Abstract
Ozone is known to produce an acute influx of neutrophils, and alveolar epithelial cells can secrete chemokines and modulate inflammatory processes. However, direct exposure of alveolar epithelial cells and macrophages to ozone (O3) produces little chemokine response. To determine if cell–cell interactions might be responsible, we investigated the effect of alveolar macrophage–conditioned media after ozone exposure (MO3CM) on alveolar epithelial cell chemokine production. Serum-free media were conditioned by exposing a rat alveolar macrophage cell line NR8383 to ozone for 1 hour. Ozone stimulated secretion of IL-1α, IL-1β, and IL-18 from NR8383 cells, but there was no secretion of chemokines or TNF-α. Freshly isolated type II cells were cultured, so as to express the biological markers of type I cells, and these cells are referred to as type I–like cells. Type I–like cells were exposed to diluted MO3CM for 24 hours, and this conditioned medium stimulated secretion of cytokine-induced neutrophil chemattractant-1 (CXCL1) and monocyte chemoattractant protein-1 (CCL2). Secretion of these chemokines was inhibited by the IL-1 receptor antagonist. Although both recombinant IL-1α and IL-1β stimulated alveolar epithelial cells to secrete chemokines, recombinant IL-1α was 100-fold more potent than IL-1β. Furthermore, neutralizing anti-rat IL-1α antibodies inhibited the secretion of chemokines by alveolar epithelial cells, whereas neutralizing anti-rat IL-1β antibodies had no effect. These observations indicate that secretion of IL-1α from macrophages stimulates alveolar epithelial cells to secrete chemokines that can elicit an inflammatory response.
Keywords: alveolar epithelial cells, cytokine-induced neutrophil chemoattractant, monocyte chemoattractant protein-1, IL-1α, cell–cell interactions
CLINICAL RELEVANCE
The acute inflammatory response produced by ozone is likely due to cell–cell interactions, whereby one cell signals another to secrete chemokines. The clinical implication is that the inflammatory response could be blocked by interrupting this paracrine signaling with drugs like IL-1 antagonists.
Ozone is a prominent air pollutant in temperate urban settings and has been reported to have significant adverse human health effects. The effect of ozone on the pulmonary system has been studied extensively in both human and animal models (1–3). In vivo, exposure to ozone induces acute injury and an influx of neutrophils. Ozone has been reported to stimulate resident lung cells to secrete a variety of chemoattractants in vivo (4–6). However, ozone is highly reactive and penetrates biologic fluids poorly. Pryor estimated the diffusion distance for ozone in lung lining fluid to be only 0.1 μm (7). Hence, surface cells such as alveolar epithelial cells, alveolar macrophages, and epithelial cells of the conducting airway are among the few cell types that come in direct contact with inhaled ozone. These cells should be involved in eliciting the acute inflammatory response.
In our previous studies in vitro, we found that exposure to ozone induces toxicity in alveolar epithelial cells but without a substantial increase in chemokine production (8–10). Isolated human alveolar macrophages and THP-1 cells derived from monocytic leukemic cells also fail to secrete chemokines in response to ozone (11, 12). However, others have shown a modest secretion of chemokines and cytokines by epithelial cells in response to ozone in A549 cells, BEAS-2B cells, and human nasal epithelial cells (12–15). Hence, there appears to be a discrepancy between the limited chemokine production by direct ozone exposure in vitro and the obvious neutrophil influx in vivo (16).
The present study was designed to demonstrate if chemokine secretion was due to a cell–cell interaction as opposed to a direct ozone effect. Specifically, we sought to identify the soluble factors released from alveolar macrophages that stimulate chemokine production from alveolar epithelial cells. One of these factors was found to be IL-1α. To our knowledge, this is the first study to demonstrate cross talk between alveolar macrophages and alveolar epithelial cells to produce chemokines that are important for recruitment of neutrophils in response to ozone.
MATERIAL AND METHODS
Cell Lines
NR8383 rat alveolar macrophages were purchased from American Type Culture Collection (Rockville, MD) and maintained in Dulbecco's Modified Minimal Essential Medium (DMEM) plus 10% fetal bovine serum (FBS) and antibiotics.
