Abstract
The mechanisms used by alveolar type I pneumocytes for maintenance of the lipid homeostasis necessary to sustain these large squamous cells are unknown. The processes may involve the ATP-binding cassette transporter A1 (ABCA1), a transport protein shown to be crucial in apolipoprotein A-I (apoA-I)–mediated mobilization of cellular cholesterol and phospholipid. Immunohistochemical data demonstrated the presence of ABCA1 in lung type I and type II cells and in cultured pneumocytes. Type II cells isolated from rat lungs and cultured for 5 days in 10% serum trans-differentiated toward cells with a type I–like phenotype which reacted with the type I cell–specific monoclonal antibody VIIIB2. Upon incubation of the type I–like pneumocytes with agents that up-regulate the ABCA1 gene (9-cis-retinoic acid [9cRA] and 22-hydroxycholesterol [22-OH, 9cRA/22-OH]), ABCA1 protein levels were enhanced to maximum levels after 8 to 16 hours and remained elevated for 24 hours. In the presence of apoA-I and 9cRA/22-OH, efflux of radioactive phospholipid and cholesterol from pneumocytes was stimulated 3- to 20-fold, respectively, over controls. Lipid efflux was inhibited by Probucol. Sucrose density gradient analysis of the media from stimulated cells incubated with apoA-I identified heterogeneous lipid particles that isolated at a density between 1.063 and 1.210 g/ml, with low or high apoA-I content. Thus, pneumocytes with markers for the type I phenotype contained functional ABCA1 protein, released lipid to apoA-I protein, and were capable of producing particles resembling nascent high-density lipoprotein, indicating an important role for ABCA1 in the maintenance of lung lipid homeostasis.
Keywords: lung, cholesterol, high-density lipoprotein, apolipoprotein A-I, phospholipids
CLINICAL RELEVANCE
Type I pneumocytes cover most of the internal surface of the alveolar space. This article describes the role of the ABCA1 transporter in these cells for the maintenance of lipid homeostasis and for the formation of nascent high density lipoprotein particles.
Pulmonary alveoli are the sites for gas exchange between the blood and the atmosphere and are composed of two types of alveolar epithelial cells, type I and type II pneumocytes. Type II cells are responsible for the production and turnover of pulmonary surfactant, the protein–lipid mixture that serves to reduce the surface tension in the alveolus allowing for even ventilation of the lung. These cuboidal cells, located at the corners of the alveoli, are filled with lamellar bodies, the organelles used for surfactant storage and release. Type I cells are large (∼ 75 μm in diameter and 2,500 μm3 in volume) squamous cells that cover about 95% of the internal surface of the lung (for review see Ref. 1). Type I pneumocytes have an average thickness of 0.3 μm and a basal lamina that is separated from the capillary endothelium by no more than a basement membrane in most sections of lung alveoli (2). Since type I cells do not proliferate, when damaged they are replaced by type II cells that transdifferentiate into type I cells (3). The ability of type II cells to transform toward a type I cell–like phenotype also has been demonstrated in primary cultures of isolated type II cells under appropriate culture conditions (4, 5).
Phospholipid turnover in the lung is well characterized due to the importance of the phospholipid-rich surfactant for normal lung function. However, cholesterol metabolism in the lung is less well understood. Cholesterol has a major structural and functional role in pneumocytes and in lung surfactant. It has been shown that the lung is capable of cholesterol synthesis, although the bulk of lung cholesterol is derived from serum lipoproteins (6). As for cholesterol export, one of the major proteins involved in receptor-mediated cholesterol efflux, the ATP-binding cassette transporter A1 (ABCA1), has been identified in the lung in pulmonary macrophages and in type II cells (7, 8). ABCA1 is a transmembrane protein composed of two similar structural units, each with a nucleotide-binding domain and a domain with six membrane-spanning peptides (9). As with all other ABC transporters, it uses ATP to generate the energy needed for substrate transport. The function of ABCA1 is to move phosphatidylserine from the internal to the external leaflet of the cell membrane and to mediate the release of phospholipid and cholesterol to lipid-free apolipoproteins that contain amphipathic α-helices, such as apolipoprotein A-I (apoA-I), the predominant apoprotein constituent of high-density lipoproteins (HDL) (for review see Ref. 10). The result of this ABCA1 activity is the generation of small nascent HDL particles with pre-β electrophoretic mobility. These lipid-poor HDL are subsequently converted to larger mature lipoproteins forms with α mobility through the action of plasma lecithin-cholesterol acyl-transferase, which esterifies free cholesterol carried in the core of the lipoprotein (11). HDL is felt to be important in reverse cholesterol transport, a process that results in the movement of cholesterol from peripheral cells to the liver. Many reports support the notion that HDL plays a critical role in the prevention of atherosclerosis, as the incidence of the disease is inversely proportional to the level of plasma HDL (11). Impairment of ABCA1 function leads to Tangier disease in humans, characterized by a loss of circulating HDL, an accumulation of cholesterol in macrophages, and premature atherosclerosis (for review see Ref. 12). Gene-targeted disruption of ABCA1 in mice results in the virtual ablation of HDL and pathologic lesions in the lung. In these animals lacking ABCA1, there is an accumulation of cholesterol in alveolar macrophages and type II cells, alveolar proteinosis, and respiratory distress characterized by rapid, shallow breathing (13, 14). These changes indicate the importance of ABCA1 function in this organ.
Recent studies examining the function of ABCA1 in primary cultures of type II pneumocytes or in a type II–like cell line have demonstrated a role for this transporter in type II cells and surfactant lipid turnover (7, 15). The nuclear liver X receptor (LXR) agonist 22-hydroxycholesterol (22-OH) in combination with the retinoid X receptor (RXR) agonist 9-cis-retinoic acid (9cRA) have been shown to act on the promoter region of the ABCA1 gene (for review see Ref. (10). The two agents were efficient in inducing ABCA1 mRNA expression and protein levels in type II cells, as has been found for many other cell types (7, 10, 15). Incubation of stimulated type II cells with lipid-free apoA-I resulted in the release of phospholipid and cholesterol, consistent with ABCA1 biologic activity (7). ABCA1-mediated efflux of phospholipid from a mouse type II–like lung epithelial cell line (MLE-12) was shown to be basolateral, the surface that is in close contact with the pulmonary capillary system (15). Stimulation of phosphatidylcholine synthesis promoted ABCA1 phospholipid efflux in MLE-12 cells, while up-regulation of ABCA1 inhibited secretagogue-stimulated surfactant secretion from primary cultures of rat type II cells, evidence that supports an important role for this transporter in the regulation of alveolar surfactant homeostasis (7, 16).
Type I pneumocytes act both as a barrier between the alveolar space and the pulmonary capillaries and as a regulator of alveolar fluid and ion homeostasis (17, 18). Type I cells have been shown to exhibit Na+-K+-ATPase activity and express the epithelial Na+ channel for active trans-alveolar ion transport, as well as aquaporin 5 for the regulation of water transport (17–19). A characteristic feature of these cells is their many flask-shaped plasmalemmal invaginations, or caveolae, indicative of a possible role in alveolar protein transcytosis (20). In addition, recent evidence has suggested that type I pneumocytes may be mechanosensors and indirectly regulate surfactant secretion by type II pneumocytes. In response to lung inflation, the type I cell increase in cytosolic calcium was transmitted to adjacent type II cells (21), and in co-cultures of pneumocytes subjected to mechanical stretch, the ATP released from type I cells provided a paracrine stimulation of surfactant secretion from the type II cells (22). Gene expression profiling has suggested that type I cells also may play a role in lung development, protection from injury, and host defense (23, 24).
Given the extensive amount of type I cell plasma membrane and cholesterol-enriched caveolar invaginations, we hypothesized that the cholesterol turnover in type I cells is tightly regulated, and that ABCA1 should play an important role in this process. Using a cell culture system of type II cells stimulated to transdifferentiate toward a type I–like phenotype, we characterized the regulation of ABCA1 protein expression and function after exposure of the cells to LXR/RXR agonists. We found ABCA1-mediated lipid export from the type I cell–like pneumocytes in the presence of lipid-free apoA-I, with the resultant production of particles in the HDL density range.
