Abstract
Rationale: Although oxidative stress is a cardinal feature of asthma, the roles of oxidant air pollutants and antioxidant genes heme oxygenase 1 (HMOX-1), catalase (CAT), and manganese superoxide dismutase (MNSOD) in asthma pathogenesis have yet to be determined.
Objectives: We hypothesized that the functional polymorphisms of HMOX-1 ([GT]n repeat), CAT (−262C>T −844C>T), and MNSOD (Ala-9Val) are associated with new-onset asthma, and the effects of these variants vary by exposure to ozone, a potent oxidant air pollutant.
Methods: We assessed this hypothesis in a population-based cohort of non-Hispanic (n = 1,125) and Hispanic white (n = 586) children who resided in 12 California communities and who were followed annually for 8 years to ascertain new-onset asthma.
Measurements and Main Results: Air pollutants were continuously measured in each of the study communities during the 8 years of study follow-up. HMOX-1 “short” alleles (<23 repeats) were associated with a reduced risk for new-onset asthma among non-Hispanic whites (hazard ratio [HR], 0.64; 95% confidence interval [CI], 0.41–0.99). This protective effect was largest in children residing in low-ozone communities (HR, 0.48; 95% CI, 0.25–0.91) (interaction P value = 0.003). Little evidence for an association with HMOX-1 was observed among Hispanic children. In contrast, Hispanic children with a variant of the CAT-262 “T” allele (CT or TT) had an increased risk for asthma (HR, 1.78; P value = 0.01). The effects of these polymorphisms were not modified by personal smoking or secondhand-smoke exposure.
Conclusions: Functional promoter variants in CAT and HMOX-1 showed ethnicity-specific associations with new-onset asthma. Oxidant gene protection was restricted to children living in low-ozone communities.
Keywords: asthma, catalase, heme oxygenase-1, MnSOD, oxidative stress, ozone
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
The selected genes HMOX-1, CAT, and MNSOD are key candidate genes in the oxidative stress pathway; the latter has been shown to play an important role in asthma development.
What This Study Adds to the Field
We observed differential effect of HMOX-1 and CAT on asthma by ethnicity. Shorter repeat lengths of the HMOX-1 gene is strongly protective for asthma among non-Hispanic whites. This reduced risk was restricted to children living in low-ozone communities.
Airway oxidative stress is a cardinal feature of asthma and is believed to play a central role in its pathogenesis (1, 2). The evidence for a role of oxidative stress in the development of asthma is growing and includes studies showing increased oxidative stress and reduced antioxidant activity among patients with (3) and studies showing that the risk of developing asthma is greater among people with decreased ability to detoxify reactive oxygen species (ROS) (4).
Cells and other structural elements of the airways are continuously exposed to oxidants produced by cellular metabolism and from exogenous sources, including ambient air pollutants such as ozone and tobacco smoke, that lead to ROS production (5). Excess ROS production may produce oxidative stress and can lead to lipid peroxidation and oxidation products that can result in further oxidative stress (6).
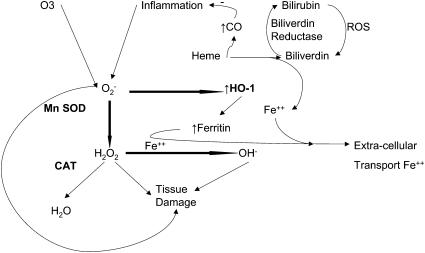
Defenses to prevent excess oxidative stress exist in the airways and include small-molecule antioxidants and antioxidant enzymes, such as heme oxygenase (HO), superoxide dismutases (SODs), and catalase (5). HO provides the first line of defense against oxidative stress because it rapidly responds to oxidants (Figure 1). HO-1 is the inducible form of HO and is present in many tissues, including the lung (7). The HO-1 gene (HMOX-1) expression is up-regulated by stressors that increase oxidant burden (7). SOD provides cytoprotection by catalyzing the reaction of super oxides that result from oxidative stress to hydrogen peroxide (H2O2) (Figure 1). Manganese SOD (MnSOD), an important mitochondrial SOD, appears to play a role in lung disease (8). ROS detoxification is completed when H2O2 is reduced to H2O by a third enzyme, catalase (Figure 1).
Figure 1.
Cellular anti-oxidant defense. Reactive oxygen species (ROS), including O2−, are generated within cells in response to exogenous (i.e., O3) and endogenous (i.e., inflammation) factors. O2−, which can directly cause tissue damage, is initially reduced to unstable H2O2 by manganese super oxide dismutase (MnSOD). This cytotoxic H2O2 is then reduced to H2O by the action of catalase (CAT). O2− also induces heme oxygenase (HMOX-1), leading to catabolism of heme protein to biliverdin with the production of CO and Fe2+. In the presence of biliverdin reductase, biliverdin is reduced to bilirubin, which, after taking up ROS, is then oxidized back to bilirubin. In the presence of biliverdin reductase and ROS, this process continues in a redox cycle, neutralizing cellular ROS.
Given the potential importance of oxidant defenses in asthma pathogenesis, genetic variants in these key oxidant defense genes that alter the activity of the encoded enzymes may modulate the risk for new-onset asthma. One common variant in HMOX-1, a (GT)n dinucleotide tandem repeat of the 5′ flanking region, has been identified, and functional studies have been reported. Functional studies have shown that inducibility of HMOX-1 is inversely related to the number of (GT)n repeats (9–11). On the basis of the distribution and classification by Chen and colleagues (10), we categorized alleles with 23 or fewer (GT)n repeats as “short” (S). MNSOD has a commonly studied polymorphism, a T to C substitution in codon 16, that changes the amino acid at −9 position in the signal peptide from valine (Val) to alanine (Ala). This polymorphism (Ala-9Val) has been proposed to affect the enzyme targeting and function (12, 13). Of the several polymorphisms of the catalase gene (CAT), two common polymorphisms in the promoter region have been studied in this study. One, located 262 bp upstream to the transcription site (CAT-262C>T), has been reported to be associated with catalase expression (14–16) and asthma risk (17). Another potentially functional polymorphism, located 844 bp upstream to the transcription site (CAT-844C>T), has been reported to be associated with essential hypertension (18).
On the basis of the role of oxidative stress in asthma and the function of the three genes in antioxidant defenses, we hypothesized that the aforementioned variants in HMOX-1, CAT, and MNSOD are associated with the risk of new-onset asthma during adolescence. Furthermore, we also hypothesized that these polymorphisms affect antioxidant responses to environmental ozone, a common environmental oxidant (Figure 1). To test these hypotheses, we examined health, genetic, and exposure data collected from 1,711 children of Hispanic and non-Hispanic white ethnicity who participated in the Children's Health Study (CHS), a longitudinal study of respiratory health among children in 12 Southern California communities (19, 20). Some of the results have been reported in abstract form (21).
METHODS
Subjects and Materials
Details concerning the design, methods, and characteristics of the CHS cohort have been presented in previous publications (19, 20). Briefly, fourth-, seventh-, and tenth-grade children were enrolled into the study from 12 communities of Southern California (selected primarily based on differential ambient pollution levels). All children were followed annually until high school graduation (see details in the online supplement).