Rat Alveolar Epithelial Cell Isolation and Culture
Alveolar type II cells were isolated from pathogen-free adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) by dissociation with porcine pancreatic elastase (Roche Molecular Biochemicals, Indianapolis, IN) and partial purification on discontinuous density gradients by standard methods (10, 17). Type II cells were plated on 6-well tissue culture plastic plates. One million freshly isolated viable type II cells were plated in 2 ml of DMEM containing 5% rat serum (RS) (Pel-Freez Biologicals, Rogers, AR), 2 mM glutamine, 2.5 μg/ml amphotericin B, 100 μg/ml streptomycin, 100 units/ml penicillin G (all from GIBCO BRL, Life Technologies Inc., Rockville, MD), and 10 μg/ml gentamicin (Sigma-Aldrich, St. Louis, MO). After attachment for 24 hours, the cells were rinsed twice with DMEM. To establish a type I–like cell phenotype, the alveolar epithelial cells were cultured in DMEM supplemented with 5% FBS for an additional 4 days before treatment.
Rat Alveolar Macrophage Isolation and Culture
Alveolar macrophages were isolated from pathogen-free adult male Sprague-Dawley rats (Harlan) by lavage with 150 mM sodium chloride, 5 mM HEPES, and 2 mM EDTA (pH 7.4). The cells were centrifuged at 250 × g for 10 minutes, and the resulting pellet was resuspended in D-10 medium. To increase the adherence and yield of rat alveolar macrophages, the cells were plated on 60-mm culture dishes coated with 0.5 mg/ml rat IgG. After 4 hours of incubation, the nonadherent cells were removed by washing with 5 ml of serum-free DMEM. Fresh DMEM medium supplemented with 5% FBS was added to each plate and incubated for 40 hours before ozone exposure.
In Vitro Ozone Exposure
Cells were exposed to ozone in an in vitro fully humidified exposure chamber that has a precisely regulated concentration of ozone (18). Medical grade oxygen was passed through ozone generator (Model OZ2SS-SS; Ozotech, Yreka, CA) to generate ozone. This ozone stream was then divided using a four-way manifold, and each of the sub-streams was regulated by one of four mass flow controllers (Sierra Instruments, Monterey, CA), which in turn was controlled by a Sierra Instruments flow box. Four specifically designed 3.6-L glass chambers were used to expose the cultured cells. One of these chambers was used as a control chamber and received humidified and warm air/CO2 mixtures. The other three chambers received a specified concentration of ozone. All chambers were fitted with a rocking platform to ensure uniform exposure of the monolayer with a minimum of overlying fluid. Ozone concentrations from each chamber were cyclically measured once every 2.5 minutes by an API 400A ozone analyzer (Teledyne Instruments, San Diego, CA). This information was recorded by a Macintosh G-3 computer and sent to the Sierra Instruments flow box, which automatically adjusted the flow of ozone to achieve the desired concentration. For the exposure, 2.0 to 3.0 ml serum-free media was added on top of 150-mm culture dishes and the cultures were rocked to minimize the fluid over the cell layer as described previously (10). Cultured cells were exposed to different concentrations of ozone or air for 1 hour.
Generation of Conditioned Media
Cells from the rat macrophage cell line NR8383 were plated in 150-mm culture dishes and cultured for 1 day in DMEM supplemented with 10% FBS. After 1 day of culture, cells were washed with serum-free DMEM and 3 ml of serum-free DMEM was added to the culture plates before ozone exposure. Cells were exposed to ozone for 1 hour, and the culture vessels were rocked. Media were collected after ozone exposure and spun at 2,700 × g for 10 minutes and kept frozen at −20°C until assayed or used to treat rat alveolar epithelial cells.
