Abstract
One of the major aspects of airway remodeling in asthma is the development of mucous cell metaplasia (MCM). The role of cytokines in the generation and resolution of MCM has been studied in mice and in isolated airway epithelial cells in culture. However, studies using organ cultures that keep the tubular structure of the airways intact and allow studies in the absence of inflammatory cells have not been reported. We established an organ culture system that replicates the allergen-induced MCM in mice and analyzed the role of Bax in the IFN-γ–induced resolution of MCM. IL-9 or IL-13 induced MCM independently, but a combined IL-9/IL-13 treatment enhanced MCM synergistically. Addition of IFN-γ at 0.1 ng/ml concentration further increased MCM to levels observed in allergen-exposed mice in vivo. However, MCM was reduced when explants were treated with 50 ng/ml IFN-γ after MCM was established. While IL-9/IL-13 induced MCM in bronchioles microdissected from bax+/+ and bax−/− mice to a similar extent, IFN-γ treatment reduced MCM only in bronchioles from bax+/+ but not in bax−/− bronchioles. Restoration of Bax expression in bax−/− bronchioles using an adenoviral expression system reduced IL-9/IL-13–induced MCM while MCM was similar in noninfected or adenoviral green fluorescent protein–infected bax−/− bronchioles. Furthermore, expressing Bax using an adenoviral expression system reduced allergen-induced MCM in mice. These studies show that allergen-induced MCM is a response to a combination of various cytokines at defined concentrations and that IFN-γ requires Bax for the resolution of MCM.
Keywords: lung airway cells, Th1 and Th2 cytokines, apoptosis, bronchiolar organ culture, adenoviral expression
CLINICAL RELEVANCE
Our studies show that in mice, mucous cell metaplasia in an asthma setting is reduced by IFN-γ through a cell death regulator, Bax. Therefore, delivery of Bax to airway cells could be an effective way to reduce mucous secretions in clinical asthma.
Excess mucus production and secretion can be fatal for patients with asthma, but controlling mucous hypersecretion remains a difficult challenge. Several inflammatory mediators have been implicated in the overproduction of mucus in allergic asthma (1). Overexpression of Th2 cytokines such as IL-4 (2, 3), IL-5 (4), IL-9 (5), and IL-13 (6) leads to mucous cell metaplasia (MCM) in animal models. However, Th2 transfer experiments with IL-4−/− (7) or IL-5−/− (8) T cells and MUC5AC promoter activity assays (9) revealed that IL-4 and IL-5 are not required for the development of MCM and mucin biosynthesis and storage. IL-13 is sufficient for the generation of MCM in a manner that is independent of immunoglobulin (Ig)E and eosinophils (10, 11), and transgenic mice overexpressing IL-13 in their lungs show extensive MCM (6). IL-13 induces MCM in cultured primary normal human bronchial epithelial cells (NHBEs) (12, 13) and mouse tracheal epithelial cells (MTECs) (14, 15). Similarly, localized overexpression of IL-9 in the lung causes massive airway inflammation and MUC2 and MUC5AC gene expression (16), and extensive MCM in the airways (5). In addition, instillation of IL-9 directly affects airway epithelial cells to express MUC2 and MUC5AC transcription (9, 16). However, whether IL-9 plays a dominant or a helper role for the development of MCM remains unclear because IL-9−/− mice develop MCM in response to allergen (17). Furthermore, the combined effect of IL-13 and IL-9 is largely unknown.
How IFN-γ, a major Th1 cytokine, affects the Th2-induced MCM is an unresolved question. Using recombinant cytokines, Ford and coworkers (18) show that IFN-γ has double-sided effects on IL-13–induced lung injury. IFN-γ inhibited IL-13–induced goblet cell hyperplasia and the infiltration of the airways with eosinophils and neutrophils but enhanced IL-6 levels and other effects. We also find that IFN-γ mediates the reduction of allergen-induced MCM by inducing programmed cell death (19, 20). Such a double-sided effect of IFN-γ (inhibiting some, potentiating others) on IL-13–induced changes in the lungs has been reported by various groups (21, 22). However, these studies do not address the possibility that the observed differences may be due to variations in local concentrations of these cytokines.
Numerous analyses of airway epithelial cell function have been conducted on human and murine tissues, primary cultures, and cell lines. The use of nonpolarized and or poorly differentiated primary cultures and immortalized cell lines is suboptimal for epithelial cell research because some functional properties of epithelial cells are dependent upon differentiation and appropriately polarized cell organization. These properties rely on extracellular signals, which are generated from cell to cell and cell to substratum interactions. It is increasingly becoming clear that the epithelial–mesenchymal trophic unit plays a role in the response of epithelial cells (23) and that retaining the ultrastructure of the original tissue should be considered when analyzing the response to certain cytokines. Therefore, to elucidate the effect of cytokines on the development and resolution of MCM, we used the culture of microdissected murine terminal bronchioles that maintains the mesenchymal trophic unit without damaging the epithelial integrity. We show that this method enables a physiologically relevant analysis of murine airway epithelial cells in vitro and allows utilization of transgenic mouse models. Our data show that a combination of IL-9 and IL-13 (IL-9/IL-13) synergistically increase MCM, and that IFN-γ at low concentrations potentiates MCM while it inhibits MCM when present at higher concentrations through a Bax-mediated pathway. The possibility of restoring Bax expression and thereby reducing allergen-induced MCM in mice was explored by intratracheal instillation of an adenoviral expression system for Bax into mice.
MATERIALS AND METHODS
Animals
C57BL/6 mice, 6 to 8 weeks of age, were purchased from Jackson Laboratory (Bar Harbor, ME) and housed in isolated cages under specific pathogen–free conditions. Bax+/− mice on C57BL/6 background were received from Dr. S. Korsmeyer (Dana-Farber Institute, Boston, MA) and bax−/− and bax+/+ littermates were bred and genotyped, as previously described (24). All experiments were approved by the Institutional Animal Care and Use Committee and were performed at Lovelace Respiratory Research Institute, a facility approved by the Association for the Assessment and Accreditation for Laboratory Animal Care International.
