Abstract
Rationale: Smoking is a primary risk factor for chronic bronchitis, emphysema, and chronic obstructive pulmonary disease, but since not all smokers develop disease, it has been suggested that some individuals may be more susceptible to exogenous factors, such as smoking, and that this susceptibility could be genetically determined.
Objectives: The aim of the present study was to assess, in a population-based sample of twins, the following: (1) to what extent genetic factors contribute to the development of chronic bronchitis, including emphysema, taking sex into consideration, and (2) whether the genetic influences on chronic bronchitis, including emphysema, are separate from those for smoking behavior.
Methods: Disease cases and smoking habits were identified in 44,919 twins older than 40 years from the Swedish Twin Registry. Disease was defined as self-reported chronic bronchitis or emphysema, or recurrent cough with phlegm. Individuals who had smoked 10 pack-years or more were defined as smokers. Univariate and bivariate structural equation models were used to estimate the heritability specific for chronic bronchitis and that in common with smoking.
Measurements and Main Results: The heritability estimate for chronic bronchitis was a moderate 40% and only 14% of the genetic influences were shared with smoking.
Conclusions: Genetic factors independent of those related to smoking habits play a role in the development of chronic bronchitis.
Keywords: chronic bronchitis, smoking, chronic obstructive pulmonary disease, twin study, genes
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
The relative importance of the role that genetic factors play in the development of chronic bronchitis has not been quantified. It is unclear if the genetic factors influencing chronic bronchitis are the same as those contributing to smoking behavior.
What This Study Adds to the Field
Genetic factors independent of those related to smoking habits play a role in the development of chronic bronchitis.
Chronic bronchitis is a disease manifested by cough and sputum, but not necessarily airflow obstruction (1). In a review of 38 studies, the pooled prevalence of chronic bronchitis in the adult population was 6.4% (2). The prevalence increases with age (3, 4), and although in some individuals the symptoms are of less severity and are relatively stable over time, others experience an increase in severity and develop airflow obstruction. Obstructive chronic bronchitis and emphysema are the main conditions included as chronic obstructive pulmonary disease (COPD), currently the fourth leading cause of death in the world (5).
Smoking is a primary risk factor for chronic bronchitis and emphysema, but because not all smokers develop disease, it has been suggested that some individuals may be more susceptible to exogenous factors, such as smoking, leading to disease and that this susceptibility could be genetically determined. Genetic influence on chronic bronchitis has previously been demonstrated (6–12), although the methods did not allow for quantification of the relative importance of environmental and genetic factors. Women more often show symptoms of chronic bronchitis, and it has been suggested that women may be more susceptible to tobacco smoking–associated respiratory disease than men (13–16), although it has not been shown whether this sex difference is genetically mediated.
Dependence on tobacco is a complex behavior, with both genetic and environmental factors contributing to population variation. In a recent twin study of heritability for lifetime regular smoking, the estimates ranged from 46 to 57%, depending on sex (17). A common genetic basis for smoking and smoking-associated disease, such as lung cancer, was suggested in the 1950s by Fisher (18). However, methods to separate heritability for smoking from heritability for disease were not available for previous studies, and it has still not been shown whether the genetic influence on chronic bronchitis is mediated through the genetic influences on smoking behavior.
The aim of the present study was to assess, in a population-based sample of twins, the following: (1) to what extent genetic factors contribute to the development of chronic bronchitis, including emphysema, taking sex into consideration, and (2) whether the genetic influences on chronic bronchitis, including emphysema, are separate from those for smoking behavior.
Some of the results of this study have been previously reported in the form of abstracts (19, 20).
METHODS
Participants
The Swedish Twin Registry (STR) contains information on more than 80,000 twin pairs born from 1886 to 2000 (21). Between 1998 and 2002, all living twins in the STR born in 1958 or earlier were contacted using a computer-assisted telephone interview in the Screening Across the Lifespan Twin (SALT) study (22). The SALT interview included introductory items concerning zygosity and a checklist of common diseases. A series of questions related to respiratory symptoms and chronic bronchitis was also included. Special emphasis was put on diagnostic items that could determine whether a twin was likely to have a disease, rather than simply asking the twin if he or she had a disease. Informed verbal consent was obtained before the interview. The study was approved by the Swedish Data Inspection Authority and the ethics review board of the Karolinska Institute, Stockholm, Sweden.