Measurements of Cytokines and Chemokines
A multiplex assay was performed with the Luminex-100 system and the XY Platform (Luminex Corporation, Austin, TX) Fluorescently labeled microsphere beads for standards and detection were purchased from Linco Research, Inc. under Luminex's patent rights, and the sheath fluid was purchased from Luminex Corporation. Granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-1α, IL-1β, IFN-γ, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, IL-18, TNF-α, monocyte chemoattractant protein 1 (MCP-1), cytokine-induced neutrophil chemoattractant 1 (CINC-1), and regulated on activation, normal T-cell expressed and secreted (RANTES) were analyzed using a rat cytokine multiplex assay according to the manufacturer's instructions. In brief, 25 μl of the cell supernatant was incubated with the antibody-coated bead mix for 20 hours at 4°C. The beads were washed twice and incubated with secondary biotinylated antibody mix for 1 hour at room temperature. Streptavidin-phycoerythrin was added and incubated for an additional 30 minutes at room temperature. The beads were washed three times, and then analyzed on a Luminex 100 platform (Luminex). Cytokine concentrations were calculated from standard curves. Acquired fluorescence data were analyzed to calculate cytokine concentrations with StatLIA immunoassay curve fitting software (Brendan Technologies, Inc., Carlsbad, CA). Rat CINC-1 was also measured by enzyme-linked immunosorbent assay kits developed by ELISA Tech, Inc. (Aurora, CO) according to the manufacturer's instructions.
Stimulation of Alveolar Epithelial Cells with IL-1α, IL-1β, and IL-18
Type I–like cells were incubated with different concentrations of recombinant rat IL-1α, IL-1β, and IL-18 (R&D Systems, Minneapolis, MN) in DMEM with 5% FBS for 24 hours. Media were collected, spun at 2,700 × g for 10 minutes, and kept frozen at −20°C until they were assayed.
Inhibition by IL-1 Receptor Antagonist and Neutralizing Anti-Rat IL-1 Antibodies
Type I–like cells were cultured for 4 days in DMEM supplemented with 5% FBS. The cells were left untreated or pretreated with 10 μg/ml of IL-1 receptor antagonist (IL-1Ra) (R&D Systems) for 1 hour, and then the cells were treated with conditioned media for 24 hours. To perform the neutralizing antibodies experiment, 3 μg/ml of specific neutralizing antibodies to rat IL-1α, IL-1β, and isotype-matched IgG (R&D Systems) were preincubated with condition media for 1 hour at room temperature and subsequently added in culture for 24 hours (19). This media was then collected, spun at 2,700 × g for 10 minutes, and kept frozen at −20°C until assayed.
Statistical Analyses
Data are represented as the mean ± SEM. For statistical analysis, the test used depended on the experimental design. For the effect of ozone on the two phenotypes, a paired two-tailed t test was used. For the dose–response relationships the results were analyzed by ANOVA and Tukey's test. A P value of < 0.05 was considered to indicate a significant difference between the groups.
RESULTS
Ozone Increases IL-1α, IL-1β, IL-18, IL-2, and GM-CSF in NR8383 Cells
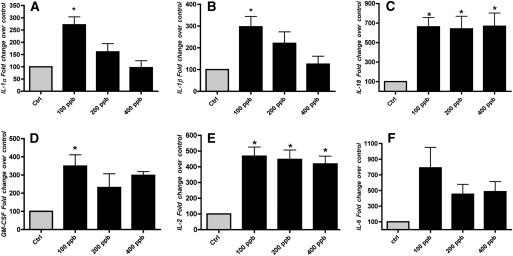
The cultured NR8383 cells were exposed to air and different concentrations of ozone for 1 hour, and immediately after ozone exposure, the media were collected. There was no obvious morphologic effect on the cells due to the ozone exposure. There was a significant increase in IL-1α, IL-1β, IL-18, GM-CSF, and IL-2 in supernatant of NR8383 cells exposed to 100 ppb ozone when compared with cells exposed to air (Figures 1A–1E). There was also a small increase in Interleukin 12a, 70-kD glycoprotein (IL-12P70) from less than 4.9 pg/ml to about 12 pg/ml. However, the direct ozone effect was apparent only at 100 ppb, except for IL-2 and IL-18, which remain elevated at all ozone concentrations. Unexpectedly, release or secretion of IL-1α and IL-1β was less in NR8383 cells exposed to higher concentrations of ozone, whereas there was no significant reduction of IL-18, GM-CSF, or IL-2. Ozone did not significantly increase the levels of CINC-1 (CXCL1), MCP-1 (CCL2), IL-6, and TNF-α in these experiments. Levels of IL-4, IL-5, IL-10, and IFN-γ were below the level of detection.