Microdissected Airway Cultures
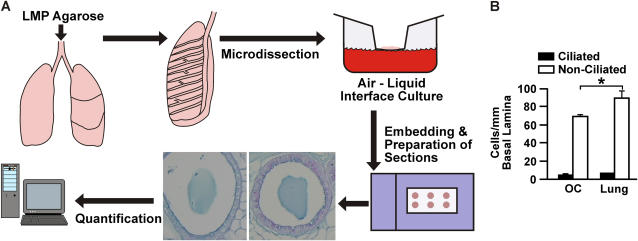
Distal airway bronchioles were removed by microdissection and maintained in culture essentially as described (25, 20). Lungs were inflated with Compatigel low–melting temperature agarose (FMC Bioproducts, Rockland, ME) and immersed in F12 medium during dissection. Distal airway branches were dissected starting from their branch point with the major (axial) airway. Bronchioles from at least three mice were prepared for each treatment to normalize for mouse-to-mouse variability. Up to 16 bronchioles were obtained from the left and right lung lobes of each mouse, and four bronchioles were used for each treatment. Bronchioles were cultured at an air–liquid interface by placing them in the center of the mesh inserts with the long axis of the cylindrical bronchiole fully extended in the plane of the insert membrane. To the bottom chamber 400 μl medium was added, while 60 μl was added to the upper compartment containing the bronchioles to provide a thin film of liquid over the explants. Bronchioles were kept in culture at the air–liquid interface for 7 days while being treated with IL-9 and/or IL-13 at 1, 10, or 20 ng/ml and IFN-γ at 0 or 0.1 ng/ml. On Day 6, 50 ng/ml of IFN-γ were added to the combined IL-9/IL-13 treatment for the last day in culture. Explants were fixed in zinc formalin for 1 to 3 days and pre-embedded in 1% agarose before paraffin embedding and preparation of tissue sections (5 μm) for staining procedures. Figure 1A shows a schematic for processing of lungs. In pilot studies, we compared the number of ciliated and nonciliated cells per mm basal lamina in explants that were maintained in culture for 7 days to the numbers found in bronchioles of similar size in in vivo lungs. Cell numbers remained identical for ciliated cells, and while the number of nonciliated cells per mm basal lamina was significantly higher in airways from in vivo mice, the increase was only by 10% compared with bronchioles maintained in cultures (Figure 1B). Mucous cells were essentially absent in nontreated airways, suggesting that the nonciliated cells represent Clara cells. These findings suggested that the culture conditions did not change the state of differentiation of epithelial cells and that this culture system is well suited for studying the changes in the state of differentiation in response to specific cytokines. During these pilot studies, we decided to exclude from analyses mice that showed mucous cells in control medium without IL-9 and IL-13 treatment, because unknown inflammatory conditions in these mice may skew the effects of treatment with IL-9 and IL-13 in our experiments.
Figure 1.
(A) Schematic describing the process of preparation, processing, and quantification of the bronchiolar organ cultures. (B) The state of differentiation of airway epithelial cells was only minimally affected by maintaining bronchioles in cultures for 7 days, compared with bronchioles in in vivo lung. The numbers of ciliated and nonciliated cells were compared in bronchioles maintained in organ culture (OC) to the numbers in bronchioles of similar size in in vivo lungs (Lung).
Infection with Adenoviral Expression Constructs and Allergen Exposure of Mice
Bronchial explants were infected with an inducible recombinant adenovirus encoding heamagglutinin (HA)-tagged Bax (Ad-Bax) or green fluorescent protein (GFP) (Ad-GFP) (26) at a multiplicity of infection of 200 for the last 2 days of culture by adding the virus to the culture media.
Sensitization and exposure of mice to allergen were as previously described (19). For adenoviral infection of mice exposed to allergen, male C57BL/6 mice (6 wk old) were immunized by intraperitoneal injection with 1 μg of ovalbumin mixed with 100 μg of Al(OH)3 in a volume of 0.5 ml on Days 1 and 7. On Day 14, mice were exposed to ovalbumin aerosol (2 mg/m3) in whole-body exposure chambers for 6 hours per day on 4 consecutive days. The next day, all mice were removed from the exposure chambers, anesthetized, and intranasally instilled with the Ad-GFP as control or with Ad-Bax in 50 μl of saline. Mice were again anesthetized, intranasally instilled with the same expression vectors 24 hours later, and killed the following day. Four mice each were instilled with 107 or 109 plaque-forming units (pfu) of Ad-Bax or Ad-GFP; however, only the results for mice infected with 109 pfu are shown because the HA-tagged Bax could not be detected in mice instilled with 107 pfu, and no difference between the Ad-GFP and Ad-Bax was observed in mice infected at this titer.
Histologic Evaluation
Tissue sections were stained with Alcian Blue and periodic acid Schiff's (AB/PAS) or hematoxylin and eosin (H&E) as described (27, 28). The number of cells per millimeter basal lamina (BL) and the volume (cubic millimeters) of mucus per unit area (nanoliter) of basement membrane (volume density [Vs]) was quantified using an Olympus BH-2 light microscope equipped with the National Institutes of Health image analysis system (Bethesda, MD) as described previously (29). Morphometry in all sections was performed by a person unaware of the exposure history of mice from which the airway tissues were taken.
Immunohistochemistry
Detection of Bax in tissue sections was as described previously (20).
SDS-PAGE and Immunoblotting
Cells were processed for SDS-PAGE as described (30). Protein concentration was determined using the BCA assay (Pierce, Rockford, IL), and equal amounts of protein were electrophoresed unless otherwise stated. Relative protein amounts were determined by densitometry using the GS800 imaging system (Bio-Rad, Hercules, CA). Detection of adenoviral expressed HA-tagged Bax was performed using anti-HA antibodies (Stressgen, San Diego, CA). Antibodies to Bax were purchased from PharMingen (San Diego, CA).