Study Cohort
At baseline, parental questionnaire responses were used to classify subjects (n = 6,259) according to lifetime history of asthma and wheeze. Children with any history of asthma (n = 1,018), wheezing (n = 1,098), or missing information for wheeze or asthma history at baseline (n = 369) were excluded from this analysis. We further excluded another 776 children who had less than two follow-up visits, which were needed to assess new-onset asthma. Genetic data were available from 66.9% (n = 2,005) of the remaining eligible 2,998 children. Analyses were restricted to children of Hispanic (n = 576) or non-Hispanic white ethnicity (n = 1,125) due to insufficient representation of other races.
New-Onset Asthma
Children with no prior history of asthma at study entry who subsequently reported physician-diagnosed asthma at annual follow-up were classified as having new-onset asthma. The date of onset was assigned to be the midpoint of the interval between the interview date when asthma diagnosis was first reported and the previous interview date.
Air Pollution Data
Ambient levels of ozone (O3), nitrogen dioxide (NO2), particulate matter with an aerodynamic diameter of less than 10 μm (PM10) and 2.5 μm (PM2.5), acid vapor, and elemental and organic carbon in each of the 12 communities were measured at air-monitoring sites from 1994 onward. We calculated long-term mean pollutant levels (from 1994 through 2003) to assign exposure to children in each community for use in the statistical analysis. For the purposes of examining genetic susceptibility, communities were classified as “higher” or “lower” by numerically ranking the 12 communities for each ambient pollutant of interest and dividing the list in half. The ozone levels (10 a.m.–6 p.m.) ranged from 46.5 to 64.9 ppb in the higher ozone communities (mean = 55.2 ppb) and 28.6 to 45.5 ppb in the lower ozone communities (mean = 38.4 ppb) (for details see the online supplement).
Sociodemographic and Medical History Information
Personal information, such as ethnicity, birth weight, premature birth, maternal smoking during pregnancy, and allergy history, was collected at study entry. Family history of asthma was defined as asthma in any of the biological parents. Ethnicity was defined based on parental response to the baseline questionnaire. “Hispanicity” was based on parental response to the question, “Is your child of Hispanic or Spanish descent?” Parents who reported that their children were white and not of “Hispanic or Spanish” descent were considered non-Hispanic whites. Hispanic whites included children who were of “Hispanic or Spanish” descent and whites. We categorized body mass index (BMI) into age- and sex-specific percentiles based on the Centers for Disease Control and Prevention BMI growth charts using 1-month age intervals (22). Participants with BMI at or above the 85th percentile were classified as overweight/at risk of overweight. This personal information, household and indoor exposures (pets, pests, humidifier use, and household smoking), and air pollution were considered as potential effect modifiers as well as confounders in this analysis (for details see the online supplement).
Genotyping
Details of sample collection, processing, and genotyping have been provided earlier (23) and are included in the online supplement. In brief, genomic DNA was extracted from buccal mucosal cells using a Puregene DNA purification kit (Gentra Systems, Minneapolis, MN). Genotyping of MNSOD Ala-9Val (rs4880), CAT-262C>T (rs1001179), and CAT-844C>T (rs769214) was performed using the TaqMan Allelic Discrimination (AD) assay (Applied Biosystems, Foster City, CA). For HMOX-1, the size of (GT)n repeats in each fluorescence-labeled polymerase chain reaction product was determined with GeneScan Analysis software (Applied Biosystems). Five samples with known HMOX-1 (GT)n repeats were run in each experiment as positive controls. Genotypes of 12 homozygous samples were confirmed by an automatic sequencing (BigDye version 3.1, 377XL DNA sequencer; Applied Biosystems). In each run, 10% of the samples were randomly selected and used for quality control.
Statistical Methods
On the basis of the distribution and classification by Chen and coworkers (10), we categorized alleles with 23 or fewer (GT)n repeats as short (S). An association test between the tandem repeat polymorphism and asthma was performed by comparing children with at least one S-allele with those without any S-allele. We also calculated the biallelic average of (GT)n repeats for each individual as the mean of the number of tandem repeats at both alleles. Using the biallelic average number of repeats, asthma risk in children with the average at or below the 25th percentile value was compared with those greater than the 25th percentile. On the basis of a previous publication, CAT-262C>T and MNSOD Ala-9Val polymorphisms were treated as dominant genetic models in this analysis (17). The best genetic model for CAT-844C>T and asthma association was selected based on the model with the lowest Aikaike information criterion (AIC) value (24).
We fitted Cox proportional hazard regression models with the time scale defined as the follow-up time to investigate the association between specific genotype and newly diagnosed asthma expressed as hazard ratio (HR) and 95% confidence interval (95% CI). All models were adjusted for community as a fixed effect, and race/ethnicity with sex-specific and age-specific (integer years) baseline hazard. Additional covariates identified a priori as potential confounders were considered for inclusion in the model based on whether their inclusion changed the HR by more than 10%. By comparing appropriate models with and without interaction terms using likelihood ratio tests, we assessed heterogeneity of associations among subgroups. Sensitivity analyses were conducted by limiting the case definition to those who reported inhaled medication use.
To assess the effect of ambient air pollution on the relationship between genetic polymorphisms and new-onset asthma, we accounted for the clustering effect of children in communities by fitting a hierarchical two-stage model to these time-dependent data (25) (details in the online supplement). In these models, the community-specific average air pollutant levels were fitted as continuous variables together with the appropriate interaction terms for genes and air pollutants. The communities were treated as random effects in these models. From our initial analysis, we noted that the choice of fixed or random effect of the communities does not substantially affect the estimates for the gene effects (data not shown). To compare the effect of genotype on the risk of new-onset asthma in communities with higher/lower levels of ambient pollutants, we also performed a stratified analysis using Cox proportional hazards models.
To assess whether the results could be replicated in independent groups of children, we performed a stratified analysis for the two independent fourth-grade cohorts of the current study population recruited in 1993 and 1996. These two cohorts have the same age structure, ethnic distributions, and air pollution exposure profiles because they represent the fourth-graders of the same study communities and schools recruited 3 years apart.
All analyses, except the hierarchical two-stage model, were conducted using SAS software version 9 (SAS Institute, Cary, NC). The hierarchical two-stage model was conducted in R-program using COXP procedure (26, 27). All hypothesis testing was conducted using a 0.05 significance level and a two-sided alternative hypothesis. Because the genes were selected based on specific hypotheses defined by biologically relevant antioxidative pathways (Figure 1) and only four functional polymorphisms were tested, the significance levels for the a priori hypothesis tests were not adjusted for multiple testing.
RESULTS
Participant Characteristics
Over the follow-up period, 160 new cases of asthma were diagnosed, for an overall crude incidence rate (IR) of 16.6 per 1,000 person-years. The IRs did not differ between non–Hispanic-white (IR = 16.7/1,000 person-years) and Hispanic (IR = 16.6/1,000 person-years) white children. A number of baseline characteristics varied between those with and without genetic data (Tables E3 and E4 of the online supplement); however, except for current maternal smoking and smokers at home, the magnitude of difference between the two groups was too small to affect the overall association. Furthermore, none of those factors were risk factors for newly diagnosed asthma in either of the populations (Tables 1 and 2).
