Abstract
Single-dose administration of beta-adrenoceptor agonists produces bronchodilation and inhibits airway hyperresponsiveness (AHR), and is the standard treatment for the acute relief of asthma. However, chronic repetitive administration of beta-adrenoceptor agonists may increase AHR, airway inflammation, and risk of death. Based upon the paradigm shift that occurred with the use of beta-blockers in congestive heart failure, we previously determined that chronic administration of beta-blockers decreased AHR in a murine model of asthma. To elucidate the mechanisms for the beneficial effects of beta-blockers, we examined the effects of chronic administration of several beta-adrenoceptor ligands in a murine model of allergic asthma. Administration of beta-blockers resulted in a reduction in total cell counts, eosinophils, and the cytokines IL-13, IL-10, IL-5, and TGF-β1 in bronchoalveolar lavage, and attenuated epithelial mucin content and morphologic changes. The differences in mucin content also occurred if the beta-blockers were administered only during the ovalbumin challenge phase, but administration of beta-blockers for 7 days was not as effective as administration for 28 days. These results indicate that in a murine model of asthma, chronic administration of beta-blockers reduces inflammation and mucous metaplasia, cardinal features of asthma that may contribute to airflow obstruction and AHR. Similar to heart failure, our results provide a second disease model in which beta-blockers producing an acutely detrimental effect may provide a therapeutically beneficial effect with chronic administration.
Keywords: beta-blockers, beta-adrenoceptor, asthma, mucin, airway inflammation
CLINICAL RELEVANCE
This research may result in a paradigm shift in the treatment of asthma. This research demonstrates the importance that duration of beta-blocker therapy has on clinical and physiologic responses.
Asthma mortality rates in the United States have risen by 60% in the last 25 years despite the widespread chronic therapeutic use of beta-adrenoceptor agonists (1). Recently, a large clinical trial using a long-acting beta-adrenoceptor agonist, salmeterol, was stopped due to an increased incidence of death and serious asthma-related events (2). Clearly there is a need for a different approach to chronic asthma therapy. Analogous to asthma, a different approach was needed in congestive heart failure (CHF) because the administration of beta-adrenoceptor agonists also produced acutely beneficial but chronically detrimental effects (3). Recently, drugs classified as beta-blockers (beta-adrenoceptor antagonists and inverse agonists) were introduced into therapy for CHF and have now become a part of first-line therapy (4). These drugs were once contraindicated in CHF because acute administration produced negative inotropic effects and decreased cardiac output (3). However, large clinical trials have shown that chronic administration improved cardiac output and decreased mortality in patients with CHF (4, 5). Notably, chronic administration of beta-blockers with partial agonist properties did not decrease mortality in CHF (6–8).
Presently, the administration of beta-blockers is contraindicated in asthma because their acute administration may cause increased airway resistance (9, 10). The effects of chronic administration of β-blockers in asthma have not been closely examined, perhaps because of the assumption that the chronic and acute effects of these drugs are similar (3, 11, 12). To determine if the assumption is incorrect, we previously investigated whether chronic administration of beta-blockers with inverse agonist properties could be useful in treating asthma (15). Beta-adrenoceptor inverse agonists are a subset of beta-blockers that, like all beta-blockers, can inhibit agonist-induced signaling, but inverse agonists can also inhibit signaling produced by constitutively active receptors (13). For the parameters that have been examined, agonists and inverse agonists produce the exact opposite effects (14). We therefore hypothesized that the reciprocal effects of agonists and inverse agonists may extend to their effect on signaling (14); specifically, that chronic agonist exposure can produce desensitization and inhibit signaling, while chronic treatment with inverse agonists may produce sensitization and enhance signaling. Our initial studies using a murine model of asthma showed that acute administration of beta-blockers with inverse agonist properties was detrimental and increased AHR, but chronic administration significantly decreased AHR (15). These data indicate the important role that duration of drug therapy can have on the observed physiologic or clinical response. Further support for our hypothesis is that as with CHF, chronic administration of a β-blocker with partial agonist properties did not decrease AHR in the murine model of asthma (6–8, 15).
In this study we have examined the effect of chronic treatment with beta-blockers on airway inflammation in an antigen-driven murine model of asthma. The inflammatory process in asthma involves the interaction between different immune cells such as lymphocytes and eosinophils, and parenchymal cells such as airway epithelial and smooth muscle cells. The nature of the interaction between these cells contributes to airflow obstruction, bronchoconstriction (including AHR), airway edema, and mucin hypersecretion (16, 17). Here we specifically measured the number of inflammatory cells and the level of the cytokines IL-13, IL-10, IL-5, transforming growth factor-β1 (TGF-β1), and regulated on activation, T cell expressed and secreted (RANTES) in bronchoalveolar lavage (BAL) fluid, and determined the degree of mucous metaplasia by airway epithelial cells.
MATERIALS AND METHODS
Mice
Six- to twelve-week old BALB/cJ (male) mice (Jackson Animal Laboratory, Bar Harbor, ME) and C57BL/6J (male and female) mice (Harlan Sprague Dawley, Indianapolis, IN) were housed under specific pathogen–free conditions in accordance with the Institutional Animal Care and Use Committee of the University of Houston and the M.D. Anderson Cancer Center, respectively.