Statistical Analysis
Grouped results from at least four different mice were expressed as mean ± SEM. Results grouped by time point and genotype were analyzed using two-way ANOVA. When significant main effects were detected (P < 0.05), Fisher's least significant difference test was used to determine differences between groups. A P < 0.05 was considered to indicate statistical significance.
RESULTS
IL-9 Enhances IL-13–Induced MCM
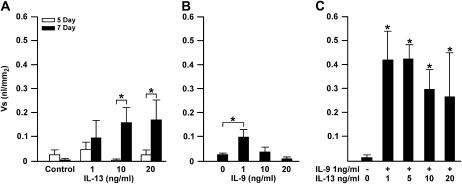
To optimize the induction of MCM in the organ culture system, initially, we treated the bronchiolar explants with increasing concentrations of IL-13 for 5 and 7 days. Results showed that little MCM was observed at 5 days, but significantly higher levels were observed at 7 days in cultures treated with 10 or 20 ng/ml IL-13 (Figure 2A). Reduced amount of stored mucosubstances was observed when explants were treated with 50 ng/ml of IL-13 (data not shown).
Figure 2.
IL-9 and IL-13 are effective inducers of mucous cell metaplasia (MCM). (A) Explant cultures show maximum MCM when cultured for 7 days in the presence of IL-13. Microdissected bronchioles were placed in culture and treated with IL-13 at 0, 1, 10, and 20 ng/ml for 5 or 7 days and processed for Alcian Blue and periodic acid Schiff's (AB/PAS) staining. The development of MCM required maintaining cultures for 7 days. (B) MCM was increased in a dose-dependent manner when treated with 10 or 20 ng/ml of IL-13, but was reduced when treated with 10 or 20 ng/ml of IL-9. Bronchiolar cultures were treated with IL-9 or IL-13 at 0, 1, 10, or 20 ng/ml and harvested after 7 days of culture. (C) IL-9 and IL-13 have a synergistic effect in inducing MCM. Organ cultures were treated with nothing or with a combination of 1 ng/ml IL-9 and either 1, 5, 10, or 20 ng/ml IL-13. A combination of IL-9/IL-13 at 1 ng/ml each induced maximum MCM, as did a combination of 1 ng/ml IL-9 with 5 ng/ml IL-13. Error bars, group means ± SEM (n = 9 mice/group). Asterisk represents a significant difference (P < 0.05).
IL-9 increases MUC5AC gene expression (9) and modulates mucus production (31), and administration of antibody to IL-9 inhibits the development of MCM (32) by a pathway different from that of IL-13 (33, 34). To explore the role of IL-9 on the induction of MCM, explant cultures were treated with increasing concentrations of IL-9. Interestingly, MCM was highest at 1 ng/ml IL-9, but was reduced with increasing IL-9 concentrations at 10 and 20 ng/ml (Figure 2B). Various concentrations of IL-4 had no effect on the development of MCM in this culture system (data not shown), suggesting that IL-13 and IL-9 were the main players for inducing MCM by directly affecting airway epithelial cells.
We further tested whether IL-13 together with IL-9 may have additive or synergistic effects in inducing MCM. Because a concentration higher than 1 ng/ml IL-9 appeared to have a suppressive effect on MCM, we kept IL-9 at a concentration of 1 ng/ml and tested whether increasing the concentration of IL-13 would enhance the levels of intraepithelial stored mucosubstances. IL-9 and IL-13 at 1 ng/ml each, or a combination of 1 ng/ml IL-9 with 5 ng/ml IL-13, showed maximum MCM that was double the levels induced by IL-13 only at 20 ng/ml (Figure 2C). It appeared that the combination of these cytokines at low concentrations has a synergistic effect on MCM.
Low Concentrations of IFN-γ Enhance MCM when Added Together with IL-9/IL-13
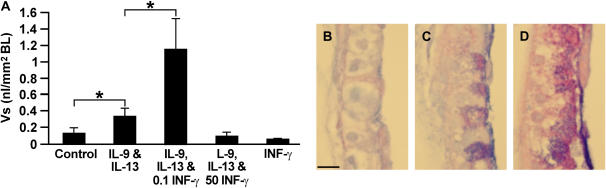
To determine the role of low IFN-γ concentrations on IL-9/IL-13–induced MCM, bronchioles were treated with the IL-9/IL-13 (1 ng/ml each) and 0.1 ng/ml IFN-γ from Day 1. Interestingly, IFN-γ at 0.1 but not at 50 ng/ml enhanced the IL-9/IL-13–induced MCM (Figures 3A–3D). In control studies, IFN-γ alone at 0.1 or at higher concentrations (data not shown) had no visible effect on airway epithelia. In addition, higher concentrations of IFN-γ when added to either IL-9 or IL-13 from Day 1 of culture did not enhance the IL-9/IL-13–induced MCM (data not shown).
Figure 3.
IFN-γ at low concentration enhances IL-9 and IL-13-induced MCM. (A) Microdissected airway bronchioles were placed in culture in an air–liquid interface and treated for 7 days with IL-9 and IL-13 at 1 ng/ml each, or with IL-9/IL-13 together with 0.1 or 50 ng/ml IFN-γ, and with 0.1 IFN-γ or vehicle as controls. Tissues were processed for AB/PAS staining, and the volume density (Vs) per square millimeter basal lamina (nl/mm2 BL) was quantified by morphometry. Bars = group means ± SEM (n = 6−8 mice/group). *Significantly different from untreated controls (P < 0.05). (B) Representative photomicrographs of AB/PAS-stained tissues; vehicle or 0.1 ng/ml IFN-γ–treated controls (B), IL-9/IL-13–treated (C), and IL-9/IL-13/IFN-γ–treated (D) tissue sections are shown.
