Abstract
Intracoronary testosterone infusions induce coronary vasodilatation and increase coronary blood flow. Longer term testosterone supplementation favorably affected signs of myocardial ischemia in men with low plasma testosterone and coronary heart disease. However, the effects on myocardial perfusion are unknown. Effects of longer term testosterone treatment on myocardial perfusion and vascular function were investigated in men with CHD and low plasma testosterone. Twenty-two men (mean age 57 ± 9 [SD] years) were randomly assigned to oral testosterone undecanoate (TU; 80 mg twice daily) or placebo in a crossover study design. After each 8-week period, subjects underwent at rest and adenosine-stress first-pass myocardial perfusion cardiovascular magnetic resonance, pulse-wave analysis, and endothelial function measurements using radial artery tonometry, blood sampling, anthropomorphic measurements, and quality-of-life assessment. Although no difference was found in global myocardial perfusion after TU compared with placebo, myocardium supplied by unobstructed coronary arteries showed increased perfusion (1.83 ± 0.9 vs 1.52 ± 0.65; p = 0.037). TU decreased basal radial and aortic augmentation indexes (p = 0.03 and p = 0.02, respectively), indicating decreased arterial stiffness, but there was no effect on endothelial function. TU significantly decreased high-density lipoprotein cholesterol and increased hip circumference, but had no effect on hemostatic factors, quality of life, and angina symptoms. In conclusion, oral TU had selective and modest enhancing effects on perfusion in myocardium supplied by unobstructed coronary arteries, in line with previous intracoronary findings. The TU-related decrease in basal arterial stiffness may partly explain previously shown effects of exogenous testosterone on signs of exercise-induced myocardial ischemia.
Cross-sectional studies suggest an inverse association between coronary heart disease (CHD) incidence and/or severity and endogenous testosterone levels irrespective of age,1 and testosterone treatment increases coronary blood flow and improved signs on myocardial ischemia in men with CHD.2,3 The aim of the present study was to investigate the hypothesis that longer term oral testosterone undecanoate (TU) treatment beneficially affects myocardial perfusion in men with established CHD and low testosterone. Second, we investigated effects on vascular and endothelial function, selected metabolic risk factors for CHD, quality of life, and angina symptoms.
Methods
Men aged 40 to 75 years with angiographically proven CHD (≥70% lesion in ≥1 major coronary artery or major branch) were recruited from the outpatient departments, cardiac catheterization lists, and hospital databases of the Royal Brompton and Harefield Hospitals, London, United Kingdom. All patients had plasma testosterone ≤12 nmol/L and normal prostate-specific antigen (normal range 0 to 4 μg/L). Exclusion criteria included myocardial infarction, coronary intervention, or thoracic or abdominal surgery in the previous 3 months; hypertension; history of testosterone treatment or similar hormone therapy; hemoglobin >16 g/dl; hematocrit >50%; history of hormone-dependent cancer; permanent pacemaker; intolerance of confined spaces; or participation in another research study within the previous 60 days. The study complied with the Declaration of Helsinki, the protocol was approved by the local research ethics committee, and all patients gave written informed consent.
The study used a randomized, placebo-controlled, crossover design. After consenting to the study, a screening blood sample was obtained to measure serum testosterone, prostate-specific antigen, and full blood count. Patients fulfilling the inclusion criteria then returned for a randomization visit, at which anthropomorphic measurements were obtained, quality-of-life questionnaires were completed, and study drug was dispensed (oral TU, 80 mg twice daily [Andriol Testocaps, Organon, Oss, The Netherlands] or identical placebo). After 8 weeks, an evaluation visit was conducted that included measurement of myocardial perfusion, endothelial function, quality-of-life assessment, and blood sampling for measurement of hormone, lipid, and hemostatic profiles. Patients then crossed over to the opposite treatment and returned for identical repeated testing after an additional 8 weeks at the same time of day as the first evaluation visit. Patients completed a symptom diary throughout the study.
Patients were permitted to use current antianginal therapy throughout the randomized treatment period, but stopped vasoactive therapy (including statins) for 1 week before each evaluation visit. Sublingual glyceryl trinitrate was permitted for treatment of anginal attacks during this time. However, if used on the day of the evaluation visit, it was rescheduled. Patients fasted for ≥6 hours, and caffeine-containing beverages were prohibited for ≥12 hours before each evaluation visit.
