«One does not see anything until one sees its beauty.»
Oscar Wilde (1854–1900)
Invisible by design [1, 2], vitreous is often overlooked as an important structure, until “one sees its beauty”. This remarkable structure is an extracellular matrix which has considerable importance in ocular health and disease [3]. Composed primarily of water, vitreous is nonetheless a solid gel in youth (Fig. 1), owing to the intricate association of collagen, hyaluronan, and other molecular components [4]. In youth, this exquisite structure is firmly adherent to the retina in a fascial (as opposed to focal) manner [5]. During aging, there are changes in vitreous macromolecular interactions that result in the formation of liquid vitreous, mostly comprised of hyaluronan and water, because the former is very hydrophyllic. Concurrently, there is aggregation of collagen fibrils into bundles of parallel fibers [6]. Vitreous liquefaction, known as “synchisis”, destabilizes vitreous and promotes its collapse, known as “syneresis” [7]. The impact of vitreous syneresis ranges from negligible to significant, depending upon the state of the vitreo–retinal interface [8].
Fig. 1.
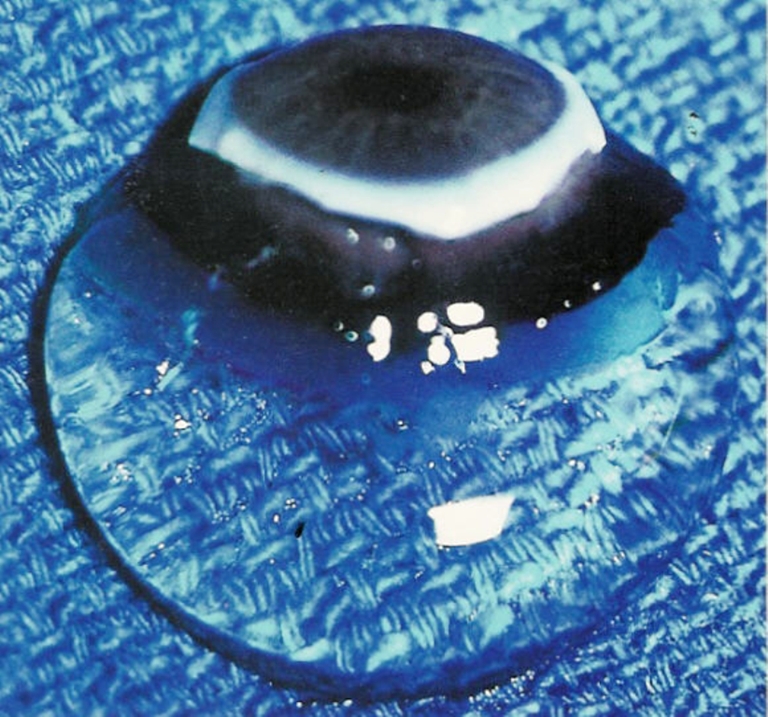
Human vitreous. The sclera, choroid, and retina were dissected off the vitreous in this autopsy specimen obtained form a 9-month-old girl. The vitreous remains attached to the anterior segment. Although the specimen rests upon a surgical towel in room air, the exquisite gel structure is maintained, owing to the young age of the donor. (Specimen courtesy of the New England Eye Bank)
Previous theories of vitreo-retinal interface anatomy proposed that the collagen fibrils of the posterior vitreous cortex insert directly into the retina. However, it is currently recognized that interposed between the internal limiting lamina of the retina and the posterior vitreous cortex is an extracellular matrix ‘glue’ that consists of fibronectin, laminin, opticin, chondroitin sulfate, heparan sulfate, and other extracellular matrix constituents [4]. Another previously held belief was that the posterior vitreous cortex is a single layer of densely-packed collagen fibrils. However, recent studies by Hageman have shown that this may not always be the case. Indeed, immunohistochemistry has shown that the outer layer of vitreous has a lamellar structure, as shown in Fig. 2.
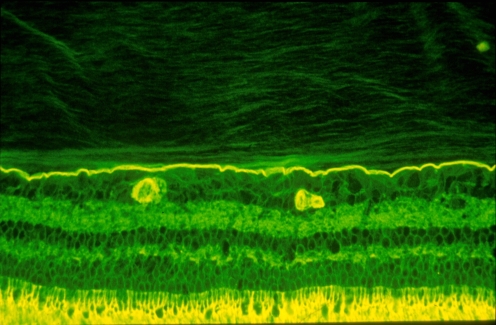
Fig. 2.
