Abstract
Objective To evaluate the effectiveness of a structured group education programme on biomedical, psychosocial, and lifestyle measures in people with newly diagnosed type 2 diabetes.
Design Multicentre cluster randomised controlled trial in primary care with randomisation at practice level.
Setting 207 general practices in 13 primary care sites in the United Kingdom.
Participants 824 adults (55% men, mean age 59.5 years).
Intervention A structured group education programme for six hours delivered in the community by two trained healthcare professional educators compared with usual care.
Main outcome measures Haemoglobin A1c levels, blood pressure, weight, blood lipid levels, smoking status, physical activity, quality of life, beliefs about illness, depression, and emotional impact of diabetes at baseline and up to 12 months.
Main results Haemoglobin A1c levels at 12 months had decreased by 1.49% in the intervention group compared with 1.21% in the control group. After adjusting for baseline and cluster, the difference was not significant: 0.05% (95% confidence interval −0.10% to 0.20%). The intervention group showed a greater weight loss: −2.98 kg (95% confidence interval −3.54 to −2.41) compared with 1.86 kg (−2.44 to −1.28), P=0.027 at 12 months. The odds of not smoking were 3.56 (95% confidence interval 1.11 to 11.45), P=0.033 higher in the intervention group at 12 months. The intervention group showed significantly greater changes in illness belief scores (P=0.001); directions of change were positive indicating greater understanding of diabetes. The intervention group had a lower depression score at 12 months: mean difference was −0.50 (95% confidence interval −0.96 to −0.04); P=0.032. A positive association was found between change in perceived personal responsibility and weight loss at 12 months (β=0.12; P=0.008).
Conclusion A structured group education programme for patients with newly diagnosed type 2 diabetes resulted in greater improvements in weight loss and smoking cessation and positive improvements in beliefs about illness but no difference in haemoglobin A1c levels up to 12 months after diagnosis.
Trial registration Current Controlled Trials ISRCTN17844016.
Introduction
Type 2 diabetes mellitus affects around 5% of European populations and is responsible for a disproportionate use of health service resources.1 In the short term diabetes may lead to symptoms and debility and in the long term can lead to serious complications such as blindness, renal failure, and amputation.2 Furthermore, diabetes is associated with increased morbidity and premature death from cardiovascular disease, including stroke and myocardial infarction. In clinical practice in the United Kingdom primary care teams are now financially rewarded for achieving tight glycaemic and metabolic targets in patients under their care and this has led to improved levels of glycaemic control, particularly in patients with type 2 diabetes.3 Although the diabetes national service framework has made recommendations for wider provision of group structured education, currently no evidence supports the belief that structured education provides added benefit for patients from the point of diagnosis.
Despite the initial successful impact of oral medication, patients find it difficult to implement and sustain the treatment and lifestyle advice given by healthcare professionals.4 This may in part relate to traditional approaches to management in which patients are passive recipients of care. The acquisition of the relevant skills for successful self management may play a key role in tackling beliefs about health and optimising metabolic control, risk factors, and quality of life.5 6 7 Educating patients about diabetes may have a pivotal role in encouraging and supporting them to assume active responsibility for the day to day control of their condition.8 9 10 11 12
Several educational programmes have been developed in Europe13 14 and North America.15 However, the National Institute for Health and Clinical Excellence (NICE) found little evidence in the UK for the effectiveness of any educational approach in people with type 2 diabetes,11 a view reinforced in other recent reviews.6 16 17 Some evidence shows that education programmes with a theoretical basis and using cognitive reframing are associated with improved outcomes.18 19 Furthermore, few programmes have been developed in a primary care setting and none has been designed specifically for patients from the point of diagnosis.
The development, piloting, and subsequent randomised controlled trial of the diabetes education and self management for ongoing and newly diagnosed (DESMOND) structured education programme aimed to tackle this gap. The work followed the Medical Research Council framework,20 which promotes a systematic approach to interventions such as educational programmes, which are complex and hard to describe and therefore difficult to replicate.21
The preliminary phases of the trial have been described elsewhere.22 A pilot phase informed the effect size and power for the current randomised controlled trial, which evaluated the effectiveness of the structured group education programme on biomedical, psychosocial, and lifestyle measures in people with newly diagnosed type 2 diabetes.
Methods
The trial was carried out in 13 sites in primary care, involving 17 primary care organisations across England and Scotland. Randomisation was at practice level, with stratification by training status and type of contract with the primary care organisation (General Medical Services or Personal Medical Services). Randomisation was undertaken independently at the University of Sheffield using Random Log (D Machin, University of Southampton).
Participants with type 2 diabetes were referred within four weeks of diagnosis, with those in the intervention arm attending a structured group education programme within 12 weeks of diagnosis. We excluded participants if they were aged less than 18 years, had severe and enduring mental health problems, were not primarily responsible for their own care, were unable to participate in a group programme (for example, housebound or unable to communicate in English), or were participating in another research study. Participants gave informed consent in accordance with the guidelines from the International Conference on Harmonisation and WHO good clinical practice standards.
Sample size
Assuming a standard deviation of haemoglobin A1c levels of 2%,23 an intraclass correlation of 0.05, and an average of 18 participants per practice, we calculated that we needed 315 per study arm to detect a clinically relevant difference in haemoglobin A1c levels of 1%, with 90% power at the 5% significance level. Assuming a failure to consent rate of 20% (not eligible as well as declining to participate) and a dropout rate of 20%, 1000 participants (500 in each arm) needed to be referred.
Study management
At each site a local coordinator oversaw the trial, recruited and trained practices, and maintained contact with practice staff. Performance of the sites and local coordinators was monitored regularly, with each site receiving a visit before the trial and a minimum of one monitoring visit per year. Practice staff sent biomedical data to the local coordinator for forwarding to the central coordinating centre.
Participating practices represented the wide spectrum of routine care currently available in the UK, with none providing any structured education that had been described and evaluated. Most offered access to some kind of diabetes education, either one to one with the practice nurse or dietician or by referral to ad hoc group education initiatives. Patients in both arms of the study therefore had access to some form of education. In addition the practices were provided with a pack containing evidence based default clinical guidelines in the form of treatment algorithms; guidance notes on “breaking bad news,” developed for the study by a clinical psychologist; and sample patient resources. Control practices were resourced to enable them to provide contact time with healthcare professionals equivalent to that provided by the structured group education programme. The practices were allowed to use the resources as they saw fit within their usual care routine. Participating practices in the control arm were therefore resourced to provide a robust comparator to the intervention and so received “enhanced” standard care.