Of the 61,767 twins born in 1958 or earlier who were alive in March 1998 when the SALT study started, 44,919 twins participated either themselves or through a proxy (if the twin was unable to take part due to cognitive impairment or hearing problems, the interviewers asked to talk to a family member or personnel at institutions), corresponding to a response rate of 73%. A total of 42,706 individuals had complete answers to the respiratory symptom screening questions and could be classified by zygosity. The analyses of chronic bronchitis are based on 4,178 complete monozygotic (MZ) twin pairs (with both members observed), 2,249 MZ singletons (with only one member observed), 11,670 complete dizygotic (DZ) pairs, and 8,761 DZ singletons. Information about smoking habits, measured by pack-years (referring to the total smoking consumption during life), was available for 36,772 individuals, consisting of 3,430 complete MZ pairs, 2,740 MZ singletons, 9,186 complete DZ pairs, and 10,195 DZ singletons.
Definition of Chronic Bronchitis and Asthma
Individuals who responded positively to questions in SALT about recurrent cough with phlegm production and/or had self-reported chronic bronchitis were defined as having chronic bronchitis. Furthermore, individuals who answered “yes” to the question on emphysema were included in the chronic bronchitis definition, using the rationale that emphysema was unlikely to be present without prior symptoms of chronic bronchitis. Individuals with negative answers to all of the questions were considered not to have chronic bronchitis. Individuals with self-reported asthma or asthma symptoms with an onset before adulthood or those that were related to allergic disease were categorized as positive for asthma.
Determination of Twin Zygosity
Zygosity information was obtained at the time of registry compilation on the basis of questions about childhood resemblance. Four separate validation studies using serology and/or genotyping have shown that, with these questions, 95 to 98% of twin pairs are classified correctly (22).
Statistical Methods
Prevalence.
The prevalence of chronic bronchitis was calculated stratified on sex, smoking habits, and zygosity, and estimates for males and females were compared by odds ratios.
Probandwise concordance.
The probandwise concordance is defined as an individual's risk of disease—that is, the probability that one twin is affected, given that his or her cotwin is affected—and is calculated as twice the number of concordant pairs divided by twice the number of concordant pairs plus the number of discordant pairs (23, 24). It was estimated for MZ and DZ twins separately, and stratified on sex and age.
Tetrachoric correlations.
Tetrachoric correlation is a measure of the correlation between two continuous variables (e.g., liability to develop disease for two individuals) that are expressed as binary variables (presence or absence of disease for the same individuals), and was used to calculate the resemblance in liability to develop chronic bronchitis within twin pairs. The correlations were stratified by sex, age, and zygosity. Because MZ twins are genetically identical, whereas DZ twins share on average half of their segregating genes, a tetrachoric correlation that is larger for MZ twins than for DZ twins suggests a genetic influence on liability to develop chronic bronchitis. Tetrachoric correlations were also calculated after dropping all twins with asthma from the analyses.
Univariate structural equation modeling.
The relative importance of genetic and environmental influences on the liability to develop chronic bronchitis was also evaluated using structural equation models. We assume a liability-threshold model, in which the liability to develop disease is normally distributed and manifested at a certain threshold as a categorical phenotype (25). The general idea is to decompose the between-individual variability in disease liability into three parameters: components attributable to genetic factors (A), shared familial environment (C, reflecting environmental factors common to both members in a twin pair), and nonshared environment (E, reflecting environmental factors unique to each twin). The threshold was adjusted for age and sex to account for the fact that the prevalence of chronic bronchitis depends on these factors.