All cardiovascular magnetic resonance (CMR) studies were performed on a 1.5-Tesla scanner (Siemens Sonata, Siemens, Germany) with a 4-channel body-array coil. For each study, first-pass myocardial perfusion at rest and with adenosine stress was assessed. A high-resolution saturation-recovery fast low-angle shot sequence was used (field of view read 320 to 400 mm, field of view phase 75% to 100% of the field of view read, acquired voxel size 1.3 to 1.6 × 1.3 to 1.6, slice thickness 10 mm, flip angle 10°, acquisition duration 295 ms, echo time 1.11 ms, and time from saturation pulse to beginning of imaging 63 ms). Gadolinium-diethylenetriaminepentaacetic acid was injected as a bolus at 7 ml/s using a power injector (Medrad, Cambridgeshire, United Kingdom) through an 18 G intravenous cannula. Images were acquired over 50 cardiac cycles, and patients were asked to breath-hold in end-expiration for as long as was comfortable. One midventricular short-axis slice, representative of the whole ventricle, was acquired during each cardiac cycle in diastole. The stress study was performed ≥20 minutes after the study at rest. Adenosine was infused for 4 minutes at 140 μg/kg/min before stress images were acquired. On the second visit, the short-axis slice planned to be imaged was compared with the short-axis slice from the first visit to ensure an equivalent slice was studied.
Because studies were analyzed quantitatively, a dual-bolus protocol was used.4,5 For both studies at rest and with stress, patients received a low-dose (0.01 mmol/kg at 0.05 mol/L) bolus of gadolinium (Omniscan; Nycomed, Lidingö, Sweden) approximately 2 minutes before a high-dose bolus of gadolinium (0.1 mmol/kg at 0.5 mol/L). This allowed an accurate arterial input function and optimized signal in the myocardium. Blood pressure (BP) and heart rate were monitored throughout each study.
On both visits, left ventricular (LV) volume, function, and mass measurements were acquired using breath-hold segmented true fast imaging with steady-state precession cines according to a standard protocol.6
Each study was analyzed by a blinded observer using customized software (CMRtools; Cardiovascular Imaging Solutions, London, United Kingdom). For perfusion analysis, subendocardial and subepicardial myocardial borders were drawn and adjusted if necessary to compensate for cardiac and respiratory motion. Arterial input function and myocardial tissue response curves were corrected using baseline subtraction, then assumed to be linearly proportional to gadolinium concentration. An index of myocardial perfusion (a unitless measure) was calculated at rest and at stress from the respective first-pass myocardial signal intensity-time curves using Fermi deconvolution.7 Myocardial perfusion index (the ratio between stress and rest myocardial perfusion) was calculated using model-based deconvolution using the Fermi function. Each CMR image was divided into 4 (anterior, lateral, inferior, and septum), and areas of myocardium supplied by coronary arteries with and without significant (≥70% lesion) angiographically documented coronary stenosis were analyzed separately. LV mass, end-diastolic and end-systolic volumes, and ejection fraction were determined using standard manual contouring techniques.
Peripheral pressure waveforms were recorded from the right radial artery using applanation tonometry (SphgmoCor PX, version 6.31; AtCor, Sydney, Australia). BP was measured noninvasively every 5 minutes throughout the tonometry study on the contralateral arm (Dinamap, GE Healthcare, Chalfont St. Giles, Buckinghamshire, United Kingdom). Immediately after each BP measurement, radial artery pulse recordings were acquired with an averaged peripheral waveform generated from 20 sequential waveforms. Corresponding central waveforms were generated using an online validated transfer function to determine central augmentation index, central pressure, and heart rate. Augmentation index was calculated as the ratio of pulse pressure at the second systolic arterial pressure waveform peak to that of the first systolic peak and is a measure of arterial stiffness. Patients underwent measurement of endothelium-dependent and -independent responses using radial artery applanation tonometry before and after salbutamol (400 μg) and sublingual glyceryl trinitrate (250 μg) as previously described.8
Different aspects of quality of life were measured using validated questionnaires: the 36-Item Short Form Health Survey questionnaire, which measures general health (a high score indicates better functioning or more pain)9; Cardiac Health Profile, which measures cardiac well-being (a low score indicates a positive response10); and the Aging Male Symptom Scale.11
Testosterone, 17β-estradiol, sex hormone–binding globulin, follicular-stimulating hormone, luteinizing hormone, and insulin were measured using radioimmunoassay (Abbott IMX System; Abbott Diagnostics, Berkshire, United Kingdom), and glucose was measured using a standard Beckman Delta analyzer (Beckman Coulter UK Ltd, United Kingdom). Total cholesterol, high-density lipoprotein cholesterol, and triglycerides were measured using a Beckman CX7 analyzer. Low-density lipoprotein cholesterol was estimated using the formula of Friedewald. Full blood count was measured using a standard technique with a Technicon 8*1 analyzer (Bayer Technicon, United Kingdom). Fibrinogen and factor VII were measured using an automated coagulometer (Instrumentation Laboratory, United Kingdom), and plasminogen activator inhibitor-1, using chromogenic substrate assays.
