Abstract
MUC1 (MUC1 in humans and Muc1 in nonhuman species) is a transmembrane mucin-like glycoprotein expressed in epithelial cells lining various mucosal surfaces as well as hematopoietic cells. Recently, we showed that Muc1−/− mice exhibited greater inflammatory responses to Pseudomonas aeruginosa or its flagellin compared with their wild-type littermates, and our studies with cultured cells revealed that MUC1/Muc1 suppressed the Toll-like receptor (TLR) 5 signaling pathway, suggesting its anti-inflammatory role. Here we demonstrate that other TLR signaling (TLR2, 3, 4, 7, and 9) is also suppressed by MUC1/Muc1. The results from this study suggest that MUC1/Muc1 may play a crucial role during airway infection and inflammation by various pathogenic bacteria and viruses.
Keywords: MUC1/Muc1, Toll-like receptors, anti-inflammatory, NF-κB, TNF-α
CLINICAL RELEVANCE
This new finding that MUC1 can suppress the activity of all the Toll-like receptors has great potential for clinical applications in controlling excessive and prolonged airway inflammation during airway infection.
Pathogens entering the airway are trapped by airway surface fluid and removed from the lung by mucociliary clearance. Pathogens escaping this first-line defense, however, are encountered by both underlying epithelial cells and macrophages and recognized in a highly specific manner by a number of Toll-like receptors (TLRs) present in these cell types leading to airway inflammation (1).
MUC1 is a transmembrane mucin-like glycoprotein that is ubiquitously expressed on the apical surface of mucosal epithelial cells, including the mammary gland and respiratory, gastrointestinal, and reproductive tracts as well as on the hematopoietic cells (2, 3). Expression of MUC1 is extremely high in malignant cells and has been shown to be associated with tumorigenesis (4). In the respiratory tract, MUC1 is thought to serve as a physicochemical barrier against inhaled chemicals and particles, including pathogens (2). Recently, we showed that Muc1 is a specific binding site for Pseudomonas aeruginosa (5), and its binding and signaling are mediated though flagella and flagellin, respectively (6, 7). Our functional studies revealed that MUC1/Muc1 suppressed inflammatory responses induced by either P. aeruginosa or flagellin both in vivo and in vitro (8), suggesting the anti-inflammatory role of MUC1/Muc1 during airway bacterial infection, at least in part, through the suppression of TLR5 signaling.
In this study, we sought to examine whether MUC1/Muc1 has an ability to suppress other TLRs signaling. Our results showed that the suppressive effect of MUC1/Muc1 is not limited to TLR5 but applicable to other TLRs, suggesting an important role of MUC1/Muc1 during airway infection by inhaled bacteria or viruses.
MATERIALS AND METHODS
Reagents
All the reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise stated. Escherichia coli LPS (O111;B4) was re-extracted as previously described (9) and used as a TLR4 ligand. P. aeruginosa strain K (PAK) flagellin was purified as previously described (10) and used as a TLR5 ligand. All the other TLR ligands used in the present study were purchased commercially: the lipopeptide (S)-(2,3-bis(palmitoyloxy)-(2RS)-propyl)-N-palmitoyl-(R)-Cys-(S)-Ser(S)-Lys4-OH, 3HCl (Pam3Cys) for TLR2 (EMC Microcollection, Tübingen, Germany), Poly (I:C) for TLR3 (Invivogen, San Diego, CA), and Loxoribine for TLR7 (Invivogen). For TLR9 ligands, two pairs of CpG oligonucleotides were purchased from Invivogen and reconstituted in endotoxin-free water: (1) murine CpG oligonucleotide (ODN 1,826) 5′-TCCATGACGTTCCTGACGTT-3′ and murine non-CpG oligonucleotide (ODN 1,826 control) 5′-TCCATGAGCTTCCTGAGCTT-3′; (2) human CpG oligonucleotide (ODN M362) 5′-TCGTCGTCGTTCGAACGACGTTGAT-3′ and human non-CpG oligonucleotide (ODN M362 control) 5′-TGCTGCTGCTTGCAAGCAGCTTGAT-3′.