A series of models, making different assumptions about the familial influence on chronic bronchitis, were evaluated by raw maximum likelihood using the Mx program (26). By removing parameters one by one from the model and comparing its goodness of fit to the data compared with a larger model, the significance of the removed parameter can be assessed. Models with fewer parameters are preferred, as long as they do not have significantly worse goodness of fit. Confidence intervals for prevalence and variance component estimates were obtained on the basis of profile likelihood methods (27). Corrected P values were obtained by halving the P values obtained from a classical likelihood ratio test on the basis of a χ2 distribution because variance components were constrained to be nonnegative (28).
Bivariate Structural Equation Modeling
Finally, to assess to what extent the genetic influences on chronic bronchitis are associated with those for smoking behavior, a bivariate liability-threshold model for chronic bronchitis and smoking habits was formulated. This model allows a quantification of how much of the genetic (A) and environmental (E) effect is specific to chronic bronchitis and how much is due to A and E influences on smoking. The analyses were adjusted for sex differences and age differences in prevalence of chronic bronchitis and smoking habits. Gene–environment interactions were not modeled.
RESULTS
Prevalence
The crude prevalence of chronic bronchitis according to our definition in the sample of 42,706 Swedish twins was 7.1%. Of these, 2,592 subjects reported only productive cough and/or self-reported chronic bronchitis, 205 subjects reported only emphysema, and 247 reported a combination of both conditions. Prevalence estimates, stratified on smoking habits, sex, and zygosity, are presented in Table 1. Significantly higher prevalences were seen for women than men in all groups.
TABLE 1.
PREVALENCE OF CHRONIC BRONCHITIS STRATIFIED BY SEX, SMOKING HABITS, ZYGOSITY, AND ASTHMA
| All
|
Men
|
Women
|
Women vs. Men
|
|||||
|---|---|---|---|---|---|---|---|---|
| n | CB (%) | n | CB (%) | n | CB (%) | OR | 95% CI | |
| Total sample | 36,772 | 7.17 | 16,414 | 6.04 | 20,358 | 8.08 | 1.37 | 1.26–1.48 |
| Never smokers | 17,831 | 5.57 | 7,229 | 4.19 | 10,602 | 6.52 | 1.59 | 1.39–1.83 |
| Ex-smokers | 11,586 | 6.90 | 6,199 | 6.08 | 5,387 | 7.83 | 1.31 | 1.14–1.52 |
| Current smokers | 7,355 | 11.46 | 2,986 | 10.45 | 4,369 | 12.15 | 1.19 | 1.02–1.38 |
| Less than 10 pack-years | 24,070 | 5.47 | 9,844 | 4.29 | 14,226 | 6.28 | 1.50 | 1.33–1.69 |
| More than 10 pack-years | 12,702 | 10.39 | 6,570 | 8.68 | 6,132 | 12.23 | 1.47 | 1.31–1.65 |
| MZ | 9,241 | 7.02 | 4,007 | 6.11 | 5,234 | 7.72 | 1.28 | 1.09–1.51 |
| DZ SS | 14,165 | 7.07 | 6,280 | 5.89 | 7,885 | 8.00 | 1.39 | 1.22–1.59 |
| DZ OS | 13,366 | 7.38 | 6,127 | 6.15 | 7,239 | 8.41 | 1.40 | 1.23–1.60 |
| With asthma | 2,429 | 24.04 | 1,059 | 18.79 | 1,370 | 28.10 | 1.69 | 1.39–2.05 |
Definition of abbreviations: CB = chronic bronchitis; CI = confidence interval; DZ OS = dizygotic opposite-sex twins; DZ SS = dizygotic same-sex twins; MZ = monozygotic.
Concordance Rates and Tetrachoric Correlations
The numbers of concordant and discordant pairs, probandwise concordance rates, and tetrachoric correlations, stratified by zygosity, sex, and age, are presented in Table 2. Probandwise concordance and tetrachoric correlation were higher for MZ than DZ twin pairs for all groups. The differences were more pronounced in the younger group than in the older group, and more pronounced in males than in females. Both probandwise concordance and tetrachoric correlation were similar for same- and opposite-sex DZ pairs. Excluding all subjects with asthma symptoms had no substantial effect on the tetrachoric correlations (MZ, 0.44; DZ, 0.18; compared with Table 2).