MATERIALS AND METHODS
Materials
The following were purchased from Sigma (St. Louis, MO): fetal bovine serum (FBS), gentamycin, rat immunoglobulin G (IgG), aprotinin, leupeptin, pepstatin, phenylmethylsulfonyl fluoride (PMSF), dimethylsulfoxide (DMSO), 22-OH, and 9cRA. Organic solvents and scintillation fluid were purchased from Fisher Scientific (Pittsburgh, PA). [1, 2-3H] cholesterol (specific activity = 45 Ci/mol) was from NEN Life Science Products, Inc. (Waltham, MA); [3H]- methyl-choline chloride (specific activity = 90 mCi/mmole) was from Amersham (Arlington Heights, IL). Plastic tissue culture dishes (35 mm) were from Costar (Cambridge, MA). Eagle's minimum essential medium (MEM) and PBS were purchased from CellGro (Herndon, VA). Fatty acid–free bovine serum albumin (BSA) was from Intergen (Purchase, NY).
Antibodies
The rabbit polyclonal antibody made to a partial peptide sequence of human ABCA1 was purchased from Novus Biologicals (Littleton, CO). This antibody specifically recognizes a single 220-kD ABCA1 protein in isolated epithelial cells cultured for 5 days with only minor amounts of other proteins visible and has been used for immunohistochemical purposes (25). The polyclonal goat anti-mouse apolipoprotein A-I antibody was from US Biological (Swampscott, MA). Monoclonal antibody VIIIB2 that recognizes an epitope in the plasma membrane of alveolar type I cells, and monoclonal antibody 3C9 that recognizes ABCA3 in type II cell lamellar bodies, have been described previously (26, 27). Alexa-labeled secondary antibodies were from Molecular Probes (Eugene, OR). Anti–β-actin antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA).
Animals
Pathogen-free Sprague-Dawley male rats (200–250 g) and C57/BL6 male mice (between 5 and 6 wk of age and weighing 20–25 g) were obtained from Charles River (Wilmington, MA). All protocols adhered to the principles of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Isolation and Culture of Alveolar Epithelial Cells
For rat cells, intratracheal elastase (3 units/ml; Worthington, Inc., Lakewood, NJ) was instilled into the lungs of pathogen-free male Sprague-Dawley rats (300 g) as previously described (28, 29). The isolated cells were filtered and, in order to remove macrophages, were placed on rat IgG-coated bacteriological plastic plates for 1 hour. Partially purified type II cells were seeded at 2 × 106 cells/35 mm dish (Costar) and incubated at 37°C in 5% CO2 with MEM supplemented with 10% FBS and gentamycin. After removal of nonadherent cells, the purity of the type II cells after overnight culture was greater than 90% by modified Papanicolaou stain (29).
For mouse cells, we used the procedure described by Warshamana and coworkers (30) with dispase, as described previously (7). Briefly, dispase (100 units) was instilled into cleared mice lungs, the lungs were incubated for 45 minutes at room temperature, lung tissue was separated from large bronchi by mechanical means, and tissue transferred to a Petri dish containing DMEM with 0.01% DNase I for 10 minutes at 37°C. The cells were filtered, centrifuged, and resuspended for sequential plating on mouse IgG (0.75 mg/ml)–coated Petri dishes followed by cell culture dishes, each at 37°C for 1 hour, in order to remove macrophages and fibroblasts, respectively. The final cell isolates were seeded on Type I collagen-coated 35-mm dishes in Ham's F12 culture medium supplemented with 15 mM HEPES, 0.8 mM CaCl2, 0.25% BSA, 5 μg/ml insulin, 5 μg/ml transferrin, 5 ng/ml sodium selenite, and 2% mouse serum. After 24 hours, the isolated type II cells were 95% pure (7).
Rodent type II cells were allowed to transdifferentiate to type I–like cells over 4 to 6 days in 10% FBS. During this incubation period, type II cells lose their well-defined characteristics of lamellar bodies, surfactant proteins, and microvilli and begin expressing type I cell markers, including aquaporin 5. Characteristics of these preparations of cytokeratin- and T1α-positive type I–like epithelial pneumocytes have been reported previously (5, 31, 32). This preparation will be referred to as type I–like cells hereafter, adopting the nomenclature of other investigators using similar culture systems (33, 34).
Apolipoproteins
Lipid-free human apoA-I was isolated by anion-exchange chromatography on Q-Sepharose from delipidated human HDL as described previously (35). ApoA-I was solubilized in 6 M guanidine-HCl overnight at 4°C. This solution was extensively dialyzed against a buffer of 10 mM Tris-HCl, 0.15 M NaCl, and 1 mM EDTA (disodium), filtered (0.45 μm; Millipore, Bedford, MA), and stored at 4°C. 14C-radiolabeled apoA-I (specific activity of ∼ 1μCi/mg) was produced by reductive methylation using 14C-formaldehyde (36). The procedure has no effect on apoA-I lipid binding properties or ability to form HDL particles (35, 36).
Measurement of Cholesterol and Phospholipid Release into Media
Two separate sets of type I-like alveolar epithelial cells (after 5 d in culture) were plated overnight in MEM supplemented with 10% FBS containing either [3H]choline chloride (0.7 μCi/dish, to label phosphatidylcholine) or [3H]cholesterol (2 μCi/dish) for 18 hours as described previously for type II cells (7). Next, the cells were washed and incubated with 0.2% BSA with or without 22-R-hydroxycholesterol (22-OH, 6.2 μM, in ethanol) and 9-cis-retinoic acid (9cRA, 5 μM in DMSO) for the indicated time. When the two agents were present in combination, they are referred to as 9cRA/22-OH. Preliminary experiments determined that this concentration of 22-OH and 9cRA produced the maximum response without toxicity (as determined by lack of release of lactic dehydrogenase or lack of change in cell protein/dish with cells exposed to agonists as compared to controls without additions). Control cells were incubated in equivalent levels of BSA and diluent with a final concentration of DMSO and ethanol of less than 0.03%. Next, human apoA-I (10 μg/ml, 1 ml/plate) was added to the cells in MEM for an additional time as indicated. To terminate the experiment, the media were removed and centrifuged to pellet detached cells. For phospholipid efflux, methanol was added to the cell monolayers, and the cells were scraped from the dish. The cells and the media were extracted separately using chloroform:methanol:water (2:2:1.8, vol/vol) (37) with 500 μg egg phosphatidylcholine added as carrier. The [3H]-phospholipid in the chloroform layer from the cells or the media was counted. For cholesterol efflux, cell monolayers were washed with PBS, dried, extracted with isopropyl alcohol, and the [3H]-cholesterol was counted. For the media, radioactivity was determined in 100 μl aliquots by liquid scintillation counting. The value for the % lipid (cholesterol or phospholipid) efflux was determined by the dpm of radiolabeled lipid (cholesterol or phospholipid) recovered from the media divided by the total dpm of lipid in the dish (cells plus media) × 100. Each experiment was performed in duplicate or triplicate.
Western Analysis
Tissue and cellular extracts were lysed in buffer (0.5% NP-40, 10 mM Tris, 1 mM MgCl2, 1% Triton X-100, pH 7.5) supplemented with protease inhibitors aprotinin (10 μg/ml), leupeptin (1 μg/ml), pepstatin (1 μg/ml), and PMSF (0.2 mM), then centrifuged at 12,000 rpm for 4 minutes to remove debris. Lysates (40 μg protein) were loaded onto the gel, electrophoresed under reducing conditions on 3 to 8% NuPAGE Tris-Acetate gels with Tris-Acetate running buffer (Novex, San Diego, CA), and transferred to nitrocellulose membranes. ABCA1 was detected with a rabbit polyclonal primary antibody to human ABCA1 (1:1,000 dilution). Membranes were then incubated with goat anti-rabbit IgG conjugated to horseradish peroxidase with visualization by enhanced chemiluminescence ECL Plus (Amersham Pharmacia Biotech, Piscataway, NJ) according to the manufacturer's instructions.