Plasmid Constructs
The ELAM-1-luciferase reporter plasmid (pELAM1-luc) was generated as described elsewhere (11) by cloning a fragment (−241 to −54 bp) of human E-selectin promoter into the pGL3 reporter plasmid (Promega, Madison, WI). All human MUC1 plasmid constructs were inserted into the mammalian expression vector pcDNA3.1 (Invitrogen, Carlsbad, CA). For the construction of the MUC1-ΔEC plasmid containing MUC1 transmembrane and cytoplasmic regions, two MUC1 fragments were amplified by PCR using human MUC1 in pcDNA3.1 (pMUC1 plasmid) as a template and ligated into pcDNA3.1. To amplify the first fragment containing signal peptide, PCR was performed with sense (T7) primer, 5′-TAATACGACTCACTATAGGG-3′, and antisense primer, 5′-CTCCTGCAGG GGTAGAGCTTGCATGACCAGAACC-3′ including the Sse83871 recognition sequence. For the second fragment containing cytoplasmic tail (CT), sense primer, 5′-CCAGTCTCCTGCAGGGGTGCCAGGCTGGGGCATCG-3′, including the Sse83871 recognition sequence and antisense primer, 5′-GATCAGCGGGTTTAAACGGGCC-3′, were used. The PCR products were digested with HindIII/Sse83871 and Sse83871/EcoRI, respectively, and then the two fragments were ligated into the HindIII/EcoRI-digested pcDNA3.1. The MUC1-ΔCT plasmid containing the MUC1 extracellular and transmembrane regions was constructed by BamHI/KpnI digestion of the full-length MUC1 encoding plasmid, purification of the 3.3-kb fragment, and in-frame ligation with an oligonucleotide linker (sense primer, 5′-CATTGCCTTGGCTGTCTAG-3′; antisense primer, 5′-AATTCTAGACAGC-3′). Human TLR2 and human MD-2 were inserted into pcDNA3.1. Human TLR4 was inserted into pcDNA3-YFP (Invitrogen). Human TLR5 inserted into pEF6/V5-His (Invitrogen) was kindly provided by Andrew T. Gewirtz (Emory University, Atlanta, GA) (10). Expression plasmids for human TLR3, TLR7, and TLR9 were constructed into the pFLAG-CMV1 vector as previously described (12). All plasmid DNAs were isolated with Endo-free Maxi-prep columns from Qiagen (Valencia, CA).
Cell Lines, Macrophages, and Tracheal Surface Epithelial Cells
The RAW264.7 murine macrophage cell line and human embryonic kidney (HEK) 293T cell line were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Gibco-BRL, Gaithersburg, MD), 100 U/ml penicillin, and 100 μg/ml streptomycin (Cellgro; Mediatech Inc., Herndon, VA). Alveolar macrophages (AM) and peritoneal macrophages (PM) were obtained from Muc1+/+ and Muc1−/− FVB mice (13) as previously described (8). Muc1−/− FVB mice were backcrossed with Muc1+/+ FVB mice (Jackson Laboratory, Bar Harbor, ME) at least five times before maintaining both Muc1−/− and their wild-type (WT) littermates used in this experiment. PMs were harvested after treating mice intraperitoneally with 3% thioglycollate (Becton-Dickinson, San Jose, CA) for 3 days, and erythrocytes were lysed with 10 mM Tris-HCl (pH 7.2) containing 150 mM NH4Cl. The AMs and PMs were seeded in 24-well tissue culture plates at 4.5 × 105, 1.0 × 105 cells/well, respectively, in DMEM supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin and allowed to adhere for 24 hours before treatment with various TLR ligands. Mouse tracheal surface epithelial (TSE) cells from Muc1+/+ and Muc1−/− FVB mice were harvested and cultured at an air–liquid interface as previously described (14). All animal experiments were conducted in accordance with the guidelines provided by the Institutional Animal Care and Use Committee of the Lovelace Respiratory Research Institute.
Enzyme-Linked Immunosorbent Assay
Mouse TNF-α and KC (mouse ortholog of human IL-8) were quantified by enzyme-linked immunosorbent assay (ELISA) using commercially available antibodies: anti-mouse TNF-α (eBioscience, San Diego, CA); anti-mouse KC (R&D Systems, Minneapolis, MN); recombinant mouse TNF-α (eBioscience), recombinant mouse KC (eBioscience), and peroxidase-labeled streptavidin Ab (KPL, Gaithersburg, MD). The XTT assay (Roche Applied Science, Indianapolis, IN) was performed to normalize the variations in cell density among the culture wells. A standard ELISA curve was generated for each plate.