TABLE 2.
ESTIMATES OF PROBANDWISE CONCORDANCE RATES, AND TETRACHORIC CORRELATIONS, FOR CHRONIC BRONCHITIS
| Twin Group | Concordant Unaffected | Discordant | Concordant Affected | Probandwise Concordance Rate (SE) | Tetrachoric Correlation (SE) |
|---|---|---|---|---|---|
| Total sample | |||||
| MZ | 3,699 | 414 | 65 | 0.239 (0.024) | 0.445 (0.042) |
| DZ | 10,209 | 1,368 | 93 | 0.120 (0.011) | 0.178 (0.031) |
| Younger than 60 yr | |||||
| MZ | 2,485 | 245 | 40 | 0.246 (0.032) | 0.477 (0.052) |
| DZ | 6,726 | 743 | 35 | 0.086 (0.014) | 0.123 (0.045) |
| 60 yr or older | |||||
| MZ | 1,214 | 169 | 25 | 0.228 (0.038) | 0.388 (0.070) |
| DZ | 3,483 | 625 | 58 | 0.157 (0.018) | 0.197 (0.044) |
| Sex | |||||
| MZ M | 1,625 | 148 | 28 | 0.275 (0.041) | 0.531 (0.061) |
| MZ F | 2,074 | 266 | 37 | 0.218 (0.030) | 0.385 (0.056) |
| DZ M | 2,351 | 253 | 14 | 0.100 (0.025) | 0.173 (0.074) |
| DZ F | 2,826 | 423 | 32 | 0.131 (0.021) | 0.180 (0.055) |
| DZ OS | 5,032 | 692 | 47 | 0.120 (0.016) | 0.175 (0.044) |
Definition of abbreviations: DZ = dizygotic; DZ OS = dizygotic opposite-sex twins; F = female; M = male; MZ = monozygotic.
Univariate Structural Equation Modeling
The results from structural equation modeling are shown in Table 3. The comparisons of models indicated that, for chronic bronchitis, (1) the same genes are of importance in men and women (model 2 vs. 1), (2) the relative importance of genes is the same in men and women (model 3 vs. 2), and that shared familial environmental influences are not of importance (model 4 vs. 3). The genetic effect was statistically significant (model 5 vs. 4: likelihood ratio test [LRT], 115.528; P value < 0.001). Models 1 and 4 are presented in detail in Table 4. In the most parsimonious model (model 4), 40% of the variance was attributed to genetic factors, and the rest (60%) to nonshared environment.
TABLE 3.
GOODNESS-OF-FIT STATISTICS FROM STRUCTURAL EQUATION MODELING OF CHRONIC BRONCHITIS
| Model | −2log–likelihood | LRT* | P Value |
|---|---|---|---|
| 1 | 15,540.658 | ||
| 2 | 15,541.315 | 0.657 | 0.418 |
| 3 | 15,543.867 | 2.552 | 0.466 |
| 4 | 15,543.867 | 0 | 0.500† |
| 5 | 15,659.395 | 115.528 | <0.001† |
Models: (1) rg (genetic correlation) free for DZ OS (dizygotic opposite-sex twins), ACE different for males and females; (2) rg fixed at 0.5 for DZ OS, ACE different for males and females; (3) rg fixed at 0.5 for DZ OS, ACE same for males and females; (4) rg fixed at 0.5 for DZ OS, AE; (5) rg fixed at 0.5 for DZ OS, E.
Likelihood ratio test for comparing the model versus the proceeding model.
Accounting for the fact that a test of a variance component is a boundary problem.
TABLE 4.