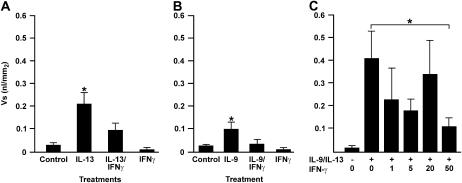
IFN-γ Reduces Established IL-9/IL-13–Induced MCM
We have shown that during prolonged exposure to allergen, high concentrations of IFN-γ are responsible for reducing MCM (19). Therefore, we investigated whether increasing IFN-γ concentrations causes a reduction in MCM when present after IL-13/IL-9–induced MCM is established. Bronchioles were treated for 7 days with IL-9 or with IL-13, and 50 ng/ml IFN-γ was added on the last day of culture. IFN-γ reduced MCM that was induced by 20 ng/ml IL-13 (Figure 4A) or 1 ng/ml IL-9 (Figure 4B). Furthermore, IFN-γ was also effective in reducing IL-9/IL-13–induced MCM (Figure 4C). Treatment of these cultures with IFN-γ at 50 ng/ml most consistently reduced MCM, while lower concentrations were not as effective (Figure 4C).
Figure 4.
Treatment of bronchiolar cultures with 50 ng/ml IFN-γ on Day 6 of culture reduced IL-9– and IL-13–induced MCM. Bronchial explants were either left untreated or treated with 20 ng/ml IL-13 (A) or 1 ng/ml IL-9 (B) for 7 days and with 50 ng/ml IFN-γ on the last day of culture. As a further control, explants were treated with 50 ng/ml IFN-γ on the last day of culture. (C) Microdissected bronchioles were treated with either nothing or with IL-9/IL-13 at 1 ng/ml each and with 1, 5, 20, or 50 ng/ml IFN-γ on the last day of culture. Error bars, group means ± SEM (n = 6 mice/group). Asterisk represents a significant difference (P < 0.05).
Bax Mediates IFN-γ–Induced Reduction of IL-9/IL-13–Induced MCM
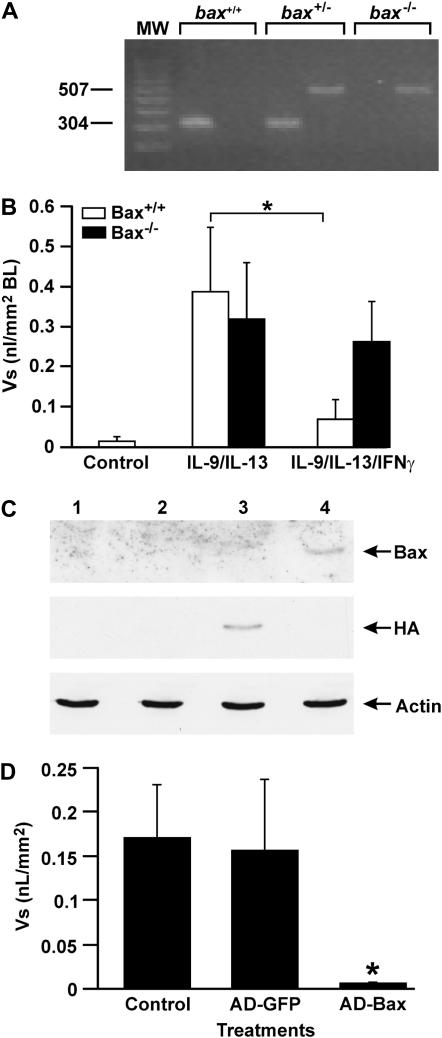
Our previous studies have suggested that IFN-γ induces cell death in proliferating airway epithelial cells through STAT1 by a Bax-mediated pathway (19, 20). To determine whether IFN-γ may be acting through Bax to reduce IL-9/IL-13–induced MCM, we microdissected bronchioles from bax−/− mouse lungs and treated them with nothing as control or with IL-13/IL-9 at 1 ng/ml each for 7 days. A PCR analysis from genomic DNA was used to identify bax−/− mice from the bax+/− and bax+/+ progenitors (Figure 5A). IL-9/IL-13 induced MCM in bax+/+ and bax−/− bronchioles to a similar extent (Figure 5B). However, addition of IFN-γ to these explant cultures significantly reduced MCM in bax+/+ but not in bax−/− bronchioles (Figure 5B).
Figure 5.
Resolution of IL-9/IL-13–induced MCM is dependent on Bax. (A) Representative figure showing a PCR analysis of genomic DNA from bax+/+, bax+/−, and bax−/− mice. (B) Bronchioles were microdissected from bax+/+ and bax−/− mice, placed in culture, and treated with IL-9 and IL-13 for 6 days, followed by 50 ng/ml IFN-γ treatment on Day 7. (C) Bronchioles were harvested and processed for quantification of MCM. Lanes 1 and 4 represent proteins from explants that were from bax+/+ or bax−/− mice, respectively. Bronchioles from bax−/− mice were infected with adenoviral expression system for Bax (lane 3) or green fluorescent protein (GFP) (lane 2) as control, harvested 2 days later, and analyzed by Western blotting. Hemagglutinin (HA)-tagged Bax was detected only in bronchioles infected with Ad-Bax. (D) Bronchioles from bax+/+ and bax−/− mice were placed in culture, treated with IL-9/IL-13 at 1 ng/ml each, and infected with Ad-Bax or Ad-GFP. Bronchioles were harvested, processed for AB/PAS staining, and analyzed for MCM. Error bars, group means ± SEM (n = 4 mice/group). Asterisk represents a significant difference (P < 0.05).