NF-κB Reporter Luciferase Assay
HEK293T cells were plated in 48-well tissue culture plates (5.2 × 104 cells/well) and incubated for 24 hours. Cells were transfected with 100 ng/well pELAM-luc reporter construct and 5 ng/well phRL-TK (a Renilla luciferase control reporter vector by Promega) using 1.2 μL/well Lipofectamine 2,000 according to the manufacturer's protocol (Invitrogen). phRL-TK was used to normalize the transfection efficiency. In addition, the cells were also transfected with 10 ng/well TLR2 pcDNA3.1, 50 ng/well FLAG-TLR3, 60 ng/well TLR4-YFP, and 30 ng/well human MD-2 pcDNA3.1, 25 ng/well TLR5, 50 ng/well FLAG-TLR7 or 50 ng/well FLAG-TLR9 together with 50, 100, or 200 ng/well full-length MUC1 plasmid (pMUC1). The empty vector pcDNA3.1 (Invitrogen) was used as a control to normalize the total DNA amount for all of the transfection reactions. Twenty-four hours after transfection, the cells were treated with various TLR ligands or “nonspecific” agents (EGF and PMA) for another 24 hours or 6 hours before lysing the cells with a passive lysis buffer (Promega) followed by assaying for firefly and Renilla luciferase activities using the Dual-luciferase Reporter System (Promega). The luciferase activity in each sample was quantified by L-Max II (Molecular Devices, Sunnyvale, CA).
Transfection of pMUC1 and Verification of MUC1 Expression
Cell suspension of RAW264.7 cells (5 × 105 cells/well) were transfected with 1 μg of the full-length MUC1 plasmid (pMUC1), EC deleted MUC1 plasmid (pMUC1-ΔEC), or CT deleted MUC1 plasmid (pMUC1-ΔCT) using 3.5 μl/well of Lipofectamine 2000 (Invitrogen) in 24-well tissue culture plates. DNA3.1 was used to normalize the total DNA amount for all of the transfection reactions. After 6 hours, the cells were washed twice with culture medium and incubated for 18 hours before stimulation with TLR ligands. Cells transfected with pMUC1 were lysed using an RIPA buffer, pH 7.4, containing 1.0% NP-40, 0.1% sodium deoxycholate, 150 mM NaCl, 50 mM Tris-HCl, pH7.5, and 1.0% protease inhibitor cocktail. The lysates were subjected to SDS-PAGE followed by immunoblot analysis with a GP1.4 antibody (Biomeda, Foster City, CA). A band located at greater than 250 kD was identified as the large subunit of MUC1 using GP1.4 antibody that recognizes the EC domain of MUC1.
Statistical Analysis
Differences among the groups were assessed by comparing their means using either Student's t test or ANOVA and were considered significant if P < 0.05.
RESULTS
Airway Epithelial Cells and Macrophages from Muc1−/− Mice Exhibit Enhanced Pro-Inflammatory Cytokine Production in Response to Various TLR Ligands Compared with Those from Their WT Litter Mates
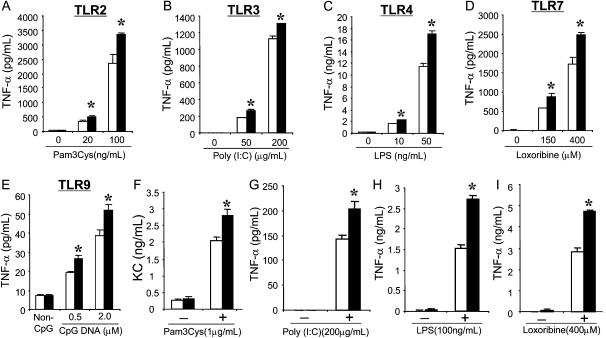
We recently showed that both cultured TSE cells and AMs from Muc1−/− mice (FBV background) released greater amounts of TNF-α and KC compared with those from their WT littermates in response to P. aeruginosa flagellin, a specific TLR5 ligand (8). In this experiment, we determined whether the enhanced pro-inflammatory response by the Muc1-deficient cells is also present in response to other TLR ligands. Three different cell types from both Muc1−/− mice and their WT littermates were cultured and treated with various TLR ligands, and the release of their major inflammatory mediators was measured: TNF-α for both AMs and PMs and KC for TSE cells. As shown in Figures 1A–1E, PM from Muc1−/− mice produced significantly higher levels of TNF-α in response to Pam3Cys (TLR2), Poly I:C (TLR3), LPS (TLR4), loxoribine (TLR7), and CpG DNA (TLR9). The whole experiment was repeated using a different mouse strain (C57BL/6 background), and similar results were obtained (data not shown). In addition, two major inflammatory cells in the lung, namely, TSE cells and AMs, also responded in a similar way (i.e., the enhanced release of KC and TNF-α from Muc1−/− TSE cells and AMs, respectively) (Figures 1F and 1G–1I). Baseline mRNA levels of all these TLRs derived from PMs were not different between Muc1−/− mice and their WT littermates (data not shown). These results indicate that inflammatory cells from Muc1−/− mice exhibit greater pro-inflammatory responses not only to TLR5 but also to other TLR ligands, suggesting that the presence of Muc1 in these cells suppresses the pro-inflammatory responses resulting from TLR activation.