The intervention
The structured group education programme is based on a series of psychological theories of learning: Leventhal’s common sense theory,24 the dual process theory,25 and the social learning theory.26 The philosophy of the programme was founded on patient empowerment, as evidenced in published work.27 28
The intervention was devised as a group education programme, with a written curriculum suitable for the broadest range of participants, to be deliverable in a community setting and to be integrated into routine care. Registered healthcare professionals received formal training to deliver the programme and were supported by a quality assurance component of internal and external assessment to ensure consistency of delivery. The programme was six hours long, deliverable in either one day or two half day equivalents and facilitated by two educators. Learning was elicited rather than taught, with the behaviour of the educators promoting a non-didactic approach. The contents of the curriculum were aimed specifically at participants attending within 12 weeks of diagnosis. Most of the curriculum was focused on lifestyle factors, such as food choices, physical activity, and cardiovascular risk factors. Attendees were encouraged to consider medication as an option in their self management strategy. The programme activates participants to consider their own personal risk factors and, in keeping with theories of self efficacy,26 to choose a specific, achievable goal of behaviour change to work on. The broad content of the curriculum and an overview of the quality assurance have been reported elsewhere.22
The programme module was intended as the first step in an ongoing cycle of diabetes care, integrating education with clinical management. The randomised controlled trial therefore had three important functions: an evaluation of the intervention itself and its generalisability, an assessment of the effectiveness of providing structured group education at diagnosis, and showing at what point any benefits of education begin to diminish.
Outcome measures
We collected outcome measures at baseline and at 4, 8, and 12 months. Biomedical data were collected at practice visits. Questionnaire data were collected from participants at the beginning of the study and by postal questionnaire at 4, 8, and 12 months. We sent out a reminder and a further copy of the questionnaire if the original was unreturned after two weeks.
Metabolic control and other biomedical measures
We measured haemoglobin A1c levels, blood pressure, and body weight at baseline and at 4, 8, and 12 months; blood lipid levels (total cholesterol, high density lipoprotein cholesterol, low density lipoprotein cholesterol, and triglycerides) and waist circumference were measured at baseline and at eight and 12 months. We collected data according to standard operating procedures. Samples were drawn from a venous sample and assayed locally in an accredited laboratory that was part of the national external quality assurance programme, with haemoglobin A1c levels measured using an aligned method produced by the diabetes control and complications trial. Practices recorded details of prescribed medication.
Lifestyle and psychosocial data
The questionnaires included lifestyle questions on smoking from the summary of diabetes self care activities questionnaire29 and physical activity from the international physical activity questionnaire.30 Frequency of physical activity was categorised into vigorous and moderate activity and walking. We assessed quality of life with the short version of the World Health Organization’s quality of life instrument WHOQOL-BREF,31 which has been validated in people with type 2 diabetes.32 We used the illness perceptions questionnaire—revised33 to assess people’s perception that they understood their diabetes (illness coherence), perception of the duration of their illness (timeline), and perception of their ability to affect the course of their diabetes (personal control). We assessed the perceived seriousness and the perceived impact of diabetes using the diabetes illness representations questionnaire.34 This was developed from the illness perceptions questionnaire35 and the personal models of diabetes interview.36 37 The problem areas in diabetes scale38 assessed emotional distress specific to diabetes and the hospital anxiety and depression scale39 measured depression.
Statistical analysis
Statistical analysis was carried out on an intention to treat basis. Results are reported according to consolidated standards of reporting trials guidelines for cluster randomised trials.40 We summarised continuous variables using means, standard deviations, medians, and ranges, and categorical variables using counts and percentages. Missing outcomes were not replaced and we derived an average over time of continuous outcomes. This procedure measures the cumulative effect of the treatment and has the maximum number of participants. To adjust for a potential clustering effect we used robust generalised estimating equations41 with exchangeable correlation structure. For binary outcomes we used a logit link with a binomial distribution for the outcome, and for continuous outcomes we used an identity link with a normal distribution. For ordinal outcomes we used an ordinal regression model with proportional odds assumption, adjusted for clusters.42 To investigate whether changes in illness beliefs are predictive of changes in outcome variable, we carried out multiple regressions with adjustment for age, sex, and baseline value of the outcome variable. Variables were entered in specified sequence, and we report standardised regression weights (β). Adjustments were not made for multiple testing. All the results from planned analyses are reported and small P values are interpreted taking into account the overall pattern of the results. Statistical significance was set at 5%. Data were analysed independently at the University of Sheffield using Stata version 9.
Results
Overall, 207 general practices (105 control, 102 intervention) were recruited from 13 sites in England and Scotland; 67% (139 practices) had General Medical Service contracts and 34% (70 practices) were involved with general practice vocational training. The total list size was 1 487 592, with practice list sizes ranging from 847 to 34 324. In total, 162 practices (77 control, 85 intervention) actively referred participants. Referral of patients with newly diagnosed type 2 diabetes began on 1 October 2004 and ended on 31 January 2006. Using practice list sizes at the beginning of the study, referral rate in the active practices was 0.96 per 1000 patient years. Site referral rates ranged from 0.69 to 1.64 per 1000 patient years. In the intervention arm the mean number of participants attending a programme was 5 (range 3-11). Participants were invited to bring a guest and the mean total number of participants and guests attending was 8 (range 4-16).
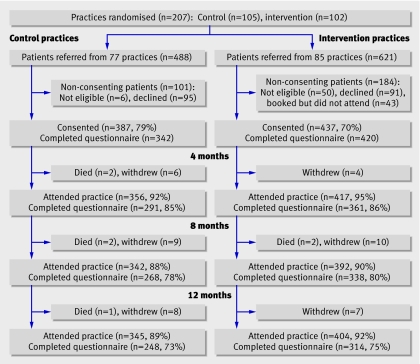
The figure shows the flow of participants through the trial. In total, 1109 patients were referred and 824 consented to take part. The overall consent rate was 74% and was lower in the intervention arm than in the control arm (70% v 79%). The mean (standard deviation) age was significantly higher in the consented group (60.3 years (12.2) v 56.5 years (13.0); P<0.001). The groups showed no statistically significant difference according to sex. Both groups had a good return of follow-up data.
Referral numbers and flow of patients through trial
Table 1 shows the characteristics of participants at baseline. The mean (standard deviation) levels of haemoglobin A1c were significantly higher in the intervention group than in the control group: 8.3% (2.2) v 7.9% (2.0). A significantly higher proportion of participants in the intervention group were prescribed oral hypoglycaemic agents than in the control group (17% v 12%). The proportion of women was significantly higher in the intervention group than in the control group (47% v 43%).
Table 1.
Baseline characteristics of participants with newly diagnosed type 2 diabetes allocated to usual care (control) or to a structured group education programme. Values are means (standard deviations) unless stated otherwise
| Characteristics | Control group (n=387) | Intervention group (n=437) |
|---|---|---|
| Mean (range) age (years) | 60.0 (29-87) | 59.0 (28-87) |
| % (No) women* | 43 (168) | 47 (204) |
| % (No) white European | 94 (327) | 94 (398) |
| Haemoglobin A1c* | 7.9 (2.0) | 8.3 (2.2) |
| Body weight (kg) | 91.6 (20.2) | 91.8 (19.2) |
| Total cholesterol (mmol/l) | 5.4 (1.2) | 5.4 (1.4) |
| High density lipoprotein cholesterol (mmol/l) | 1.2 (0.4) | 1.2 (0.5) |
| Low density lipoprotein cholesterol (mmol/l) | 3.3 (1.1) | 3.1 (1.1) |
| Triglycerides (mmol/l) | 2.5 (1.7) | 2.6 (2.4) |
| Systolic blood pressure (mm Hg) | 140.0 (16.6) | 141.1 (18.5) |
| Diastolic blood pressure (mm Hg) | 81.0 (10.5) | 82.4 (10.5) |
| Waist circumference (cm) | 106.6 (13.6) | 105.6 (14.7) |
| Body mass index (kg/m2) | 32.4 (6.5) | 32.3 (6.1) |
| % (No) smokers | 16 (53) | 14 (57) |
| % (No) prescribed oral hypoglycaemic agents*† | 12 (47) | 17 (94) |
*Groups differed significantly for sex, haemoglobin A1c level, and use of oral hypoglycaemic agents (P<0.05).