Animal Sensitization and Challenge
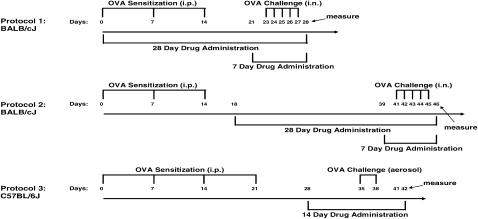
In the first set of experiments with BALB/cJ mice (Figure 1, protocol 1), antigen-challenged animals were sensitized (three intraperitoneal ovalbumin [OVA] injections weekly on Days 0, 7, and 14) and challenged (once daily intranasally for 5 d on Days 23–27) with OVA as previously described (18). In the second set of experiments (Figure 1, protocol 2), antigen-challenged BALB/cJ mice were sensitized (weekly intraperitoneal injections, on Days 0, 7, and 14) and challenged (once daily intranasally for 5 d on Days 41–45) with OVA. Thus, the only difference between the two protocols was the time of challenge relative to the sensitization injections. Control mice received OVA during sensitizations and saline during the challenges. Because our results were unexpected, a second laboratory performed the experiments using C57BL/6J mice and a different sensitization and challenge protocol (Figure 1, protocol 3). Antigen-challenged C57BL/6J mice were sensitized (intraperitoneal OVA injection four times, weekly on Days 0, 7, 14, and 21) and challenged (twice, on Days 35 and 38) with aerosolized OVA (19).
Figure 1.
Schematic diagram illustrating the protocols for ovalbumin (OVA) sensitization, challenges, and drug administration to BALB/cJ and C57BL/6J mice.
Drug Administration
The beta-blockers ICI 118,551 and nadolol were chosen because a previous study using transgenic mice with cardiac overexpression of the human beta2-adrenoceptor revealed these two drugs to be full inverse agonists at this receptor (13). However, nadolol has equal affinity for both beta1- and beta2-adrenoceptors, while ICI 118,551 has an approximately 3,000 selectivity for the beta2-adrenoceptor. In the first set of experiments (Figure 1, protocol 1), a group of antigen-challenged BALB/cJ mice were fed (ad libitum) mouse chow containing the nonselective beta-adrenoceptor antagonist nadolol (13). Drug was administered on Days 0 to 28; at a concentration of 250 ppm in the mouse chow. Other groups of antigen-challenged mice received vehicle or the selective beta2-adrenoceptor antagonist ICI 118,551 ([±]-1-[2,3-(Dihydro-7-methyl-1H-inden-4-yl)oxy]-3-[(1-methyl-ethyl)amino]-2-butanolhydro chloride) for either 7 or 28 days (drug was administered for 7 days on Days 21 to 28, at 8 mg/kg/day; or drug was administered for 28 days on Days 0 to 28; at 2 mg/kg/d) or the beta2-adrenoceptor agonist salbutamol for 28 days (Days 0–28; at 0.5 mg/kg/d) by subcutaneous mini-osmotic pump implantation (model 2001 delivers drug for 7 d and model 2004 delivers drug for 28 d, Alzet; Durect Corporation, Cupertino, CA). Experimental mice were killed on Day 28. Due to their short half-life, salbutamol and ICI 118,551 were administered by mini-osmotic pump, while nadolol's half-life and pharmacokinetic profile are suitable for dosing orally in the chow. In the second set of experiments (Figure 1, protocol 2), antigen-challenged BALB/cJ mice began drug (salbutamol, ICI 118,551, or nadolol) administration after the sensitization process was completed. Drugs were administered on Days 18 to 46 for the 28-day treatment or Days 39 to 46 for the 7-day treatment, and were killed on Day 46. Drug dosage and routes of administration were kept the same as in the previous set of experiments. This protocol of treatment (Figure 1, protocol 2) with nadolol from Days 18 to 46 was used in the experiments measuring cytokines. Untreated saline-challenged or antigen-challenged mice were fed with normal mouse chow. We again varied the experiments with the C57BL/6J mice by using a treatment time of 14 days (Figure 1, protocol 3). Antigen-challenged C57BL/6J mice received vehicle or ICI 118,551 (8 mg/kg/d) for 14 days between Days 28 and 42 (the mini-osmotic pump model 2001 delivers drug or vehicle for 7 d, hence two pumps were implanted one week apart [on Days 28 and 35] to provide the 14 d of treatment), and were killed on Day 42. For all mice, mini-osmotic pumps were implanted under anesthesia after an intraperitoneal injection of 4 to 5 μl/g of a solution containing 40 mg/ml ketamine and 6 mg/ml xylazine. Absences of corneal reflex and motor response to nocioceptive stimuli were verified before the surgical procedures. All drugs were purchased from Sigma (St. Louis, MO).
Bronchoalveolar Lavage
Cold phosphate-buffered saline (1 ml PBS) was infused and drawn back through the tracheal cannula from killed (pentobarbital 0.1 ml of 65 mg/ml) BALB/cJ mice, and repeated once. The total leukocyte count in the BAL was determined using a Coulter counter (Beckman Coulter, Fullerton, CA). Cytospins were prepared from 200 μl of BAL at 500 rpm for 5 minutes and stained with Hema-3 for enumeration of cell types. Eosinophils, monocytes, lymphocytes, or neutrophils were identified by standard morphologic criteria. At least 200 cells were counted from cytospin preparations. BAL was also obtained as described above through the tracheal cannula in euthanized C57BL/6J mice. The total leukocyte count was determined using a hemaytometer, and cell populations were determined by cytocentrifugation of 300 μl of BAL using a Cytospin 4 (Thermo Electron Corporation, Waltham, MA) at 2,000 rpm for 5 minutes, followed by Wright-Giemsa staining.