ESTIMATES OF PERCENTAGES OF VARIANCE EXPLAINED BY GENETIC FACTORS (A), SHARED FAMILIAL ENVIRONMENT (C), AND NONSHARED ENVIRONMENT REFLECTING ENVIRONMENTAL FACTORS (E) AND 95% CONFIDENCE INTERVALS
| Males
|
Females
|
||||||
|---|---|---|---|---|---|---|---|
| A | C | E | A | C | E | rg | |
| Full model (model 1) | 0.49 (0.29–0.60) | 0 (0–0.15) | 0.51 (0.40–0.58) | 0.38 (0.12–0.47) | 0 (0–0.20) | 0.63 (0.53–0.73) | 0.38 (0.10–0.50) |
| Most parsimonious model (model 4) | 0.40 (0.33–0.47) | 0.60 (0.53–0.60) | |||||
Definition of abbreviation: rg = genetic correlation.
Bivariate Structural Equation Modeling
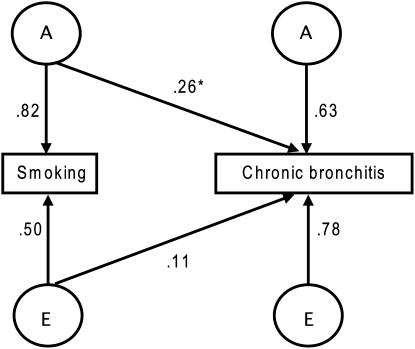
The factors contributing to the association between smoking behavior and chronic bronchitis were investigated using an AE (shared genetic [additive] and nonshared environmental effects) bivariate liability-threshold model. We found that a genetic path mediating the association could not be excluded (LRT, 58.252; P value < 0.001). Thus, the genes involved in smoking behavior are partly associated with the genes influencing the development of chronic bronchitis. However, they account only for a modest portion (14%) of the genetic component for chronic bronchitis. In other words, most of the genes that are important for the development of chronic bronchitis are independent of those that are important for smoking behavior. The bivariate model and parameter estimates are illustrated in Figure 1.
Figure 1.
Bivariate path diagram describing the genetic (A) and nonshared environmental (E) effects that are common to smoking and chronic bronchitis, as well as the genetic and nonshared environmental effects that are unique to each phenotype, adjusted for prevalence differences related to age and sex. The observed traits (smoking and chronic bronchitis) are represented by rectangles, whereas unobserved (latent) genetic and nonshared environmental variables are represented by circles. The parameter estimates (arrows) are path coefficients, indicating the relative importance of the latent variables A and E to smoking and chronic bronchitis. The square of these estimates represents the variance of the trait accounted for by that specific latent factor. *Common genetic path.
DISCUSSION
The current study on the population-based STR, showing a moderate genetic effect for the development of chronic bronchitis that does not differ by sex, is to our knowledge the first to quantify heritability for the disease. Because smoking behavior has a known genetic component and smoking is the primary risk factor for chronic bronchitis, the possibility of an overlap of the genetic factors for disease being the same as the genetic factors for smoking behavior was tested by bivariate structural equation modeling. Results show that the genetic effects for the liability to develop chronic bronchitis were largely independent from those for smoking behavior, although some associations were found.
Higher prevalence of chronic bronchitis in women compared with men has previously been reported. Some have suggested that this is due to more women smoking, or because women would be more aware of symptoms than men and therefore report more chronic bronchitis (4). However, in the current study, more men than women had smoked at least 10 pack-years. Disease was also more common in females in opposite-sex twin pairs, which indicates that it is not simply a reflection of the higher age in women. It is well known that women are more susceptible to environmental factors, such as tobacco smoke, than men (13–16).
Higher concordances and correlations were seen for MZ than for DZ twins in the present study, indicating that genetic factors are of importance for the development of chronic bronchitis. This was further supported by the results from the structural equation modeling and is in agreement with results from early studies on chronic bronchitis and regular cough (6–8).