TABLE 1.
SELECTED CHARACTERISTICS OF NON-HISPANIC WHITE CHILDREN AT STUDY ENTRY
| No Asthma (n = 1,022) | Newly Diagnosed Asthma (n = 103) | P Value* | |
|---|---|---|---|
| Age group, yr | |||
| 7–9 | 520 (50.9) | 65 (63.1) | 0.003 |
| 10–11 | 217 (21.2) | 24 (23.3) | |
| >11 | 285 (27.9) | 14 (13.6) | |
| Boys | 481 (47.1) | 38 (36.9) | 0.05 |
| Overweight/at risk of overweight | 97 (9.5) | 16 (15.7) | 0.06 |
| Parental history of asthma | 149 (15.2) | 25 (25.2) | 0.01 |
| History of allergy | 232 (23.3) | 26 (26.0) | 0.55 |
| Humidifier use | 297 (29.8) | 33 (32.7) | 0.54 |
| Smokers at home | 177 (17.5) | 13 (12.7) | 0.21 |
| Maternal smoking during pregnancy | 175 (17.5) | 17 (16.7) | 0.83 |
| Maternal smoking at study entry | 102 (10.1) | 7 (6.9) | 0.28 |
| Pests of any kind | 851 (86.7) | 78 (76.5) | 0.009 |
| Pets at home | 920 (90.0) | 96 (93.2) | 0.28 |
| Health insurance | 909 (90.3) | 93 (90.3) | 0.99 |
| Income, $† | |||
| ≤14,999 | 69 (7.8) | 5 (5.1) | 0.63 |
| 15,000–49,999 | 347 (39.1) | 38 (39.2) | |
| ≥50,000 | 470 (53.0) | 54 (55.6) | |
| Highest parental education level† | |||
| Less than high school | 56 (5.5) | 2 (2.0) | 0.21 |
| College | 776 (77.0) | 85 (83.3) | |
| Graduate | 177 (17.5) | 15 (14.7) | |
| Community ozone status, higher | 539 (52.7) | 47 (45.6) | 0.17 |
| Community PM10 status, higher | 468 (45.8) | 42 (40.8) | 0.33 |
Values indicate the number of children (%) available in the specific group.
Chi-square P value determining the difference in distribution between children with and without newly diagnosed asthma during follow-up.
The numbers do not add up to the total due to missing data.
TABLE 2.
SELECTED CHARACTERISTICS OF HISPANIC WHITE CHILDREN AT STUDY ENTRY
| No Asthma (n = 519) | Newly Diagnosed Asthma (n = 57) | P Value* | |
|---|---|---|---|
| Age group, yr | |||
| 7–9 | 296 (57.0) | 38 (66.7) | 0.08 |
| 10–11 | 107 (20.6) | 13 (22.8) | |
| >11 | 116 (22.3) | 6 (10.5) | |
| Boys | 215 (41.4) | 23 (40.3) | 0.87 |
| Overweight/at risk of overweight | 94 (18.4) | 13 (23.2) | 0.38 |
| Parental history of asthma | 60 (12.3) | 6 (11.1) | 0.80 |
| History of allergy | 65 (13.0) | 8 (14.3) | 0.79 |
| Humidifier use | 74 (15.3) | 14 (25.4) | 0.07 |
| Smokers at home | 53 (10.5) | 8 (14.5) | 0.38 |
| Maternal smoking during pregnancy | 36 (7.1) | 5 (9.3) | 0.57 |
| Maternal smoking at study entry | 25 (4.9) | 1 (1.8) | 0.25 |
| Pests of any kind | 308 (69.5) | 41 (77.4) | 0.23 |
| Pets at home | 342 (65.9) | 41 (71.9) | 0.35 |
| Health insurance | 353 (69.3) | 45 (80.4) | 0.08 |
| Income, $† | |||
| ≤14,999 | 103 (24.7) | 8 (16.7) | 0.15 |
| 15,000–49,999 | 215 (51.8) | 23 (47.9) | |
| ≥50,000 | 97 (23.5) | 17 (35.4) | |
| Highest parental education level† | |||
| Less than high school | 158 (32.8) | 10 (18.5) | 0.09 |
| College | 293 (61.0) | 39 (72.2) | |
| Graduate | 30 (6.2) | 5 (9.3) | |
| Community ozone status, higher | 263 (50.1) | 27 (47.4 | 0.63 |
| Community PM10 status, higher | 272 (52.4) | 32 (56.1) | 0.59 |
Values indicate the number of children (%) available in the specific group.
Chi-square P value determining the difference in distribution between children with and without newly diagnosed asthma during follow-up.
The numbers do not add up to the total due to missing data.
Allele Frequencies
The number of (GT)n repeats of the HMOX-1 gene showed a bimodal distribution with the two peaks being 23 and 30 repeats in both ethnic groups (see Figure E1). Hispanics were more likely to have a higher number of repeats compared with non-Hispanic whites (P value < 0.001) (Table 3). The biallelic average number of repeats was also significantly less among non-Hispanic whites compared with Hispanics (P value < 0.01) (see Figure E2). Both polymorphisms of CAT and the Ala-9Val polymorphism of MNSOD were in Hardy-Weinberg equilibrium in both ethnic groups.
TABLE 3.
DISTRIBUTION OF HMOX-1, CATALASE, AND MNSOD GENOTYPE AMONG HISPANIC AND NON-HISPANIC WHITE CHILDREN
| Genotype | Non-Hispanic White | Hispanic White |
|---|---|---|
| HMOX-1 | ||
| ≤23 (S)* | 469 (22.3) | 154 (14.7) |
| >23 (No-S) | 1,631 (77.7) | 892 (85.3) |
| At least one ‘S’ | 421 (40.1) | 148 (28.3) |
| CAT-262C>T | ||
| CC | 694 (62.4) | 417 (73.2) |
| CT | 381 (34.2) | 135 (23.6) |
| TT | 38 (3.4) | 18 (3.2) |
| CAT-844C>T | ||
| CC | 494 (44.9) | 162 (29.2) |
| CT | 474 (43.2) | 261 (47.1) |
| TT | 131 (11.9) | 131 (23.6) |
| MNSOD–Ala-9Val | ||
| Ala/Ala (CC) | 262 (24.2) | 188 (34.0) |
| Ala/Val (CT) | 531 (49.1) | 281 (51.0) |
| Val/Val (TT) | 289 (26.7) | 83 (15.0) |
Total number and percentages in parentheses are provided for the allele frequencies. The total number varies by genotype due to incomplete data for individual genotype. The distribution of the alleles between Hispanic and non-Hispanic whites varied significantly for all of the genes (P value < 0.01).
Biallelic distribution of “S” or “no-S” allele.