Sample-size calculation was determined by the primary end point of myocardial perfusion. Our intracoronary testosterone study showed an increase in blood flow to testosterone of approximately 10% to 15%.2 A mean difference of 15% in myocardial perfusion index with an SD of 0.5 requires 22 patients, with assumptions of a 5% significance level, 80% power to detect a difference between groups, and randomization to the 2 groups in equal proportions. Allowing for a 20% drop-out rate, 28 patients were required for enrollment.
First, the size of the period-by-treatment interaction (carryover effect) was examined, and no carryover effect was found for any variable. Second, the difference between treatment groups was analyzed by subtracting results of the second period from results from the first period, and a t test was performed to compare these differences. An advantage of this method compared with a paired t test is that it removes the effect of period from the analysis. When data were not normally distributed, analysis was performed using Mann-Whitney test. Significance was set at p ≤0.05. Data are presented as mean ± SD or mean difference (95% confidence interval).
For myocardial perfusion data, each CMR image was divided into 4 (as described). Because this violates the assumption of independence, data were analyzed in 2 ways. First, a t test was used (which assumes independence), then linear regression with robust SEs to allow for repeated observations from the same subjects.
Results
Approximately 4,000 patients were contacted by post, and 50% of patients expressed interest in participating in the study. Of these, approximately 80% were excluded because of hypertension. One hundred nine patients attended for a consent and screening appointment. Half those screened had testosterone greater than the inclusion criteria.
Twenty-eight patients were enrolled in the study, and 23 completed the protocol. Two patients were randomly assigned, but withdrew consent before taking the study drug (1 moved away and 1 repeatedly did not attend the randomization visit), and 1 patient was withdrawn after a few days on study medication (TU) subsequent to hospitalization for a suspected stroke. One patient withdrew consent after the first treatment phase (placebo) because of depression, and 1 patient withdrew for personal reasons. Characteristics of the 25 patients who had at least 1 evaluation visit are listed in Table 1. Although not actively questioned about symptoms of hypogonadism, 1 patient was known to have previously used testosterone treatment (stopped 3 months before randomization). In addition, prerandomization Aging Male Symptom Scale scores indicated that 22 patients (88%) had moderate or severe impairment of sexual function. This score was not changed by either testosterone or placebo.
Table 1.
Patient characteristics (n = 25)
| Variable | |
|---|---|
| Age (yrs) | 57 ± 8 (36–67) |
| No. significantly diseased major coronary arteries | |
| 1 | 6 (24%) |
| 2 | 9 (36%) |
| 3 | 10 (40%) |
| Coronary intervention | |
| Percutaneous coronary intervention | 17 (68%) |
| Coronary artery bypass surgery | 10 (40%) |
| Time since intervention (yrs) | |
| Percutaneous coronary intervention | 3 ± 3 (0.4–11) |
| Coronary artery bypass surgery | 4 ± 4 (0.4–12) |
| Myocardial infarction | 14 (56%) |
| Time since myocardial infarction (yrs) | 5 ± 6 (0.4–17) |
| Diabetes | 3 (12%) |
| Smoking history | |
| Current | 2 (8%) |
| Ex | 17 (68%) |
| Never | 6 (24%) |
| Statin use | 25 (100%) |
| Systolic BP (mm Hg) | 132 ± 20 |
| Diastolic BP (mm Hg) | 76 ± 12 |
| Heart rate (beats/min) | 60 ± 8 |
| Weight (kg) | 91.5 ± 13 |
| Waist-hip ratio | 1.01 ± 0.06 |
| Body mass index (kg/m2) | 30.5 ± 3.5 |
| Body surface area (m2) | 2.9 ± 3.7 |
| Total testosterone (nmol/L) | 9.4 ± 2 |
| Prostate-specific antigen (μg/L) | 0.88 ± 0.36 |
Values expressed as mean ± SD (range) or number (percent).
Hormone levels are listed in Table 2. TU did not significantly change serum total testosterone or 17β-estradiol; however, percentages of free and bioavailable testosterone and dihydrotestosterone levels increased (all p <0.001). Conversely, TU treatment was associated with a significant decrease in sex hormone–binding globulin, follicular-stimulating hormone, and luteinizing hormone (all p <0.001).
Table 2.