Vitreo–retinal interface. Imunohistochemical studies of the monkey vitreo–retinal interface employed fixation in 4% paraformaldehyde and staining with fluorescein-conjugated ABA lectin. The retina is at the bottom and the vitreous is at the top of this image. The intensely-stained, horizontal linear structure is the internal limiting lamina (ILL) of the retina. Above the ILL is the posterior vitreous cortex. The lamellar structure is clearly evident. Effective magnification ∼400x. (Courtesy of Greg Hageman, Ph.D.)
The primary reason vitreous plays an important role in retinal diseases is that it is attached to the retina. Thus, the aging changes in vitreous that result in gel liquefaction may have untoward effects upon the retina in many cases. It is not known what initiates vitreous gel liquefaction, but some invoke metabolic and light-induced reactive oxygen species as the cause of conformational changes in vitreous macromolecules, resulting in dissociation of collagen from hyaluronan and the formation of liquid vitreous [9]. It is also not known what changes occur at the interface to weaken vitreo-retinal adhesion and promote vitreo-retinal dehiscence in the majority of individuals. However, what is known is that both of these two processes must occur simultaneously and in concert to result in an innocuous posterior vitreous detachment (PVD). Individuals who experience gel liquefaction without concurrent dehiscence at the vitreo-retinal interface may experience anomalous PVD [10].
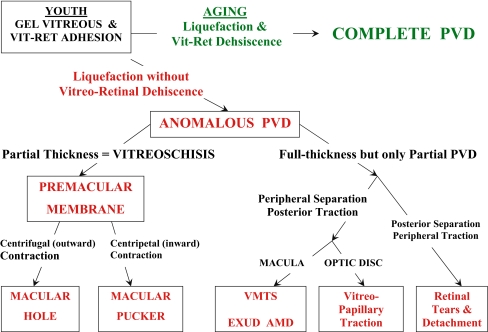
Anomalous PVD can have a variety of manifestations (Fig. 3), depending upon where the gel vitreous is liquefied and where vitreo-retinal adhesion is strong. In the peripheral fundus, advanced gel liquefaction in the presence of strong vitreo-retinal adhesion causes retinal tears and detachments. Retinal lattice with overlying pockets of liquefied vitreous is a good example of peripheral anomalous PVD. At the optic disc, anomalous PVD can induce various vitreo-papillopathies, as well as play a role in promoting neovascularization and vitreous hemorrhage in ischemic retinopathies. At the macula, anomalous PVD can cause a variety of pathologies, depending upon whether or not the posterior vitreous cortex is intact (full thickness) or split (partial thickness). Full-thickness vitreous cortex adherence to the macula with peripheral vitreo-retinal separation can induce vitreo-macular traction, known as the vitreo-macular traction syndrome (VMTS). There is a broad, full-thickness posterior vitreous adhesion in VMTS, while the vitreo-foveal traction syndrome is caused by a more focal adhesion. In both instances, there is peripheral vitreo-retinal separation but persistent adhesion at the macula. The tractional forces are primarily antero-posterior, which induce retinal thickening with edema in the VMTS and a central cyst in the vitreo-foveal traction syndrome. Recent studies by Binder and colleagues in Vienna [11] have shown that in AMD, persistent vitreo-macular adhesion (that on OCT resembles VMTS) is associated with a significantly higher incidence of exudative AMD as compared to dry AMD (36% vs 7% respectively; P < 0.0001). A total PVD was found by ultrasonography in 72% of the eyes with dry AMD, as compared to 34% with exudative AMD (P = 0.0002) [11].
Fig. 3.