Histochemistry
For staining with periodic acid fluorescent Schiff's (PAFS) reagent to examine intracellular mucin, lungs were fixed with 4% paraformaldehyde in PBS (pH 7.0) infused through a tracheal cannula at room temperature, then removed from the thoracic cavity and further fixed overnight at 4°C, embedded in paraffin, sectioned, and stained for light microscopy as previously described (19).
Fluorescence Microscopy
For the quantification of mucin, PAFS-stained slides were examined under a ×40 objective. Images of 10 fields from the axial bronchi were captured, and camera settings were managed using MagnaFire 2.1 (Optronics, Goleta, CA) (19). The volume density of mucin in the airway epithelium was then measured using ImagePro Plus and calculated as previously described (19). Images were acquired before any measurements and analyzed by blinded investigators.
Immunohistochemistry
Immunohistochemical staining was performed on 5-μm paraffinized sections of C57BL/6J mice lung tissue after paraformaldehyde (Sigma) fixation. Sections dewaxed with xylene (Sigma-Aldrich) were placed in 100% ethanol for 2 × 5 minutes, followed by 3% H2O2 (Sigma) in methanol for 10 minutes to quench endogenous peroxidase activity, and then fully rehydrated. Antigen retrieval was performed in citrate buffer 10 mM, pH 6.0 for 10 minutes at 100°C. Goat serum (Vector Laboratories, Burlingame, CA) diluted in PBS was used to block nonspecific binding sites. For Muc5ac, the primary antibody used was chicken polyclonal anti-Muc5ac (20) at a 1:2,000 dilution. After 1 hour of incubation at 23°C, the biotinylated secondary antibody goat anti-chicken (Invitrogen, Carlsbad, CA) was applied at 1:500 for 30 minutes at 23°C. For Muc5b, primary antibody used was rabbit anti-Muc5B (from C.E. and M.K.) at 1:10,000 for 1 hour at 23°C followed by incubation with secondary antibody goat anti-rabbit –horseradish peroxidase at 1:200 for 30 minutes at 23°C. The Muc5b antibody was raised against a 15-aa synthetic peptide corresponding to a sequence that was repeated six times in the tandem repeats of Muc5b between aa1560 and aa3370. Bound antibody was visualized using the Vectastain ABC staining kit (Vector Laboratories). Slides were examined under a ×60 objective.
RNA Isolation and RT-PCR
Lungs were removed from killed mice, washed in PBS, and snap-frozen in liquid nitrogen. For RNA extraction, tissues were homogenized in TRIZOL reagent (Invitrogen). Five micrograms of total RNA was reverse transcribed using random 9-mers as complimentary DNA (cDNA) primers in a 20-μl reaction. For quantitative PCR, we used TaqMan Gene Expression Master Mix (Applied Biosystems, Foster City, CA) and TaqMan Gene Expression Assays (Applied Biosystems) containing a mixture of unlabeled PCR primers and FAM dye-labeled TaqMan probes for Muc5ac (Assay Mm 01,276,725 × g1) and Muc5b (Assay Mm 00,466,376 m1), and VIC dye-labeled TaqMan probe for 18S (Assay 4319413E). Quantitative PCR was performed using 100 ng of cDNA in a 20-μl reaction, using an ABI PRISM 7000 Sequence Detection System (Applied Biosystems). A standard curve was generated by running in parallel synthetic single stranded oligonucleotide templates for each transcript at dilutions of 10 to 0.001nM. The results were expressed as number of mucin gene copies per 18S copies.
Cytokine and Chemokine Levels
The levels of IL-13, IL-10, IL-5, TGF-β1, and RANTES in BAL obtained 24 hours after the last antigen challenge were measured by Pierce Searchlight (Woburn, MA) using multiplex enzyme-linked immunosorbent assay. The samples were provided to Pierce Searchlight in blinded fashion.
Statistical Analysis
Quantitative data are presented as mean ± SEM (expressed as the percent standard error of the mean). Statistical analysis for multiple groups was performed using one-way ANOVA followed by Dunnett's multicomparison test. Unpaired t test was performed for analysis of two groups (Prism, GraphPad software, CA). P < 0.05 was considered statistically significant.