Two previous studies, a twin study by Cederlöf and colleagues (6) and a family study by Higgins and Keller (8), have shown that genetic influences seem to be strongest in the younger age groups. Higgins and Keller suggested that this could be explained by heredity being less important in older ages, or that fewer hazardous environmental exposures were shared between offspring and parents, or that the offspring over 40 years with surviving parents could be a healthier, unrepresentative segment of the population. The fact that the prevalence of chronic bronchitis increases by age had to be accounted for in the current study to avoid an overestimation of shared environment.
The design of previous studies has not allowed for exploration of how the familial influences for chronic bronchitis can be separated from those for smoking, although attempts were made to control for smoking habits (6, 10, 12), indicating that the results were not only a reflection of heredity for smoking habits. The present study used bivariate analyses, adjusted for age and sex differences in prevalence, to show a significant association between the genetic influences on chronic bronchitis and smoking behavior. Nevertheless, this correlation only accounted for 14% of the genetic variance for chronic bronchitis. Therefore, chronic bronchitis has a moderate genetic influence that is largely independent from that for smoking behavior. This should, however, not be interpreted to mean that smoking has no effect in certain individuals, keeping in mind that the prevalence of chronic bronchitis in smokers was twice as high as in nonsmokers. Smoking may directly influence development of chronic bronchitis regardless of the (genetic) reasons for smoking.
The prevalence of chronic bronchitis strongly depends on how the disease is defined, the age of the population, and smoking habits. A previous Swedish study of an adult population aged 40–59 years, with 30–38% smokers and 24–34% ex-smokers, reported self-reported chronic bronchitis prevalences of between of 3.8 and 6.1% in males and 5.5 and 6.5% in females (4). The current study had similar disease prevalence (7%) and smoking habits (32% smokers and 20% ex-smokers). Although it should be noted that the population is older and wider inclusion criteria were used, it should be reasonable to assume that the twin population can be regarded as representative of the rest of the population (22).
The present study has some limitations. First, it should be noted that the results are based on questionnaire-based telephone interview data only, and not obtained by objective examination. The criterion used to define chronic bronchitis was wider than the classical criterion of “cough with phlegm production for at least 3 months during at least 2 consecutive years” (29). The use of a wider criterion might better mirror the respiratory problems of the population, but also falsely classify healthy subjects as diseased. The stricter criteria might exclude true disease and, from the questionnaire data, very few twins fulfilled the classical criteria. However, because the data were used for heritability estimates, the misclassification will only be a problem if it is differential between MZ and DZ twins. Furthermore, we chose to include emphysema in the definition of chronic bronchitis. The rationale for this was not only to mirror what we today would call COPD but also to ensure that subjects who might have passed from chronic bronchitis to emphysema were not falsely excluded, as they might mention “COPD” or “emphysema” rather than chronic bronchitis. Second, smoking was dichotomized, using 10 pack-years as a cutoff, which means that light smokers were classified as nonsmokers. The motivation for using this measure was to make sure that those classified as smokers had smoked enough to damage the lungs. Again, misclassification of smoking habits will only be a problem if the misclassification is differential between MZ and DZ twins. Third, it is conceivable that part of the genetic influence attributed to chronic bronchitis could partly be due to heritability for atopy, which is a known risk factor for the development of asthma. However, the exclusion of all subjects with asthma symptoms did not influence the heritability estimates for chronic bronchitis substantially.
From the results of this study, we conclude that heritability has a moderate influence on the development of chronic bronchitis, and that the genes that are involved are largely independent of those related to smoking habits. We have also confirmed that chronic bronchitis seems to be more prevalent among females. The cohort will also be suitable for studies of markers for development of COPD, although the diagnosis needs to be confirmed by spirometry.