It is known that deficiency in one protein may cause overexpression of other proteins, or cells activate other compensatory mechanisms that could mislead interpretations of results as to the importance of the targeted protein. To fully determine whether Bax is responsible for the lack of resolution, we reconstituted its expression in bax−/− bronchioles. To determine whether Bax expression reduces MCM, the bax−/− bronchioles were infected with an adenoviral expression vector at a multiplicity of infection of approximately 200 for the last 2 days of culture. Infection of these cultures was determined by detecting Ad-Bax by Western blot analysis in tissues transfected with an adenoviral expression vector but not in control tissues infected with Ad-GFP (Figure 5C). As expected, HA and Bax were detected only in Ad-Bax–infected bax−/− bronchioles, and only Bax but not HA was detected in bronchiolar cultures from bax+/+ mice (Figure 5C). In a separate set of experiments, bax−/− bronchiolar tissues were processed for AB/PAS, and MCM was quantified by morphometry. Results showed that MCM was present in epithelia treated with IL-9 and IL-13 or with control Ad-GFP, while MCM was absent in bronchioles infected with Ad-Bax (Figure 5D). These studies suggest that Bax is crucial for IFN-γ–induced resolution of allergen-induced MCM.
Expression of Bax Reduces Allergen-Induced MCM in C57BL/6 Mice
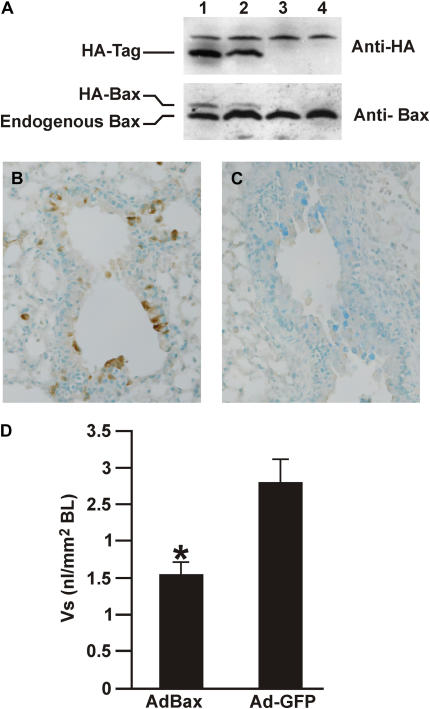
To investigate whether the expression of Bax would reduce allergen-induced MCM in vivo, mice were exposed to allergen for 5 days, when MCM is maximum (19), and were instilled with 109 pfu Ad-Bax or Ad-GFP as control. Lungs from Ad-Bax– and Ad-GFP–instilled mice were analyzed by Western blotting for expression of the HA tag and for Bax. While Bax was expressed in all mice, the HA-tagged Bax that runs as a larger protein was only observed in mice that were instilled with Ad-Bax (Figure 6A), but not in mice instilled with Ad-GFP (Figure 6A).
Figure 6.
Mice instilled with allergen for 5 days were infected with Ad-GFP or Ad-Bax. (A) Western blot analysis showing expression of HA-tagged Bax in 2 representative lungs of Ad-Bax- (lanes 1 and 2) but not in Ad-GFP–instilled (lanes 3 and 4) mice. A protein that cross-reacts with the HA-tag antibody was visible in all lanes. HA was detected by immunohistochemistry in epithelial cells of Ad-Bax– (B) but not Ad-GFP (C)–instilled mice. (D) MCM was significantly reduced in the airways of mice instilled with Ad-Bax compared with those instilled with Ad-GFP. Error bars, group means ± SEM (n = 4 mice/group). *Significantly different from Ad-Bax (P < 0.05).
To determine which cells were expressing the HA-tagged Bax, lung tissue sections were analyzed by immunohistochemistry using anti-HA antibodies. Immunohistochemistry with antibodies against the HA-tag identified that the HA-tag was primarily observed in airway epithelial cells (AECs), but peripheral lung cells were also staining positive for the HA-tag as shown by the brown staining (Figure 6B) in Ad-Bax–instilled mice. The HA-tag was not detected in mice infected with Ad-GFP (Figure 6C). This finding demonstrated that the adenovirus had successfully expressed the HA-Bax in epithelial cells. Further evidence for the effect of expressing Bax in epithelial cells stemmed from the quantifications for MCM. Both the Vs of the amount of intraepithelial stored mucosubstances (Figure 6D) and the mucous cells per millimeter basal lamina (data not shown) showed that MCM was reduced with Ad-Bax compared with those bronchioles instilled with Ad-GFP.
DISCUSSION
The present studies show that the organ culture system with microdissected bronchioles is an effective approach to study the role of cytokines on airway epithelial cells without the potential confounding factors emanating from inflammatory cells present in whole animal models. Using this system, we show that IL-13 when present alone induces MCM in a concentration-dependent manner. Interestingly, a high concentration of this cytokine has an inhibitory effect on MCM. These findings were also noted by other investigators using well-differentiated airway epithelial cultures isolated from humans (12) or mice (14). IL-13 activates the IL-13Rα1 and IL-4Rα to activate STAT6 and cause MCM (6, 10, 15). Other STAT6-independent pathways mediated by activating the phosphatidylinositol 3-kinase and the mitogen-activated protein kinase (MAPK) have also been proposed for IL-13–induced mucin gene expression in primary epithelial cells (12, 35, 36). We were unable to induce MCM in STAT1−/− bronchioles, suggesting that STAT1 may be involved in the development of MCM by IL-9/IL-13 (data not shown).
Similar to IL-13, IL-9 increased MCM at a low concentration but had an inhibitory effect at higher concentrations. Interestingly, IL-9 alone only minimally increased MCM, suggesting that it is not as prominent as IL-13. However, this cytokine enhanced IL-13–induced MCM in a synergistic way. The minor role of IL-9 in inducing MCM when present alone is consistent with previous studies demonstrating that the development of allergen-induced MCM is not affected in IL-9–deficient mice because IL-13 may have been present to induce MCM (17). Furthermore, a recent study suggests that IL-9 promotes asthma through IL-13–independent pathways via expansion of mast cells, eosinophils, and B cells, and requires the production of IL-13 by hematopoietic cells for mucus production and recruitment of eosinophils by lung epithelial cells (37). However, several other studies show that IL-9 affects airway epithelial cells directly. Instillation of IL-9 causes MUC5AC expression before the presence of IL-13 mRNA in lung tissues or in the lavage fluid (38). Furthermore, IL-9 increases MUC2 and MUC5AC transcription in the mouse epidermoid carcinoma cell line NCI-H292 and primary NHBEs (9), suggesting that IL-9 can have a direct effect in inducing MCM.