RESULTS
Chronic Administration of Beta-Blockers Reduces Airway Eosinophilia and Cytokine Levels
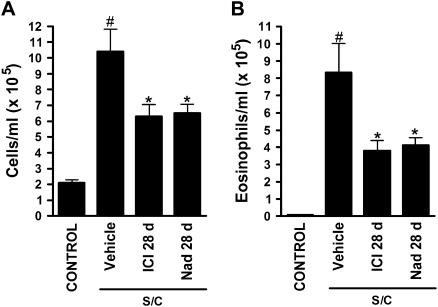
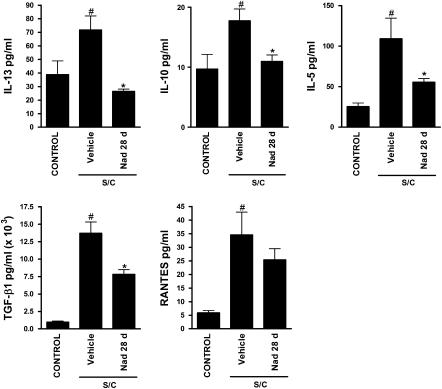
The total cell count in BAL was greatly increased in antigen-challenged immunized BALB/cJ mice compared with saline-challenged mice (Figure 2A). Chronic administration of the selective β2-adrenoceptor antagonist ICI 118,551 (28 d), or the nonselective β-adrenoceptor antagonist nadolol (13) (28 d), significantly reduced total cell counts in antigen-challenged mice (Figure 2A). BAL eosinophil numbers were also greatly increased in antigen-challenged mice compared with saline-challenged mice (Figure 2B), and again, chronic administration with ICI 118,551 or nadolol significantly reduced BAL eosinophil numbers (Figure 2B). A similar effect was observed by a second laboratory using a different strain of mice (C57BL/6J instead of BALB/cJ and a different sensitization and challenge protocol; see Materials and Methods), with total cell counts reduced by approximately 80% (from 1.4 × 105 ± 6.5% to 0.3 × 105 ± 11%) and eosinophils reduced by approximately 90% (from 1.0 × 105 ± 9.6% to 0.1 × 105 ± 38.6%) (n = 3, P < 0.05 for each comparison) after chronic administration of ICI 118,551 (14 d). These findings suggest that chronic administration of β-blockers modifies eosinophilic airway inflammation in a murine model of asthma. Antigen-challenged mice also had elevated levels of the cytokines IL-13, IL-10, IL-5, TGF-β1, and RANTES in BAL compared with saline-challenged mice (Figure 3). Consistent with the changes in cellular inflammation, chronic administration of nadolol resulted in a reduction in the levels of the cytokines IL-13, IL-10, IL-5, and TGF-β1 (Figure 3).
Figure 2.
Effects of chronic administration of beta-blockers on cell count and eosinophils in bronchoalveolar lavage (BAL) of antigen-sentized and -challenged BALB/cJ mice. (A) Total cell count and (B) eosinophil numbers in BAL from saline-challenged mice (CONTROL), antigen-challenged mice (S/C) administered vehicle, the selective beta2-adrenoceptor antagonist ICI 118,551 for 28 days (ICI 28 d), or the nonselective beta-adrenoceptor antagonist nadolol for 28 days (Nad 28 d) (Figure 1, protocol 1). BAL was collected 24 hours after the last challenge. Values are the means ± SEM of data from 6 to 12 mice in each group (n = 6–12). #P < 0.05, significantly different compared with saline-challenged mice; *P < 0.05, significantly different compared with antigen-challenged mice.
Figure 3.
Effects of chronic administration of nadolol on cytokines levels in the BAL of BALB/cJ mice. The concentration of IL-13, IL-10, IL-5, TGF-β1, and RANTES was measured by multiplex enzyme-linked immunosorbent assay analysis of BAL harvested 24 hours after the last challenge from saline-challenged mice (CONTROL), antigen-challenged mice (S/C) administered vehicle or nadolol for 28 days (Nad 28 d) (Figure 1, protocol 2). Values are the means ± SEM of data from seven to eight mice in each group (n = 7–8). #P < 0.05, significantly different compared with saline-challenged mice; *P < 0.05, significantly different compared with antigen-challenged mice.
Chronic Administration of Beta-Blockers Attenuates Airway Epithelial Cell Mucin Production
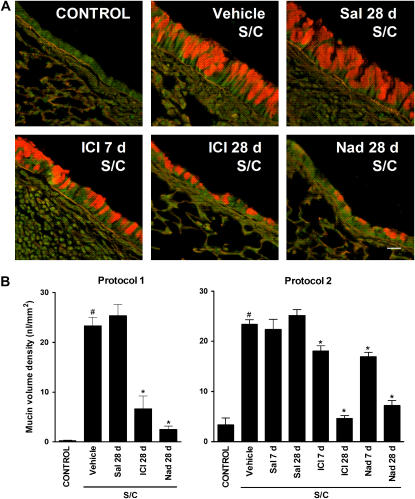
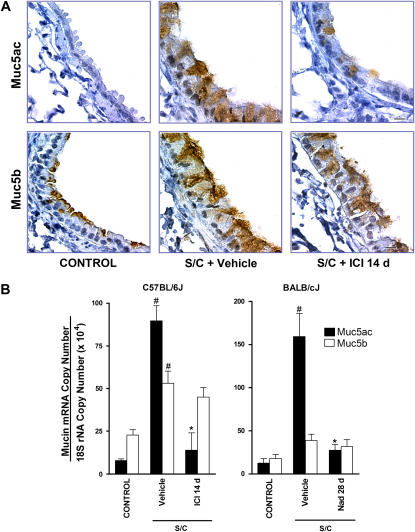
To determine whether chronic administration of beta-blockers affects mucin content, sections from the axial bronchi of experimental mice were assessed using PAFS staining. Minimal intracellular mucin was observed in saline-challenged mice, whereas in antigen-challenged mice there was increased mucin content and airway epithelial cell height (mucous metaplasia) (Figure 4). Within the antigen-challenged mouse group, chronic administration of the beta2-adrenoceptor agonist salbutamol had no significant effect on mucous metaplasia (Figure 4). However, chronic administration of ICI 118,551 or nadolol for 28 days decreased mucin content and partially reversed the changes in airway epithelial cell morphology (Figure 4). A similar effect was observed in antigen-challenged C57BL/6J mice, with mucin volume density reduced by more than 90% from 14.7 nl/mm2 ± 6.3% to 1.2 nl/mm2 ± 55.6% (n = 3, P < 0.05) after chronic administration of ICI 118,551 (14 d).
