer plus 4.0 g of CsCl were added to each sonicate to provide a density of ∼1.39-1.40. The chromatin suspensions were placed in polyallomer tubes in a SW 41 rotor, filled to the top with mineral oil, and centrifuged in a Beckmann L90 centrifuge at 28,000 rpm for 72 h at 22°C. Fractions of ∼300 μl were collected from the bottom of each centrifuge tube. The density of each fraction was determined by weighing 100 μl aliquots. Each fraction was dialyzed against sonication buffer supplemented with 10% glycerol at 4° to remove CsCl.
Immunoblot analysis
Aliquots of 10-20 μl of dialyzed chromatin fractions were heated for 45 min at 95°in LDS sample buffer (Invitrogen) to reverse cross-links and denature proteins. Proteins were resolved on NuPage 4-12% Bis-Tris gels and transferred to nitrocellulose membranes (Invitrogen). Immunoblots were probed with E1A polyclonal antibody (Santa Cruz SC-430) and developed using enhanced chemiluminescence reagents (SuperSignal West Femto, Pierce). Semi-quantitative immunoblot analysis was performed by developing blots with ECF reagent (GE Healthcare) followed by analysis on a Storm 840 Phosphorimager (GE Healthcare).
Acknowledgements
We thank Ling Zhao for critical reading of the manuscript and Carolyn Mulhall for insightful editorial assistance. This work was supported by Research Career Award AI-04739 and Public Health Service grant CA29561 to M.G. from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avvakumov N, Sahbegovic M, Zhang A, Shuen M, Mymryk JS. Analysis of DNA binding by the adenovirus type 5 E1A oncoprotein. J. Gen. Virol. 2002;83:517–524. doi: 10.1099/0022-1317-83-3-517. [DOI] [PubMed] [Google Scholar]
- Berk AJ. Recent lessons in gene expression, cell cycle control, and cell biology from adenovirus. Oncogene. 2005;24:7673–7685. doi: 10.1038/sj.onc.1209040. [DOI] [PubMed] [Google Scholar]
- Boyd JM, Loewenstein PM, Tang Q-Q, Yu L, Green M. Adenovirus E1A N-terminal amino acid sequence requirements for repression of transcription in vitro and in vivo correlate with those required for E1A interference with TBP-TATA complex formation. J. Virol. 2002;76:1461–1474. doi: 10.1128/JVI.76.3.1461-1474.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee PK, Bruner M, Flint SJ, Harter ML. DNA binding properties of an adenovirus 289R E1A protein. EMBO Journal. 1988;7:835–841. doi: 10.1002/j.1460-2075.1988.tb02882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnadura G. In: CtBP family proteins. Chinnadura G, editor. Landes Biosciences; Georgetown, TX: 2006. pp. 1–17. [Google Scholar]
- Frisch SM, Mymryk JS. Adenovirus-5 E1A: paradox and paradigm. Nature Reviews Molecular Cell Biology. 2002;3:441–452. doi: 10.1038/nrm827. [DOI] [PubMed] [Google Scholar]
- Ghosh MK, Harter ML. A viral mechanism for remodeling chromatin structure in G0 cells. Mol. Cell. 2003;12:255–260. doi: 10.1016/s1097-2765(03)00225-9. [DOI] [PubMed] [Google Scholar]
- Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Green M, Loewenstein PM. Current Protocols in Microbiology. John Wiley and Sons, Inc; 2005. Human adenoviruses: propagation, purification, quantification, and storage; pp. 14C1.1–14C.1.19. [DOI] [PubMed] [Google Scholar]
- Howe JA, Mymryk JS, Egan C, Branton PE, Bayley ST. Retinoblastoma growth suppressor and a 300-kDa protein appear to regulate cellular DNA synthesis. Proc. Natl. Acad. Sci. USA. 1990;87:5883–5887. doi: 10.1073/pnas.87.15.5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis N, Evelegh C, Graham FL. Cloning and sequencing of the cellular-viral junctions from the human adenovirus type 5 transformed 293 cell line. Virology. 1997;233:423–429. doi: 10.1006/viro.1997.8597. [DOI] [PubMed] [Google Scholar]
- Loewenstein PM, Arackal S, Green M. Mutational and functional analysis of an essential sub-domain of the adenovirus E1A N-terminal transcription repression domain. Virology. 2006;351:312–321. doi: 10.1016/j.virol.2006.03.041. [DOI] [PubMed] [Google Scholar]
- Orlando V, Strutt H, Paro R. Analysis of chromatin structure by in vivo formaldehyde cross-linking. Methods. 1997;11:205–214. doi: 10.1006/meth.1996.0407. [DOI] [PubMed] [Google Scholar]
- Orlando V. Mapping chromosomal proteins in vivo by formaldeyde-crosslinked-chromatin immunoprecipitation. Trends Biochem Sci. 2000;25:99–104. doi: 10.1016/s0968-0004(99)01535-2. [DOI] [PubMed] [Google Scholar]
- Shenk T. Fundamental Virology. Lippincott, Williams and Wilkins; New York, N.Y.: 2001. Adenoviridae: The Viruses and Their Replication; pp. 1053–1088. [Google Scholar]
- Song C-Z, Tierney CJ, Loewenstein PM, Pusztai R, Symington JS, Tang Q, Toth K, Bayley ST, Green M. Transcriptional repression by human adenovirus E1A N-terminus/conserved domain 1 polypeptides in vivo and in vitro in the absence of protein synthesis. J. Biol. Chem. 1995a;40:23263–23267. doi: 10.1074/jbc.270.40.23263. [DOI] [PubMed] [Google Scholar]
- Song C-Z, Loewenstein PM, Green M. Repression in vitro, by human adenovirus E1A protein domains, of basal or Tat-activated transcription of the human immunodeficiency virus type 1 long terminal repeat. J. Virol. 1995b;69:2907–2911. doi: 10.1128/jvi.69.5.2907-2911.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C-Z, Loewenstein PM, Toth K, Green M. TFIID is a direct functional target of the adenovirus E1A transcription-repression domain. Proc. Natl. Acad. Sci. USA. 1995c;92:10330–10333. doi: 10.1073/pnas.92.22.10330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C-Z, Loewenstein PM, Toth K, Tang Q, Nishikawa A, Green M. The adenovirus E1A repression domain disrupts the interaction between the TATA binding protein and the TATA box in a manner reversible by TFIIB. Mol. Cell. Biol. 1997;17:2186–2193. doi: 10.1128/mcb.17.4.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Rayman JB, Dynlacht BB. Analysis of promoter binding by the E2F and pRB families in vivo: distinct E2F proteins mediate activation and repression. Genes Dev. 2000;14:804–816. [PMC free article] [PubMed] [Google Scholar]