Detailed studies on the combined effects of cytokines on the development of MCM have not been reported. Our studies suggest that IL-9 and IL-13 may independently regulate mucin biosynthesis and storage by directly affecting airway epithelial cells. The fact that IL-9 and IL-13 showed a synergistic effect on MCM in our studies supports the idea that IL-9 acts through a pathway different from IL-13 to induce MCM. Our findings are consistent with reports that IL-9 and IL-13 during injury and repair show stark differences in inducing MCM (33). IL-13 confers the asthma-like phenotype and MCM through the IL-4Rα chain (39, 40) using the STAT6-dependent pathway (35, 41). Furthermore, several studies suggest the IL-13–induced MCM also involves the p38α MAPK (36, 42). Because the signaling pathway(s) responsible for IL-9–induced MCM are currently not known, it is not clear how the synergy between these two cytokines is processed.
MCM was enhanced when IFN-γ was present at low concentrations with IL-9/IL-13 before MCM was established. These MCM levels were similar to what is occasionally observed in mice after exposure to allergen for 5 days (19). In the current study, when mice were exposed to allergen and instilled with Ad-GFP, they appeared to have increased MCM compared with what was observed in previous studies without viral instillation. The enhancing effect of IFN-γ on IL-9/IL-13–induced MCM is consistent with our previous report that showed allergen-induced MCM to be 3-fold higher in bim−/− compared with bim+/+ mice despite similar levels of IL-13 (43). IFN-γ levels in the lung tissue of naive bim−/− mice were higher compared with bim+/+ mice because of the higher number of T cells present as Bim regulates T cell death (44, 45). Our previous study with bim−/− mice suggested that the presence of IFN-γ may have enhanced Th2-induced MCM. IFN-γ primarily signals through STAT1 (46), but some of its signaling is also STAT1-independent (47). Whether the role of IFN-γ–enhancing MCM is mediated by STAT1 is currently unknown. Understanding the pathway by which IL-9 acts, and the role of STAT6 activation in combination with activation of the STAT1 and MAPK pathways, is essential to completely explain the development of MCM in asthma.
MCM was reduced when IFN-γ was added at higher concentrations on the last day of culture. This finding replicates our previous observation that IFN-γ levels during prolonged exposure to allergen are increased at 10 days of allergen exposure and that instillation of increasing IFN-γ concentrations reduces allergen-induced MCM (19). Our studies suggest that essentially all the cell death–inducing activity of IFN-γ is mediated through STAT1 (19). Furthermore, that IFN-γ was ineffective in reducing MCM in bax−/− bronchioles confirms our previous observation based on prolonged exposure of bax+/+ and bax−/− mice (20). The present studies show that deficiency of Bax in epithelial cells is responsible for the lack of IFN-γ, thereby causing a reduction of MCM. Furthermore, restoration of Bax expression using the adenoviral expression system causes a reduction of IL-9– and IL-13–induced MCM. However, identifying the cells that undergo apoptosis has been difficult. This could be due to the speed by which the dying cells are removed or due to the fact that they may be undergoing a type of apoptosis that is not classic in that the dying cells show condensed nuclei. Future studies will address this problem.
Instilling adenoviral expression vectors for Bax reduced the number of metaplastic mucous cells compared with the Ad-GFP–instilled controls. Interestingly, while the Bax protein is present in the AECs as detected by Western blot analysis, the endogenous Bax appears to be inactive. However, the Bax derived from the adenoviral expression system appears to be in an active form for reducing MCM.
Furthermore, HA-tagged Bax was not only detected in mucous airway epithelial cells but also in other cell types. Interestingly, in both the organ cultures and in in vivo mice instilled with Ad-Bax, the integrity of the epithelium did not seem to be disrupted because no edema or complete denudation of the epithelium was observed. These studies suggest that the majority of epithelial cells are resistant to Bax-induced cell death. This observation is supported by the fact that only proliferating AECs are susceptible to IFN-γ–induced cell death, while confluent cultures are minimally affected (19). It is possible that hyperplastic mucous cells represent hyperplastic epithelial cells, and expressing Bax may selectively target hyperplastic cells to cause cell death while resting AECs are not affected. The resistance of resting AECs to Bax-induced cell death may ultimately result in the reduction of MCM without affecting the integrity of the epithelium.
In summary, bronchiolar cultures that do not compromise the differentiation of the airway epithelium, the subepithelial structures, or the tubular structures of the airways are useful to elucidate the development of MCM in response to a combination of cytokines that represent the milieu in an asthmatic condition. Furthermore, the use of bronchioles from transgenic mice allowed us to study the importance of IFN-γ to resolve IL-9/IL-13–induced MCM by a Bax-mediated pathway. The importance of Bax in resolving MCM was verified in allergen-exposed mice.
Acknowledgments
The authors thank Yoneko Knighton and Lois Herrera for preparing tissue samples for light microscopic analyses.
These studies were supported by grants from the National Institutes of Health (HL068111 and ES09237 to Y.T.).