Figure 4.
Effects of chronic administration of beta-adrenoceptor ligands on mucin content in the airway epithelium. Mucin content in the airway epithelia of BALB/cJ mice was measured using periodic acid fluorescent Schiff from (A) saline-challenged mice (CONTROL), antigen-challenged mice (S/C) administered vehicle, the beta2-adrenoceptor agonist salbutamol for 28 days (Sal 28 d), the selective beta2-adrenoceptor antagonist ICI 118,551 for 7 or 28 days (ICI 7 or 28 d), or the nonselective beta-adrenoceptor antagonist nadolol for 28 days (Nad 28 d) (Figure 1, protocol 1). Scale bar, 20 μm. (B) Morphometric quantification of the mucin volume density was assessed from the various groups (left panel using protocol 1 of Figure 1). A second set of experiments was performed to assess the effect of beta-adrenoceptor ligands administration during the challenge phase only (right panel using protocol 2 of Figure 1). Values are the means ± SEM of data from 3 to 10 mice in each group (n = 3–10). #P < 0.05, significantly different compared with saline-challenged mice; *P < 0.05, significantly different compared with antigen-challenged mice.
Chronic Administration of Beta-Blockers during the Challenge Phase Alone Reduces Airway Inflammation and Mucin Production
In our initial experiments, the 28-day administration of beta-adrenoceptor ligands (salbutamol, ICI 118,551, or nadolol) occurred during both the OVA sensitization and challenge phases (Figure 1, protocol 1). To determine which phase is critically affected by beta-blockers, a second set of experiments was performed in which exposure to the beta-adrenoceptor ligands was begun after completion of the sensitization process (Figure 1, protocol 2). Administration of beta-blockers for 28 days beginning after the completion of sensitization produced similar reductions in mucous metaplasia (compare Figure 4B, protocols 1 and 2), thus ruling out interference by the beta-adrenoceptor ligands with the sensitization process as a cause of the observed decreases in mucous metaplasia. Furthermore, the administration of ICI 118,551 for 7 days beginning after the completion of sensitization reduced BAL total cell counts from 10.4 × 105 ± 13.6% to 5.4 × 105 ± 16.6% (n = 5–12, P < 0.05), a reduction very similar in degree to that observed with the administration of beta-blockers for 28 days during both sensitization and challenge (Figure 2). Also, the results with C57BL/6J mice, in which exposure to ICI 118,551 occurred after completion of the sensitization process (Figure 1, protocol 3), parallel the results observed in the BALB/cJ mice. Surprisingly, 7 days of beta-blocker during the challenge phase alone did not reduce mucous metaplasia to the same extent as 28 days of beta-blocker during the challenge phase (Figure 4B, protocol 2). Decreased mucin production was confirmed by immunohistochemical staining in C57BL/6J mice (Figure 5A) and by quantification of Muc5ac transcripts in both C57BL/6J and BALB/cJ mice (Figure 5B). Muc5ac is the predominant mucin upregulated in airway epithelial cells in patients with asthma and in the ovalbumin mouse models of asthma (21, 22). The results show that Muc5ac staining and transcripts are markedly increased in antigen-challenged mice compared with saline-challenged mice, but both are decreased in antigen-challenged mice treated with ICI 118,551 or nadolol (28 d) (Figure 5). We also performed immunohistochemical staining (Figure 5A) and quantification of transcripts for Muc5b (Figure 5B). This mucin does not change substantially in antigen-induced mucous metaplasia (21, 22), and, as expected, antigen sensitization and challenge produced only a small change in Muc5b. By immunohistochemical staining, Muc5b was apparent at the apical pole of epithelial cells in saline-challenged mice, became redistributed throughout the distended cytoplasm in antigen-challenged mice, and did not change appreciably in antigen-challenged mice treated with ICI 118,551 (Figure 5).
Figure 5.
Effect of chronic administration of beta-blockers on Muc5ac and Muc5b expression. (A) Muc5ac and Muc5b glycoprotein expression was assessed in C57BL/6J mice using immunohistochemical staining of saline-challenged mice (CONTROL), and antigen-challenged mice administered vehicle (S/C + vehicle) or ICI 118,551 for 14 days (S/C + ICI 14 d) (Figure 1, protocol 3). Scale bar, 10 μm. (B) Muc5ac and Muc5b transcripts were quantified by using quantitative PCR of RNA from the lungs of both C57BL/6J (left panel) and BALB/cJ (right panel) mice. Saline-challenged mice (CONTROL), and antigen-challenged mice (S/C) were administered vehicle or ICI 118,551 for 14 days (ICI 14 d) (Figure 1, protocol 3) or nadolol for 28 days (Nad 28 d) (Figure 1, protocol 1). Values are the means ± SEM of data from three to five mice in each group (n = 3–5). #P < 0.05, significantly different compared with saline-challenged mice; *P < 0.05 significantly different compared with antigen-challenged mice.