Anomalous PVD. This schematic diagram demonstrates the various possible manifestations of anomalous PVD [10]. When gel liquefaction and weakening of vitreo–retinal adhesion occur concurrently, the vitreous separates away from the retina without sequelae (top of diagram). If the separation of vitreous from retina is full-thickness but incomplete, there can be different forms of partial PVD (right side of diagram). Posterior separation with persistent peripheral vitreo–retinal attachment can induce retinal breaks and detachments. Peripheral vitreo–retinal separation with persistent full-thickness attachment of vitreous to the retina posteriorly can induce traction upon the macula, known as the vitreo–macular traction syndrome (VMTS). This phenomenon appears to be highly associated with exudative AMD [11]. Persistent attachment to the optic disc can induce vitreo-papillopathies and also contribute to neovascularization and vitreous hemorrhage in ischemic retinopathies. If during PVD the posterior vitreous cortex splits (vitreoschisis), there can be differences depending upon the level of the split. Vitreoschsis anterior to the level of the hyalocytes leaves a relatively thick, cellular membrane attached to the macula. Inward (centripetal) contraction of this membrane induces macular pucker. If the split occurs at a level posterior to the hyalocytes, the remaining premacular membrane is relatively thin and hypocellular. Outward (centrifugal) tangential traction can induce a macular hole
A split in the posterior vitreous cortex, known as vitreoschisis, induces a different set of vitreo–macular pathologies. Vitreoschisis is the consequence of anomalous PVD [10] with such strong vitreo–macular adhesion that when syneresis occurs, the posterior vitreous cortex splits, leaving the outermost layer attached to the macula, while the remainder of the vitreous collapses forward. This is believed to happen, at least in part, because of the underlying lamellar structure of the posterior vitreous cortex (Fig. 2). Balazs [12] first coined the term “vitreoschisis”, although what he described was actually not true vitreoschisis. Schepens and colleagues [13] were the first to diagnose vitreoschisis in patients with retino-vascular diseases using clinical biomicroscopy. Studies employing ultrasonography [14] and histopathology [15] have also detected vitreoschisis in proliferative diabetic retinopathy, and there is even a report of vitreoschisis in Eale’s disease [4, 16].
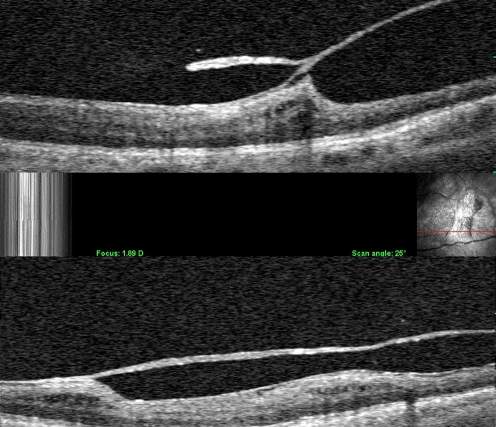
Recently, combined optical coherence tomography/scanning laser ophthalmoscopy (OCT/SLO; OPKO, Inc. Miami) was able to identify vitreoschisis clinically (Fig. 4). Indeed, studies [17] at the VMR Institute found that combined OCT/SLO imaging detected vitreoschisis in 53% of patients with macular holes and in 43% of patients with macular pucker. These studies led to the proposal that if the split occurs anterior to the level of hyalocytes, the remaining layer of vitreous will be relatively thick, hypercellular, and contractile. Contraction of this tissue causes inward (centripetal) tangential traction upon the underlying retina and results in macular pucker. If the split occurs posterior to the level of hyalocytes, a thin hypocellular membrane remains attached to the macula. If also attached to the optic disc, it may induce outward (centrifugal) tangential contraction, causing a macular hole. Fundamental to the theory of anomalous PVD with vitreoschisis is the hypothesis that the posterior vitreous cortex can indeed split into layers.
Fig. 4.
Clinical vitreoschisis. Combined OCT/SLO imaging detected a split in the posterior vitreous cortex. In these two cases, the outer layer of the split posterior vitreous cortex remains adherent to the retina. The point where the two layers re-join into one full-thickness layer is often the site of significant traction upon the retina. Studies have shown that about half of patients with macular hole and macular pucker have vitreoschisis
In this issue of Graefe’s Archive for Clinical and Experimental Ophthalmology, Yamashita, Uemura, and Sakamoto present the first in vivo, intra-operative evidence of the occurrence of vitreoschisis. In this important study, 80% of eyes with macular pucker were found to develop vitreoschisis during vitrectomy surgery employing intra-operative triamcinolone to enhance visualization. Amongst those eyes that developed vitreoschisis intra-operatively, 58% had a visible ‘hole’ in the posterior vitreous cortex, while 42% of such eyes had no visible hole, suggesting that vitreoschisis can occur at various levels within the posterior vitreous cortex. This is consistent with the underlying multi-lamellar anatomy of the posterior vitreous (Fig. 2). These findings not only significantly contribute to our understanding of pathogenic mechanisms in vitreo-retinal disorders, but could also influence the performance of vitreo-retinal surgery. Knowing that the posterior vitreous cortex can split into layers (either pre-operatively or during surgery) can influence how aggressively one searches for membranes during membrane peeling. A higher index of suspicion in certain cases could also assist in the decision as to whether or not to employ surgical adjuncts like colored stains, such as indocyanine green and trypan blue, or particulate suspensions, such as the triamcinolone used by Yamashita and colleagues in their study. Selectively, more aggressive membrane dissection could also favorably impact upon the incidence of failed surgery and recurrences.