Figure 1.
Effects of various Toll-like receptor (TLR) ligands on the release of inflammatory mediators from cells derived from Muc1−/− mice or their wild-type littermates. Three different types of cells obtained from Muc1+/+ (open bars) and Muc1−/− (solid bars) mice were treated with the specific TLR ligands for 24 hours, and the aliquots of spent media were subjected to enzyme-linked immunosorbent assay (ELISA) as described in Materials and Methods. (A–E) Peritoneal macrophages. (F) Confluent tracheal surface epithelial cells grown at an air–liquid interface. (G–I) Alveolar macrophages. Each bar represents a mean ± SEM (n = 4). *P < 0.05, compared with Muc1+/+. The results are representative of three independent experiments.
MUC1 Suppresses NF-κB Activation Induced by TLR Ligands
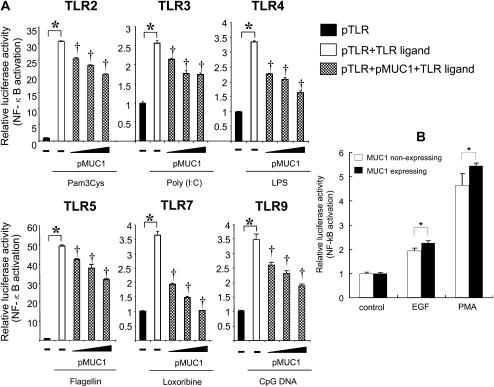
While there are a number of TLRs recognizing their specific ligands, referred to as pathogen-associated molecular patterns (PAMPs), all TLR signaling, either MyD88-dependent or TRIF-dependent, converges on NF-κB (15), which is a key regulator for the production of the major inflammatory mediators including KC and TNF-α (1). Therefore, we examined whether the presence of MUC1 suppresses the activation of NF-κB induced by activation of various TLRs. HEK293T cells were transfected with three different plasmids: (1) pELAM1-luc to measure the activation of NF-κB; (2) an expression plasmid containing either human TLR2, 3, 4, 5, 7, or 9; and (3) varying concentrations of pMUC1 or pcDNA3.1 as a control. The transfected cells were treated with various TLR ligands, and luciferase activity was measured. Our results showed that NF-κB activation was drastically induced not only by flagellin (Figure 2D), a specific TLR5 ligand, but also by all TLR ligands tested. Furthermore, the increased NF-κB activation was suppressed by MUC1 in a dose-dependent fashion (Figures 2A–2F). Activation of NF-κB by the TLR ligands and its inhibition by MUC1 were confirmed by measuring degradation of IkB-α (data now shown). Thus, the results suggest that the reduced pro-inflammatory responses in cells derived from Muc1+/+ mice compared with those from Muc1−/− mice was most likely due to the suppression of TLR-mediated NF-κB activation by MUC1. An alternative explanation might be the dose-dependent, suppressive effect of pMUC1 on the expression of TLRs. This possibility, however, was ruled out by using pFlag-TLR9 as a representative whose expression was not affected when varying concentrations of pMUC1 were co-transfected (data not shown). Finally, to determine whether or not the suppressive effect of MUC1 on TLR-mediated NF-κB activation is “nonspecific,” HEK293 cells stably expressing MUC1 or its empty vector were transiently transfected with pELAM1-luc before treatment with either EGF or PMA, and the degree of NF-κB activation by these agents was measured by luciferase assay. As shown in Figure 2B, activation of NF-κB by either of these non–TLR-mediating agents was not affected by the expression of MUC1, indicating that the inhibitory effect of MUC1 on TLR signaling was rather specific.