†Metformin, sulphonlyureas, or glitazone.
Biomedical outcomes
Table 2 shows the mean (95% confidence interval) change in biomedical outcomes in the study groups at 4, 8, and 12 months’ follow-up. The mean change in haemoglobin A1c levels from baseline to 12 months was higher in the intervention group than in the control group: −1.49% (95% confidence intervals −1.69% to −1.29%) compared with −1.21% (−1.40% to −1.02%). Adjustment for baseline and cluster effect, however, indicated that the difference was not statistically significant (P=0.52 at 12 months). Further analyses of haemoglobin A1c levels with an additional adjustment for oral hypoglycaemic agents showed no significant difference between the groups at all time points (P=0.64 at 12 months). Both groups showed a loss in body weight over the 12 months; the mean change was greater in the intervention group than in the control group: −2.98 kg (−3.54 to −2.41) compared with −1.86 kg (−2.44 to −1.28). The differences in weight, after adjusting for baseline and cluster effect, were significant at four and 12 months (P=0.024 and P=0.027). The intervention group showed a significant reduction in triglyceride levels at eight months (P=0.008). Both groups showed improvements in the remaining biomedical measures over the 12 months; however the differences between the groups were not statistically significant at the 5% level.
Table 2.
Changes in biomedical outcomes by follow-up times and treatment differences between participants with newly diagnosed type 2 diabetes allocated to a structured group education programme or to usual care (control)
| Variables | Change† (95% CI) | Model summary‡ | P value§ | |
|---|---|---|---|---|
| Intervention group | Control group | Coefficient (95% CI) | ||
| Haemoglobin A1c level (%): | ||||
| 4 months | −1.23 (−1.41 to −1.06) | 0.93 (−1.10 to −0.76) | 0.02 (−0.14 to 0.19) | 0.78 |
| 8 months | −1.50 (−1.69 to −1.31) | −1.11 (−1.29 to −0.92) | −0.03 (−0.18 to 0.13) | 0.74 |
| 12 months | −1.49 (−1.69 to −1.29) | −1.21 (−1.40 to −1.02) | 0.05 (−0.10 to 0.20) | 0.52 |
| Overall¶ | — | — | 0.01 (−0.12 to 0.14) | 0.88 |
| Body weight (kg): | ||||
| 4 months | −2.84 (−3.24 to −2.45) | −2.05 (−2.47 to −1.63) | −0.72 (−1.35 to −0.10) | 0.024* |
| 8 months | −3.08 (−3.62 to −2.54) | −2.29 (−2.81 to −1.77) | −0.76 (−1.63 to 0.09) | 0.081 |
| 12 months | −2.98 (−3.54 to −2.41) | −1.86 (−2.44 to −1.28) | −1.01 (−1.91 to −0.12) | 0.027* |
| Overall¶ | — | — | −0.83 (−1.55 to −0.11) | 0.025* |
| Total cholesterol (mmol/l): | ||||
| 8 months | −0.89 (−1.02 to −0.77) | −0.88 (−1.01 to −0.75) | −0.12 (−0.29 to 0.05) | 0.17 |
| 12 months | −0.95 (−1.09 to −0.81) | −0.94 (−1.08 to −0.81) | −0.08 (−0.25 to 0.08) | 0.33 |
| Overall¶ | — | — | 0.05 (−0.19 to 0.29) | 0.70 |
| High density lipoprotein cholesterol (mmol/l): | ||||
| 8 months | 0.02 (−0.01 to 0.05) | −0.01 (−0.04 to 0.03) | 0.04 (−0.01 to 0.09) | 0.14 |
| 12 months | 0.05 (0.004 to 0.09) | 0.03 (−0.01 to 0.07) | 0.02 (−0.05 to 0.08) | 0.62 |
| Overall¶ | — | — | 0.01 (−0.06 to 0.08) | 0.75 |
| Low density lipoprotein cholesterol (mmol/l): | ||||
| 8 months | −0.59 (−0.72 to −0.46) | −0.78 (−0.95 to −0.61) | −0.02 (−0.18 to 0.14) | 0.81 |
| 12 months | −0.75 (−0.89 to −0.61) | −0.90 (−1.07 to −0.72) | 0.001 (−0.19 to 0.19) | 0.99 |
| Overall¶ | — | — | −0.04 (−0.30 to 0.22) | 0.78 |
| Triglyceride (mmol/l): | ||||
| 8 months | −0.57 (−0.71 to −0.42) | −0.34 (−0.53 to −0.15) | −0.33 (−0.58 to −0.09) | 0.008** |
| 12 months | −0.51 (−0.66 to −0.36) | −0.48 (−0.66 to −0.29) | −0.15 (−0.37 to 0.07) | 0.18 |
| Overall¶ | — | — | −0.11 (−0.42 to 0.21) | 0.50 |
| Systolic blood pressure (mm Hg): | ||||
| 4 months | −4.99 (−6.63 to −3.34) | −5.04 (−6.70 to −3.38) | 0.4 (−1.7 to 2.4) | 0.72 |
| 8 months | −7.99 (−9.63 to −6.35) | −5.93 (−7.93 to −3.93) | −1.4 (−3.8 to 1.0) | 0.25 |
| 12 months | −6.12 (−8.01 to −4.22) | −6.24 (−8.04 to −4.44) | 0.7 (−2.0 to 3.5) | 0.60 |
| Overall¶ | — | — | 0.1 (−1.8 to 2.0) | 0.93 |
| Diastolic blood pressure (mm Hg): | ||||
| 4 months | −3.62 (−4.62 to −2.62) | −2.91 (−4.00 to −1.83) | −0.1 (−1.4 to 1.3) | 0.94 |
| 8 months | −4.55 (−5.62 to −3.48) | −3.49 (−4.66 to −2.32) | −0.2 (−1.6 to 1.2) | 0.78 |
| 12 months | −4.17 (−5.26 to −3.07) | −3.43 (−4.58 to −2.28) | 0.3 (−1.1 to 1.7) | 0.65 |
| Overall¶ | −0.1 (−1.2 to 1.0) | 0.85 | ||
| Waist circumference (cm): | ||||
| 8 months | −2.95 (−3.62 to −2.27) | −2.42 (−3.07 to −1.76) | −0.65 (−1.74 to 0.45) | 0.25 |
| 12 months | −2.85 (−3.66 to −2.03) | −2.79 (−3.50 to −2.08) | −0.08 (−1.32 to 1.15) | 0.90 |
| Overall¶ | — | — | −0.28 (−1.38 to 0.81) | 0.61 |
*P<0.05.
**P<0.01.
†Change from baseline (95% confidence interval) unadjusted.
‡Difference between treatment groups, adjusted for baseline value and cluster effect.