Microscopy and Immunofluorescence
Mouse or rat lungs were perfused with PBS to remove blood followed by infusion with 4% paraformaldehyde in 0.1 M sodium cacodylate buffer. Five-micrometer cryosections of lung were incubated with 0.01 M sodium borohydride in PBS for 15 minutes and 0.3% glycine in PBS for 20 minutes to reduce tissue autoflourescence and remove formaldehyde. The tissue samples were placed in blocking solution (5% BSA + 5% normal goat serum [NGS] + 0.3% Triton X-100 in PBS) for 1 hour. The samples were incubated with primary antibodies (goat anti-mouse apoA-I polyclonal antibody at 1:200; rabbit anti-ABCA1 antibody; or mouse anti-ABCA3 monoclonal antibody 3C9 at 1:200) in the incubation solution (1% BSA + 2% NGS in PBS) for 3 hours. Murine monoclonal antibody VIIIB2 was used as described (26). After washing five times in PBS the appropriate secondary antibody at 1:250 dilution was applied for 1 hour. The secondary antibodies used were: Alexa 488–conjugated goat anti-mouse antibody; Alexa 594–conjugated rabbit anti-goat antibody; and Alexa 568– or Alexa 594–conjugated goat anti-rabbit antibody. After further washing with PBS and distilled water, the slides were mounted with Vectashield mounting medium. Alveolar epithelial cells were grown on glass coverslips in 35-mm dishes for 1 or 5 days and were fixed overnight with 4% paraformaldehyde in PBS. The cells then were stained as described above for tissue samples. Lung sections or cell samples incubated without primary antibody were used as controls. Cell and tissue samples were examined using a laser confocal microscope, BIORAD 2000 (Carl Zeiss Microimaging, Thornwood, NY) using “Laser Sharp 2000” software. The microscope is equipped with a ×60 oil objective (Nikon, Melville, NY).
Sucrose Density Ultracentrifugation Analysis
Pooled conditioned media from labeled cells were filtered through a 0.45-μm filter (Millex-HV; Millipore) and the density of the lipid-containing particles determined using a modification of the method of Hara and Yokoyama (38). A sucrose solution of density = 1.1g/m (9 ml) was placed over a sucrose solution of d = 1.3 g/ml (2 ml), followed by overlay of the conditioned medium (7 ml). After centrifugation in a Beckman ultracentrifuge (Beckman, Fullerton, CA) using a TL 50.2 rotor at 50,000 rpm at 14°C for 30 hours, the fractions (1 ml) were removed manually from the top to avoid cross-contamination of proteins. The density was measured in each fraction by refractometry. The fractions were either lipid extracted (for 14C-cholesterol, or 3H-phospholipid) or counted directly (14C-apoA-I) for radioactivity quantitation.
Statistical Analysis
Results are reported as mean ± SE. Statistical significance was determined by two-tailed Student's t tests (GraphPad Prism version 3.0; GraphPad Software, San Diego, CA) or paired t tests with Sigma Stat for Windows (Jandel, San Raphael, CA). Statistical significance is taken as P < 0.05. n = number of separate experiments performed.
RESULTS
Pneumocyte ABCA1 in the Lung and in the Cell Culture System
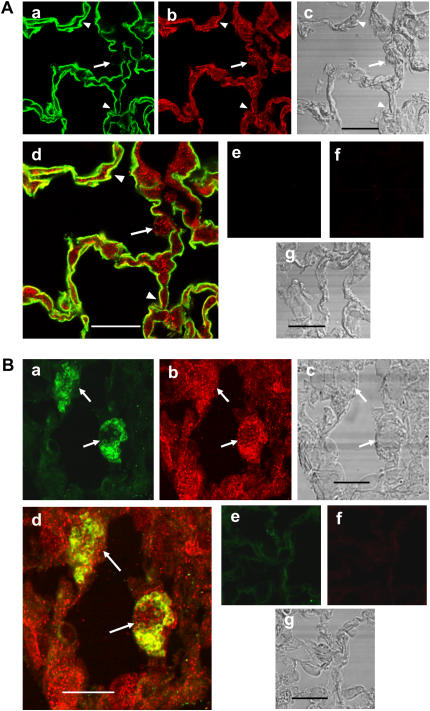
The presence of ABCA1 protein in the lung and isolated pneumocytes has been shown by Western blot procedures (7, 8). In order to demonstrate the presence of ABCA1 protein in pneumocytes in the intact lung, lung sections were stained with anti-ABCA1 antibody and either the type I cell–specific antibody VIIIB2, which has been shown to recognize an epitope on rat type I cell membranes, or anti-ABCA3 antibody, 3C9, which has been shown to recognize the lamellar body membrane-localized ABCA3 protein in type II cells (26, 27). Figure 1A demonstrates co-localization of ABCA1 protein in type I cells in rat lung that are reactive with VIIIB2 (arrowheads) and in anti-ABCA3 antibody-positive type II cells (Figure 1B, arrows) in mouse lung. In order to characterize ABCA1 in type I cells, we used a well-developed in vitro model in which type II cells in culture for 5 days transdifferentiate toward a type I cell–like phenotype, losing their cuboidal shape, lamellar bodies, and ability to synthesize surfactant proteins while gaining type I cell–specific markers (4, 5, 39). The type I–like alveolar cells acquire a squamous cell shape, express aquaporin 5, and exhibit other type I cell characteristics (5, 31, 32). We have shown previously that rat type II cells in culture for 3 to 5 days have SP-A, -B, and -C mRNA levels less than 1% of freshly isolated cells. The cells have a flattened morphology and demonstrate only minor reactivity with monoclonal antibody 3C9, specific for ABCA3 in lamellar bodies (40, 41). Using culture conditions identical to our own, Oswari and colleagues (42) demonstrated that the type I–like cells lost pro–surfactant protein (SP)-C protein and gained RTI40 reactivity, a type I cell–specific protein (43). Figures 2a–2c demonstrate that the rat type I–like cells after 5 days of culture in 10% FCS have a flattened morphology and stain positively for ABCA1 protein as well as the VIIIB2 epitope that has been demonstrated to be specific for type I cells (26). Type II cells isolated from rat lungs after 24 hours in culture containing ABCA3-positive lamellar bodies and ABCA1 protein are shown in Figures 2d–2f.
Figure 1.
ABCA1 protein in (A) VIIIB2-reactive type I cells in rat lung or in (B) anti-ABCA3 (3C9) antibody-reactive type II cells in mouse lung. (A) Rat lung sections (a–g) were stained with the type I cell–specific monoclonal antibody VIIIB2 (a and d) and anti-ABCA1 antibody (b and d) and probed with Alexa 488– (green) or Alexa 568 (red)–conjugated secondary antibody, respectively. d shows overlay of VIIIB2 and ABCA1 signals. c shows corresponding phase images for a, b, and d. e, f, and g are the control sections without primary antibodies. Type I cells shown by arrowheads stain positively for both ABCA1 and the VIIIB2 epitope. A cuboidal type II cell (arrow) is positive for ABCA1 and lacks VIIIB2 staining. Scale bar, 10 μm. (B) Mouse lung sections (a–g) were stained with the type II cell–specific anti-ABCA3 antibody (a and d) and anti-ABCA1 antibody (b and d) and probed with Alexa 488– (green) or Alexa-594 (red)–labeled secondary antibody, respectively. d shows overlay of ABCA3 and ABCA1 signals. c shows corresponding phase images of a, b, and d. e, f, and g are the control sections without primary antibodies. The ABCA3-positive type II cells (arrows) contain ABCA1. Scale bar, 3 μm.
Figure 2.
Immunostaining for ABCA1 protein in rat alveolar pneumocytes in culture. (a–c) Cultures of rat alveolar type II cells transdifferentiated to type I–like cells after 5 days. Pneumocytes were stained with VIIIIB2 (a and c) and anti-ABCA1 antibody (b and c) and probed with Alexa 488– (green) or Alexa 568 (red)–conjugated secondary antibody, respectively. c shows overlay of a and b. d–f show rat alveolar type II cells in culture for 1 day stained with anti-ABCA3 (d and f) and anti-ABCA1 antibodies (e and f) and probed with Alexa 488– (green) or Alexa 594 (red)–labeled secondary antibody, respectively. f shows overlay of d and e. The flattened VIIIB2-positive type I–like cells and the cuboidal ABCA3-positive type II cells stain for ABCA1 protein. Scale bar, 10 μm.