Figure 2.
Effect of MUC1 overexpression on TLR-induced NF-κB activation in HEK293T cells. (A) HEK293T cells were transiently transfected with four different plasmids as described in Materials and Methods: (1) pELAM-luc reporter construct, (2) phRL-TK, (3) one of the pTLRs, and (4) varying concentrations of pMUC1 (0, 50, 100, 200 ng per well). pcDNA empty vector was used to normalize the total amount of DNA added to each well. Twenty-four hours after transfection, the cells were treated for an additional 24 hours with the following TLR ligands: Pam3Cys (1 μg/ml), Poly (I:C) (300 μg/ml), LPS (100 ng/ml); flagellin (10 ng/ml), Loxoribine (300 μM), or CpG DNA (1 μM). Each bar represents a mean ± SEM (n = 3). *P < 0.05, compared with control (without TLR ligand); †P < 0.05, compared with control (without pMUC1). The results are representative of three independent experiments. (B) HEK293 cells stably transfected with or without MUC1 were transiently transfected with pELAM-luc and phRL-TK for 24 hours before treatment with EGF (100 ng/ml) or PMA (100 nM) for 6 hours. Cell lysates were prepared and subjected to luciferase assay as described in Materials and Methods. Each bar represents a mean ± SEM (n = 3). *P > 0.05. The results are representative of two independent experiments.
The Presence of MUC1 CT Is Required for the Anti-inflammatory Activity of MUC1 during TLR Activation
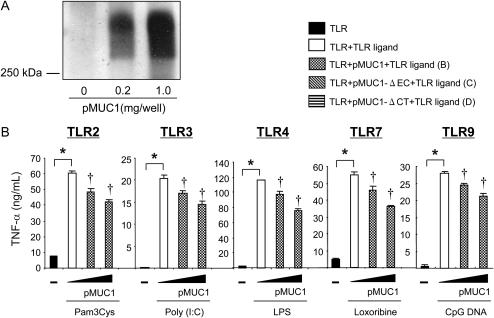
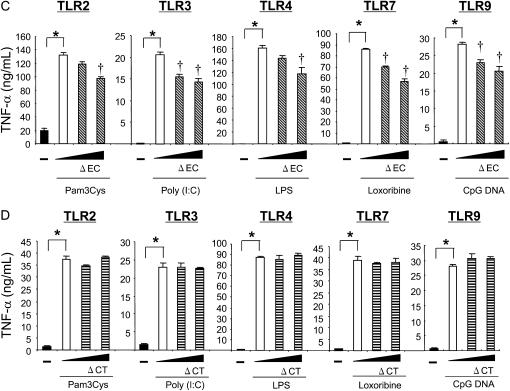
The amino acid sequences of MUC1 CT are highly conserved among species (16) and contain numerous binding sites for various signaling molecules or adaptor proteins (17). Our recent results showed that the presence of the CT domain is required for the suppression of IL-8 release by TLR5 in HEK293T cells treated with flagellin (8). To examine whether or not the CT domain is also required for the anti-inflammatory activity of MUC1 during TLR activation, we employed a mouse macrophage cell line RAW264.7, which endogenously expresses a relatively small amount of Muc1 while expressing all the TLRs except for TLR5 (data not shown). RAW264.7 cells were transiently transfected with pMUC1, pMUC1-ΔEC, or pMUC1-ΔCT before stimulation with various TLR ligands (notice that human MUC1 was expressed in this mouse cell line). First, we confirmed the expression of MUC1 in the cells transfected with pMUC1 using immunoblotting (Figure 3A). While the untransfected RAW264.7 cells responded to all the TLR ligands tested with a significant release of TNF-α, the cells transfected with varying concentrations of pMUC1 showed a dose-dependent suppression of the enhanced TNF-α release by each TLR ligand (Figure 3B). The anti-inflammatory responses of MUC1 were retained in the cells transfected with pMUC1-ΔEC (Figure 3C) but completely lost in the cells transfected with pMUC1-ΔCT (Figure 3D). These results indicate that the CT domain of MUC1 is required for the anti-inflammatory effect of MUC1 not only against TLR5 (8) but also all the other TLRs, suggesting crosstalk between MUC1 CT and TLRs as a potential mechanism for the anti-inflammatory effect of MUC1.