Hormone levels
| Variable | Baseline | Oral TU | Placebo |
|---|---|---|---|
| Total testosterone (nmol/L) | 9.4 ± 2 | 10.2 ± 3.9 | 10.4 ± 2.4 |
| Free testosterone (nmol/L) | 0.23 ± 0.06 | 0.27 ± 0.1 | 0.25 ± 0.06 |
| (%) | 2.3 ± 0.5 | 2.7 ± 0.4⁎ | 2.4 ± 0.4 |
| Bioavailable testosterone (nmol/L) | 5.2 ± 1.6 | 6.3 ± 2.5 | 5.6 ± 1.4 |
| (%) | 52 ± 12 | 62 ± 10⁎ | 54 ± 10 |
| Dihydrotestosterone (nmol/L) | 1.18 ± 0.33 | 2.2 ± 1⁎ | 1.1 ± 0.3 |
| Sex hormone–binding globulin (nmol/L) | 29 ± 17 | 18.6 ± 7.7⁎ | 25.3 ± 10.8 |
| 17β-Estradiol (pmol/L) | 123 ± 75 | 141 ± 64 | 176 ± 102 |
| Follicular-stimulating hormone (IU/L) | 5.9 ± 2 | 4.2 ± 1.6⁎ | 5.8 ± 1.8 |
| Luteinizing hormone (IU/L) | 3.9 ± 2 | 2.1 ± 1.1⁎ | 3.8 ± 1.5 |
Values expressed as mean ± SD.
p <0.01 compared with placebo.
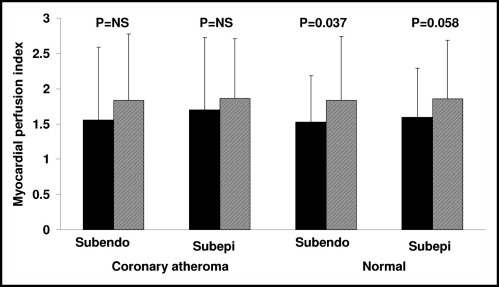
Twenty-two patients had assessable CMR data for both evaluation visits. Global myocardial perfusion was not significantly different between treatments transmurally (1.8 ± 0.7 vs 1.6 ± 0.7, TU vs placebo; p = 0.28) or in the subendocardium (1.8 ± 0.7 vs 1.5 ± 0.6, TU vs placebo; p = 0.17) or subepicardium (1.8 ± 0.7 vs 1.6 ± 0.7, TU vs placebo; p = 0.33). When analyzed by segments, no significant difference in perfusion was shown in the subendocardium (1.83 ± 0.92 vs 1.54 ± 0.88, TU vs placebo, p = 0.2) or subepicardium (1.86 ± 0.83 vs 1.65 ± 0.89, TU vs placebo, p = 0.54) after adjustment for repeated measures. However, myocardial segments supplied by coronary arteries without significant obstruction showed significant improvement in the myocardial perfusion index of the subendocardium (1.83 ± 0.9 vs 1.52 ± 0.65, TU vs placebo, p = 0.037; Figure 1) and borderline improvement in the subepicardium (1.85 ± 0.82 vs 1.59 ± 0.69, TU vs placebo, p = 0.058; Figure 1) in patients administered TU versus placebo. Myocardial segments supplied by coronary arteries with significant coronary atherosclerosis showed no difference in myocardial perfusion indexes in the subendocardium (1.83 ± 0.94 vs 1.55 ± 1.03, TU vs placebo respectively; p = 0.11) or subepicardium (1.86 ± 0.84 vs 1.69 ± 1.02, TU vs placebo; p = 0.29). Adenosine did not decrease heart rate or cause atrioventricular block in any patient.
Figure 1.
Bar graph shows mean myocardial perfusion index for placebo (black bars) and TU (hatched bars) for myocardial regions associated with angiographically documented significant coronary atheroma or perfusion defect on preenrollment thallium scan and remaining “normal” regions. Subendo = subendocardial; subepi = subepicardial.
Sixteen patients had LV data for both visits. LV ejection fraction was significantly higher after testosterone treatment compared with placebo (67 ± 8% vs 65 ± 8%; p = 0.05). However, stroke volume (89 ± 12 vs 88 ± 14 ml; p = 0.61), end-systolic volume (45 ± 14 vs 47 ± 14 ml; p = 0.1), end-diastolic volume (135 ± 19 vs 134 ± 18 ml; p = 0.97), and mass (170 ± 33 vs 169 ± 34 g; p = 0.73) were not different.