Association between Candidate Genes and Asthma
Among non-Hispanic whites, children with at least one S-allele were at a reduced risk of asthma compared with those who had no S-allele (HR, 0.64; 95% CI, 0.41–0.99) (Table 4). A similar protective effect was observed for children with a biallelic average number of repeats in the lowest quartile (≤26.5 repeats; HR, 0.56; 95% CI, 0.36–0.89) compared with those with a biallelic average number of repeats greater than 26.5. No statistically significant associations between this tandem repeat polymorphism of HMOX-1 and the risk of new-onset asthma were observed among Hispanic children.
TABLE 4.
ASSOCIATION OF HMOX-1 (GT-REPEATS), CATALASE, AND MNSOD GENOTYPE WITH NEW-ONSET ASTHMA AMONG HISPANIC AND NON-HISPANIC WHITE CHILDREN
| Model | Genes | Non-Hispanic HR (95% CI) | Hispanics HR (95% CI) |
|---|---|---|---|
| HMOX-1 (GT-repeats) | |||
| Any S | No-S | 1 | 1 |
| At least one S | 0.64 (0.41–0.99)* | 1.25 (0.64–2.47) | |
| Biallelic average | >26.5 | 1 | 1 |
| ≤26.5 | 0.56 (0.36–0.89)* | 1.31 (0.66–2.57) | |
| CAT-262C-T (rs1001179) | CC | 1 | 1 |
| CT or TT | 0.90 (0.61–1.31) | 1.93 (1.05–3.55)† | |
| CAT-844C-T (rs769214) | Numbers of ‘T’ allele | 0.77 (0.56–1.05) | 0.92 (0.59–1.42) |
| MNSOD (rs4880) | Val/Val | 1 | 1 |
| Ala/Val or Ala/Ala | 1.26 (0.78–2.02) | 0.90 (0.40–2.03) |
Definition of abbreviations: CI = confidence interval; HR = hazard ratio.
The adjusted HR and 95% CI are reported from Cox's proportional hazard model controlling for communities with age- and sex-specific baseline hazard. We fitted the dominant model for CAT-262C-T and MNSOD, and the additive model for CAT-844C-T.
P value < 0.01.
P value < 0.05.
In contrast to HMOX-1, we found that the CAT-262C>T variant was associated with asthma in Hispanic-white children but was not associated with asthma in non-Hispanic white children. Among Hispanic children, carriers of the variant T allele (CT or TT) of CAT-262C>T were at an increased risk of new-onset asthma compared with the children who were homozygous for the common allele (CC) (HR, 1.93; P value = 0.01). CAT-262C>T was not associated with asthma risk among non-Hispanic white children. No statistically significant association between asthma and the CAT-844C>T or Ala-9Val polymorphism of MNSOD was observed in either ethnic group.
In further analysis, these genetic associations were neither confounded nor modified by parental history of asthma, history of allergy, secondhand smoke (SHS), current SHS exposure, in utero exposure to maternal smoking, personal smoking, physical activity, socioeconomic status (SES), health insurance, or overweight/at risk of overweight (Table E5, model 1). As a sensitivity analysis, we restricted the asthma definition to those new-onset asthma cases who also used an inhaler (n = 121). The use of this restricted case definition resulted in little change in the estimated genetic effects for all the loci examined. For example, in analyses using the restricted case definition, the HR associated with S-alleles among non-Hispanic whites and the CT or TT alleles of the CAT-262C>T polymorphism among Hispanics was 0.63 (95% CI, 0.38–1.04) and 2.13 (95% CI, 1.09–4.18), respectively (Table E5, model 2).
To investigate whether the associations could be replicated in an independent population, we performed a stratified analysis for the two fourth-grade cohorts of the current study population recruited in 1993 and 1996 (Table 5). The ethnicity-specific genetic-effect estimates for the genes in each of these cohorts were consistent in both cohorts of children.
TABLE 5.
ASSOCIATION OF HMOX-1 (GT-REPEATS) AND CAT-262C-T (rs1001179) WITH NEW-ONSET ASTHMA AMONG HISPANIC AND NON-HISPANIC WHITE FOURTH-GRADERS, STRATIFIED BY YEAR OF RECRUITMENT
| Model | Genes | Non-Hispanic HR (95% CI) | Hispanics HR (95% CI) |
|---|---|---|---|
| Recruited in 1993 | n = 401 | n = 167 | |
| Any S | LL/LM/MM | 1 | 1 |
| SS/SM/SL | 0.64 (0.3–1.2)* | 1.87 (0.6–5.7) | |
| CAT-262C-T (rs1001179) | CC | 1 | 1 |
| CT or TT | 0.75 (0.4–1.4) | 1.76 (0.6–5.3) | |
| Recruited in 1996 | n = 382 | n = 253 | |
| Any S | LL/LM/MM | ||
| SS/SM/SL | 0.68 (0.3–1.4)* | 1.59 (0.6–4.0) | |
| CAT-262C-T (rs1001179) | CC | 1 | 1 |
| CT or TT | 1.11 (0.6–2.2) | 2.33 (1.0–5.4)† |
Definition of abbreviations: CI = confidence interval; HR = hazard ratio.
The adjusted HR and 95% CI are reported from Cox's proportional hazard model controlling for communities with age- and sex-specific baseline hazard.
P value < 0.1.
P value < 0.05.
Gene–Air Pollution Interaction
We found evidence that the effect of variation in the HMOX-1 gene on the risk of new-onset asthma differed by ambient ozone level (interaction P value = 0.003; Table 6). The largest protective effect of the (GT)n repeat polymorphism of HMOX-1 was observed for children who were S-allele carriers and resided in low-ozone communities (HR, 0.44; 95% CI, 0.23–0.83). The relative ratio of HR of S-allele carriers who resided in high-ozone communities (HR, 0.88) was twofold greater than in those who resided in the low-ozone communities (HR, 0.44). The observed pattern did not vary by children's participation in sports (data not shown) or time spent outdoors (see Table E6). Adjusting for individual level covariates, such as SES, parental education, health insurance, and SHS exposure, as well as community level PM10, also did not change the modifying effect of ambient ozone (data not shown). No significant interaction was observed between nonozone pollutants, such as PM10, and the HMOX-1 gene (Table 6). We found little evidence for interactions between any of the other polymorphisms and ambient pollutant levels.
TABLE 6.
EFFECT OF HMOX-1 (GT-REPEATS) ON NEW-ONSET ASTHMA AMONG NON-HISPANIC WHITE CHILDREN IN HIGHER AND LOWER OZONE AND PM10 CHILDREN'S HEALTH STUDY COMMUNITIES
| Community | No S-Allele HR (95% CI) | S-Allele HR (95% CI) | P Value* |
|---|---|---|---|
| Low ozone | 1 | 0.44 (0.23–0.83) | 0.003 |
| High ozone | 0.94 (0.36–2.43) | 0.88 (0.33–2.34) | |
| Low PM10 | 1 | 0.94 (0.54–1.62) | 0.18 |
| High PM10 | 1.79 (0.69–4.61) | 0.62 (0.20–1.87) |
Definition of abbreviations: CI = confidence interval; HR = hazard ratio; PM10 = particulate matter with an aerodynamic diameter of less than 10 μm.