Figure 3.
Analysis of MUC1 domains responsible for suppression of TLR-induced TNF-α release in RAW264.7 cells. RAW264.7 cells were transiently transfected with 0, 0.2, 1.0 μg/well pMUC1 (B), pMUC1-ΔEC (C), and pMUC1-ΔCT (D) as described in Materials and Methods. pcDNA3.1 empty vector was used to normalize the total amount of DNA. Twenty-four hours after transfection, the cells were treated for 12 hours with various TLR ligands at the concentrations described in Figure 2. (A) The expression of MUC1 following pMUC1 transfection was verified by immunoblotting using GP1.4, an anti-MUC1 antibody that recognizes the EC domain. TNF-α levels in spent media were measured by ELISA. Each bar represents a mean ± SEM (n = 3). * Significantly different (P < 0.05) from control (no TLR ligand). † Significantly different (P < 0.05) from control (no pMUC1). The results are representative of three independent experiments.
DISCUSSION
Recently we demonstrated that the inflammatory responses mediated through the activation of TLR5 were suppressed by MUC1/Muc1 based on both in vivo and in vitro experiments (8). In this study, we determined whether the anti-inflammatory activity of MUC1/Muc1 is also applicable to other TLRs. Individual TLRs were stimulated using their specific ligands, while the levels of TLRs and MUC1/Muc1 were manipulated in three different cell culture systems. The results from this study clearly indicated that the anti-inflammatory activity of MUC1/Muc1 is not limited to TLR5 but also applies to other TLRs, including TLR2, 3, 4, 7, and 9.
How can a single molecule like MUC1/Muc1 suppress the function of a whole family of membrane receptors that possess totally different ligand specificities? Although these individual TLRs are different in their structure and ligand specificity, their signaling shares some common pathway. Upon activation by their specific ligands, all these TLRs form a TRAF6–TAK1 complex, eventually leading to NF-κB activation (15). Based on our results that MUC1/Muc1 suppresses NF-κB activation induced by activation of various TLRs (Figure 2), the site of interaction with MUC1/Muc1 appears to be between the TRAF6–TAK1 complex and NF-κB activation. Given that MUC1 CT contains multiple binding motifs for the proteins involved in TLR signaling, including PI3K, Shc, Src, β-catenin, GSK-3β, ErbB receptor family, PKCδ, PLCγ1, and Grb-2/Sos (17–19), it is possible that the anti-inflammatory activity of MUC1/Muc1 is mediated through one or a combination of these signaling molecules that interact with the common TLR signaling pathway. Recently, PI3K has been shown to suppress pro-inflammatory responses elicited by TLR2, 3, 4, 5, and 9 (20, 21), suggesting that it could be a mechanism driving the anti-inflammatory activity of MUC1/Muc1 during TLR5 activation. Our recent study, however, ruled out that possibility because we found that, while MUC1 had an ability to activate the PI3K–AKT pathway, the pharmacological inhibition of the PI3K activity failed to block the suppressive effect of MUC1/Muc1 on TLR-mediated inflammatory responses (22). Another candidate could be GSK-3β, which has been shown to suppress pro-inflammatory cytokine production through the regulation of a multitude of transcription factors, including NF-κB (23). Finally, it was shown that c-Src, a member of a family of nonreceptor tyrosine kinases, is involved in pro-inflammatory cytokine production induced by the activation of TLR2 and TLR4 in murine macrophage cell lines (24, 25). c-Src was shown to be involved also in TLR2- and TLR9-dependent NF-κB activation (26) as well as IL-1–induced, MyD88-dependent NF-κB activation (27). Therefore, it might be possible that the concentrations of cytosolic c-Src available for TLR signaling were reduced by overexpression of MUC1 CT through sequestration. Crosstalk between MUC1/Muc1 and TLR signaling in the context of the anti-inflammatory activity of MUC1/Muc1 during TLR activation is currently under investigation in our laboratory.