Up-Regulation of ABCA1 Protein by 9cRA and 22-OH
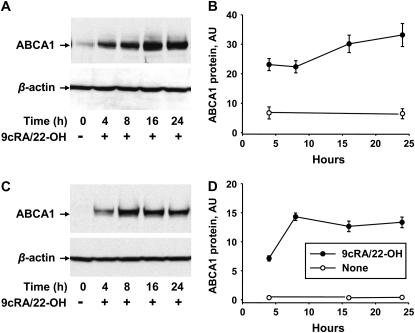
To examine the time course of expression of ABCA1 in pneumocytes with type I cell phenotypic characteristics, type II cells were isolated from rodent lungs and placed in culture for 4 to 7 days in 10% FCS. Expression of ABCA1 was enhanced by incubation of the cells with the ligands for the nuclear receptors LXR and RXR, 22-OH (6 μM) and 9cRA (5 μM), respectively. Examination of the time course of stimulated expression of ABCA1 protein in rodent type I–like cells by Western blot is shown in Figure 3. Representative immunoblots are shown on the left (Figures 3A and 3C) and quantitative changes in ABCA1 content obtained from densitometric analysis of blots from separate experiments expressed relative to β-actin are shown on the right (Figures 3B and 3D). After 5 days in culture with FCS, ABCA1 protein in the alveolar cells was present but at a low concentration in pneumocytes isolated from rats (Figure 3A, time 0) and was below the level of detection in cells isolated from mice (Figure 3C). The transporter was up-regulated upon incubation of the type I–like alveolar cells with 9cRA/22-OH in a time-dependent fashion. ABCA1 protein was elevated after 4 hours in cells from both species, enhanced to a maximum after 8 hours (mice) or 16 hours (rats) of incubation, and remained robust after 24 hours of exposure (Figures 3A and 3B). Previous data obtained from rat type II cells incubated under identical conditions indicated that ABCA1 protein levels peaked at 16 hours and fell at 24 hours (7). A direct comparison of ABCA1 protein levels in the two types of rat pneumocytes by Western analysis determined that, after 16 hours of induction, the levels of ABCA1 protein in the rat type I–like alveolar cells isolated from rats were 2.5 ± 0.1- fold higher (n = 3) than levels in rat type II cells based on equal protein loading. In these experiments, the density of the ABCA1 protein band on the Western blots was expressed relative to a loading standard (ABCA1 protein in WI38VA13 human fibroblasts [44]).
Figure 3.
Time course of up-regulation of ABCA1 protein in type I-like cells after exposure to LXR/RXR agonists using immunoblot analysis. Total cell lysates were prepared at different hours after the addition of 9-cis-retinoic acid (9cRA) (5 μM)/22-hydroxycholesterol (22-OH) (6 μM). Forty micrograms of protein were run per lane. β-actin was used as a control for protein loading. (A, C) Typical immunoblots of the time course of changes in ABCA1 and β-actin in epithelial type I-like cells isolated from (A) rat or (C) mouse lungs and exposed to 9cRA/22-OH. (B, D) Quantitation of separate experiments showing the amount of ABCA1 protein relative to β-actin in arbitrary units (AU) from type I–like cells from rats (B, n = 4) or C57Bl6 mice (D, n = 3). Data are shown as mean ± SE. A and B, rats; C and D, mice.
Biological Activity of ABCA1
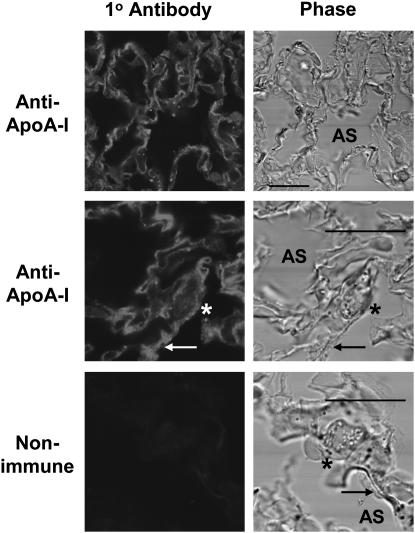
The role of ABCA1 in the formation of nascent HDL particles depends on the interaction of apoA-I with the transporter. In order to demonstrate the presence of apoA-I in the lung, mouse lungs were perfused with PBS to remove blood, then perfused further with 4% paraformaldehyde, fixed, and permeabilized. Cryosections were stained with rabbit anti-mouse apoA-I antibody. As seen in Figure 4, there was substantial antibody reactivity in the mouse lung in the interalveolar septai, surrounding the cuboidal type II cells (arrowhead), and in the area of the adjacent thin type I cells (arrows) in the alveoli. Thus there was an ample supply of apoA-I in the lung tissue available for interaction with pneumocyte ABCA1.
Figure 4.
Apolipoprotein A-I (apoA-I) in mouse lung. Lung cryosections of perfused, perfusion-fixed C57Bl6 mouse lungs were labeled with anti–apoA-I antibody or nonimmune IgG (Control). Low and high magnification views are shown of lung sections stained with anti–apoA-I antibody and the complimentary phase. Lamellar body–filled, cuboidal type II cells are indicated by asterisk. Adjacent flat type I–like cells are indicated by arrow. AS, alveolar space. Scale bar, 10 μm.
Since the transdifferentiation of type II to type I–like cells isolated from rats is well characterized, the remaining experiments were performed in cells obtained from rat lungs. The biological activity of ABCA1 was determined in the type I–like cells in culture by measuring the ABCA1-dependent release of lipid from the isolated cells to exogenously added lipid-poor apoA-I in the media. Type I–like cells isolated from rats were incubated with [3H]-choline or [3H]-cholesterol for 24 hours to label the phospholipid or cholesterol pools, respectively, in the cells. The labeled type I–like cells were incubated without or with 9cRA/22-OH to up-regulate ABCA1 followed by addition of apoA-I (10 μg/ml) for 16 hours and lipid efflux to the media measured. As shown in Fig 5, the greatest stimulation of lipid efflux to apoA-I was from cells pre-exposed to LXR/RXR agonists with enhanced levels of ABCA1 protein. After incubation with 9cRA/22-OH, efflux of either radioactive phospholipid or cholesterol from type I–like pneumocytes to apoA-I was stimulated 3.3- or 5.5-fold, respectively, over controls without additions (Figure 5). In a series of experiments, apoA-I–mediated efflux of phospholipid under unstimulated conditions was 2.5 ± 0.4% and with up-regulation of ABCA1 was 7.0 ± 1.7% (mean ± SE, n = 4, P < 0.01) while efflux of cholesterol under unstimulated or stimulated conditions was 4.3 ± 0.7% or 11.8 ± 1.8% (mean ± SE, n = 17, P < 0.001), respectively.
Figure 5.
Release of lipid to apoA-I from rat type I–like cells after up-regulation of ABCA1. Type I–like cells labeled with [3H]-cholesterol or [3H]-phospholipid were incubated for 16 hours without or with 9cRA/22-OH. ApoA-I was added to some cells, and efflux of label into the media after 7 hours was quantitated. Data are shown as mean ± SE from one cholesterol or one phospholipid experiment performed in triplicate and are representative of the 17 or 4 experiments, respectively, performed. * Significantly different from all other values, P < 0.05.
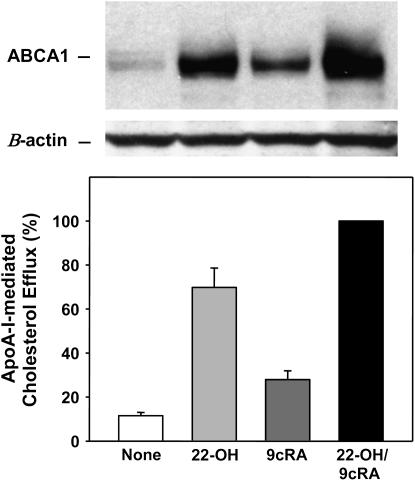
ABCA1 protein levels are known to be differentially regulated depending on the cell type (45). To explore which of the agonists was the most potent for type I–like cells, the LXR agonist, 22-OH, and the RXR agonist, 9cRA, alone or in combination, were incubated with [3H]-cholesterol-labeled rat type I–like cells for 16 hours followed by incubation with apoA-I for 6 or 7 hours. The LXR agonist was more effective than the RXR agonist in the up-regulation of ABCA1 protein as measured by Western blot (Figure 6, top) and by apoA-I mediated cholesterol efflux (Figure 6, bottom). The amount of ABCA1 protein and the degree of efflux was higher upon co-incubation of the two agonists with type I–like cells (Figure 6). The data demonstrate the direct correlation between the levels of ABCA1 and the release of cholesterol to apoA-I.
Figure 6.
22-OH cholesterol is more potent than 9cRA in the stimulation of ABCA1 protein levels. Top: Western blot analysis of rat type I–like cell lysates (40 μg of protein) after incubation of the cells with 22-OH or 9cRA, alone or in combination. The blots are representative of the three performed. β-actin serves as a loading control. Bottom: ApoA-I–mediated efflux of cholesterol from the cells treated as shown in the Western blot. Type I–like cells were pre-labeled with [3H]-cholesterol and incubated without (None) or with 22-OH (6 μM) and 9cRA (5 μM) alone or together for 16 hours. ApoA-I (10 μg/ml) was added, and efflux into the media over the next 6 or 7 hours was measured. Background efflux from untreated cells in the absence of apoA-I was subtracted from the data (< 3% efflux). Data are shown as mean ± SE of four experiments performed in duplicate or triplicate. The data are expressed as a percentage of the cholesterol efflux found with incubation of apoA-I plus 9cRA/22-OH, which was set equal to 100% in each experiment. Cholesterol efflux for apoA-I plus 9cRA/22-OH was 8.3 ± 1.7% (mean + SE, n = 4). All data bars are significantly different from each other, P < 0.01, n = 4.