Interaction P value is reported from hierarchical two stage Cox's proportional hazard model fitting the community-specific ozone and PM10 levels (continuous) and controlling for random effect of the communities. The HRs are based on Cox's proportional hazard model with age- and sex-specific baseline hazard and adjusting for communities.
DISCUSSION
Oxidative stress is believed to contribute to asthma pathogenesis (2, 4, 28). In this study, we show that for new-onset asthma during adolescence, variants in genes that encode antioxidant enzymes contribute to variations in asthma occurrence. Although these variants appear to modulate asthma risk, their associations depended on ethnicity and level of oxidant exposure. Shorter (GT)n repeats in the promoter region of the HMOX-1 gene appeared to be protective among non-Hispanic whites, and CAT-262C>T was a risk factor among Hispanics. Furthermore, the effect of the (GT)n repeat polymorphism of HMOX-1 also varied by the ambient ozone level.
The ethnicity-specific effect of HMOX-1 repeats and the CAT-262C>T polymorphism is consistent with some previous reports and emphasizes the importance of evaluating the ethnicity-specific effect of genetic polymorphisms in assessing gene–disease associations. An ethnicity-specific effect of the (GT)n repeat polymorphism has been observed in coronary artery disease, for which an association was observed among Asians (10) but not in whites (29). Similarly, the CAT-262C>T polymorphism has been shown to affect the catalase activity among whites but not among African Americans (16).
It is unlikely that population admixture can explain the observed ethnic differences because the incident rates of new-onset asthma did not vary substantially among the ethnic groups. The linkage disequilibrium (LD) pattern of HMOX-1 is very similar for Hispanic and non-Hispanic whites, although some variation was observed for the CAT gene between the two ethnic groups (see Figures E3–E6). Thus, a difference in the regional LD pattern is an unlikely explanation for the observed ethnic differences for HMOX-1, although it is a possibility for the ethnicity-specific effect of variants in the CAT gene. It is possible that the relevant genetic background varies in Hispanic and non-Hispanic whites and that the relative contributions of genes involved in maintaining the antioxidant balance differs among ethnic groups.
It is difficult to determine whether the observed differences in the allele frequencies and biological effects of the polymorphisms in Hispanic and non-Hispanic whites were due to direct effect of anti-/prooxidative environment exposures or due to some other indirect exposures. From an evolutionary biological perspective, it is possible that the S-allele of HMOX-1 (protective factor in asthma) is underselected in Hispanic whites compared with non-Hispanic whites (28.3 vs. 40.1%), whereas the CC genotype of CAT-262C>T plays a compensatory role in Hispanic whites compared with non-Hispanic whites (73.2 vs. 62.4%). It is likely that studies also need to consider genes in related pathways to understand the ethnic differences in gene associations. For example, biliverdin is synthesized by HO-1 and can contribute to antioxidative activity only in the presence of sufficient activity of biliverdin reductase (Figure 1). A differential activity of biliverdin reductase in Hispanic and non-Hispanic whites could contribute to the differential role of HMOX-1 by ethnicity. Furthermore, although we examined a number of common environmental exposures and found no evidence that exposures account for the ethnic differences in associations, we cannot rule out an influence of ethnic variation in diet or other unmeasured lifestyle factors and environmental exposures. On the basis of our previous and ongoing research, no ethnicity-specific difference was observed for genes involved in other parts of the oxidative pathway, such as GSTP1, GSTM1, and GSTT1 (23, 30, 31); however, an ethnicity-specific effect has been reported for other genes in relation to asthma (32).
Our findings for HMOX-1 in non-Hispanic whites are consistent with the known functionality of the enzyme and previous studies. Although multiple studies have shown that the presence of long (“long” has been defined by differing cutoffs in different studies) (GT)n repeat polymorphisms of HMOX-1 are associated with an increased risk of different oxidative stress–mediated respiratory (11, 33–35) and cardiovascular conditions (10), its role in asthma has not been reported. Emerging evidence indicates that HO-1 plays a significant protective role for oxidative stress and subsequent pulmonary inflammation (36). HO-1 degrades heme to biliverdin, which is readily converted to bilirubin, a potent antioxidant (37, 38). During this process, free iron and CO are produced, and HO-1 exerts a cytoprotective effect by regulating cellular iron (39). CO has been shown to act as a signaling molecule to activate guanyl cyclase (40), thus mediating several physiologic antioxidative processes (40) and smooth muscle relaxation (41). HO-1 has also been shown to inhibit airway smooth muscle proliferation in humans, a process that is central to asthma pathogenesis (42). This array of possible protective mechanisms of HO-1 against asthma supports a role for the GT repeat polymorphism of the HMOX-1 gene that up-regulates the enzymatic expression or activity of HO-1. The functional effect of (GT)n repeat sizes on the transcriptional activity of HMOX-1 promoter has been reported from studies using luciferase promoter constructs and transient transfection (10, 11). Both of these previous studies showed that (GT)n repeats fewer than 23 were associated with the greatest promoter activity. A novel study using lymphoblastoid cell lines established from persons of known (GT)n repeats showed that cell lines homozygous for (GT)n repeats of fewer than 27 were associated with 2.4- ± 0.6-fold increased HO-1 enzyme activity under oxidative stress, whereas the increase under similar conditions was 1 ± 0.2 for cells that were homozygous for (GT)n repeats of more than 32 (9). On the basis of the functional effect of the (GT)n repeat polymorphism on HMOX-1 expression and its association in different disease conditions, our finding is biologically plausible.
We defined the short allele as repeat numbers 23 or less based on the greatest promoter activity (10, 11) and the distribution in our cohort (<2% had repeats < 23). Given the additive effect of the number of repeats in each allele in the antioxidative activity of the HMOX-1 as shown by Chen and colleagues (10), the biallelic average number of repeats categorized at the 25th percentile value (≤26.5) should also be associated with high HO-1 activity as shown by Hirai and coworkers (9). We observed a strong protective effect of the shorter length of the HMOX-1 polymorphism on asthma risk using both of these categorizations among non-Hispanic whites.
Catalase is involved in reducing H2O2 to H2O (Figure 1) and plays a significant antioxidative role in human lungs, especially at the alveolar level (6). The presence of the minor allele (T) of the CAT-262C>T polymorphism has been shown to reduce the enzymatic activity of catalase in red blood cells (14–16). A single hospital-based case-control study has shown that the presence of the minor allele (CT or TT) is associated with asthma among Chinese patients with asthma. However, the presence of the T allele was associated with a protective effect against asthma despite reduced catalase activity (17), and no association was observed when cases and control subjects were stratified by atopic status. Our finding that the presence of the minor allele (CT or TT) of the CAT-262C>T polymorphism increases the risk of new-onset asthma is consistent with the observation that the presence of CT or TT decreases the antioxidative activity of catalase. This association was not modified by atopic status or family history of asthma.