§Significance of intervention term in model.
¶Sum of outcomes at all follow-up points.
United Kingdom prospective diabetes study risk engine
Although the risk estimate for cardiovascular disease from the United Kingdom prospective diabetes study43 was not a specified end point it was calculated at baseline and 12 months for the participants with complete data for the required variables (146 in control group, 180 in intervention group). The median 10 year risk estimate of coronary heart disease or stroke at baseline was 17.7% (interquartile range 11.6%-29.1%) for the control group and 18.9% (11.2%-31.8%) for the intervention group. The equivalent risks at 12 months in the control and intervention groups were 13.6% (7.6%-20.2%) and 10.9% (6.7%-19.1%). The intervention group showed a significantly greater improvement in 10 year risk status than the control group (P<0.002). The percentage with a less than 15% risk at 10 years increased from 39% to 56% in the control group and from 41% to 65% in the intervention group.
Lifestyle outcomes
The intervention group showed a greater reduction in smoking status at all time points (table 3). At 12 months the odds of not smoking in the intervention group was 3.56 (95% confidence interval 1.11 to 11.45) higher than that of the control group, after adjusting for baseline and cluster effect. The results for smoking status were significant at eight and 12 months (P=0.033 for both time points). Participants in the intervention group showed a greater increase in physical activity at all time points, and this was significant at four months (P=0.046, table 3).
Table 3.
Summary of lifestyle outcomes by follow-up times for participants with newly diagnosed type 2 diabetes allocated to a structured group education programme or to usual care (control). Values are percentages (numbers) of participants unless stated otherwise
| Outcomes | Control group | Intervention group | % difference (95% CI)* | Model summary† | |
|---|---|---|---|---|---|
| Odds ratio (95%CI)‡ | P value§ | ||||
| Smoking status¶: | |||||
| Baseline | 16 (53) | 14 (57) | — | — | — |
| 4 months | 14 (39) | 13 (43) | −1.6 (−7.0 to 3.8) | 3.09 (0.99 to 9.61) | 0.052 |
| 8 months | 14 (35) | 10 (33) | −3.7 (−9.1 to 1.7) | 2.97 (1.09 to 8.08) | 0.033 |
| 12 months | 16 (37) | 11 (32) | −5.1 (−11.2 to 1.0) | 3.56 (1.11 to 11.45) | 0.033 |
| Physical activity**: | |||||
| Baseline | 92 (302) | 93 (363) | — | — | — |
| 4 months | 91 (259) | 95 (336) | −4.3 (−8.3 to −0.3) | 2.17 (1.01 to 4.66) | 0.046 |
| 8 months | 93 (245) | 94 (305) | −6.8 (−9.9 to −3.8) | 1.18 (0.61 to 2.26) | 0.63 |
| 12 months | 96 (233) | 96 (293) | −0.6 (−4.0 to 2.8) | 1.11 (0.47 to 2.65) | 0.81 |
*Difference in proportion: (control)−(intervention).
†Estimates are derived using robust generalised estimating equations. Results are adjusted for baseline values and cluster effect.
‡Odds for not smoking in intervention group compared with control group.
§Significance of intervention term in model.
¶Reported in previous week.
**Any reported in previous week.
Psychosocial measures
Adjusted analyses showed that differences between the groups in four illness belief scores (coherence, timeline, personal responsibility, and seriousness) were all highly significant (P<0.001, table 4). The directions of change were positive showing that the intervention group had greater understanding of their illness and its seriousness. The intervention group also showed a better perception of the duration of their diabetes and of their ability to affect the course of their disease, as indicated by the increase in these scores at 12 months (table 4).
Table 4.
Scores for belief in illness by follow-up times for participants with newly diagnosed type 2 diabetes allocated to a structured group education programme or to usual care (control)
| Outcomes | Median (interquartile range) | Model summary* | |||
|---|---|---|---|---|---|
| Control | Intervention | Coefficient (95% CI) | P value† | ||
| Illness coherence‡: | |||||
| Baseline | 15 (12-19) | 14 (12-18) | −0.80 (−1.49 to −0.11) | ||
| 4 months | 18 (14-20) | 19 (16-20) | 2.18 (1.60 to 2.76) | <0.001 | |
| 8 months | 19 (14-20) | 20 (16-20) | 1.50 (0.90 to 2.09) | <0.001 | |
| 12 months | 19 (15-20) | 20 (17-20) | 1.22 (0.61 to 1.83) | <0.001 | |
| Overall§ | 18.3 (14-20) | 19.5 (16.3-20.5) | 1.78 (1.25 to 2.31) | <0.001 | |
| Timeline¶: | |||||
| Baseline | 20 (15-24) | 20 (17-25) | 0.88 (0.29 to 1.47) | ||
| 4 months | 20 (19-25) | 22 (20-25) | 0.60 (0.08 to 1.12) | 0.023 | |
| 8 months | 20 (17-25) | 22 (20-25) | 1.09 (0.41 to 1.77) | 0.002 | |
| 12 months | 20 (19-25) | 22 (20-25) | 0.69 (0.04 to 1.37) | 0.036 | |
| Overall§ | 20.4 (18-23) | 22 (20-24.7) | 0.83 (0.38 to 1.29) | <0.001 | |
| Personal responsibility¶: | |||||
| Baseline | 24 (22-26) | 24 (22-26) | −0.06 (−0.58 to 0.47) | ||
| 4 months | 24 (22-26) | 25 (24-28) | 1.20 (0.79 to 1.62) | <0.001 | |
| 8 months | 24 (22-26) | 25 (23-29) | 0.84 (0.40 to 1.27) | <0.001 | |
| 12 months | 24 (23-26) | 24 (23-28) | 0.51 (−0.002 to 1.027) | 0.051 | |
| Overall§ | 24 (22.5-26.3) | 25 (23.3-27.3) | 0.92 (0.56 to 1.29) | <0.001 | |
| Impact**: | |||||
| Baseline | 14 (12-17) | 14 (12-17) | −0.20 (−0.79 to 0.39) | ||
| 4 months | 13 (12-16) | 13 (11-16) | −0.07 (−0.46 to 0.32) | 0.72 | |
| 8 months | 13 (12-15) | 13 (11-16) | 0.33 (−0.16 to 0.83) | 0.19 | |
| 12 months | 13 (12-15) | 14 (12-15) | 0.04 (−0.50 to 0.58) | 0.89 | |
| Overall§ | 13 (11.3-15.7) | 13.3 (11.5-15.3) | 0.05 (−0.34 to 0.43) | 0.82 | |
| Seriousness¶: | |||||
| Baseline | 16 (15-18) | 16 (15-18) | 0.22 (−0.10 to 0.54) | ||
| 4 months | 16 (14-18) | 17 (15-19) | 1.05 (0.70 to 1.39) | <0.001 | |
| 8 months | 16 (14-18) | 17 (16-19) | 1.05 (0.66 to 1.44) | <0.001 | |
| 12 months | 16 (14-18) | 17 (15-19) | 0.85 (0.43 to 1.27) | <0.001 | |
| Overall§ | 16 (14.5-18.0) | 17.2 (15.7-19.0) | 0.97 (0.67 to 1.27) | <0.001 | |
*Coefficient represents change in mean in intervention group compared with control group. Estimates are derived using robust generalised estimating equations. Results are adjusted for baseline values and cluster effect.