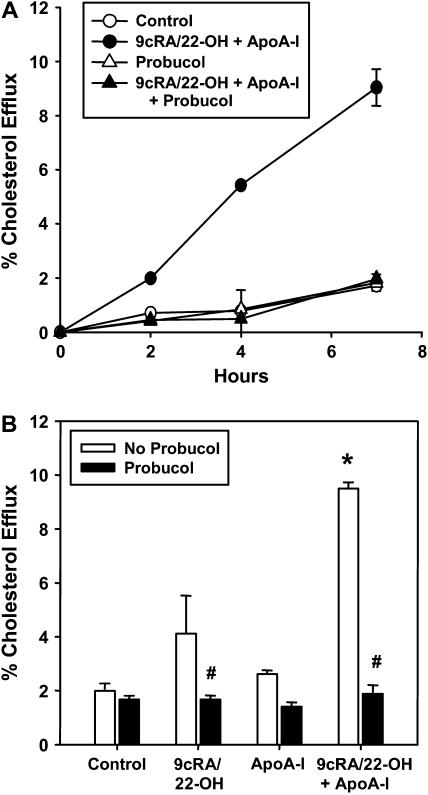
Treatment with 9cRA/22-OH induces the expression of other genes besides ABCA1. Therefore, a known inhibitor of ABCA1 activity, Probucol, was used to establish the specific role of this transporter (46, 47). Probucol inhibits ABCA1-mediated lipid efflux by inactivation of ABCA1, resulting in blockage of apoA-I binding to the cell surface (46, 47). [3H]-cholesterol-labeled type I–like cells that had or had not been treated with 9cRA/22-OH were incubated without or with Probucol and efflux of cholesterol to apoA-I followed. Probucol treatment had no effect on the basal rate of cholesterol efflux that occurs in the absence of apoA-I or ABCA1 (control), but had a pronounced inhibitory effect on the release of cholesterol to apoA-I in cells exposed to 9cRA/22-OH (Figure 7). The effect was seen at the first time point measured (2 h) and continued over the entire 7 h experiment (Figure 7A). In three experiments, the 5-fold stimulation of cholesterol efflux due to the up-regulation of ABCA1 and the presence of apoA-I was completely abolished in the presence of Probucol (Figure 7B). These observations suggest that ABCA1 was primarily responsible for the observed apoA-I–dependent lipid efflux.
Figure 7.
Inhibition of ABCA1-mediated cholesterol efflux from type I–like cells by Probucol. Type I–like rat cells labeled with [3H]-cholesterol were incubated without or with Probucol (20 μM) and or 9cRA/22-OH for 16 hours, then washed and incubated with apoA-I (10 μg/ml) for the indicated time period. (A) Time course of the release of [3H]-cholesterol. Data are shown as mean ± range of a single experiment performed in duplicate and representative of the three performed. (B) Type I–like cells were incubated as described in A and harvested after 7 hours. Data are shown as mean ± SE of three experiments performed in duplicate. # Significantly different from No Probucol, P < 0.05. * Significantly different from Control, P < 0.05.
Formation of Particles in the Density Range of HDL by Alveolar Cells in Culture
The interaction of apoA-I and various cell types, including macrophages and fibroblast cell lines, results in the release of phospholipid and cholesterol and the formation of nascent HDL particles (35). In order to determine whether alveolar cells in culture for 5 days are capable of the production of HDL, sucrose density gradient analysis was performed on media without or containing apoA-I after a 7-hour incubation with [3H]-cholesterol-labeled rat pneumocytes incubated without or with RXR/LXR agonists to up-regulate ABCA1 protein. The cholesterol, released from stimulated cells in the presence of apoA-I, was recovered from the sucrose density gradient between 1.063-1.21 g/ml, the density of HDL (Figure 8A). Little (cells exposed to agonists alone) or no (cells exposed to no additions or apoA-I alone) cholesterol was recovered in this density range in the absence of agonists or apolipoprotein acceptor.
Figure 8.
Generation of particles in the density range of HDL by rat alveolar epithelial cells. (A) 3H-cholesterol. Alveolar epithelial cells, labeled with [3H]-cholesterol, were incubated for 16 hours without or with 9cRA/22-OH, then incubated with apoA-I for 7 hours. The conditioned media were collected, centrifuged, and filtered to remove cells and debris, and analyzed by sucrose density gradient centrifugation. Each fraction was analyzed for density and cholesterol content. The density of HDL (1.063–1.21) is marked by dotted lines. Data shown are from a representative experiment of the five performed. Closed triangle, control; open triangle, apoA-I; open circle, 9cRA/22-OH; closed circle, 9cRA/22-OH + apoA-I. (B) ApoA-I and lipids. Type I–like cells were labeled either with [3H]-phospholipid or [3H]-cholesterol, incubated with 9cRA/22-OH for 16 hours, then incubated with [14C]-apoA-I for 7 hours. The conditioned media were analyzed by sucrose density gradient centrifugation. Density was measured in each fraction. The samples were either counted directly (apoA-I), or extracted and counted (phospholipid or cholesterol). Data are representative of the three experiments performed. Open square, phospholipid; open triangle, cholesterol; closed circle, apoA-I.
Our laboratories have shown previously that [14C]-labeling of apoA-I does not destroy its ability to promote lipid efflux from cells in culture (35, 48), results that were confirmed in the type I–like epithelial cells. Upon induction of ABCA1 levels by 9cRA/22-OH, [14C]-apoA-I stimulated the release of cholesterol from 3H-cholesterol labeled alveolar cells by 4-fold compared with controls (% cholesterol efflux, 7 h = 8.5 ± 0.2% with [14C]-apoA-I and 9cRA/22-OH versus 2.1 ± 0.1% controls, n = 5, P < 0.001). Media were collected from parallel experiments using pneumocytes labeled with either [3H]-cholesterol or [3H]-phospholipid, exposed to RXR/LXR agonists and incubated with [14C]-apoA-I for 7 hours. After centrifugation to remove detached cells, the media were placed on a sucrose gradient, centrifuged, and analyzed. The majority of the lipid and protein associated with particles was isolated at a density between 1.05 and 1.21, but the composition of the particles appeared heterogeneous (Figure 8B). Some lipid was found in the less dense fractions (density < 1.07) that contained only minor amounts of apoA-I, while apoA-I protein and lipid were associated together in the density between 1.10 and 1.20. Little material was recovered at d>1.15, where the bulk of the BSA is found. Collectively, these data show that particles consistent with the density of nascent HDL were recovered from the media of alveolar cells incubated with apoA-I.
DISCUSSION
Type I cells are an important component of lung alveoli, playing a critical role in barrier function and fluid transport, and acting as a mechanosensor of lung alveolar inflation (1). Little is known with regard to the lipid metabolism of these large, thin epithelial cells. Immunohistochemical data demonstrated the presence of ABCA1 protein in type I and type II pneumocytes in the lung and in isolated pneumocytes. ABCA1 is a transport protein that is known to play a role in apoA-1–mediated lipid efflux and nascent HDL formation (10). Using a cell culture model for type I cells where type II cells transdifferentiate toward a type I cell phenotype, we found that these type I–like pneumocytes contained ABCA1 protein that was stimulated by LXR/RXR agonists. Incubation of agonist-stimulated cells with lipid-free apoA-I resulted in the release of cholesterol and phospholipid into the media. The extent of lipid release was proportional to the levels of intracellular ABCA1 and was completely inhibited by exposure of the cells to Probucol, an agent known to block the biologic activity of ABCA1. Finally, in the presence of lipid-free apoA-I, the type I–like cells were capable of producing particles with densities similar to nascent HDL containing apoA-I, cholesterol, and phospholipid. These observations indicate an important role of ABCA1 in the modulation of lipid efflux and the formation of HDL by these pneumocytes.