Originally Published in Press as DOI: 10.1165/rcmb.2007-0078OC on September 27, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Fahy JV, Corry DB, Boushey HA. Airway inflammation and remodeling in asthma. Curr Opin Pulm Med 2000;6:15–20. [DOI] [PubMed] [Google Scholar]
- 2.Rankin JA, Picarella DE, Geba GP, Temann UA, Prasad B, DiCosmo B, Tarallo A, Stripp B, Whitsett J, Flavell RA. Phenotypic and physiologic characterization of transgenic mice expressing interleukin 4 in the lung: lymphocytic and eosinophilic inflammation without airway hyperreactivity. Proc Natl Acad Sci USA 1996;93:7821–7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dabbagh K, Takeyama K, Lee HM, Ueki IF, Lausier JA, Nadel JA. IL-4 induces mucin gene expression and goblet cell metaplasia in vitro and in vivo. J Immunol 1999;162:6233–6237. [PubMed] [Google Scholar]
- 4.Lee JJ, McGarry MP, Farmer SC, Denzler KL, Larson KA, Carrigan PE, Brenneise IE, Horton MA, Haczku A, Gelfand EW, et al. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J Exp Med 1997;185:2143–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Temann UA, Geba GP, Rankin JA, Flavell RA. Expression of interleukin 9 in the lungs of transgenic mice causes airway inflammation, mast cell hyperplasia, and bronchial hyperresponsiveness. J Exp Med 1998;188:1307–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest 1999;103:779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohn L, Homer RJ, Marinov A, Rankin J, Bottomly K. Induction of airway mucus production by T helper 2 (Th2) cells: a critical role for interleukin 4 in cell recruitment but not mucus production. J Exp Med 1997;186:1737–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohn L, Homer RJ, MacLeod H, Mohrs M, Brombacher F, Bottomly K. Th2-induced airway mucus production is dependent on IL-4R alpha, but not on eosinophils. J Immunol 1999;162:6178–6183. [PubMed] [Google Scholar]
- 9.Longphre M, Li D, Gallup M, Drori E, Ordonez CL, Redman T, Wenzel S, Bice DE, Fahy JV, Basbaum C. Allergen-induced IL-9 directly stimulates mucin transcription in respiratory epithelial cells. J Clin Invest 1999;104:1375–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science 1998;282:2258–2261. [DOI] [PubMed] [Google Scholar]
- 11.Zuhdi Alimam M, Piazza FM, Selby DM, Letwin N, Huang L, Rose MC. Muc-5/5ac mucin messenger RNA and protein expression is a marker of goblet cell metaplasia in murine airways. Am J Respir Cell Mol Biol 2000;22:253–260. [DOI] [PubMed] [Google Scholar]
- 12.Atherton HC, Jones G, Danahay H. IL-13-induced changes in the goblet cell density of human bronchial epithelial cell cultures: MAP kinase and phosphatidylinositol 3-kinase regulation. Am J Physiol Lung Cell Mol Physiol 2003;285:L730–L739. [DOI] [PubMed] [Google Scholar]
- 13.Martin LD, Norford D, Voynow J, Adler KB. Response of human airway epithelium In vitro to inflammatory mediators: dependence on the state of cellular differentiation. Chest 2000;117:267S. [DOI] [PubMed] [Google Scholar]
- 14.Lankford SM, Macchione M, Crews AL, McKane SA, Akley NJ, Martin LD. Modeling the airway epithelium in allergic asthma: interleukin-13- induced effects in differentiated murine tracheal epithelial cells. In Vitro Cell Dev Biol Anim 2005;41:217–224. [DOI] [PubMed] [Google Scholar]
- 15.Kuperman DA, Huang X, Koth LL, Chang GH, Dolganov GM, Zhu Z, Elias JA, Sheppard D, Erle DJ. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med 2002;8:885–889. [DOI] [PubMed] [Google Scholar]
- 16.Louahed J, Toda M, Jen J, Hamid Q, Renauld JC, Levitt RC, Nicolaides NC. Interleukin-9 upregulates mucus expression in the airways. Am J Respir Cell Mol Biol 2000;22:649–656. [DOI] [PubMed] [Google Scholar]
- 17.McMillan SJ, Bishop B, Townsend MJ, McKenzie AN, Lloyd CM. The absence of interleukin 9 does not affect the development of allergen-induced pulmonary inflammation nor airway hyperreactivity. J Exp Med 2002;195:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ford JG, Rennick D, Donaldson DD, Venkayya R, McArthur C, Hansell E, Kurup VP, Warnock M, Grunig G. Il-13 and IFN-gamma: interactions in lung inflammation. J Immunol 2001;167:1769–1777. [DOI] [PubMed] [Google Scholar]
- 19.Shi ZQ, Fischer MJ, De Sanctis GT, Schuyler M, Tesfaigzi Y. IFNγ but not Fas mediates reduction of allergen-induced mucous cell metaplasia by inducing apoptosis. J Immunol 2002;168:4764–4771. [DOI] [PubMed] [Google Scholar]
- 20.Tesfaigzi Y, Fischer MJ, Green FHY, De Sanctis GT, Wilder JA. Bax is crucial for IFNγ-induced resolution of allergen-induced mucous cell metaplasia. J Immunol 2002;169:5919–5925. [DOI] [PubMed] [Google Scholar]
- 21.Randolph DA, Stephens R, Carruthers CJ, Chaplin DD. Cooperation between Th1 and Th2 cells in a murine model of eosinophilic airway inflammation. J Clin Invest 1999;104:1021–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen G, Berry G, DeKruyff RH, Umetsu DT. Allergen-specific Th1 cells fail to counterbalance Th2 cell-induced airway hyperreactivity but cause severe airway inflammation. J Clin Invest 1999;103:175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knight DA, Lane CL, Stick SM. Does aberrant activation of the epithelial-mesenchymal trophic unit play a key role in asthma or is it an unimportant sideshow? Curr Opin Pharmacol 2004;4:251–256. [DOI] [PubMed] [Google Scholar]
- 24.Knudson CM, Tung KS, Tourtellotte WG, Brown GA, Korsmeyer SJ. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science 1995;270:96–99. [DOI] [PubMed] [Google Scholar]