†Significance of intervention term in model.
‡Score range 5-25.
§Sum of outcomes at 4, 8, and 12 months.
¶Score range 5-30.
**Score range 5-35.
Symptoms indicative of depression (depression score ≥8 on hospital anxiety and depression scale) were reported by 16% of women and 8% of men at baseline, values that compare with published normative data from the UK.44 Depression scores were lower in the intervention group than in the control group at all time points, and the difference between the groups was significant at 12 months (P=0.032, table 5). The groups did not differ significantly for emotional impact of diabetes at eight and 12 months (P=0.97 and P=0.91, table 5).
Table 5.
Depression and emotional impact of diabetes by follow-up times for participants with newly diagnosed type 2 diabetes allocated to a structured group education programme or to usual care (control)
| Outcomes | Median (interquartile range) | Model summary* | |||
|---|---|---|---|---|---|
| Control group | Intervention group | Coefficient (95% CI) | P value† | ||
| Depression‡: | |||||
| Baseline | 3 (1-5) | 2 (1-5) | −0.28 (−0.83 to 0.27) | — | |
| 4 months | 2 (1-5) | 2 (1-4) | −0.28 (−0.66 to 0.10) | 0.15 | |
| 8 months | 2 (1-6) | 2 (1-5) | −0.25 (−0.64 to 0.14) | 0.21 | |
| 12 months | 2 (1-6) | 2 (1-5) | −0.50 (−0.96 to −0.04) | 0.032 | |
| Overall§ | — | — | −0.46 (−0.80 to −0.13) | 0.007** | |
| Emotional impact of diabetes¶: | |||||
| 8 months | 14.1 (4.7-26.6) | 14.1 (4.7-28.1) | −0.05 (−2.73 to 2.63) | 0.97 | |
| 12 months | 12.5 (4.7-28.1) | 14.1 (6.3-28.1) | 0.19 (−3.20 to 3.59) | 0.91 | |
| Overall§ | — | — | −0.10 (−2.90 to 2.70) | 0.95 | |
**P<0.01.
*Coefficient represents change in mean in the intervention group compared to the control group. Estimates are derived using robust generalised estimating equations. Results are adjusted for baseline values and cluster effect.
†Significance of intervention term in model.
‡Score range 0 to 21.
§Sum of outcomes at all follow-up points.
¶Score range 0 to 100.
Quality of life
The groups did not differ significantly in any of the scores for six dimensions of quality of life (two overall scores and four subscale scores for physical, psychological, social, and environmental). The results of the analyses are available at www.leicestershirediabetes.org.uk.
Association of changes in illness beliefs with biomedical outcomes
After controlling for weight at baseline, sex, and age, the change in perceived responsibility correlated with weight loss at four months (β=0.13; P<0.002) and 12 months (β=0.12; P<0.008). That is, people who reported a greater increase in their perceived responsibility for the course of their diabetes lost more weight.
Discussion
A group structured education programme focused on behaviour change can successfully engage those with newly diagnosed type 2 diabetes in starting additional effective lifestyle changes sustainable over 12 months from diagnosis. Although clinically significant improvements were found in haemoglobin A1c levels, lipid profile, body weight, and blood pressure in both the intervention and control groups (usual care), after adjusting for cluster and baseline values we found no statistically significant differences between the groups, apart from a greater reduction in triglyceride levels in the intervention group at eight months and in body weight at four and 12 months. The modest (1.1 kg) but statistically significant difference in weight loss between the groups was sustained to 12 months. A significantly greater reduction was found in the number of people in the intervention group who reported smoking, and this was sustained to 12 months. Self reported physical activity was greater in the intervention group at four months; this difference was not present at eight and 12 months. There was a greater improvement in the risk score for coronary heart disease from the United Kingdom prospective diabetes study at 12 months, with significantly more participants in the intervention group having a 10 year risk score of less than 15%.
Key health beliefs differed significantly between the groups after the intervention, with participants in the intervention group showing greater improvement in beliefs about diabetes related illness. This confirms previous findings from the pilot phase of the study.45 Depression scores decreased significantly at 12 months in the intervention group but no difference was found in diabetes specific emotional distress, the values for which were similar to those collected in a study on patients with screen detected type 2 diabetes.46 These results are reassuring as they indicate that despite the intervention group reporting increased personal responsibility for their diabetes, a greater belief in its seriousness, and that it would last for life, they experienced less depression and no difference in emotional distress.
Across the whole cohort, after adjusting for body weight at baseline, a significant association was found between change in perceived personal responsibility and weight loss at four and 12 months. Although this analysis does not establish causality, data from the pilot study22 indicate that changes in these beliefs about illness are evident immediately after the intervention and therefore precede changes in weight loss.
Strengths and limitations of the study
The limitations and difficulties of doing pragmatic intervention trials in a primary care setting are well recognised.47 48 Intervention and control participants were well matched for variables except for haemoglobin A1c levels and sex. Such anomalies are not, however, uncommon in a pragmatic cluster randomised controlled trial.49 One reason for a higher level of haemoglobin A1c in the intervention group could be differential referral rates, with intervention practices more enthusiastic to refer patients with higher levels. The introduction of the quality and outcomes framework may have incentivised treatment to target, particularly where haemoglobin A1c is concerned.50 The lack of difference in quality of life between the groups may result from a lack of sensitivity in the tool used.
The study has several strengths. Firstly, it has widespread generalisability as the sample size was large and representative of patients with newly diagnosed type 2 diabetes. Secondly, the intervention was pragmatically designed for implementation in a primary care setting in the UK. Up to 10 newly diagnosed people can attend each group intervention allowing its delivery to large numbers of people. Thirdly, the trial had a robust cluster design to reduce contamination between practices, with high recruitment and retention rates of participants. Finally, we applied few exclusion criteria, and validated generic and disease specific questionnaires were used in the evaluation.
The UK now has some of the best data in the world for process in diabetes care,3 51 with over 90% of patients with diabetes having biomedical variables recorded and translated into good outcomes in achieving targets for haemoglobin A1c levels, blood pressure, and lipid levels. For example, 59% of people with diabetes achieve a haemoglobin A1c level of less than or equal to 7.4%, 71% a blood pressure of less than or equal to 145/85, and 72% a cholesterol of less than or equal to 5 mmol/l. Therefore shortly after diagnosis, when medical outcomes are being aggressively and effectively targeted, it becomes harder to show the additional benefits of providing structured education.
It is also important to remember that by design the structured group education approach encourages people to choose their own risk factors for action. Furthermore, a major proportion of the programme focuses on the importance of diet, lifestyle, and physical activity and includes the importance of managing cardiovascular risk factors, with participants encouraged to focus on the risk factor of most importance to them.
As this intervention is offered early in the course of type 2 diabetes, it is not surprising that people focused on factors such as weight loss, physical activity, and smoking rather than biomedical variables that may already be at target levels. Overall mean levels for the cohort at 12 months were 6.7% for haemoglobin A1c, 4.4 mmol/l for cholesterol, 1.2 mmol/l for high density lipoprotein cholesterol, 134 mm Hg for systolic blood pressure, and 77 mm Hg for diastolic blood pressure. All these outcome measures are well below those levels advocated by the quality and outcomes framework.