The well-characterized cell system used in this study takes advantage of the ability of isolated type II cells to transdifferentiate into cells with type I characteristics. During this process the type II cells loose their lamellar bodies, surfactant proteins, and cuboidal shape and transition into type I–like cells that express type I cell–specific markers (26, 49), become morphologically larger and flattened, and demonstrate the presence of caveolae and caveolin 1 (4). The use of this in vitro model to study the properties of type I cells is supported by evidence that type II cells serve as the progenitors for injured type I cells in vivo (3; for review see Ref. 1). This cell system has been employed by several laboratories investigating the characteristics of type I pneumocytes, including, for example, effects of mechanical deformation (31), epithelial barrier properties (5), and role in lung ion and fluid transport (17). Improved isolation protocols developed for primary cultures of type I cells will allow future studies of freshly isolated type I cells, although many primary cells loose differentiated characteristics after culture (50).
In primary cultures of lung pneumocytes, the ABCA1 transporter in both type I–like and type II cells responded to co-incubation with 9cRA/22-OH, as was found to be the case for other primary cells in culture (human skin fibroblasts and human macrophages [35, 48], for example) as well as various transformed cell lines including a mouse lung epithelial cell line, MLE-12 (16). The stimulated levels of ABCA1 protein in type I-like pneumocytes were maintained for 24 hours in the absence of apoA-I or other helical proteins known to stabilize the transporter and protect it from degradation (51), while ABCA1 levels in type II cells began to fall after 16 hours of exposure to agonist (7). The regulation of ABCA1 protein levels has been shown to differ between cell types (45, 52). In type I–like cells, the LXR agonist, 22-OH, proved to be more potent than 9cRA in stimulating both ABCA1 protein levels and biologic activity, as also was found to be the case with type II pneumocytes (data not shown). In addition, the ability of ABCA1 to stimulate apoA-I–mediated lipid efflux was proportional to the levels of ABCA1 protein in both type I–like and type II cells (7). Pneumocytes join the catalogue of cell types, such as mouse peritoneal macrophages and WI-38 fibroblasts, that release both phospholipid and cholesterol to apoA-I (53). Other cells, such as L929 fibroblasts and Chinese hamster ovary (CHO-K1) cells, release only phospholipid, while COS-7 monkey kidney cells have no ABCA1 and do not produce HDL (53).
Several transporter proteins are stimulated by LXR/RXR agonists, some of which are involved in lipid efflux, and those that are present in the pneumocytes may have contributed to the data. However, ABCG5 and ABCG8 have not been identified in the lung, and ABCG1-mediated lipid release occurs to HDL, not apoA-I (54–56). Notably, ABCA7 is found in the lung and mediates apoA-I–dependent lipid efflux, but this ABC transporter is not sensitive to induction by LXR agonists (57). Finally, the observed lipid efflux was completely ablated by Probucol, a compound that is known to inhibit the function of ABCA1 in the plasma membrane (46, 47). Scavenger receptor class B type I (SR-BI) is present in type II cells, but selectively releases cholesterol to larger, phospholipid-rich, HDL and is not affected by Probucol treatment (44). Since lipid efflux was stimulated by both LXR and RXR agonists, occurred in the presence of lipid-poor apoA-I, was blocked by Probucol, and resulted in the formation of HDL-size particles, the data taken together point to the importance of ABCA1 in the lipid release process of type I–like cells. However, contributions of other proteins to lipid efflux from type I–like cells cannot be entirely ruled out.
Upon interaction of lipid-poor apoA-I with type I–like cells in culture, this study showed that particles were generated with a density consistent with that of nascent HDL. These particles contained apoA-I, cholesterol, and phospholipid. Our laboratory has shown previously that the interaction of apoA-I with ABCA1 on fibroblasts and J774 macrophages in culture generates nascent discoidal HDL particles predominantly 9 and 12 nm in diameter with 2 to 4 molecules of apoA-I per particle (35, 48). In addition, plasma membrane vesicles or microparticles are formed that lack apoA-I but contain proteins consistent with a lipid raft origin (35, 48). Since the two types of particles formed in parallel, the findings were consistent with a concurrent release of particles from the cells and not a result of precursor–product relationships (48). While a more detailed analysis of the particles formed by type I–like pneumocytes is necessary, the density profile and composition of the vesicles produced by type I–like cells was consistent with those found with fibroblasts and macrophages. At least two types of particles in the HDL density range were recovered from the media of the pneumocytes. The less dense particles consisted primarily of cholesterol and phospholipid with little apoA-I and the other, more dense, particles were composed of both lipids and apoA-I. Apolipoprotein E (ApoE) is another serum lipoprotein that is capable of binding lipids and forming particles. The evidence that type I cells synthesize apoE (24) adds an additional dimension to the available mechanisms for HDL generation by type I–like pneumocytes and would explain the presence of some cholesterol-containing particles in the HDL density range seen in the absence of added apoA-I.
All of the components necessary for efficient efflux of cholesterol and phospholipid to lipid-poor apolipoproteins are present in the lung. First, there is abundant opportunity for interactions between alveolar cells and circulating lipoproteins since: (1) the basal lamina of the type I cells is often fused with the capillary endothelium for the purpose of gaseous exchange; (2) the entire cardiac output flows through the lung; (3) the capillary bed of the lung accounts for 1/3 of its mass; and (4) the pulmonary vascular endothelium accounts for approximately 30% of the vascular bed of the body (2). Secondly, organ screening for gene expression of ABCA1 found the lung to have the second highest amount of ABCA1 mRNA, after the liver (58). By immunohistochemical methods, this study found abundant amounts of apoA-I in the lung and significant levels of ABCA1 in type I and type II cells in both the lung and in isolated cells. Further, our current report on type I–like cells, together with our recent studies on type II cells, demonstrate that ABCA1 in both types of pneumocytes is inducible (7). Finally, of all the organs screened in the ABCA1 gene-targeted mouse, the lung had the most predominant pathology, implicating the importance of ABCA1 function in this organ (13, 14). Whether or not the observed respiratory distress of the ABCA1-deficient mouse is due to affected type I cells will be of interest (14). Thus, it is clear that all of the pathways needed for ABCA1 biological activity are available for the maintenance of lung lipid homeostasis. Recent reports have determined that the liver and intestine are important organs in the production of HDL particles, while other organ systems are felt to play an important role in the transfer of cholesterol to nascent HDL particles (59–61). Whether the lung remodels HDL or contributes to circulating plasma HDL levels remain intriguing questions.
Due to the importance of the cholesterol and phospholipid components of surfactant lipid in maintenance of proper surface tension and viscosity, as well as the role of lipids in the maintenance of cell surface membrane integrity of pneumocytes, the lung has a need for the precise regulation of lipid turnover. Our previous and current results support the crucial role of ABCA1 in the lung in the regulation of lipid homeostasis (7, 14). For type I–like pneumocytes, we found that ABCA1 protein levels were inducible through exposure to LXR/RXR agonists. Incubation with apoA-I, the major apolipoprotein component of HDL, resulted in the ABCA1-mediated production of heterogeneous particles that isolate in the density range of HDL. The present findings add type I–like pneumocytes to the inventory of extra-hepatic, nonmacrophage cells capable of the assembly of HDL with exogenous apoA-I. The subsequent fates of these newly produced lipoprotein particles, whether they reach the circulation and/or add to plasma HDL levels, remain to be determined. Should these observations with type I–like cells in culture be substantiated by type I cells in vivo, the ability to assemble particles comparable to nascent HDL will add to the growing list of physiologic functions attributable to type I cells, pneumocytes which were once thought to serve only as an inert barrier between the circulation and the air space in the lung.
Acknowledgments
The authors thank Dr. Aron B. Fisher for helpful discussions and Drs. Sissel Lund-Katz and Michael C. Phillips for the 14C-labeled apoA-I. Portions of the manuscript were presented at the ATS 2006 San Diego International Conference and published in abstract form in Proc Am Thorac Soc 2006;3:A760.
This work was funded by: AHA 0355695U, University of Pennsylvania Research Foundation Award, HL 22633, HL 63768 (to G.H.R.), HL38578, HL 38621 (to E.D.C.), and the Hastings Foundation.