Figure 1.
Ozone increases inflammatory cytokine secretion in NR8383 cells. NR8383 cells were cultured in 150-mm dishes and exposed to air and different concentrations of ozone for 1 hour. The media were collected immediately after exposure and the cytokines were measured by a multiplex assay as described in Materials and Methods. The results are shown as percent (%) change over control. The control values for these cytokines were (A) IL-1α, 113 pg/ml; (B) IL-1β, 26.7 pg/ml; (C) IL-18, 599 pg/ml; (D) granulocyte-macrophage colony-stimulating factor, 7.2 pg/ml; (E) IL-2, 6.2 pg/ml; and (F) IL-6, 12.7 pg/ml. The results are the means of ± SEM of three independent experiments. The asterisk signifies an increase compared to the air control (P < 0.05).
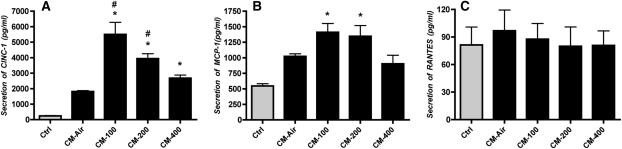
Conditioned Media from Ozone-Exposed NR8383 Cells Increase Secretion of CINC-1 and MCP-1 by Alveolar Epithelial Cells
The purpose of this experiment was to determine if macrophage-conditioned media would stimulate chemokine production from alveolar epithelial cells. In previous experiments, we found that direct ozone exposure of alveolar epithelial cells did not produce a significant chemokine response (10). The NR8383-conditioned media were diluted with four volumes of fresh media and added to the alveolar type I–like cells for 24 hours. Supernatants from the epithelial cells were analyzed for different cytokines and chemokines by the multiplex assay. There was an increase in CINC-1 and MCP-1, but not RANTES (Figure 2). There was also an increase in IL-1α, IL-1β, and IL-18 in these analyses, but these increases could be attributed to the levels of those cytokines in the NR8383-conditioned media. The TNF-α concentration was less than 4.9 pg/ml and did not increase with macrophage-conditioned medium. In other experiments, we treated isolated primary rat alveolar macrophages with conditioned media from NR8383 cells and found no increase in CINC-1 secretion, indicating that the epithelial cells were much more sensitive to the factors in the conditioned media than alveolar macrophages.
Figure 2.
NR8383 cell conditioned media increases cytokine-induced neutrophil chemoattractant (CINC-1) and monocyte chemoattractant protein (MCP-1) secretion from rat type I–like cells. Alveolar type II cells were cultured with fetal bovine serum on plastic plates to transdifferentiate into type I–like cells. Conditioned media from NR8383 cells supplemented with fresh media were added to the type I–like cells on Day 5 of culture, and the media were collected 24 hours later. Conditioned media increased the secretion of the chemokines (A) CINC-1 and (B) MCP-1, but (C) RANTES remained unchanged. The original values are shown as pg/ml. The results are the means of ± SEM of three independent experiments. * An increase compared with the untreated control; # an increase compared with air-exposed conditioned media (P < 0.05).