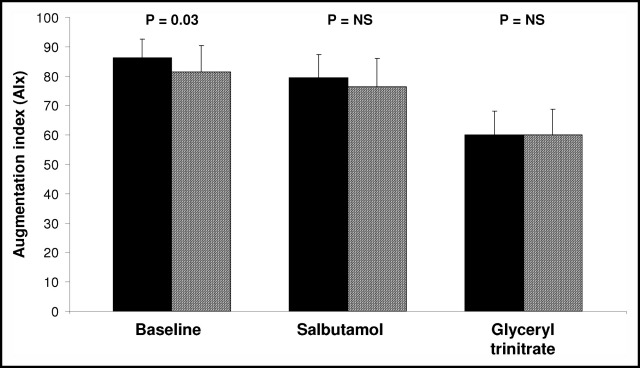
Seventeen patients completed both endothelial function assessments. TU treatment was associated with significantly lower baseline radial augmentation (p = 0.03; Figure 2) and aortic augmentation indexes (141 ± 12 vs 147 ± 9, TU vs placebo, respectively; p = 0.02) compared with placebo, indicating a testosterone-induced decrease in basal arterial stiffness. However, testosterone did not affect salbutamol-induced changes in radial augmentation indexes compared with placebo (p = 0.48; Figure 2), signifying no effect on endothelium-dependent responses. Glyceryl trinitrate–induced decreases in radial augmentation indexes were similar after both treatments (p = 0.15; Figure 2). Time to return of reflected waves, an estimate of pulse-wave velocity,12 was prolonged after TU treatment compared with placebo (142.4 ± 16.6 vs 137.2 ± 9.3 ms; p = 0.04). Brachial systolic BP was not altered throughout the tonometry study (148 ± 16 vs 147 ± 17 mm Hg, TU vs placebo; p = 0.71). Similarly, there was no significant difference in central pulse pressure (52 ± 10 vs 52 ± 13 mm Hg, TU vs placebo, respectively; p = 0.88) or heart rate at rest (62 ± 7 vs 60 ± 7 beats/min, TU vs placebo, respectively; p = 0.08).
Figure 2.
Bar graph shows radial augmentation index at baseline, 20 minutes after inhaled salbutamol, and 5 minutes after sublingual glyceryl trinitrate for placebo (black bars) and TU (hatched bars).
TU treatment was associated with significantly lower high-density lipoprotein cholesterol and apolipoprotein-A1 and higher high-density lipoprotein–low-density lipoprotein ratio compared with placebo (Table 3). Apolipoprotein-A1: apolipoprotein-B ratio decreased with borderline statistical significance, and glucose and insulin were not different between treatments (Table 3).
Table 3.
Metabolic and hemostatic variables
| Variable | Baseline | Oral TU | Placebo |
|---|---|---|---|
| Total cholesterol (mmol/L) | 4.3 ± 1.3 | 4.9 ± 1.5 | 5.2 ± 1.2 |
| (mg/dl) | 168 ± 51 | 191 ± 58 | 203 ± 47 |
| High-density lipoprotein cholesterol (mmol/L) | 0.99 ± 0.2 | 0.9 ± 0.3⁎ | 1.1 ± 0.2 |
| (mg/dl) | 40 ± 8 | 35 ± 12 | 43 ± 8 |
| Low-density lipoprotein cholesterol (mmol/L) | 2.5 ± 1 | 3.1 ± 1.5 | 3.1 ± 1.1 |
| (mg/dl) | 97 ± 39 | 120 ± 58 | 121 ± 43 |
| High-density–low-density lipoprotein cholesterol ratio | 4.37 ± 0.77 | 5.3 ± 1.3† | 4.9 ± 1 |
| Triglycerides (mmol/L) | 1.68 ± 0.68 | 2.1 ± 1.7 | 2.1 ± 1 |
| Lipoprotein(a) (mg/L) | Not done | 276 ± 338 | 323 ± 362 |
| Apolipoprotein A1 (g/L) | Not done | 1.2 ± 0.3† | 1.3 ± 0.2 |
| Apolipoprotein B (g/L) | Not done | 1 ± 0.2 | 1 ± 0.2 |
| Apolipoprotein A:Apolipoprotein B | Not done | 1.2 ± 0.2 | 1.3 ± 0.2 |
| Glucose (mmol/L) | 5.9 ± 1.3 | 5.9 ± 1 | 5.9 ± 0.9 |
| Insulin (mU/L) | Not done | 9.7 ± 4 | 10 ± 4 |
| Plasminogen activator-1 (ng/ml) | 92 ± 41 | 89 ± 39 | 92 ± 52 |
| Fibrinogen (g/L) | 3.4 ± 0.7 | 3.2 ± 0.7 | 3.2 ± 0.8 |
| Factor VII (u/dl) | 116 ± 28 | 105 ± 26 | 109 ± 25 |
Values expressed as mean ± SD.
p <0.001 compared with placebo.
p = 0.01 compared with placebo.
There was no difference in plasminogen activator inhibitor-1, fibrinogen, or factor VII after treatment with TU or placebo (Table 3). Hematocrit slightly but significantly increased after TU treatment (44.5 ± 1.8% vs 43.5 ± 1.9%, TU vs placebo, respectively; p = 0.002). However, hemoglobin was not affected (15 ± 0.6 vs 14.9 ± 0.7 g/dl, TU vs placebo, respectively; p = 0.22).