Originally Published in Press as DOI: 10.1165/rcmb.2007-0020OC on September 20, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.McElroy MC, Kasper M. The use of alveolar epithelial type I cell-selective markers to investigate lung injury and repair. Eur Respir J 2004;24:664–673. [DOI] [PubMed] [Google Scholar]
- 2.Crapo JD, Barry BE, Gehr P, Bachofen M, Weibel ER. Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis 1982;126:332–337. [DOI] [PubMed] [Google Scholar]
- 3.Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol 1975;22:142–150. [DOI] [PubMed] [Google Scholar]
- 4.Fuchs S, Hollins AJ, Laue M, Schaefer UF, Roemer K, Gumbleton M, Lehr CM. Differentiation of human alveolar epithelial cells in primary culture: morphological characterization and synthesis of caveolin-1 and surfactant protein-C. Cell Tissue Res 2003;311:31–45. [DOI] [PubMed] [Google Scholar]
- 5.Cheek JM, Evans MJ, Crandall ED. Type I cell-like morphology in tight alveolar epithelial monolayers. Exp Cell Res 1989;184:375–387. [DOI] [PubMed] [Google Scholar]
- 6.Hass MA, Longmore WJ. Regulation of lung surfactant cholesterol metabolism by serum lipopoteins. Lipids 1980;15:401–406. [DOI] [PubMed] [Google Scholar]
- 7.Bortnick AE, Favari E, Tao JQ, Francone OL, Reilly M, Zhang Y, Rothblat GH, Bates SR. Identification and characterization of rodent ABCA1 in isolated type II pneumocytes. Am J Physiol Lung Cell Mol Physiol 2003;285:L869–L878. [DOI] [PubMed] [Google Scholar]
- 8.Lawn RM, Wade DP, Couse TL, Wilcox JN. Localization of human ATP-binding cassette transporter 1 (ABC1) in normal and atherosclerotic tissues. Arterioscler Thromb Vasc Biol 2001;21:378–385. [DOI] [PubMed] [Google Scholar]
- 9.Fitzgerald ML, Mendez AJ, Moore KJ, Andersson LP, Panjeton HA, Freeman MW. ATP-binding cassette transporter A1 contains an NH2-terminal signal anchor sequence that translocates the protein's first hydrophilic domain to the exoplasmic space. J Biol Chem 2001;276:15137–15145. [DOI] [PubMed] [Google Scholar]
- 10.Oram JF, Heinecke JW. ATP-binding cassette transporter A1: a cell cholesterol exporter that protects against cardiovascular disease. Physiol Rev 2005;85:1343–1372. [DOI] [PubMed] [Google Scholar]
- 11.Fielding CJ, Fielding PE. Molecular physiology of reverse cholesterol transport. J Lipid Res 1995;36:211–228. [PubMed] [Google Scholar]
- 12.Oram JF. ATP-binding cassette transporter A1 and cholesterol trafficking. Curr Opin Lipidol 2002;13:373–381. [DOI] [PubMed] [Google Scholar]
- 13.McNeish J, Aiello RJ, Guyot D, Turi T, Gabel C, Aldinger C, Hoppe KL, Roach ML, Royer LJ, de Wet J, et al. High density lipoprotein deficiency and foam cell accumulation in mice with targeted disruption of ATP-binding cassette transporter-1. Proc Natl Acad Sci USA 2000;97:4245–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bates SR, Tao JQ, Collins HL, Francone OL, Rothblat GH. Pulmonary abnormalities due to ABCA1 deficiency in mice. Am J Physiol Lung Cell Mol Physiol 2005;289:L980–L989. [DOI] [PubMed] [Google Scholar]
- 15.Agassandian M, Mathur SN, Zhou J, Field FJ, Mallampalli RK. Oxysterols trigger ABCA1-mediated basolateral surfactant efflux. Am J Respir Cell Mol Biol 2004;31:227–233. [DOI] [PubMed] [Google Scholar]
- 16.Zhou J, You Y, Ryan AJ, Mallampalli RK. Upregulation of surfactant synthesis triggers ABCA1-mediated basolateral phospholipid efflux. J Lipid Res 2004;45:1758–1767. [DOI] [PubMed] [Google Scholar]
- 17.Johnson MD, Widdicombe JH, Allen L, Barbry P, Dobbs LG. Alveolar epithelial type I cells contain transport proteins and transport sodium, supporting an active role for type I cells in regulation of lung liquid homeostasis. Proc Natl Acad Sci USA 2002;99:1966–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nielsen S, King LS, Christensen BM, Agre P. Aquaporins in complex tissues. II. Subcellular distribution in respiratory and glandular tissues of rat. Am J Physiol 1997;273:C1549–C1561. [DOI] [PubMed] [Google Scholar]
- 19.Borok Z, Liebler JM, Lubman RL, Foster MJ, Zhou B, Li X, Zabski SM, Kim KJ, Crandall ED. Na transport proteins are expressed by rat alveolar epithelial type I cells. Am J Physiol Lung Cell Mol Physiol 2002;282:L599–L608. [DOI] [PubMed] [Google Scholar]
- 20.Newman GR, Campbell L, von Ruhland C, Jasani B, Gumbleton M. Caveolin and its cellular and subcellular immunolocalisation in lung alveolar epithelium: implications for alveolar epithelial type I cell function. Cell Tissue Res 1999;295:111–120. [DOI] [PubMed] [Google Scholar]
- 21.Ashino Y, Ying X, Dobbs LG, Bhattacharya J. [Ca(2+)](i) oscillations regulate type II cell exocytosis in the pulmonary alveolus. Am J Physiol Lung Cell Mol Physiol 2000;279:L5–L13. [DOI] [PubMed] [Google Scholar]
- 22.Patel AS, Reigada D, Mitchell CH, Bates SR, Margulies SS, Koval M. Paracrine stimulation of surfactant secretion by extracellular ATP in response to mechanical deformation. Am J Physiol Lung Cell Mol Physiol 2005;289:L489–L496. [DOI] [PubMed] [Google Scholar]
- 23.Dahlin K, Mager EM, Allen L, Tigue Z, Goodglick L, Wadehra M, Dobbs L. Identification of genes differentially expressed in rat alveolar type I cells. Am J Respir Cell Mol Biol 2004;31:309–316. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Chen Z, Chintagari NR, Bhaskaran N, Jin N, Narasaraju T, Liu L. Alveolar type I cells protect rat lung epithelium from oxidative injury. J Physiol 2006;572:625–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landry YD, Denis M, Nandi S, Bell S, Vaughan AM, Zha X. ATP-binding cassette transporter A1 expression disrupts raft membrane microdomains through its ATPase-related functions. J Biol Chem 2006;281:36091–36101. [DOI] [PubMed] [Google Scholar]
- 26.Danto SI, Zabski SM, Crandall ED. Reactivity of alveolar epithelial cells in primary culture with type I cell monoclonal antibodies. Am J Respir Cell Mol Biol 1992;6:296–306. [DOI] [PubMed] [Google Scholar]
- 27.Zen K, Notarfrancesco K, Oorschot V, Slot JW, Fisher AB, Shuman H. Generation and characterization of monoclonal antibodies to alveolar type II cell lamellar body membrane. Am J Physiol 1998;275:L172–L183. [DOI] [PubMed] [Google Scholar]
- 28.Dobbs LG, Gonzalez R, Williams MC. An improved method for isolating type II cells in high yield and purity. Am Rev Respir Dis 1986;134:141–145. [DOI] [PubMed] [Google Scholar]
- 29.Bates SR, Tao JQ, Notarfrancesco K, DeBolt K, Shuman H, Fisher AB. Effect of surfactant protein A on granular pneumocyte surfactant secretion in vitro. Am J Physiol Lung Cell Mol Physiol 2003;285:L1055–L1065. [DOI] [PubMed] [Google Scholar]
- 30.Warshamana GS, Corti M, Brody AR. TNF-alpha, PDGF, and TGF-beta(1) expression by primary mouse bronchiolar-alveolar epithelial and mesenchymal cells: tnf-alpha induces TGF-beta(1). Exp Mol Pathol 2001;71:13–33. [DOI] [PubMed] [Google Scholar]
- 31.Tschumperlin DJ, Margulies SS. Equibiaxial deformation-induced injury of alveolar epithelial cells in vitro. Am J Physiol 1998;275:L1173–L1183. [DOI] [PubMed] [Google Scholar]
- 32.Borok Z, Lubman RL, Danto SI, Zhang XL, Zabski SM, King LS, Lee DM, Agre P, Crandall ED. Keratinocyte growth factor modulates alveolar epithelial cell phenotype in vitro: expression of aquaporin 5. Am J Respir Cell Mol Biol 1998;18:554–561. [DOI] [PubMed] [Google Scholar]
- 33.Bhaskaran M, Kolliputi N, Wang Y, Gou D, Chintagari NR, Liu L. Trans-differentiation of alveolar epithelial type II cells to type I cells involves autocrine signaling by transforming growth factor beta 1 through the Smad pathway. J Biol Chem 2007;282:3968–3976. [DOI] [PubMed] [Google Scholar]
- 34.DeBiase PJ, Lane K, Budinger S, Ridge K, Wilson M, Jones JC. Laminin-311 (Laminin-6) fiber assembly by type I-like alveolar cells. J Histochem Cytochem 2006;54:665–672. [DOI] [PubMed] [Google Scholar]
- 35.Liu L, Bortnick AE, Nickel M, Dhanasekaran P, Subbaiah PV, Lund-Katz S, Rothblat GH, Phillips MC. Effects of apolipoprotein A-I on ATP-binding cassette transporter A1-mediated efflux of macrophage phospholipid and cholesterol: formation of nascent high density lipoprotein particles. J Biol Chem 2003;278:42976–42984. [DOI] [PubMed] [Google Scholar]
- 36.Krebs KE, Ibdah JA, Phillips MC. A comparison of the surface activities of human apolipoproteins A-I and A-II at the air/water interface. Biochim Biophys Acta 1988;959:229–237. [DOI] [PubMed] [Google Scholar]
- 37.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Med Sci 1959;37:911–917. [DOI] [PubMed] [Google Scholar]
- 38.Hara H, Yokoyama S. Interaction of free apolipoproteins with macrophages: formation of high density lipoprotein-like lipoproteins and reduction of cellular cholesterol. J Biol Chem 1991;266:3080–3086. [PubMed] [Google Scholar]
- 39.Voelker DR, Mason RJ. Alveolar type II epithelial cells. Lung cell biology, lung biology health and disease series. New York: Marcel Dekker; 1989.