The data collection in SALT was supported by grants from the Swedish Research Council and NIH grant AG 08724. Support also provided by AstraZeneca and the Swedish Heart-Lung Foundation.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200704-565OC on November 29, 2007
Conflict of Interest Statement: J.H. received fees for lectures (<$1,000) and attended one course organized and financed by GlaxoSmithKline. A.D. is an employee of AstraZeneca. U.K.E. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.G.d.V. is an employee of AstraZeneca and owns stock as part of AstraZeneca's bonus program. N.L.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.D. is an employee of AstraZeneca and owns stock as part of AstraZeneca's bonus program. U.N. is an employee of AstraZeneca and owns stock as part of AstraZeneca's bonus program. T.H. is an employee of AstraZeneca and owns stock as part of AstraZeneca's bonus program. M.S. has been reimbursed by GlaxoSmithKline for attending American Thoracic Society conferences. M.S. received $3,000 from Pfizer for serving on an advisory board regarding pulmonary risk associated with treatment with inhaled insulin. M.S. received more than $2,000 from Pfizer for speaking at sponsored educational meetings regarding inhaled insulin, Exubera, and more than $500 from GlaxoSmithKline for education regarding environmental factors causing COPD.
References
- 1.American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1995;152:S77–S121. [PubMed] [Google Scholar]
- 2.Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM. Global burden of COPD: systematic review and meta-analysis. Eur Respir J 2006;28:523–532. [DOI] [PubMed] [Google Scholar]
- 3.Lundback B, Nystrom L, Rosenhall L, Stjernberg N. Obstructive lung disease in northern Sweden: respiratory symptoms assessed in a postal survey. Eur Respir J 1991;4:257–266. [PubMed] [Google Scholar]
- 4.Montnemery P, Adelroth E, Heuman K, Johannisson A, Johansson SA, Lindholm LH, Lundback B, Lofdahl CG. Prevalence of obstructive lung diseases and respiratory symptoms in southern Sweden. Respir Med 1998;92:1337–1345. [DOI] [PubMed] [Google Scholar]
- 5.Calverley PM, Walker P. Chronic obstructive pulmonary disease. Lancet 2003;362:1053–1061. [DOI] [PubMed] [Google Scholar]
- 6.Cederlof R, Edfors ML, Friberg L, Jonsson E. Hereditary factors, “spontaneous cough” and “smoker's cough”: a study on 7,800 twin-pairs with the aid of mailed questionnaires. Arch Environ Health 1967;14:401–406. [DOI] [PubMed] [Google Scholar]
- 7.Cederlöf R, Friberg L, Hrubec Z. Cardiovascular and respiratory symptoms in relation to tobacco smoking: a study on American twins. Arch Environ Health 1969;18:934–940. [DOI] [PubMed] [Google Scholar]
- 8.Higgins M, Keller J. Familial occurrence of chronic respiratory disease and familial resemblance in ventilatory capacity. J Chronic Dis 1975;28:239–251. [DOI] [PubMed] [Google Scholar]
- 9.Speizer FE, Rosner B, Tager I. Familial aggregation of chronic respiratory disease: use of National Health Interview Survey data for specific hypothesis testing. Int J Epidemiol 1976;5:167–172. [DOI] [PubMed] [Google Scholar]
- 10.Tager I, Tishler PV, Rosner B, Speizer FE, Litt M. Studies of the familial aggregation of chronic bronchitis and obstructive airways disease. Int J Epidemiol 1978;7:55–62. [DOI] [PubMed] [Google Scholar]
- 11.Silverman EK, Chapman HA, Drazen JM, Weiss ST, Rosner B, Campbell EJ, O'Donnell WJ, Reilly JJ, Ginns L, Mentzer S, et al. Genetic epidemiology of severe, early-onset chronic obstructive pulmonary disease: risk to relatives for airflow obstruction and chronic bronchitis. Am J Respir Crit Care Med 1998;157:1770–1778. [DOI] [PubMed] [Google Scholar]