Putting the study in context
One study reported on long term non-pharmacological weight loss in adults with type 2 diabetes using data from 22 studies with a follow-up of 1-5 years.52 The pooled weight loss for any intervention compared with usual care was 1.7 kg. One conclusion was that weight loss strategies including dietary, physical activity, or behavioural interventions produced small improvements in weight between the groups, similar to our results. Within our study (which did not specifically target weight loss), after adjustment for clustering the intervention group lost an additional 1.1 kg. For changes in self reported smoking status, a recent systematic review and meta-analysis of psychosocial interventions for smoking cessation for patients with coronary heart disease53 showed overall a positive effect of the intervention on abstinence after 6-12 months, with an odds ratio of 1.66. In comparison, our study reported an odds ratio of 3.56 (95% confidence interval 1.11 to 11.45).
Since the last NICE review of effectiveness of structured education in people with established type 2 diabetes,11 two key studies have been published. The Turin study54 reported on a group education intervention in people with established diabetes (about nine years). Although the study was relatively small, with only 120 participants, at five years biomedical outcomes, knowledge about diabetes, and quality of life differed significantly between the control and intervention groups. This study was carried out in a single specialist centre in Italy. The findings have informed the rethink organisation to improve education and outcomes study,55 which is being done across several sites and will tackle the generalisability and replicability of the programme.
The expert patient education versus routine treatment study56 was carried out in a single primary care trust, with the programme delivered by one experienced educator to people with established diabetes (314 participants). At 14 months there was a reduction in haemoglobin A1c levels and significant improvements in knowledge about diabetes, physical activity levels, and satisfaction with treatment, with no difference in quality of life between the groups. The generalisability of this programme has yet to be tested.
It is difficult to compare our structured group education programme directly with the two key studies because of the different populations (newly diagnosed diabetes compared with established diabetes) and because our study concerned multiple sites and educators. As the intervention was delivered in 13 geographical locations forming a representative sample drawn from primary care, it provides data on the largest cohort of this particular population. The study was robustly carried out according to an evaluation framework widely accepted as providing scientific rigour for complex interventions of this kind.20 The attention to the quality indicators recommended by NICE and the Department of Health for structured education ensured that previously neglected areas were dealt with, such as systematic training and quality assurance for facilitators delivering the programme. Our programme involved 34 educators, trained for two days. Quality assurance was provided during the trial to ensure consistency in the quality and integrity of the intervention delivered. As a result, this intervention is replicable.
We found significant reductions in haemoglobin A1c levels in both arms of our study. Although the intervention group had a higher baseline and an absolute decrease at 12 months that was 0.4% greater than the control group, both groups had haemoglobin A1c levels well below the national target of 7.5%.50 In the UK prospective diabetes study, which also recruited a newly diagnosed cohort, after three months of dietary treatment and from a higher baseline than the present study, haemoglobin A1c levels decreased from 9.1% to 7.2%.57 In those randomised to treatment with either a sulphonylurea or insulin, a further decrease in haemoglobin A1c levels to 6.1% and 6.8%, respectively, was reported at 12 months whereas a modest rise was observed in those who were randomised to diet alone. Therefore it is usual for noticeable reductions to occur in levels shortly after diagnosis and in terms of showing a difference in levels between groups, patients with newly diagnosed type 2 diabetes may be the most difficult in which to demonstrate this. To investigate this further, we will be collecting follow-up data at three years.
Implications
The significance of our results to clinical practice lies in their generalisability. The most recent figure for incidence of type 2 diabetes in England and Wales is 2.21 per 1000 patient years.58 Comparison of the rates of referral and consent (per 1000 patient years) from our study with this recent incidence data shows that around 55% of predicted incident cases were referred.
The results provide evidence that structured education meeting national service framework and NICE quality standards can provide added benefit to medical optimisation. Additional benefits shown in our intervention group were improvements in weight loss, self reported smoking status, and physical activity levels, and a change in illness beliefs that was associated with these behavioural changes. Depression in people with diabetes is associated with poor glycaemic control59 and increased mortality.60 However, our intervention also led to a decrease in depression scores.
In summary, our structured group education programme encapsulates a patient centred approach to diabetes care. Taking place at a time when the quality and outcomes framework targets aggressively promote medical therapies to reach glycaemic targets, it is perhaps not surprising that levels of haemoglobin A1c were not significantly different between the groups. However, pharmacological treatments by their nature cannot tackle markers of successful long term control such as beliefs about illness and attitudes to diabetes, which influence behaviour and lifestyle change and sustain motivation. Our trial has filled a gap in the evidence base on structured education in people with newly diagnosed type 2 diabetes, and has shown that group structured education that focused on behaviour change can successfully engage patients in starting additional effective lifestyle changes sustainable over 12 months from diagnosis.
What is already known on this topic
The diabetes national service framework promotes structured education for all from diagnosis of diabetes
However, until now, there has been no scientific evaluation and no programmes demonstrably meeting all the quality criteria
What this study adds
A structured group education programme for patients with newly diagnosed type 2 diabetes was associated with benefits in illness beliefs, weight loss, physical activity, smoking status, and depression but not in haemoglobin A1c levels
Most of the changes were sustained over 12 months without further reinforcement
The intervention is generalisable and replicable
Contributors: MJD, SH, MJC, MEC, HMD, HD, SE, CF, KR, GR, TCS, and KK wrote the paper on behalf of the Diabetes Education and Self Management for Ongoing and Newly Diagnosed Collaborative. The Collaborative Steering Group comprised MJD, S Roberts, S Amiel, F Arundel, MEC, D Cavan, SC, HMD, HD, YD, SE, A Farooqi, CF, G Hawthorne, SH, P James, K Jones, E Kennedy, N Kennedy, KK, S Lucas, M MacKinnon, C Mittler, LO, GR, L Richardson, J Roddick, A Rogers, J Roland, A Setterfield, TCS, S Tesfaye, S Trowbridge, P Weir, S White, and G Wilson. The Training Strategy Group comprised SC, HD, YD, LO, and TCS. The participating primary care trusts (local coordinators) were Bath and North East Somerset (Anne Thomas; Yvonne Smith, Jody Smally); Greater Glasgow (Florence Brown, Fiona MacIntyre); Greater Peterborough primary care trust partnership (Sam Hartley); East Staffordshire (Fiona Kirkland, Kathy Rea); East Leicester and Melton Rutland and Harborough (MEC); Ipswich, Suffolk Central and Suffolk Coastal (Donna Watling, Heidi Heighton, Caroline Calver); Northampton (Penny Meade); Newcastle (Jacqui Stephenson, Michelle Hurst); North Tyneside (LO, Norma Cardill); South Leicestershire (Maxine Mays); North Sheffield and Sheffield West (Jenny Cowling, Cathy Bounekhla, Alison Iliffe); West Cumbria (Anne Eldred); and West Lothian (Reita Early, Laura Hunter). The educators were Jane Davies and Catherine Taylor (Bath and North-East Somerset); Sayjal Amin, Lorraine Martin Stacey, and Maggie Boddington (Eastern Leicester and Melton Rutland and Harborough); Anne Scott and Kath Sutton (Ipswich); Marie Caraher and Sarah White (Newcastle); Penny Meade, Kath Hall and Karen Osbourne (Northampton); LO, Susan Robinson, Anne Rodgers, and Lesley Tiffin (North Tyneside); Monica Harris and Gail Nixon (Peterborough); Pauline Cowling, Lynette Hall, and Penny Walker (Sheffield); Maxine Mays, Geri Gray and Suzanne Fenn (Leicester South); Fiona Kirkland and Karen Gale (East Staffordshire); Cheryl Taylor and Karen Rogers (West Cumbria); Debbie Halliday, May Lavelle, Katrina Phillips, and Maureen Cullen (Greater Glasgow); and Reita Early and Grace Bathgate (West Lothian). MEC was the project manager; HMD was the research associate; M Bonar, A Harding, and C Sutcliffe were the central office team; and MJC and KR were the statisticians (University of Sheffield).