- 25.Van Winkle LS, Buckpitt AR, Plopper CG. Maintenance of differentiated murine Clara cells in microdissected airway cultures. Am J Respir Cell Mol Biol 1996;14:586–598. [DOI] [PubMed] [Google Scholar]
- 26.Xiang J, Gomez-Navarro J, Arafat W, Liu B, Barker SD, Alvarez RD, Siegal GP, Curiel DT. Pro-apoptotic treatment with an adenovirus encoding Bax enhances the effect of chemotherapy in ovarian cancer. J Gene Med 2000;2:97–106. [DOI] [PubMed] [Google Scholar]
- 27.El-Zimaity HM, Ota H, Scott S, Killen DE, Graham DY. A new triple stain for Helicobacter pylori suitable for the autostainer: carbol fuchsin/Alcian blue/hematoxylin-eosin. Arch Pathol Lab Med 1998;122:732–736. [PubMed] [Google Scholar]
- 28.Spicer SS, Chakrin LW, Wardell JR Jr, Kendrick W. Histochemistry of mucosubstances in the canine and human respiratory tract. Lab Invest 1971;25:483–490. [PubMed] [Google Scholar]
- 29.Harkema JR, Hotchkiss JA. In vivo effects of endotoxin on nasal epithelial mucosubstances: quantitative histochemistry. Exp Lung Res 1991;17:743–761. [DOI] [PubMed] [Google Scholar]
- 30.Tesfaigzi J, Smith-Harrison W, Carlson DM. A simple method for reusing Western blots on PVDF membranes. Biotechniques 1994;17:268–269. [PubMed] [Google Scholar]
- 31.Townsend JM, Fallon GP, Matthews JD, Smith P, Jolin EH, McKenzie NA. IL-9-deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity 2000;13:573–583. [DOI] [PubMed] [Google Scholar]
- 32.Kung TT, Luo B, Crawley Y, Garlisi CG, Devito K, Minnicozzi M, Egan RW, Kreutner W, Chapman RW. Effect of anti-mIL-9 antibody on the development of pulmonary inflammation and airway hyperresponsiveness in allergic mice. Am J Respir Cell Mol Biol 2001;25:600–605. [DOI] [PubMed] [Google Scholar]
- 33.Vermeer PD, Harson R, Einwalter LA, Moninger T, Zabner J. Interleukin-9 induces goblet cell hyperplasia during repair of human airway epithelia. Am J Respir Cell Mol Biol 2003;28:286–295. [DOI] [PubMed] [Google Scholar]
- 34.Whittaker L, Niu N, Temann UA, Stoddard A, Flavell RA, Ray A, Homer RJ, Cohn L. Interleukin-13 mediates a fundamental pathway for airway epithelial mucus induced by CD4 T cells and interleukin-9. Am J Respir Cell Mol Biol 2002;27:593–602. [DOI] [PubMed] [Google Scholar]
- 35.Lee PJ, Zhang X, Shan P, Ma B, Lee CG, Homer RJ, Zhu Z, Rincon M, Mossman BT, Elias JA. ERK1/2 mitogen-activated protein kinase selectively mediates IL-13-induced lung inflammation and remodeling in vivo. J Clin Invest 2006;116:163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duan W, Chan JH, Wong CH, Leung BP, Wong WS. Anti-inflammatory effects of mitogen-activated protein kinase kinase inhibitor U0126 in an asthma mouse model. J Immunol 2004;172:7053–7059. [DOI] [PubMed] [Google Scholar]
- 37.Steenwinckel V, Louahed J, Orabona C, Huaux F, Warnier G, McKenzie A, Lison D, Levitt R, Renauld JC. IL-13 mediates in vivo IL-9 activities on lung epithelial cells but not on hematopoietic cells. J Immunol 2007;178:3244–3251. [DOI] [PubMed] [Google Scholar]
- 38.Reader JR, Hyde DM, Schelegle ES, Aldrich MC, Stoddard AM, McLane MP, Levitt RC, Tepper JS. Interleukin-9 induces mucous cell metaplasia independent of inflammation. Am J Respir Cell Mol Biol 2003;28:664–672. [DOI] [PubMed] [Google Scholar]
- 39.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 1998;282:2261–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grunewald SM, Werthmann A, Schnarr B, Klein CE, Brocker EB, Mohrs M, Brombacher F, Sebald W, Duschl A. An antagonistic IL-4 mutant prevents type I allergy in the mouse: inhibition of the IL-4/IL-13 receptor system completely abrogates humoral immune response to allergen and development of allergic symptoms in vivo. J Immunol 1998;160:4004–4009. [PubMed] [Google Scholar]
- 41.Kuperman D, Schofield B, Wills-Karp M, Grusby MJ. Signal transducer and activator of transcription factor 6 (Stat6)-deficient mice are protected from antigen-induced airway hyperresponsiveness and mucus production. J Exp Med 1998;187:939–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duan W, Chan JH, McKay K, Crosby JR, Choo HH, Leung BP, Karras JG, Wong WS. Inhaled p38alpha mitogen-activated protein kinase antisense oligonucleotide attenuates asthma in mice. Am J Respir Crit Care Med 2005;171:571–578. [DOI] [PubMed] [Google Scholar]
- 43.Pierce J, Rir-Sima-Ah J, Estrada I, Wilder JA, Strasser A, Tesfaigzi Y. Loss of pro-apoptotic Bim promotes accumulation of pulmonary T lymphocytes and enhances allergen-induced goblet cell metaplasia. Am J Physiol Lung Cell Mol Physiol 2006;291:L862–L870. [DOI] [PubMed] [Google Scholar]
- 44.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, Adams JM, Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 1999;286:1735–1738. [DOI] [PubMed] [Google Scholar]
- 45.Bouillet P, Purton JF, Godfrey DI, Zhang LC, Coultas L, Puthalakath H, Pellegrini M, Cory S, Adams JM, Strasser A. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature 2002;415:922–926. [DOI] [PubMed] [Google Scholar]
- 46.Meraz MA, White JM, Sheehan KC, Bach EA, Rodig SJ, Dighe AS, Kaplan DH, Riley JK, Greenlund AC, Campbell D, et al. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell 1996;84:431–442. [DOI] [PubMed] [Google Scholar]
- 47.Ramana CV, Gil MP, Schreiber RD, Stark GR. Stat1-dependent and -independent pathways in IFN-gamma-dependent signaling. Trends Immunol 2002;23:96–101. [DOI] [PubMed] [Google Scholar]