Our finding that the Ala-9Val polymorphism of MNSOD is not associated with asthma is consistent with most of the previous reports of no association between this polymorphism and asthma (17, 43–45). However, Mak and colleagues (17) reported a protective effect of the C allele (Val/Ala or Ala/Ala) against asthma among atopic cases and control subjects who were never-smokers, yet they did not observe any overall association between this polymorphism and asthma. We cannot exclude the possibility that other polymorphisms in this gene region are associated with the risk for asthma.
Ozone, an ambient strong oxidant that induces inflammation, has been reported to be associated with asthma incidence and exacerbation (46, 47). Our research has previously shown that the protective effect of TNF-308 GG (a promoter variant of tumor necrosis factor) genotype against asthma was attenuated by ambient ozone levels (48). Similarly, in this study, we observed that the protective effect of short (GT)n repeats was observed in lower ozone communities but not in higher ozone communities. This effect did not vary by the time-activity pattern of the children (see Table E6). It appears that in environments of low ambient ozone, specific polymorphisms can increase the antioxidative activity in response to an increased oxidative stress when needed and thus can counter any temporary imbalance in an oxidant–antioxidant relationship. However, in the presence of high background ozone, those beneficial polymorphisms (S-allele in this analysis and TNF-308 GG in our earlier analysis) may already provide maximum activity, and variation in promoters no longer affects levels of expression. It is interesting to note that only ozone and not particulate matter modified the protective effect of HMOX-1; however, we note that differential effects of particulate matter and ozone on the respiratory health have been reported in the literature (49, 50). We speculate that ozone, being a stronger oxidant than particulates, has a different chemical mechanism and substrates that produce different toxic oxidation products and therefore has larger effects in genetically susceptible groups. In our CHS study, we have previously reported a differential role of ozone and particulate matters for lung function and asthma (51, 52). Further studies are required to characterize the gene expression patterns, oxidative stress levels, and acute and chronic responses in people with different HMOX-1 repeats exposed to different levels of background ozone and particulate matter, and to further replicate this finding in an independent study with adequate power and exposure contrast.
The structure of our study—a cohort study of school-age children with annual collection of their asthma diagnosis and air pollution exposure—was an effective foundation for the current study and has proved to be a strength. We had a substantial number of children with genetic data to perform ethnicity-specific analyses. Genetic data were available for 67% of the study population. There were some differences between those with and without available genetic data (Tables E3 and E4); however, except for factors associated with SHS status (current maternal smoking and numbers of smokers at home), the magnitude of difference between the two groups was too small to affect the overall association. These socioeconomic factors also varied between those who were lost to follow-up (n = 776) and the source population (n = 2,998) of this study (see Table E7). These factors are unlikely to produce a selection bias, as they are not dependent on the genotype of the children and are not independent risk factors of asthma. Exposure to SHS was higher among children of low SES in this cohort, and loss to follow-up and unavailability of genetic data were also more common among children of low SES because their parents were more likely to move for economic reasons (based on interviews). Furthermore, neither SHS nor other factors in the tables were confounders (Table E5, model 1) or effect modifiers (data not shown) for the observed associations; thus, the differences are not likely to substantially bias the findings of our study.
One potential limitation of our study could be the accuracy of the case definition for new-onset asthma because it was based on personal interview and questionnaire data. Excluding any child with a history of wheezing also minimized misclassification of asthma status at entry. A recent study noted that children as young as 7 years can provide information regarding their asthma with an acceptable level of validity and reliability (53). Furthermore, unless the diagnosis varied by genotype, error in determining asthma status would likely lead to a downward bias in genetic-effect estimates, and therefore would not explain the observed associations.
Access to care and differences in practice among physicians have the potential to influence asthma diagnoses (54). We found that adjustment for factors that mediate access to care, including family income, education, and medical insurance, did not explain our results, indicating that differential access to care did not substantially bias our results. To further investigate the potential for bias from variation in medical practices, we conducted analyses restricting cases to those who recently used inhaled medication and found little change in the risk estimates. Because the associations of the genotypes were apparent among the group of cases for which the diagnosis of asthma was most certain, our results are unlikely to be explained by misclassification of outcome.
One potential criticism of the interpretation of our finding is that we have not adjusted for multiple testing; however, we do not think it is justified to correct P values for multiple testing in this analysis because the selection of the genes was based on a priori hypotheses defined by a well-studied biological pathway (Figure 1) and reported genetic variant functionality (9–18). Adjusting for multiple testing in this analysis would have ignored the available prior knowledge. There were five hypothesis-driven tests for each ethnic group. Four tests were conducted in each ethnic group to identify the main effect of the single-nucleotide polymorphisms (SNPs). After identifying the main effects, based on our a priori hypothesis, we tested for possible effect modification by O3 with HMOX-1 among non-Hispanic whites and CAT-262C-T among Hispanic whites. Importantly, we showed a consistent pattern for the main effects in two similar populations by performing stratified analysis for the two fourth-grade cohorts of the current study population recruited in 1993 and 1996 (Table 5). The ethnicity-specific genetic-effect estimates for the genes in each of these cohorts were consistent with those in the main analysis. Because the methodology of this study (selection of genes and SNPs, selection of the genetic model, and tested interaction with ambient ozone) is based on previous findings and a defined oxidative pathway (Figure 1), the observed main effect of the genes and the modifying effect of ambient ozone are biologically plausible. Because the main effect was replicated in a cohort-specific analysis, we do not think that these findings can be explained by chance alone. Due to sample size issues, we could not test the modifying effect of ambient ozone in different cohorts, thus it needs to be confirmed in studies conducted by other investigators with similar age groups and environmental exposures.
In conclusion, promoter variants that affect expression of HMOX-1 and CAT are associated with the development of new-onset asthma in children. The ethnicity-specific association between the tandem repeats polymorphism of the promoter region of the HMOX-1 gene and the CAT-262C>T polymorphism with new-onset asthma suggests that, for some variants, it is necessary to consider the ethnicity in the study of the complex relationship between variants in antioxidant genes and asthma occurrence. The finding that beneficial polymorphisms fail to protect children from the injurious effect of ambient ozone when the levels are sufficiently elevated argues for regulations that account for the effects in genetically susceptible children.