There was a statistically significant increase in hip circumference after TU compared with placebo (108 ± 6 vs 107 ± 6 cm, respectively; p = 0.03), but no difference in body weight (92 ± 11 vs 92 ± 13 kg; p = 0.1), waist circumference (107 ± 8 vs 108 ± 10 cm; p = 0.77), waist-hip ratio (0.98 ± 0.05 vs 1 ± 0.06; p = 0.14), body surface area (2.1 ± 0.2 vs 2.1 ± 0.2 m2; p = 0.08), or body mass index (30.5 ± 3.2 vs 30.7 ± 3.6 kg/m2; p = 0.9). There was a trend toward a slight TU-induced increase in seated systolic BP (141 ± 17 vs 137 ± 16 mm Hg; p = 0.07); however, there was no difference in diastolic BP (83 ± 8 vs 83 ± 7 mm Hg; p = 0.97) or heart rate (62 ± 8 vs 64 ± 9 beats/min; p = 0.37).
There was no significant difference between treatments for any of the 36-Item Short Form Health Survey, Cardiac Health Profile, or Aging Male Symptom Scale measures examined and no difference in incidence of angina symptoms (2 ± 5 vs 1 ± 2 per 8-week treatment period, TU vs placebo, respectively). There were no other symptoms considered related to study drug. One patient who experienced “almost a depression” was administered placebo and did not cross over to TU treatment.
Discussion
Our results show that 8 weeks of oral TU in men with CHD and low testosterone modestly increased myocardial perfusion in myocardium supplied by unobstructed coronary arteries, whereas perfusion in areas of myocardium supplied by coronary arteries with significant coronary atheroma was not affected. To our knowledge, this is the first investigation of blood flow effects of testosterone in territories associated with atherosclerotic coronary disease in humans. TU treatment decreased basal peripheral and central arterial stiffness, and concurrently, there was a comparative increase in LV ejection fraction. There was a null effect on overall myocardial perfusion, global endothelial function, quality of life, and angina symptoms compared with placebo.
We report for the first time the effects of testosterone on myocardial perfusion in myocardial territories supplied by atherosclerotic coronary arteries. A previous study showed that acute intracoronary testosterone induced coronary vasodilation and increased coronary blood flow in unobstructed coronary arteries in patients with CHD in other major epicardial coronary arteries, but flow responses were not measured in significantly diseased arteries.2 In line with the intracoronary study, the present longer term study showed testosterone-related enhancement of myocardial blood flow in normally perfused regions of myocardium that may be mediated through effects on the epicardial coronary arteries or/and microvasculature. Our previous short-term intracoronary testosterone study showed no effect on coronary microvessels or coronary flow reserve.2 However, the different testosterone preparations and durations of treatment used may have had differing effects. A previous study of perfusion CMR in patients with cardiac syndrome X showed subendocardial hypoperfusion in response to adenosine, but no such effect was shown in the present study.13 It is perhaps surprising that we were unable to identify enhanced perfusion in myocardial segments associated with significant atherosclerosis because testosterone therapy in the longer term has beneficial effects on signs of exercise-induced myocardial ischemia.3 TU-related decreases in basal arterial stiffness imply that the mechanism involved in this anti-ischemic effect may at least in part involve effects on vascular tone (with no effect on BP or heart rate), thereby decreasing afterload. However, our myocardial perfusion data correspond with the null effect of testosterone treatment on angina symptoms shown in both the present study and other reports.3
Puberty in males is associated with an increase in large-vessel arterial stiffness relative to prepubertal males, related at least in part to changes in sex-steroid milieu.14 However, a recent longitudinal study in older men (mean age 68 years) reported an independent inverse relation between endogenous serum testosterone and arterial stiffness.15 Our results provide evidence that oral TU treatment has a beneficial effect on arterial stiffness in older men with testosterone at or less than the lower limit of normal range. In light of recent research showing that arterial stiffness was a predictor of cardiovascular outcomes,16,17 our results may suggest that in the longer term, oral TU could potentially confer cardiovascular benefit through its effects on arterial stiffness. The endothelium did not appear to mediate effects of oral TU on arterial stiffness or vascular tone in vivo in humans, shown by the present study and others,18 although animal studies show that nitric oxide, prostacyclin, endothelium-derived hyperpolarizing factor, endothelin, and thromboxane-A2 mediate testosterone-induced vascular effects.19,20 The lower augmentation index measured after TU treatment could be caused by a combination of decreased wave reflection (possibly from an effect on small vessels) and delayed wave reflection suggestive of lower pulse-wave velocity from an effect on larger vessels (in the absence of a BP change).
Oral TU treatment resulted in a small but significant increase in LV ejection fraction compared with placebo, with no associated difference in LV mass, stroke volume, or end-systolic or -diastolic volumes. This is interesting in light of a report of decreased LV ejection fraction in patients with low endogenous free testosterone21 and may be caused by a direct action of TU on myocardial contractility.22 Castration was associated with decreased cardiac performance in male rats with slightly improved functioning in castrated testosterone-replaced animals.23 These effects may be explained by testosterone-induced actions on calcium-myosin adenosine triphosphatase activity.24 However, data are scarce and additional studies are needed, particularly investigating the actions of physiologic testosterone replacement on cardiac function in humans.