Funding: This study was funded by Diabetes UK. The project office administration was funded by an unrestricted educational grant from Novo Nordisk. The researchers were independent of any of the study funders. The study sponsor was the University Hospitals of Leicester NHS Trust. The research team and the principle investigator were employees of the sponsor during the period of the study.
Competing interests: None declared.
Ethical approval: This study was approved by the Huntingdon local research ethics committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.International Diabetes Federation. IDF diabetes atlas—prevalence estimates of diabetes mellitus. www.eatlas.idf.org 2007.
- 2.Massi-Benedetti M. The cost of diabetes type II in Europe: the CODE-2 study. Diabetologia 2002;45:S1-4. [DOI] [PubMed] [Google Scholar]
- 3.Khunti K, Gadsby R, Millett C, Majeed A, Davies M. Quality of diabetes care in the UK: comparison of published quality-of-care reports with results of the Quality and Outcomes Framework for Diabetes. Diabetic Medicine 2007;24:1436-41. [DOI] [PubMed] [Google Scholar]
- 4.Morris AD. Considerations in assessing effectiveness and costs of diabetes care: lessons from DARTS. Diabetes Metab Res Rev 2002;18:S32-5. [DOI] [PubMed] [Google Scholar]
- 5.Skinner T, Cradock S, Arundel F, Graham W. Four theories and a philosophy: self-management education for individuals newly diagnosed with type 2 diabetes. Diabetes Spectr 2003;16:75-80. [Google Scholar]
- 6.Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control. Diabetes Care 2002;25:1159-71. [DOI] [PubMed] [Google Scholar]
- 7.Lorig K. Partnerships between expert patients and physicians. Lancet 2002;359:814-5. [DOI] [PubMed] [Google Scholar]
- 8.Department of Health. National service framework for diabetes: standards London: DoH, 2001
- 9.Department of Health. National service framework for diabetes: delivery strategy London: DoH, 2002
- 10.Audit Commission. Testing times. A review of diabetes services in England and Wales Northampton: Belmont Press, 2000
- 11.National Institute for Clinical Excellence. Guidance on the use of patient-education models for diabetes (Technology Appraisal 60) London: NICE, 2003
- 12.Rutten G. Diabetes patient education: time for a new era. Diabet Med 2005;22:671-3. [DOI] [PubMed] [Google Scholar]
- 13.Kronsbein P, Jorgens V, Muhlhauser I, Scholz V, Venhaus A, Berger M. Evaluation of a structured treatment and teaching programme on non-insulin-dependent diabetes. Lancet 1988;2:1407-11. [DOI] [PubMed] [Google Scholar]
- 14.Trento M, Passera P, Borgo E, Tomalino M, Bajardi M, Cavallo F, et al. A 5-year randomized controlled study of learning, problem solving ability, and quality of life modifications in people with type 2 diabetes managed by group care. Diabetes Care 2004;27:670-5. [DOI] [PubMed] [Google Scholar]
- 15.Wagner EH, Grothaus LC, Sandhu N, Galvin MS, McGregor M, Artz K, et al. Chronic care clinics for diabetes in primary care: a system-wide randomized trial. Diabetes Care 2001;24:695-700. [DOI] [PubMed] [Google Scholar]
- 16.Norris SL, Engelgau MM, Narayan KM. Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care 2001;24:561-87. [DOI] [PubMed] [Google Scholar]
- 17.Deakin T, McShane CE, Cade JE, Williams RD. Group based training for self-management strategies in people with type 2 diabetes mellitus. Cochrane Database Syst Rev 2005;CD003417. [DOI] [PubMed]
- 18.Glasgow RE, Hampson SE, Strycker LA, Ruggiero L. Personal-model beliefs and social-environmental barriers related to diabetes self-management. Diabetes Care 1997;20:556-61. [DOI] [PubMed] [Google Scholar]
- 19.Ellis SE, Speroff T, Dittus RS, Brown A, Pichert JW, Elasy TA. Diabetes patient counseling: a meta-analysis and meta-regression. Patient Educ Couns 2005;52:97-105. [DOI] [PubMed] [Google Scholar]
- 20.Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, et al. Framework for design and evaluation of complex interventions to improve health. BMJ 2000;321:694-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Education Group for Guidelines on Evaluation. Guidelines for evaluating papers on educational interventions. BMJ 1999;318:1265-7. [PMC free article] [PubMed] [Google Scholar]
- 22.Skinner TC, Carey ME, Cradock S, Daly H, Davies MJ, Doherty Y, et al. Diabetes education and self-management for ongoing and newly diagnosed (DESMOND): process modelling of pilot study. Patient Educ Couns 2006;64:369-77. [DOI] [PubMed] [Google Scholar]
- 23.Kinmonth AL, Woodcock A, Griffin S, Spiegal N, Campbell MJ. Randomised controlled trial of patient centred care of diabetes in general practice: impact on current wellbeing and future disease risk. BMJ 1998;317:1202-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leventhal H, Meyer D, Nerenz D. The common-sense representation of illness danger. Contributions to medical psychology 2nd ed. New York: Pergamon, 1980
- 25.Chaiken S, Wood W, Eagly A. Principles of persuasion. In: Higgih ET, Kruglanski AW, eds. Social psychology: handbook of basic principles New York: Guilford Press, 1996
- 26.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev 1977;84:191-215. [DOI] [PubMed] [Google Scholar]
- 27.Anderson RM. Patient empowerment and the traditional medical model. A case of irreconcilable differences? Diabetes Care 1995;18:412-5. [DOI] [PubMed] [Google Scholar]
- 28.Anderson RM, Funnell MM, Butler PM, Arnold MS, Fitzgerald JT, Feste CC. Patient empowerment. Results of a randomized controlled trial. Diabetes Care 1995;18:943-9. [DOI] [PubMed] [Google Scholar]
- 29.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care 2000;23:943-50. [DOI] [PubMed] [Google Scholar]
- 30.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381-95. [DOI] [PubMed] [Google Scholar]
- 31.The WHOQOL Group. The World Health Organisation Quality of Life Assessment (WHOQOL): development and general psychometric properties. Soc Sci Med 2005;46:1569-85. [DOI] [PubMed] [Google Scholar]
- 32.Rose M, Fliege H, Hildebrandt M, Schirop T, Klapp BF. The network of psychological variables in patients with diabetes and their importance for quality of life and metabolic control. Diabetes Care 2002;25:35-42. [DOI] [PubMed] [Google Scholar]