DISCUSSION
Based on the paradigm shift that occurred in the treatment of heart failure with regard to beta-adrenoceptor drugs, we hypothesized that chronic beta-blocker treatment may be beneficial in asthma (12, 15). Here we report that chronic treatment with “beta-blockers,” a class of drugs currently contraindicated in asthma, reduces airway inflammation in a murine model of asthma. A characteristic feature of asthma is the airway infiltration by inflammatory cells such as lymphocytes and eosinophils (16, 17). These cells produce a wide range of inflammatory mediators and cytotoxic proteins that are responsible for the perpetuation of airway inflammation (16, 17). Our results show that chronic treatment with nadolol or ICI 118,551 reduced the inflammatory cells in the BAL of antigen-challenged mice (Figure 2), and nadolol also reduced the levels of the cytokines IL-13, IL-10, IL-5, and TGF-β1 (Figure 3). The approximately 50% reduction in eosinophils in BAL was comparable to what we had previously reported, although in that study the reduction did not reach statistical significance (P = 0.07) (15). The effect of beta-blockers on BAL cellularity was not confined to one strain of mice, since it was observed in both BALB/cJ and C57BL/6J mice. The results with C57BL/6J mice were performed by another laboratory using a slightly different sensitization and challenge protocol. Thus the effect of beta-blockers appears to be robust and relatively insensitive to experimental conditions.
The reduction in inflammatory cells and cytokines caused by chronic administration of beta-blockers suggested that target effects of airway inflammation might also be reduced by this drug treatment. We therefore asked whether chronic administration of beta-blockers affected airway epithelial cell morphology and mucin content (17). The airway epithelium is a mechanical barrier that protects the underlying tissue from the external environment (23), but also shows great plasticity in structure and function (24–26). In asthma, the airway epithelium contains increased numbers of goblet cells filled with mucus, which contributes significantly to airflow obstruction and asthma morbidity (24, 26–28). At a molecular level, up-regulation of Muc5ac gene expression is the central event in mucous metaplasia (21). Chronic treatment with beta-blockers produced a marked time-dependent decrease in goblet cell numbers and mucin content of the airway epithelium (Figures 4 and 5). The effect of nadolol showed selectivity for Muc5ac staining and mRNA, as Muc5b, a polymeric mucin not substantially involved in the antigen-induced mucous metaplasia, was not affected by ICI 118,551 or nadolol administration (Figure 5) (22). The reduction in mucous metaplasia was not due to interference with the sensitization process, as the results were the same when treatment was begun after the sensitization process was complete (Figure 4, and the reduction in mucous metaplasia observed in the C57BL/6J mice). Furthermore, there was no significant change in serum IgE levels after chronic nadolol treatment (data not shown), which also suggests a lack of interference with the sensitization process.
The fact that the principal effect of beta-blockers is exerted during the challenge rather than the sensitization phase of our asthma models, and that the reduction in mucous metaplasia from beta-blockade is disproportionate to the reduction in lung inflammatory cells, suggests that beta-blockers exert an important effect on the epithelium itself. Supporting this hypothesis is the fact that chronic exposure to beta-blockers has been shown to produce a marked increase in beta2-adrenoceptor numbers primarily in airway epithelial cells (29), and that overexpression of the beta2-adrenoceptors in the airway epithelium reduces AHR (30). In our murine model of asthma, AHR reduction does parallel the reduction in BAL cellularity and also the mucin data to an extent. However, there are also differences. In experiments run in parallel to these but published separately (29), the observed reduction in AHR is maximal after 7 days of treatment with a beta-blocker (29) and stays reduced to the same extent at 28 days, thus following a time course similar to the cells and eosinophils in BAL, while the mucous metaplasia, though significantly reduced at 7 days, continues to decline further with 28 days of beta-blocker treatment. Comparing the effects of 7 days versus 28 days of beta-blocker treatment suggests that the reduction in mucous metaplasia from beta-blockade does not reach steady state after 7 days and that there are complex effects on epithelial cell signaling (24, 26). Mucous metaplasia is a cardinal feature of the asthma phenotype that is thought to play a central role in death from asthma (24, 25, 31). Recent studies also suggest that mucin hypersecretion can contribute significantly to AHR (27, 32, 33). Thus, the finding that chronic administration of beta-blockers reduces mucin content in airway epithelial cells suggests a possible identification of a cell type involved in the decreased AHR that we previously observed (15).
Together, these observations of the anti-inflammatory effect of beta-blockers in a murine model of asthma suggest that individuals with asthma may benefit from treatment with such drugs given their ability to reduce some indices of airway inflammation and to further reduce mucous metaplasia. Indeed, in a small (10 patients) clinical trial, treatment of subjects with mild asthma with nadolol for 9 weeks resulted in a dose-dependent increase in the concentration of methacholine required to produce a 20% fall in forced expiratory volume at 1 second (PC20 methacholine value) (34).