Plasma total testosterone levels did not increase after TU treatment because TU is absorbed into the lymphatic system with newly formed cholymicrons, then subsequently absorbed into blood from the intestine25 and rapidly converted into dihydrotestosterone.26 In this way, hepatic first pass is avoided. Dihydrotestosterone significantly increased and sex hormone–binding globulin, luteinizing hormone, and follicular-stimulating hormone decreased after TU administration.
The rationale for the 8-week treatment period was based on our knowledge of the possible longer term mechanisms of action of ovarian and related steroids on the vascular system after long-term exposure.3,27 We therefore wished to expose patients to several weeks of therapy, and the available data suggested a minimum period of 4 to 8 weeks. It is possible that testosterone therapy for longer periods may show different results. Long-term testosterone treatment is likely to affect aspects of human cardiovascular physiology that were not measured in the present study, but that may influence myocardial perfusion and vascular function. CMR perfusion techniques are rapidly developing, and although newer methods may not change the overall results, they may give more comprehensive information, for example, single versus multiple slices. The anticipated changes in myocardial perfusion and those shown were relatively small and it may be of interest to perform similar studies using different perfusion techniques.
Footnotes
This work was supported by the British Heart Foundation, United Kingdom (C. Webb) and Organon, Oss, The Netherlands. Dr. Webb is currently supported by the Victor Phillip Dahdaleh Charitable Foundation, United Kingdom. Dr. Elkington was supported by Organon to present the data in abstract form at the American Heart Association annual scientific sessions, 2004. Dr. Pennell received research support from Siemens and is a consultant to Siemens and a director of CVIS, which is a software company that markets CMRtools. Dr. Collins received research funding from Organon to support this study. There are no conflicts of interest for any other author.
References
- 1.Wu F.C., von Eckardstein A. Androgens and coronary artery disease. Endocr Rev. 2003;24:183–217. doi: 10.1210/er.2001-0025. [DOI] [PubMed] [Google Scholar]
- 2.Webb C.M., McNeill J.G., Hayward C.S., de Ziegler D., Collins P. Effects of testosterone on coronary vasomotor regulation in men with coronary artery disease. Circulation. 1999;100:1690–1696. doi: 10.1161/01.cir.100.16.1690. [DOI] [PubMed] [Google Scholar]
- 3.English K.M., Steeds R.P., Jones T.H., Diver M.J., Channer K.S. Low-dose transdermal testosterone therapy improves angina threshold in men with chronic stable angina: a randomized, double-blind, placebo-controlled study. Circulation. 2000;102:1906–1911. doi: 10.1161/01.cir.102.16.1906. [DOI] [PubMed] [Google Scholar]
- 4.Christian T.F., Rettmann D.W., Aletras A.H., Liao S.L., Taylor J.L., Balaban R.S., Arai A.E. Absolute myocardial perfusion in canines measured by using dual-bolus first-pass MR imaging. Radiology. 2004;232:677–684. doi: 10.1148/radiol.2323030573. [DOI] [PubMed] [Google Scholar]
- 5.Elkington A.G., Ablitt N.A., Yang G.Z., Firmin D.N., Pennell D.J. Interstudy reproducibility of quantitative perfusion cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2005;7:815–822. doi: 10.1080/10976640500287877. [DOI] [PubMed] [Google Scholar]
- 6.Maceira A.M., Prasad S.K., Khan M., Pennell D.J. Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2006;8:417–426. doi: 10.1080/10976640600572889. [DOI] [PubMed] [Google Scholar]
- 7.Jerosch-Herold M., Wilke N., Stillman A.E. Magnetic resonance quantification of the myocardial perfusion reserve with a Fermi function model for constrained deconvolution. Med Phys. 1988;25:73–84. doi: 10.1118/1.598163. [DOI] [PubMed] [Google Scholar]
- 8.Hayward C.S., Kraidly M., Webb C.M., Collins P. Assessment of endothelial function using peripheral waveform analysis: A clinical application. J Am Coll Cardiol. 2002;40:521–528. doi: 10.1016/s0735-1097(02)01991-5. [DOI] [PubMed] [Google Scholar]