- 12.Montnemery P, Lanke J, Lindholm LH, Lundback B, Nyberg P, Adelroth E, Lofdahl CG. Familial related risk-factors in the development of chronic bronchitis/emphysema as compared to asthma assessed in a postal survey. Eur J Epidemiol 2000;16:1003–1007. [DOI] [PubMed] [Google Scholar]
- 13.Gold DR, Wang X, Wypij D, Speizer FE, Ware JH, Dockery DW. Effects of cigarette smoking on lung function in adolescent boys and girls. N Engl J Med 1996;26;335:931–937. [DOI] [PubMed] [Google Scholar]
- 14.Prescott E, Bjerg AM, Andersen PK, Lange P, Vestbo J. Gender difference in smoking effects on lung function and risk of hospitalization for COPD: results from a Danish longitudinal population study. Eur Respir J 1997;10:822–827. [PubMed] [Google Scholar]
- 15.Langhammer A, Johnsen R, Gulsvik A, Holmen TL, Bjermer L. Sex differences in lung vulnerability to tobacco smoking. Eur Respir J 2003;21:1017–1023. [DOI] [PubMed] [Google Scholar]
- 16.Dransfield MT, Davis JJ, Gerald LB, Bailey WC. Racial and gender differences in susceptibility to tobacco smoke among patients with chronic obstructive pulmonary disease. Respir Med 2006;100:1110–1116. [DOI] [PubMed] [Google Scholar]
- 17.Madden PAF, Pedersen NL, Kaprio J, Koskenvuo MJ, Martin NG. The epidemiology and genetics of smoking initiation and persistence: cross-cultural comparisons of twin study results. Twin Res 2004;7:82–97. [DOI] [PubMed] [Google Scholar]
- 18.Fisher RA. Lung cancer and cigarettes. Nature 1958;182:108. [DOI] [PubMed] [Google Scholar]
- 19.Hallberg J, Gerhardsson de Verdier M, Nihlén U, Pedersen N, Svartengren M. Interaction between smoking and genetic factors in the development of chronic bronchitis. Eur Respir J 2003;22:388s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Svartengren M, Hallberg J, Dominicus A, Gerhardsson M, Davies C, Pedersen NL, Dahlback M, Nilhlen U, Higginbottam T. Interaction between smoking and genetic factors in the development of chronic bronchitis [abstract]. Am J Respir Crit Care Med 2007;175:A825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lichtenstein P, Sullivan PF, Cnattingius S, Gatz M, Johansson S, Carlstrom E, Bjork C, Svartengren M, Wolk A, Klareskog L, et al. The Swedish Twin Registry in the third millennium: an update. Twin Res Hum Genet 2006;9:875–882. [DOI] [PubMed] [Google Scholar]
- 22.Lichtenstein P, De Faire U, Floderus B, Svartengren M, Svedberg P, Pedersen NL. The Swedish Twin Registry: a unique resource for clinical, epidemiological and genetic studies. J Intern Med 2002;252:184–205. [DOI] [PubMed] [Google Scholar]
- 23.McGue M. When assessing twin concordance, use the probandwise not the pairwise rate. Schizophr Bull 1992;18:171–176. [DOI] [PubMed] [Google Scholar]
- 24.Witte JS, Carlin JB, Hopper JL. Likelihood-based approach to estimating twin concordance for dichotomous traits. Genet Epidemiol 1999;16:290–304. [DOI] [PubMed] [Google Scholar]
- 25.Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992.
- 26.Neale MC, Boker SM, Xie G, Maes HH. Mx: statistical modeling, 6th ed. Richmond, VA: Department of Psychiatry, Virginia Institute for Psychiatric and Behavior Genetics, Virginia Commonwealth University; 2003. Available from: http://www.vcu.edu/mx/
- 27.Neale MC, Miller MB. The use of likelihood-based confidence intervals in genetic models. Behav Genet 1997;27:113–120. [DOI] [PubMed] [Google Scholar]
- 28.Dominicus A, Skrondal A, Gjessing HK, Pedersen NL, Palmgren J. Likelihood ratio tests in behavioral genetics: problems and solutions. Behav Genet 2006;36:331–340. [DOI] [PubMed] [Google Scholar]
- 29.American Thoracic Society. Definitions and classification of chronic bronchitis, asthma, and pulmonary emphysema. Am Rev Respir Dis 1962;85:762–785. [Google Scholar]