Supplementary Material
Supported by the NIEHS (grants 5P01ES009581, 5P01ES011627, and 5P30ES007048), the U.S. Environmental Protection Agency (grants R826708-01 and RD831861-01), the National Heart, Lung, and Blood Institute (grants 5R01HL061768 and 5R01HL076647), and the Hastings Foundation.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200706-863OC on November 29, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Chung KF. Role of inflammation in the hyperreactivity of the airways in asthma. Thorax 1986;41:657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes PJ. New concepts in the pathogenesis of bronchial hyperresponsiveness and asthma. J Allergy Clin Immunol 1989;83:1013–1026. [DOI] [PubMed] [Google Scholar]
- 3.Nadeem A, Chhabra SK, Masood A, Raj HG. Increased oxidative stress and altered levels of antioxidants in asthma. J Allergy Clin Immunol 2003;111:72–78. [DOI] [PubMed] [Google Scholar]
- 4.Greene LS. Asthma and oxidant stress: nutritional, environmental, and genetic risk factors. J Am Coll Nutr 1995;14:317–324. [DOI] [PubMed] [Google Scholar]
- 5.Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J 2006;28:219–242. [DOI] [PubMed] [Google Scholar]
- 6.Rahman I, Biswas SK, Kode A. Oxidant and antioxidant balance in the airways and airway diseases. Eur J Pharmacol 2006;533:222–239. [DOI] [PubMed] [Google Scholar]
- 7.Exner M, Minar E, Wagner O, Schillinger M. The role of heme oxygenase-1 promoter polymorphisms in human disease. Free Radic Biol Med 2004;37:1097–1104. [DOI] [PubMed] [Google Scholar]
- 8.Wang LI, Neuberg D, Christiani DC. Asbestos exposure, manganese superoxide dismutase (MnSOD) genotype, and lung cancer risk. J Occup Environ Med 2004;46:556–564. [DOI] [PubMed] [Google Scholar]
- 9.Hirai H, Kubo H, Yamaya M, Nakayama K, Numasaki M, Kobayashi S, Suzuki S, Shibahara S, Sasaki H. Microsatellite polymorphism in heme oxygenase-1 gene promoter is associated with susceptibility to oxidant-induced apoptosis in lymphoblastoid cell lines. Blood 2003;102:1619–1621. [DOI] [PubMed] [Google Scholar]
- 10.Chen YH, Lin SJ, Lin MW, Tsai HL, Kuo SS, Chen JW, Charng MJ, Wu TC, Chen LC, Ding YA, et al. Microsatellite polymorphism in promoter of heme oxygenase-1 gene is associated with susceptibility to coronary artery disease in type 2 diabetic patients. Hum Genet 2002;111:1–8. [DOI] [PubMed] [Google Scholar]
- 11.Yamada N, Yamaya M, Okinaga S, Nakayama K, Sekizawa K, Shibahara S, Sasaki H. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to emphysema. Am J Hum Genet 2000;66:187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimoda-Matsubayashi S, Matsumine H, Kobayashi T, Nakagawa-Hattori Y, Shimizu Y, Mizuno Y. Structural dimorphism in the mitochondrial targeting sequence in the human manganese superoxide dismutase gene: a predictive evidence for conformational change to influence mitochondrial transport and a study of allelic association in Parkinson's disease. Biochem Biophys Res Commun 1996;226:561–565. [DOI] [PubMed] [Google Scholar]
- 13.Hiroi S, Harada H, Nishi H, Satoh M, Nagai R, Kimura A. Polymorphisms in the SOD2 and HLA-DRB1 genes are associated with nonfamilial idiopathic dilated cardiomyopathy in Japanese. Biochem Biophys Res Commun 1999;261:332–339. [DOI] [PubMed] [Google Scholar]
- 14.Nadif R, Mintz M, Jedlicka A, Bertrand JP, Kleeberger SR, Kauffmann F. Association of CAT polymorphisms with catalase activity and exposure to environmental oxidative stimuli. Free Radic Res 2005;39:1345–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nadif R, Kleeberger SR, Kauffmann F. Polymorphisms in manganese superoxide dismutase and catalase genes: functional study in Hong Kong Chinese asthma patients. Clin Exp Allergy 2006;36:1104–1105. [Author reply, 1105–1106.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahn J, Nowell S, McCann SE, Yu J, Carter L, Lang NP, Kadlubar FF, Ratnasinghe LD, Ambrosone CB. Associations between catalase phenotype and genotype: modification by epidemiologic factors. Cancer Epidemiol Biomarkers Prev 2006;15:1217–1222. [DOI] [PubMed] [Google Scholar]
- 17.Mak JC, Leung HC, Ho SP, Ko FW, Cheung AH, Ip MS, Chan-Yeung MM. Polymorphisms in manganese superoxide dismutase and catalase genes: functional study in Hong Kong Chinese asthma patients. Clin Exp Allergy 2006;36:440–447. [DOI] [PubMed] [Google Scholar]
- 18.Jiang Z, Akey JM, Shi J, Xiong M, Wang Y, Shen Y, Xu X, Chen H, Wu H, Xiao J, et al. A polymorphism in the promoter region of catalase is associated with blood pressure levels. Hum Genet 2001;109:95–98. [DOI] [PubMed] [Google Scholar]
- 19.Peters JM, Avol E, Gauderman WJ, Linn WS, Navidi W, London SJ, Lurmann F, Margolis H, Rappaport E, Vora H, et al. A study of 12 Southern California communities with differing levels and types of air pollution: II. Effects on Pulmonary Function. Am J Respir Crit Care Med 1999;159:768–775. [DOI] [PubMed] [Google Scholar]
- 20.Peters JM, Avol E, Navidi W, London SJ, Gauderman WJ, Lurmann F, Linn WS, Margolis H, Rappaport E, Gong H, et al. A study of 12 Southern California communities with differing levels and types of air pollution: I. Prevalence of respiratory morbidity. Am J Respir Crit Care Med 1999;159:760–767. [DOI] [PubMed] [Google Scholar]
- 21.Islam T, Gauderman W, McConnell R, Gilliland F. Heme oxygenase, ozone, and incident asthma [abstract]. Proc Am Thorac Soc 2007;175:A833. [Google Scholar]
- 22.A SAS program for the CDC growth charts [Internet]. Atlanta: Centers for Disease Control and Prevention; [updated 16 Dec 2005; accessed 10 Jan 2007] Available from: http://www.cdc.gov/nccdphp/dnpa/bmi
- 23.Gilliland FD, Gauderman WJ, Vora H, Rappaport E, Dubeau L. Effects of glutathione-S-transferase M1, T1, and P1 on childhood lung function growth. Am J Respir Crit Care Med 2002;166:710–716. [DOI] [PubMed] [Google Scholar]
- 24.Akaike H. Information theory and an extension of the maximum likelihood principle. In: Petrov BN, Csaki F, editors. Second International Symposium on Information Theory. Budapest: Akademia Kiado; 1973. pp. 267–281.