In addition to investigating the differences we observed on mucous metaplasia between 28 and 7 days of treatment, future studies will explore differences in the changes produced by nadolol and other beta-blockers. So far, different effects on airway resistance have been observed for various beta-blockers (15), and whether all beta-blockers confer equal benefit in asthma therapy would be a subject of importance. Within the limitations of extrapolating from a murine model of asthma, these results provide a second disease model (besides heart failure) in which compounds producing an acutely detrimental effect may provide a therapeutically beneficial effect with chronic administration, and indicate that the chronic effect of drugs cannot be predicted from their acute effects (11, 12). Furthermore, similar to the paradigm shift in the treatment of heart failure, our current results with beta-blockers could potentially lead to another paradigm shift in the treatment of another important disease, asthma.
Acknowledgments
The authors thank Sir James W. Black for his support and scientific critique of this manuscript.
This work was supported by the Sandler Program for Asthma Research (R.A.B.) and the National Institutes of Health grant R01HL72984 (B.F.D.).
Originally Published in Press as DOI: 10.1165/rcmb.2007-0279RC on December 20, 2007
Conflict of Interest Statement: L.P.N. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. O.O. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.P. is a shareholder in Inverseon, Inc. J.M.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Z.A.-A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.B.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.E. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. B.J.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.J.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. B.F.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.A.B. is scientific founder and a shareholder of Inverseon, Inc.
References
- 1.American Lung Association's Epidemiology and Statistics Unit, Best Practices and Program Services. Trends in asthma morbidity and mortality. 2002. (http://www.lungusa.org, accessed May 2007)
- 2.Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest 2006;129:15–26. [DOI] [PubMed] [Google Scholar]
- 3.Bond RA, Spina D, Parra S, Page C. Getting to the heart of asthma: can beta-blockers be used to treat asthma? Pharmacol Ther (In press) [DOI] [PubMed]
- 4.Lechat P, Packer M, Chalon S, Cucherat M, Arab T, Boissel JP. Clinical effects of beta-adrenergic blockade in chronic heart failure: a meta-analysis of double-blind, placebo-controlled, randomized trials. Circulation 1998;98:1184–1191. [DOI] [PubMed] [Google Scholar]
- 5.Hall SA, Cigarroa CG, Marcoux L, Risser RC, Grayburn PA, Eichhorn EJ. Time course of improvement in left ventricular function, mass and geometry in patients with congestive heart failure treated with beta-adrenergic blockade. J Am Coll Cardiol 1995;25:1154–1161. [DOI] [PubMed] [Google Scholar]
- 6.Maack C, Cremers B, Flesch M, Hoper A, Sudkamp M, Bohm M. Different intrinsic activities of bucindolol, carvedilol and metoprolol in human failing myocardium. Br J Pharmacol 2000;130:1131–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson JL, Krause-Steinrauf H, Goldman S, Clemson BS, Domanski MJ, Hager WD, Murray DR, Mann DL, Massie BM, McNamara DM, et al. Failure of benefit and early hazard of bucindolol for Class IV heart failure. J Card Fail 2003;9:266–277. [DOI] [PubMed] [Google Scholar]
- 8.Witchitz S, Cohen-Solal A, Dartois N, Weisslinger N, Juste K, Darmon JY. Treatment of heart failure with celiprolol, a cardioselective beta blocker with beta-2 agonist vasodilatory properties. The CELICARD Group. Am J Cardiol 2000;85:1467–1471. [DOI] [PubMed] [Google Scholar]
- 9.Singh BN, Whitlock RM, Comber RH, Williams FH, Harris EA. Effects of cardioselective beta adrenoceptor blockade on specific airways resistance in normal subjects and in patients with bronchial asthma. Clin Pharmacol Ther 1976;19:493–501. [DOI] [PubMed] [Google Scholar]
- 10.Boskabady MH, Snashall PD. Bronchial responsiveness to beta-adrenergic stimulation and enhanced beta-blockade in asthma. Respirology 2000;5:111–118. [DOI] [PubMed] [Google Scholar]
- 11.Bond RA. Can intellectualism stifle scientific discovery? Nat Rev Drug Discov 2002;1:825–829. [DOI] [PubMed] [Google Scholar]
- 12.Bond RA. Is paradoxical pharmacology a strategy worth pursuing? Trends Pharmacol Sci 2001;22:273–276. [DOI] [PubMed] [Google Scholar]
- 13.Bond RA, Leff P, Johnson TD, Milano CA, Rockman HA, McMinn TR, Apparsundaram S, Hyek MF, Kenakin TP, Allen LF, et al. Physiological effects of inverse agonists in transgenic mice with myocardial overexpression of the beta 2-adrenoceptor. Nature 1995;374:272–276. [DOI] [PubMed] [Google Scholar]
- 14.Bond RA, Evans KJ, Callaerts-Vegh Z. From inverse agonism to paradoxical pharmacology. In: Inverse agonism. Ijzerman AP, editor. Oxford, UK: Elsevier Science; 2003. pp. 27–37.