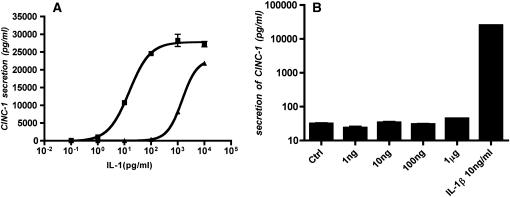
Alveolar Epithelial Cells Secrete CINC-1 in Response to Recombinant Rat IL-1α and IL-1β but Not IL-18
Previously we reported that type I–like cells secrete MCP-1 and MIP-2 in response to IL-1β (10). In the present study we found that IL-1α and IL-1β stimulated the secretion of CINC-1 by type I–like cells, whereas IL-18 did not (Figure 3). In addition, there was a difference in potency between the two IL-1 isoforms. IL-1α stimulation of CINC-1 secretion by the epithelial cells was nearly 100-fold more potent than IL-1β (Figure 3A). The observation that there was no stimulation of CINC-1 secretion by alveolar epithelial cells in response to exogenous IL-18 indicates that the IL-18 is not the source of the stimulation in the NR8383-conditioned media.
Figure 3.
IL-1α and IL-1β increase CINC-1 secretion from rat alveolar type I–like epithelial cells. Type I–like cells were cultured as described in Figure 2, and exogenous IL-1α, IL-1β, and IL-18 were added on Day 5 of culture. The media were collected 24 hours later. The values are shown as pg/ml. A shows the dose response of IL-1α (squares) and IL-1β (triangles), and B shows the effect of different concentrations of IL-18 and 10 ng/ml of IL-1β as a positive control.
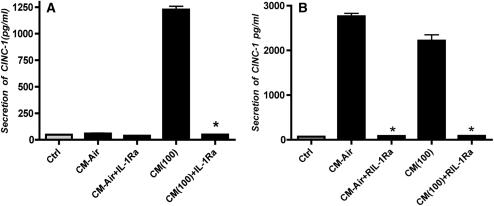
IL-1Ra Inhibits the Effect of Macrophage-Conditioned Media on Epithelial CINC-1 Secretion
Previously we have shown that 10 μg/ml of the human IL-1R antagonist inhibits rat IL-1 (19). Pretreatment of rat type I–like alveolar epithelial cells with 10 μg/ml of human IL-1Ra abolished the effect of NR8383-conditioned media on CINC-1 secretion by alveolar epithelial cells (Figure 4A). In the absence of inhibitors, the ozone-generated conditioned media stimulated the rat alveolar type I–like cells to secrete over 1,200 pg/ml of CINC-1. The IL-1Ra also blocked the secretion of CINC-1 by alveolar epithelial cells by conditioned media from primary rat alveolar macrophages. In pilot experiments, we found it was difficult to get sufficient rat macrophages to generate conditioned media. In order to increase the adherence and plating efficiency of primary alveolar rat macrophages, we coated culture plates with rat IgG. Pretreatment of the primary rat alveolar macrophage–conditioned media attenuated the effect of conditioned media on CINC-1 production (Figure 4B). The unusually high level of CINC-1 secretion from epithelial cells incubated with the air control–conditioned media from the primary alveolar macrophages was thought to be due to activation by macrophage binding to the IgG-coated dishes. This stimulation was also inhibited by the IL-1 antagonist.
Figure 4.
CINC-1 secretion by rat alveolar epithelial cells. Rat alveolar type I–like epithelial cells were cultured as described in Figure 2, and left untreated or pretreated with IL-1 antagonist (10 μg/ml) for 1 hour and then incubated with conditioned media. Media were collected 24 hours later and CINC-1 measured by enzyme-linked immunosorbent assay. A shows the results with NR8383-conditioned media and B shows the results with primary rat alveolar macrophage–conditioned media. The asterisk signifies inhibition compared with the respective control (P < 0.05).