- 25.Berhane K, Gauderman W, Stram D, Thomas D. Statistical issues in studies of the long term effects of air pollution: the Southern California Children Health Study. Stat Sci 2004;19:414–449. [Google Scholar]
- 26.Ma R, Krewski D, Burnett RT. Random effects Cox models: a Poisson modeling approach. Biometrika 2003;90:157–169. [Google Scholar]
- 27.Jerrett M, Burnett RT, Ma R, Pope CA, Krewski D, Newbold KB, Thurston G, Shi Y, Finkelstein N, Calle EE, et al. Spatial analysis of air pollution and mortality in Los Angeles. Epidemiology 2005;16:727–736. [DOI] [PubMed] [Google Scholar]
- 28.Barnes PJ. Reactive oxygen species and airway inflammation. Free Radic Biol Med 1990;9:235–243. [DOI] [PubMed] [Google Scholar]
- 29.Endler G, Exner M, Schillinger M, Marculescu R, Sunder-Plassmann R, Raith M, Jordanova N, Wojta J, Mannhalter C, Wagner OF, et al. A microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with increased bilirubin and HDL levels but not with coronary artery disease. Thromb Haemost 2004;91:155–161. [DOI] [PubMed] [Google Scholar]
- 30.Gilliland FD, Li YF, Dubeau L, Berhane K, Avol E, McConnell R, Gauderman WJ, Peters JM. Effects of glutathione S-transferase M1, maternal smoking during pregnancy, and environmental tobacco smoke on asthma and wheezing in children. Am J Respir Crit Care Med 2002;166:457–463. [DOI] [PubMed] [Google Scholar]
- 31.Gilliland FD, Rappaport EB, Berhane K, Islam T, Dubeau L, Gauderman WJ, McConnell R. Effects of glutathione S-transferase P1, M1, and T1 on acute respiratory illness in school children. Am J Respir Crit Care Med 2002;166:346–351. [DOI] [PubMed] [Google Scholar]
- 32.Hersh CP, Raby BA, Soto-Quiros ME, Murphy AJ, Avila L, Lasky-Su J, Sylvia JS, Klanderman BJ, Lange C, Weiss ST, et al. Comprehensive testing of positionally cloned asthma genes in two populations. Am J Respir Crit Care Med 2007;176:849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hersh CP, Demeo DL, Lange C, Litonjua AA, Reilly JJ, Kwiatkowski D, Laird N, Sylvia JS, Sparrow D, Speizer FE, et al. Attempted replication of reported chronic obstructive pulmonary disease candidate gene associations. Am J Respir Cell Mol Biol 2005;33:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guenegou A, Leynaert B, Benessiano J, Pin I, Demoly P, Neukirch F, Boczkowski J, Aubier M. Association of lung function decline with the heme oxygenase-1 gene promoter microsatellite polymorphism in a general population sample: results from the European Community Respiratory Health Survey (ECRHS), France. J Med Genet 2006;43:e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kikuchi A, Yamaya M, Suzuki S, Yasuda H, Kubo H, Nakayama K, Handa M, Sasaki T, Shibahara S, Sekizawa K, et al. Association of susceptibility to the development of lung adenocarcinoma with the heme oxygenase-1 gene promoter polymorphism. Hum Genet 2005;116:354–360. [DOI] [PubMed] [Google Scholar]
- 36.Almolki A, Taille C, Martin GF, Jose PJ, Zedda C, Conti M, Megret J, Henin D, Aubier M, Boczkowski J. Heme oxygenase attenuates allergen-induced airway inflammation and hyperreactivity in guinea pigs. Am J Physiol Lung Cell Mol Physiol 2004;287:L26–L34. [DOI] [PubMed] [Google Scholar]
- 37.Stocker R, Ames BN. Potential role of conjugated bilirubin and copper in the metabolism of lipid peroxides in bile. Proc Natl Acad Sci USA 1987;84:8130–8134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science 1987;235:1043–1046. [DOI] [PubMed] [Google Scholar]
- 39.Ferris CD, Jaffrey SR, Sawa A, Takahashi M, Brady SD, Barrow RK, Tysoe SA, Wolosker H, Baranano DE, Dore S, et al. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nat Cell Biol 1999;1:152–157. [DOI] [PubMed] [Google Scholar]
- 40.Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med 2000;6:422–428. [DOI] [PubMed] [Google Scholar]
- 41.Kinhult J, Uddman R, Cardell LO. The induction of carbon monoxide-mediated airway relaxation by PACAP 38 in isolated guinea pig airways. Lung 2001;179:1–8. [DOI] [PubMed] [Google Scholar]
- 42.Taille C, Almolki A, Benhamed M, Zedda C, Megret J, Berger P, Leseche G, Fadel E, Yamaguchi T, Marthan R, et al. Heme oxygenase inhibits human airway smooth muscle proliferation via a bilirubin-dependent modulation of ERK1/2 phosphorylation. J Biol Chem 2003;278:27160–27168. [DOI] [PubMed] [Google Scholar]
- 43.Gurel A, Tomac N, Yilmaz HR, Tekedereli I, Akyol O, Armutcu F, Yuce H, Akin H, Ozcelik N, Elyas H. The Ala-9Val polymorphism in the mitochondrial targeting sequence (MTS) of the manganese superoxide dismutase gene is not associated with juvenile-onset asthma. Clin Biochem 2004;37:1117–1120. [DOI] [PubMed] [Google Scholar]
- 44.Holla LI, Kankova K, Vasku A. Functional polymorphism in the manganese superoxide dismutase (MnSOD) gene in patients with asthma. Clin Biochem 2006;39:299–302. [DOI] [PubMed] [Google Scholar]
- 45.Kinnula VL, Lehtonen S, Koistinen P, Kakko S, Savolainen M, Kere J, Ollikainen V, Laitinen T. Two functional variants of the superoxide dismutase genes in Finnish families with asthma. Thorax 2004;59:116–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hiltermann JT, Lapperre TS, van Bree L, Steerenberg PA, Brahim JJ, Sont JK, Sterk PJ, Hiemstra PS, Stolk J. Ozone-induced inflammation assessed in sputum and bronchial lavage fluid from asthmatics: a new noninvasive tool in epidemiologic studies on air pollution and asthma. Free Radic Biol Med 1999;27:1448–1454. [DOI] [PubMed] [Google Scholar]
- 47.McConnell R, Berhane K, Gilliland F, London SJ, Islam T, Gauderman WJ, Avol E, Margolis HG, Peters JM. Asthma in exercising children exposed to ozone: a cohort study. Lancet 2002;359:386–391. [DOI] [PubMed] [Google Scholar]
- 48.Li YF, Gauderman WJ, Avol E, Dubeau L, Gilliland FD. Associations of tumor necrosis factor G-308A with childhood asthma and wheezing. Am J Respir Crit Care Med 2006;173:970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meng YY, Wilhelm M, Rull RP, English P, Ritz B. Traffic and outdoor air pollution levels near residences and poorly controlled asthma in adults. Ann Allergy Asthma Immunol 2007;98:455–463. [DOI] [PubMed] [Google Scholar]
- 50.Lagorio S, Forastiere F, Pistelli R, Iavarone I, Michelozzi P, Fano V, Marconi A, Ziemacki G, Ostro BD. Air pollution and lung function among susceptible adult subjects: a panel study. Environ Health 2006;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gauderman WJ, Avol E, Gilliland F, Vora H, Thomas D, Berhane K, McConnell R, Kuenzli N, Lurmann F, Rappaport E, et al. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med 2004;351:1057–1067. [DOI] [PubMed] [Google Scholar]
- 52.Islam T, Gauderman WJ, Berhane K, McConnell R, Avol E, Peters JM, Gilliland FD. Relationship between air pollution, lung function and asthma in adolescents. Thorax 2007;62:957–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olson LM, Radecki L, Frintner MP, Weiss KB, Korfmacher J, Siegel RM. At what age can children report dependably on their asthma health status? Pediatrics 2007;119:e93–e102. [DOI] [PubMed] [Google Scholar]
- 54.Samet JM. Epidemiologic approaches for the identification of asthma. Chest 1987;91(6, Suppl):74S–78S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.