- 15.Callaerts-Vegh Z, Evans KL, Dudekula N, Cuba D, Knoll BJ, Callaerts PF, Giles H, Shardonofsky FR, Bond RA. Effects of acute and chronic administration of beta-adrenoceptor ligands on airway function in a murine model of asthma. Proc Natl Acad Sci USA 2004;101:4948–4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wills-Karp M, Karp CL. Biomedicine. Eosinophils in asthma: remodeling a tangled tale. Science 2004;305:1726–1729. [DOI] [PubMed] [Google Scholar]
- 17.Barnes PJ, Chung KF, Page CP. Inflammatory mediators of asthma: an update. Pharmacol Rev 1998;50:515–596. [PubMed] [Google Scholar]
- 18.Evans KL, Bond RA, Corry DB, Shardonofsky FR. Frequency dependence of respiratory system mechanics during induced constriction in a murine model of asthma. J Appl Physiol 2003;94:245–252. [DOI] [PubMed] [Google Scholar]
- 19.Evans CM, Williams OW, Tuvim MJ, Nigam R, Mixides GP, Blackburn MR, DeMayo FJ, Burns AR, Smith C, Reynolds SD, et al. Mucin is produced by clara cells in the proximal airways of antigen-challenged mice. Am J Respir Cell Mol Biol 2004;3:382–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shekels LL, Lyftogt C, Kieliszewski M, Filie JD, Kozak CA, Ho SB. Mouse gastric mucin: cloning and chromosomal localization. Biochem J 1995;311:775–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ordonez CL, Khashayar R, Wong HH, Ferrando R, Wu R, Hyde DM, Hotchkiss JA, Zhang Y, Novikov A, Dolganov G, et al. Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. Am J Respir Crit Care Med 2001;163:517–523. [DOI] [PubMed] [Google Scholar]
- 22.Young HWJ, Williams OW, Chandra D, Bellinghausen LK, Pérez G, Suárez A, Tuvim MJ, Roy MG, Alexander SN, Moghaddam SJ, et al. Central role of muc5ac expression in mucous metaplasia and its regulation by conserved 5′ elements. Am J Respir Cell Mol Biol 2007;37:273–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogers DF. Airway mucus hypersecretion in asthma: an undervalued pathology? Curr Opin Pharmacol 2004;4:241–250. [DOI] [PubMed] [Google Scholar]
- 24.Fahy JV. Goblet cell and mucin gene abnormalities in asthma. Chest 2002;122:320S–326S. [DOI] [PubMed] [Google Scholar]
- 25.Williams OW, Sharafkhaneh A, Kim V, Dickey BF, Evans CM. Airway mucus: from production to secretion. Am J Respir Cell Mol Biol 2006;34:527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holgate ST, Holloway J, Wilson S, Bucchieri F, Puddicombe S, Davies DE. Epithelial-mesenchymal communication in the pathogenesis of chronic asthma. Proc Am Thorac Soc 2004;1:93–98. [DOI] [PubMed] [Google Scholar]
- 27.Kuperman DA, Huang X, Koth LL, Chang GH, Dolganov GM, Zhu Z, Elias JA, Sheppard D, Erle DJ. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med 2002;8:885–889. [DOI] [PubMed] [Google Scholar]
- 28.Zhen G, Park SW, Nguyenvu LT, Rodriguez MW, Barbeau R, Paquet AC, Erle DJ. IL-13 and epidermal growth factor receptor have critical but distinct roles in epithelial cell mucin production. Am J Respir Cell Mol Biol 2007;36:244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin R, Peng H, Nguyen LP, Dudekula NB, Shardonofsky F, Knoll BJ, Parra P, Bond RA. Changes in β2-adrenoceptor densities and other signaling proteins produced by chronic administration of ‘beta–blockers’ in a murine asthma model. Pulm Pharmacol Ther (In press) [DOI] [PMC free article] [PubMed]
- 30.McGraw DW, Forbes SL, Mak JC, Witte DP, Carrigan PE, Leikauf GD, Liggett SB. Transgenic overexpression of beta(2)-adrenergic receptors in airway epithelial cells decreases bronchoconstriction. Am J Physiol Lung Cell Mol Physiol 2000;279:L379–L389. [DOI] [PubMed] [Google Scholar]
- 31.Kuyper LM, Pare PD, Hogg JC, Lambert RK, Ionescu D, Woods R, Bai TR. Characterization of airway plugging in fatal asthma. Am J Med 2003;115:6–11. [DOI] [PubMed] [Google Scholar]
- 32.Wang SZ, Rosenberger CL, Espindola TM, Barrett EG, Tesfaigzi Y, Bice DE, Harrod KS. CCSP modulates airway dysfunction and host responses in an Ova-challenged mouse model. Am J Physiol Lung Cell Mol Physiol 2001;281:L1303–L1311. [DOI] [PubMed] [Google Scholar]
- 33.Agrawal A, Rengarajan S, Adler KB, Ram A, Ghosh B, Fahim M, Dickey BF. Inhibition of mucin secretion with MARCKS-related peptide improves airway obstruction in a mouse model of asthma. J Appl Physiol 2007;102:399–405. [DOI] [PubMed] [Google Scholar]
- 34.Hanania NA, Singh S, Eli-Wali R, Flashner M, Franklin AE, Garner WJ, Dickey BF, Parra S, Ruoss SJ, Shardonofsky F, et al. The safety and effects of the beta-blocker, nadolol, in mild asthma; an open-label pilot study. Pulm Pharmacol Ther (In press) [DOI] [PMC free article] [PubMed]