- 40.Bates SR, Gonzales LW, Tao JQ, Rueckert P, Ballard PL, Fisher AB. Recovery of rat type II cell surfactant components during primary cell culture. Am J Physiol Lung Cell Mol Physiol 2002;282:L267–L276. [DOI] [PubMed] [Google Scholar]
- 41.Mulugeta S, Gray JM, Notarfrancesco KL, Gonzales LW, Koval M, Feinstein SI, Ballard PL, Fisher AB, Shuman H. Identification of LBM180, a lamellar body limiting membrane protein of alveolar type II cells, as the ABC transporter protein ABCA3. J Biol Chem 2002;277:22147–22155. [DOI] [PubMed] [Google Scholar]
- 42.Oswari J, Matthay MA, Margulies SS. Keratinocyte growth factor reduces alveolar epithelial susceptibility to in vitro mechanical deformation. Am J Physiol Lung Cell Mol Physiol 2001;281:L1068–L1077. [DOI] [PubMed] [Google Scholar]
- 43.McElroy MC, Pittet JF, Hashimoto S, Allen L, Wiener-Kronish JP, Dobbs LG. A type I cell-specific protein is a biochemical marker of epithelial injury in a rat model of pneumonia. Am J Physiol 1995;268:L181–L186. [DOI] [PubMed] [Google Scholar]
- 44.Duong M, Collins HL, Jin W, Zanotti I, Favari E, Rothblat GH. Relative contributions of ABCA1 and SR-BI to cholesterol efflux to serum from fibroblasts and macrophages. Arterioscler Thromb Vasc Biol 2006;26:541–547. [DOI] [PubMed] [Google Scholar]
- 45.Bortnick AE, Rothblat GH, Stoudt G, Hoppe KL, Royer LJ, McNeish J, Francone OL. The correlation of ATP-binding cassette 1 mRNA levels with cholesterol efflux from various cell lines. J Biol Chem 2000;275:28634–28640. [DOI] [PubMed] [Google Scholar]
- 46.Wu CA, Tsujita M, Hayashi M, Yokoyama S. Probucol inactivates ABCA1 in plasma membrane with respect to its mediation of apolipoprotein binding and high density lipoprotein assembly and to its proteolytic degradation. J Biol Chem 2004;279:30168–30174. [DOI] [PubMed] [Google Scholar]
- 47.Favari E, Zanotti I, Zimetti F, Ronda N, Bernini F, Rothblat GH. Probucol inhibits ABCA1-mediated cellular lipid efflux. Arterioscler Thromb Vasc Biol 2004;24:2345–2350. [DOI] [PubMed] [Google Scholar]
- 48.Duong PT, Collins HL, Nickel M, Lund-Katz S, Rothblat GH, Phillips MC. Characterization of nascent HDL particles and microparticles formed by ABCA1-mediated efflux of cellular lipids to apoA-I. J Lipid Res 2006;47:832–843. [DOI] [PubMed] [Google Scholar]
- 49.Dobbs LG, Williams MC, Gonzalez R. Monoclonal antibodies specific to apical surfaces of rat alveolar type I cells bind to surfaces of cultured, but not freshly isolated, type II cells. Biochim Biophys Acta 1988;970:146–156. [DOI] [PubMed] [Google Scholar]
- 50.Chen J, Chen Z, Narasaraju T, Jin N, Liu L. Isolation of highly pure alveolar epithelial type I and type II cells from rat lungs. Lab Invest 2004;84:727–735. [DOI] [PubMed] [Google Scholar]
- 51.Wang N, Chen W, Linsel-Nitschke P, Martinez LO, Agerholm-Larsen B, Silver DL, Tall AR. A PEST sequence in ABCA1 regulates degradation by calpain protease and stabilization of ABCA1 by apoA-I. J Clin Invest 2003;111:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Denis M, Bissonnette R, Haidar B, Krimbou L, Bouvier M, Genest J. Expression, regulation, and activity of ABCA1 in human cell lines. Mol Genet Metab 2003;78:265–274. [DOI] [PubMed] [Google Scholar]
- 53.Yamauchi Y, Abe-Dohmae S, Yokoyama S. Differential regulation of apolipoprotein A-I/ATP binding cassette transporter A1-mediated cholesterol and phospholipid release. Biochim Biophys Acta 2002;1585:1–10. [DOI] [PubMed] [Google Scholar]
- 54.Berge KE, Tian H, Graf GA, Yu L, Grishin NV, Schultz J, Kwiterovich P, Shan B, Barnes R, Hobbs HH. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science 2000;290:1771–1775. [DOI] [PubMed] [Google Scholar]
- 55.Klucken J, Buchler C, Orso E, Kaminski WE, Porsch-Ozcurumez M, Liebisch G, Kapinsky M, Diederich W, Drobnik W, Dean M, et al. ABCG1 (ABC8), the human homolog of the Drosophila white gene, is a regulator of macrophage cholesterol and phospholipid transport. Proc Natl Acad Sci USA 2000;97:817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakamura K, Kennedy MA, Baldan A, Bojanic DD, Lyons K, Edwards PA. Expression and regulation of multiple murine ATP-binding cassette transporter G1 mRNAs/isoforms that stimulate cellular cholesterol efflux to high density lipoprotein. J Biol Chem 2004;279:45980–45989. [DOI] [PubMed] [Google Scholar]
- 57.Wang N, Lan D, Gerbod-Giannone M, Linsel-Nitschke P, Jehle AW, Chen W, Martinez LO, Tall AR. ATP-binding cassette transporter A7 (ABCA7) binds apolipoprotein A-I and mediates cellular phospholipid but not cholesterol efflux. J Biol Chem 2003;278:42906–42912. [DOI] [PubMed] [Google Scholar]
- 58.Kielar D, Dietmaier W, Langmann T, Aslanidis C, Probst M, Naruszewicz M, Schmitz G. Rapid quantification of human ABCA1 mRNA in various cell types and tissues by real-time reverse transcription-PCR. Clin Chem 2001;47:2089–2097. [PubMed] [Google Scholar]
- 59.Basso F, Freeman L, Knapper CL, Remaley A, Stonik J, Neufeld EB, Tansey T, Amar MJ, Fruchart-Najib J, Duverger N, et al. Role of the hepatic ABCA1 transporter in modulating intrahepatic cholesterol and plasma HDL cholesterol concentrations. J Lipid Res 2003;44:296–302. [DOI] [PubMed] [Google Scholar]
- 60.Brunham LR, Kruit JK, Iqbal J, Fievet C, Timmins JM, Pape TD, Coburn BA, Bissada N, Staels B, Groen AK, et al. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J Clin Invest 2006;116:1052–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singaraja RR, Van Eck M, Bissada N, Zimetti F, Collins HL, Hildebrand RB, Hayden A, Brunham LR, Kang MH, Fruchart JC, et al. Both hepatic and extrahepatic ABCA1 have discrete and essential functions in the maintenance of plasma high-density lipoprotein cholesterol levels in vivo. Circulation 2006;114:1301–1309. [DOI] [PubMed] [Google Scholar]