- 9.Stewart A.L., Hays R.D., Ware J.E., Jr The MOS short-form general health survey: Reliability and validity in a patient population. Med Care. 1988;26:724–735. doi: 10.1097/00005650-198807000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Wahrborg P., Emanuelsson H. The Cardiac Health Profile: content, reliability and validity of a new disease-specific quality of life questionnaire. Coron Artery Dis. 1996;7:823–829. [PubMed] [Google Scholar]
- 11.Daig I., Heinemann L.A., Kim S., Leungwattanakij S., Badia X., Myon E., Moore C., Saad F., Potthoff P., Thai D.M. The Aging Males’ Symptoms (AMS) Scale: review of its methodological characteristics. Health Qual Life Outcomes. 2003;1:77. doi: 10.1186/1477-7525-1-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.London G., Guerin A., Pannier B., Marchais S., Benetos A., Safar M. Increased systolic pressure in chronic uremia: Role of arterial wave reflections. Hypertension. 1992;20:10–19. doi: 10.1161/01.hyp.20.1.10. [DOI] [PubMed] [Google Scholar]
- 13.Panting J.R., Gatehouse P.D., Yang G.Z., Grothues F., Firmin D.N., Collins P., Pennell D.J. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med. 2002;346:1948–1953. doi: 10.1056/NEJMoa012369. [DOI] [PubMed] [Google Scholar]
- 14.Ahimastos A.A., Formosa M., Dart A.M., Kingwell B.A. Gender differences in large artery stiffness pre- and post puberty. J Clin Endocrinol Metab. 2003;88:5375–5380. doi: 10.1210/jc.2003-030722. [DOI] [PubMed] [Google Scholar]
- 15.Hougaku H., Fleg J.L., Najjar S.S., Lakatta E.G., Harman S.M., Blackman M.R., Metter E.J. Relationship between androgenic hormones and arterial stiffness based on longitudinal hormone measurements. Am J Physiol. 2006;290:E234–E242. doi: 10.1152/ajpendo.00059.2005. [DOI] [PubMed] [Google Scholar]
- 16.Willum-Hansen T., Staessen J.A., Torp-Pedersen C., Rasmussen S., Thijs L., Ibsen H., Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 17.Mattace-Raso F.U., van der Cammen T.J., Hofman A., van Popele N.M., Bos M.L., Schalekamp M.A., Asmar R., Reneman R.S., Hoeks A.P., Breteler M.M., Witteman J.C. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 18.Kang S.M., Jang Y., Kim J.Y., Chung N., Cho S.Y., Chae J.S., Lee J.H. Effect of oral administration of testosterone on brachial arterial vasoreactivity in men with coronary artery disease. Am J Cardiol. 2002;89:862–864. doi: 10.1016/s0002-9149(02)02202-6. [DOI] [PubMed] [Google Scholar]
- 19.Orshal J.M., Khalil R.A. Gender, sex hormones, and vascular tone. Am J Physiol. 2004;286:R233–R249. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- 20.Gonzales R.J., Ghaffari A.A., Duckles S.P., Krause D.N. Testosterone treatment increases thromboxane function in rat cerebral arteries. Am J Physiol. 2005;289:H578–H585. doi: 10.1152/ajpheart.00958.2004. [DOI] [PubMed] [Google Scholar]
- 21.Dobrzycki S., Serwatka W., Nadlewski S., Korecki J., Jackowski R., Paruk J., Ladny J.R., Hirnle T. An assessment of correlations between endogenous sex hormone levels and the extensiveness of coronary heart disease and the ejection fraction of the left ventricle in males. J Med Invest. 2003;50:162–169. [PubMed] [Google Scholar]
- 22.Golden K.L., Marsh J.D., Jiang Y., Brown T., Moulden J. Gonadectomy of adult male rats reduces contractility of isolated cardiac myocytes. Am J Physiol. 2003;285:E449–E453. doi: 10.1152/ajpendo.00054.2003. [DOI] [PubMed] [Google Scholar]
- 23.Scheuer J., Malhotra A., Schaible T.F., Capasso J. Effects of gonadectomy and hormonal replacement on rat hearts. Circ Res. 1987;61:12–19. doi: 10.1161/01.res.61.1.12. [DOI] [PubMed] [Google Scholar]
- 24.Schaible T.F., Malhotra A., Ciambrone G., Scheuer J. The effects of gonadectomy on left ventricular function and cardiac contractile proteins in male and female rats. Circ Res. 1984;54:38–49. doi: 10.1161/01.res.54.1.38. [DOI] [PubMed] [Google Scholar]
- 25.Nieschlag E., Mauss J., Coert A., Kicovic P. Plasma androgen levels in men after oral administration of testosterone or testosterone undecanoate. Acta Endocrinol. 1975;79:366–374. doi: 10.1530/acta.0.0790366. [DOI] [PubMed] [Google Scholar]
- 26.Hirschhauser C., Hopkinson C.R., Sturm G., Coert A. Testosterone undecanoate: a new orally active androgen. Acta Endocrinol. 1975;80:179–187. doi: 10.1530/acta.0.0800179. [DOI] [PubMed] [Google Scholar]
- 27.Jaffe M.D. Effect of testosterone cypionate on postexercise ST segment depression. Br Heart J. 1977;39:1217–1222. doi: 10.1136/hrt.39.11.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]