- 33.Moss-Morris R, Weinman J, Petrie KJ, Horne R, Cameron LD, Buick L. The Revised Illness Perceptions Questionnaire (IPQ-R). Psychology and Health 2002;17:1-16. [Google Scholar]
- 34.Skinner TC, Howells L, Greene S, Edgar K, McEvilly A, Johansson A. Development, reliability and validity of the diabetes illness representations questionnaire: four studies with adolescents. Diabet Med 2003;20:283-9. [DOI] [PubMed] [Google Scholar]
- 35.Weinman J, Petrie KJ, Moss-Morris R, Horne R. The illness perceptions questionnaire: a new method for assessing the cognitive presentation of illness. Psychol Health 1996;11:431-41. [Google Scholar]
- 36.Hampson SE, Glasgow RE, Toobert DJ. Personal models of diabetes and their relations to self-care activities. Health Psychol 1990;9:632-46. [DOI] [PubMed] [Google Scholar]
- 37.Skinner TC, Hampson SE, Fife-Schaw C. Personality, personal model beliefs, and self-care in adolescents and young adults with type 1 diabetes. Health Psychol 2002;21:61-70. [PubMed] [Google Scholar]
- 38.Welch GW, Jacobson AM, Polonsky WH. The problem areas in diabetes scale. An evaluation of its clinical utility. Diabetes Care 1997;20:760-6. [DOI] [PubMed] [Google Scholar]
- 39.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. [DOI] [PubMed] [Google Scholar]
- 40.Campbell MK, Elbourne DR, Altman DG. CONSORT statement: extension to cluster randomised trials. BMJ 2004;328:702-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campbell MJ, Donner A, Klar N. Developments in cluster randomized trials and statistics in medicine. Stat Med 2007;26:2-19. [DOI] [PubMed] [Google Scholar]
- 42.Harrell FE, Margolis PA, Gove S, Mason KE, Mulholland EK, Lehmann L, et al. Development of a clinical prediction model for an ordinal outcome: the World Health Organization multicentre study of clinical signs and etiologic agents of pneumonia, sepsis and meningitis in young infants. Stat Med 1998;17:90-144. [DOI] [PubMed] [Google Scholar]
- 43.Stevens RJ, Kothari V, Adler AI, Stratton IM. The UKPDS risk engine: a model for the risk of coronary heart disease in type II diabetes (UKPDS 56). Clin Sci (Lond) 2001;101:671-9. [PubMed] [Google Scholar]
- 44.Pouwer F, Beekman AT, Nijpels G, Dekker JM, Snoek FJ, Kostense PJ, et al. Rates and risks for co-morbid depression in patients with type 2 diabetes mellitus: results from a community-based study. Diabetologia 2003;46:892-8. [DOI] [PubMed] [Google Scholar]
- 45.Davies MJ, Carey ME, Dallosso HM, Heller S, Khunti K, Skinner TC. Effect of a structured education programme on illness beliefs, QOL, and physical activity in individuals newly diagnosed with type 2 diabetes: the DESMOND pilot study. Diabetologia 2006;49(suppl 1):535 [Google Scholar]
- 46.Thoolen BJ, de Ridder DT, Bensing JM, Gorter KJ, Rutten GE. Psychological outcomes of patients with screen-detected type 2 diabetes: the influence of time since diagnosis and treatment intensity. Diabetes Care 2006;29:2257-62. [DOI] [PubMed] [Google Scholar]
- 47.Moher M, Yudkin P, Wright L, Turner R, Fuller A, Schofield T, et al. Cluster randomised controlled trial to compare three methods of promoting secondary prevention of coronary heart disease in primary care. BMJ 2001;322:1338-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilson S, Delaney BC, Roalfe A, Roberts L, Redman V, Wearn AM, et al. Randomised controlled trials in primary care: case study. BMJ 2000;321:24-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khunti K, Stone M, Paul S, Baines J, Gisbourne L, Farooqi A, et al. Disease management programme for secondary prevention of coronary heart disease and heart failure in primary care: a cluster randomised controlled trial. Heart 2007;93:1398-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sigfrid LA, Turner C, Crook D, Ray S. Using the UK Primary Care Quality and Outcomes Framework to audit health care equity: preliminary data on diabetes management. J Public Health 2006;28:221-5. [DOI] [PubMed] [Google Scholar]
- 51.Campbell S, Reeves D, Kontopantelis E, Middleton E, Sibbald B, Roland M. Quality of primary care in England with the introduction of pay for performance. N Engl J Med 2007;357:181-90. [DOI] [PubMed] [Google Scholar]
- 52.Norris SL, Zhang X, Avenell A, Gregg E, Bowman B, Serdula M, et al. Long-term effectiveness of lifestyle and behavioral weight loss interventions in adults with type 2 diabetes: a meta-analysis. Am J Med 2004;117:762-74. [DOI] [PubMed] [Google Scholar]
- 53.Barth J, Critchley J, Bengel J. Efficacy of psychosocial interventions for smoking cessation in patients with coronary heart disease: a systematic review and meta-analysis. Ann Behav Med 2006;32:10-20. [DOI] [PubMed] [Google Scholar]
- 54.Trento M, Passera P, Borgo E, Tomalino M, Bajardi M, Cavallo F, et al. A 5-year randomized controlled study of learning, problem solving ability, and quality of life modifications in people with type 2 diabetes managed by group care. Diabetes Care 2004;27:670-5. [DOI] [PubMed] [Google Scholar]
- 55.Porta M, Trento M. ROMEO: rethink organization to improve education and outcomes. Diabet Med 2004;21:644-5. [DOI] [PubMed] [Google Scholar]
- 56.Deakin TA, Cade JE, Williams R, Greenwood DC. Structured patient education: the diabetes X-PERT programme makes a difference. Diabet Med 2006;23:944-54. [DOI] [PubMed] [Google Scholar]
- 57.United Kingdom Prospective Diabetes Study Group. United Kingdom prospective diabetes study (UKPDS) 13: relative efficacy of randomly allocated diet, sulphonylurea, insulin, or metformin in patients with newly diagnosed non-insulin dependent diabetes followed for three years. BMJ 1995;310:83-8. [PMC free article] [PubMed] [Google Scholar]
- 58.Forouhi NG, Merrick D, Goyder E, Ferguson BA, Abbas J, Lachowycz K, et al. Diabetes prevalence in England, 2001—estimates from an epidemiological model. Diabet Med 2006;23:189-97. [DOI] [PubMed] [Google Scholar]
- 59.Lustman PJ, Anderson RJ, Freedland KE, De Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care 2000;23:934-42. [DOI] [PubMed] [Google Scholar]
- 60.Zhang X, Norris SL, Gregg EW, Cheng YJ, Beckles G, Kahn HS. Depressive symptoms and mortality among persons with and without diabetes. Am J Epidemiol 2005;161:652-60. [DOI] [PubMed] [Google Scholar]