The results from this study may provide critical information in understanding how airway inflammation is resolved during airway infection. It is now well established that inhaled pathogens are detected by various TLRs present in the inflammatory cells, such as airway epithelial cells and macrophages (28), which in turn elicit inflammation by producing inflammatory mediators such as IL-8 and TNF-α to recruit neutrophils into the lung. We showed that inflammatory products such as neutrophil elastase and TNF-α stimulate the production of MUC1 (29, 30) whose levels on the cell surface are relatively low before inflammation (our unpublished data). The increased levels of MUC1 in these inflammatory cells will likely attenuate ongoing inflammation by suppressing further activation of TLR signaling. Thus, the expression of MUC1 mucin may be crucially important at the late stage of airway inflammation to prevent excessive and prolonged inflammation that would otherwise develop into chronic pulmonary diseases.
Acknowledgments
The authors thank Dr. Akinori Hisatsune (Kumamoto University Graduate School of Medicine and Pharmacy) for kindly providing pMUC1-ΔEC. They are also grateful to Dr. Erik Lillehoj (Department of Pediatrics, University of Maryland School of Medicine, Baltimore, MD) for his excellent review and Ms. Bonne Cleveland for editing this manuscript.
This work was supported by grants from the National Institutes of Health (RO1 HL-47125 and HL-81825 to K.C.K.).
Originally Published in Press as DOI: 10.1165/rcmb.2007-0336RC on December 13, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Basu S, Fenton MJ. Toll-like receptors: function and roles in lung disease. Am J Physiol Lung Cell Mol Physiol 2004;286:L887–L892. [DOI] [PubMed] [Google Scholar]
- 2.Gendler SJ. MUC1, the renaissance molecule. J Mammary Gland Biol Neoplasia 2001;6:339–353. [DOI] [PubMed] [Google Scholar]
- 3.Lillehoj ER, Kim KC. Airway mucus: its components and function. Arch Pharm Res 2002;25:770–780. [DOI] [PubMed] [Google Scholar]
- 4.Schroeder JA, Masri AA, Adriance MC, Tessier JC, Kotlarczyk KL, Thompson MC, Gendler SJ. MUC1 overexpression results in mammary gland tumorigenesis and prolonged alveolar differentiation. Oncogene 2004;23:5739–5747. [DOI] [PubMed] [Google Scholar]
- 5.Lillehoj EP, Hyun SW, Kim BT, Zhang XG, Lee DI, Rowland S, Kim KC. Muc1 mucins on the cell surface are adhesion sites for Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol 2001;280:L181–L187. [DOI] [PubMed] [Google Scholar]
- 6.Lillehoj EP, Kim BT, Kim KC. Identification of Pseudomonas aeruginosa flagellin as an adhesin for Muc1 mucin. Am J Physiol Lung Cell Mol Physiol 2002;282:L751–L756. [DOI] [PubMed] [Google Scholar]
- 7.Lillehoj EP, Kim H, Chun EY, Kim KC. Pseudomonas aeruginosa stimulates phosphorylation of the airway epithelial membrane glycoprotein Muc1 and activates MAP kinase. Am J Physiol Lung Cell Mol Physiol 2004;287:L809–L815. [DOI] [PubMed] [Google Scholar]
- 8.Lu W, Hisatsune A, Koga T, Kato K, Kuwahara I, Lillehoj EP, Chen W, Cross AS, Gendler SJ, Gewirtz AT, et al. Cutting edge: enhanced pulmonary clearance of Pseudomonas aeruginosa by Muc1 knockout mice. J Immunol 2006;176:3890–3894. [DOI] [PubMed] [Google Scholar]
- 9.Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol 2000;165:618–622. [DOI] [PubMed] [Google Scholar]
- 10.Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol 2001;167:1882–1885. [DOI] [PubMed] [Google Scholar]
- 11.Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem 1999;274:10689–10692. [DOI] [PubMed] [Google Scholar]
- 12.Schoenemeyer A, Barnes BJ, Mancl ME, Latz E, Goutagny N, Pitha PM, Fitzgerald KA, Golenbock DT. The interferon regulatory factor, IRF5, is a central mediator of toll-like receptor 7 signaling. J Biol Chem 2005;280:17005–17012. [DOI] [PubMed] [Google Scholar]