Vitreoschisis may be present in a variety of other vitreo-retinal diseases. It is furthermore possible that at least part of the multi-lamellar structure present at the vitreo-retinal interface in pathologic eyes is the internal limiting lamina of the retina. Advances in imaging technology will hopefully provide ever more powerful methods with which to explore these possibilities. However, it is important to emphasize that the technologic developments that will provide these new diagnostic instruments must be matched by advances in our understanding and knowledge of vitreous anatomy and pathobiology, so that the new diagnostic technology will be used intelligently and productively. Investigators in this area are thus well-advise to recall that
«What we see depends mainly on what we look for.»
Sir John William Lubbock (1803–1865)
Biography
Dr. Sebag is Professor of Clinical Ophthalmology at the University of Southern California, and Founding Director of the VMR Institute in Huntington Beach, California (www.VMRinstitute.com).
References
- 1.Sebag J (2004) Seeing the invisible: the challenge of imaging vitreous. J Biomed Opt 9:38–46 [DOI] [PubMed]
- 2.Sebag J (1997) Classifying posterior vitreous detachment—a new way to look at the invisible (Guest editorial). Brit J Ophthalmol 81:521–522 [DOI] [PMC free article] [PubMed]
- 3.Sebag J (1989) The vitreous—structure, function, and pathobiology. Springer-Verlag, New York
- 4.Sebag J (1998) Macromolecular structure of vitreous. Prog Polym Sci 23:415–446
- 5.Sebag J (1991) Age-related differences in the human vitreo-retinal interface. Arch Ophthalmol 109:966–971 [DOI] [PubMed]
- 6.Sebag J, Balazs EA (1989) Morphology and ultrastructure of human vitreous fibers. Invest Ophthalmol Vis Sci 30:1867–1871 [PubMed]
- 7.Sebag J (1992) The vitreous. In: Hart WM Jr (ed) Adler’s Physiology of the Eye. Mosby, St. Louis, pp 268–347
- 8.Green WR, Sebag J (2001) Vitreous and the vitreo-retinal interface. In: Ryan SJ (ed) Retina. Mosby, St. Louis, Vol III, pp 1882–1960
- 9.Ueno N, Sebag J, Hirokawa H, Chakrabarti B (1987) Effects of visible-light irradiation on vitreous structure in the presence of a photosensitizer. Exp Eye Res 44:863–870 [DOI] [PubMed]
- 10.Sebag J (2004) Anomalous PVD—a unifying concept in vitreo-retinal diseases. Graefes Arch Clin Exp Ophthalmol 242:690–698 [DOI] [PubMed]
- 11.Krebs I, Brannath W, Glittenberg C, Zeiler F, Sebag J, Binder S (2007) Posterior vitreomacular adhesion: a potential risk factor for exudative age-related macular degeneration? Am J Ophthalmol 144:741–746 [DOI] [PubMed]
- 12.Balazs EA (1973) The vitreous. In: Zinn K (ed) Ocular fine structure for the clinician (International Ophthalmology Clinics Vol 13 No 3). Little Brown, Boston, pp 169–187 [PubMed]
- 13.Kakehashi A, Schepens CL, de Sousa-Neto A et al (1993) Biomicroscopic findings of posterior vitreoschisis. Ophthalmic Surg 24:846–850 [PubMed]
- 14.Chu TG, Lopez P, Cano MR, et al (1996) Posterior vitreoschisis. An echographic finding in proliferative diabetic retinopathy. Ophthalmology 103:315–322 [DOI] [PubMed]
- 15.Schwartz SD, Alexander R, Hiscott P, Gregor Z (1996) Recognition of vitreoschisis in proliferative diabetic retinopathy. A useful landmark in vitrectomy for diabetic traction retinal detachment. Ophthalmology 103:323–328 [DOI] [PubMed]
- 16.Badrinath SS, Gopal L, Sharma T, et al (1999) Vitreoschisis in Eales’ disease: pathogenic role and significance in surgery. Retina 19:51–54 [DOI] [PubMed]
- 17.Sebag J, Gupta P, Rosen R, Garcia P, Sadun AA (2007) Macular holes and macular pucker – the role of vitreoschisis as imaged by optical coherence tomography/scanning laser ophthalmoscopy. Trans Am Ophthalmol Soc 105, (in press) [PMC free article] [PubMed]