- 13.Spicer AP, Rowse GJ, Lidner TK, Gendler SJ. Delayed mammary tumor progression in Muc-1 null mice. J Biol Chem 1995;270:30093–30101. [DOI] [PubMed] [Google Scholar]
- 14.Lu W, Lillehoj EP, Kim KC. Effects of dexamethasone on Muc5ac mucin production by primary airway goblet cells. Am J Physiol Lung Cell Mol Physiol 2005;288:L52–L60. [DOI] [PubMed] [Google Scholar]
- 15.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol 2004;4:499–511. [DOI] [PubMed] [Google Scholar]
- 16.Park H, Hyun SW, Kim KC. Expression of MUC1 mucin gene by hamster tracheal surface epithelial cells in primary culture. Am J Respir Cell Mol Biol 1996;15:237–244. [DOI] [PubMed] [Google Scholar]
- 17.Singh PK, Hollingsworth MA. Cell surface-associated mucins in signal transduction. Trends Cell Biol 2006;16:467–476. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Lillehoj EP, Kim KC. Identification of four sites of stimulated tyrosine phosphorylation in the MUC1 cytoplasmic tail. Biochem Biophys Res Commun 2003;310:341–346. [DOI] [PubMed] [Google Scholar]
- 19.Zrihan-Licht S, Baruch A, Elroy-Stein O, Keydar I, Wreschner DH. Tyrosine phosphorylation of the MUC1 breast cancer membrane proteins: cytokine receptor-like molecules. FEBS Lett 1994;356:130–136. [DOI] [PubMed] [Google Scholar]
- 20.Fukao T, Koyasu S. PI3K and negative regulation of TLR signaling. Trends Immunol 2003;24:358–363. [DOI] [PubMed] [Google Scholar]
- 21.Yu Y, Nagai S, Wu H, Neish AS, Koyasu S, Gewirtz AT. TLR5-mediated phosphoinositide 3-kinase activation negatively regulates flagellin-induced proinflammatory gene expression. J Immunol 2006;176:6194–6201. [DOI] [PubMed] [Google Scholar]
- 22.Kato K, Lu W, Kai H, Kim KC. Phosphoinositide 3-kinase is activated by MUC1 but not responsible for MUC1-induced suppression of TLR5 signaling. Am J Physiol Lung Cell Mol Physiol 2007;293:L686–L692. [DOI] [PubMed] [Google Scholar]
- 23.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol 2005;6:777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan H, Teti G, Ashton S, Guyton K, Tempel GE, Halushka PV, Cook JA. Involvement of G(i) proteins and Src tyrosine kinase in TNFalpha production induced by lipopolysaccharide, group B Streptococci and Staphylococcus aureus. Cytokine 2003;22:126–133. [DOI] [PubMed] [Google Scholar]
- 25.Leu TH, Charoenfuprasert S, Yen CK, Fan CW, Maa MC. Lipopolysaccharide-induced c-Src expression plays a role in nitric oxide and TNFalpha secretion in macrophages. Mol Immunol 2006;43:308–316. [DOI] [PubMed] [Google Scholar]
- 26.Chang YJ, Wu MS, Lin JT, Sheu BS, Muta T, Inoue H, Chen CC. Induction of cyclooxygenase-2 overexpression in human gastric epithelial cells by Helicobacter pylori involves TLR2/TLR9 and c-Src-dependent nuclear factor-kappaB activation. Mol Pharmacol 2004;66:1465–1477. [DOI] [PubMed] [Google Scholar]
- 27.Funakoshi-Tago M, Tago K, Andoh K, Sonoda Y, Tominaga S, Kasahara T. Functional role of c-Src in IL-1-induced NF-kappa B activation: c-Src is a component of the IKK complex. J Biochem (Tokyo) 2005;137:189–197. [DOI] [PubMed] [Google Scholar]
- 28.Sha Q, Truong-Tran AQ, Plitt JR, Beck LA, Schleimer RP. Activation of airway epithelial cells by toll-like receptor agonists. Am J Respir Cell Mol Biol 2004;31:358–364. [DOI] [PubMed] [Google Scholar]
- 29.Kuwahara I, Lillehoj EP, Hisatsune A, Lu W, Isohama Y, Miyata T, Kim KC. Neutrophil elastase stimulates MUC1 gene expression through increased Sp1 binding to the MUC1 promoter. Am J Physiol Lung Cell Mol Physiol 2005;289:L355–L362. [DOI] [PubMed] [Google Scholar]
- 30.Koga T, Kuwahara I, Lillehoj EP, Lu W, Miyata T, Isohama Y, Kim KC. TNF-α induces MUC1 gene transcription in lung epithelial cells: its signaling pathway and biological implication. Am J Physiol Lung Cell Mol Physiol 2007;293:L693–L701. [DOI] [PubMed] [Google Scholar]