Neutralization of IL-1α by Antibodies Inhibits the Effect of Macrophage-Conditioned Media on Epithelial CINC-1 Secretion
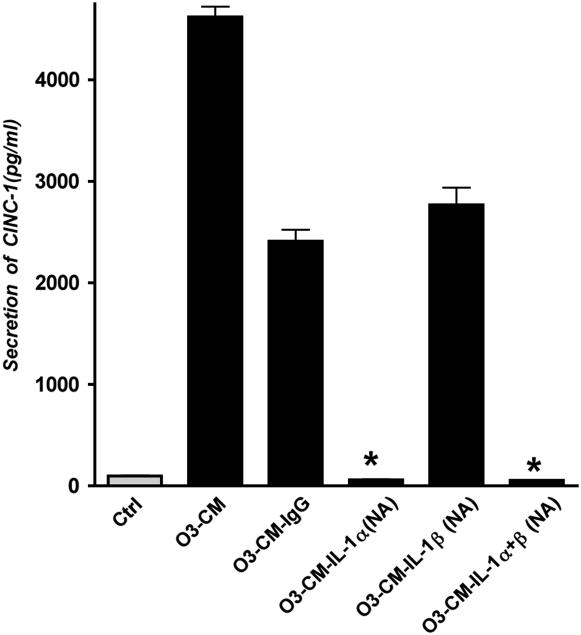
After establishing that rat IL-1 protein from rat macrophages stimulates the secretion of CINC-1, we next determined which isoform of IL-1 is responsible for CINC-1 production from the alveolar epithelium. Pretreatment of ozone-treated conditioned media with 3 μg/ml specific rat IL-1α–neutralizing IgG abolished the effect of condition media on CINC-1 production by rat alveolar epithelial cells, but IL-1β–neutralizing IgG was similar to control IgG (Figure 5). Both antibodies in combination also abolished the effect of condition media. This experiment demonstrated that the effect of conditioned media on alveolar epithelial cells for CINC-1 production was primarily due to IL-1α and not IL-1β.
Figure 5.
Macrophage IL-1α is primarily responsible for CINC-1 production from rat alveolar type I–like epithelial cells. Rat alveolar type I–like cells were cultured as described in Figure 2. Conditioned media generated from ozone-exposed macrophages were pretreated with IL-1α–selective neutralizing antibody (IL-1α NA), IL-1β NA, or control isotype match IgG at concentration of 3 μg/ml for 1 hour at room temperature before treatment of cultured cells. IL-1α NA alone as well as in combination with IL-1β NA significantly inhibited CINC-1 secretion compared with the IgG control condition (P < 0.05). In contrast, administration of IL-1β NA had no significant effect on CINC-1 secretion in these cultures.
DISCUSSION
Because ozone induces an acute inflammatory response and neutrophil influx in the distal lung, we examined how ozone might produce chemoattractants with the knowledge that direct ozone exposure to epithelial cells and macrophages produced toxicity but not robust chemokine secretion. In this study we observed that direct exposure of the alveolar macrophage cell line NR8383 did not produce significant amounts of CINC-1, TNF-α, or MCP-1. We next tested the hypothesis that chemokines are produced by cross-talk between alveolar epithelial cells and macrophages due to the release of some factor(s) from the exposed cells that would in turn stimulate unexposed cells.
It is known that acute ozone toxicity causes a neutrophilic influx in vivo. Numerous chemokines have been suggested to serve as neutrophil chemoattractants during ozone-induced injury (20, 21). In our experiments, macrophage-conditioned media after ozone exposure elicited a significant secretion of CINC-1 and MCP-1 by alveolar epithelial cells. Our results indicate that this stimulation was due to IL-1α. This conclusion is based on the finding of an increase in IL-1α in the ozone-exposed macrophage-conditioned media, a robust CINC-1 secretion by alveolar epithelial cells in response to IL-1α, an inhibition of the effect by IL-1Ra, and finally by nearly complete inhibition of CINC-1 secretion by an antibody to IL-1α but not by an antibody to IL-1β. The importance of IL-1 in the response to ozone has also been shown in vivo. Park and coworkers showed that IL-1Ra blocked the increase in chemokines in lung homogenates, neutrophil influx, and airway hyperresponsiveness (22). In that study, IL-1β increased and IL-1α was not reported. In another in vivo study, mRNA for IL-1α was not changed in response to ozone (23). In addition, Ishii and colleagues reported that ozone increased macrophage secretion of IL-β and TNF-α and that CINC levels were reduced in vitro by the combination of anti–IL-1 and anti-TNF antibodies. The individual antibodies were ineffective (24). Hence, our results are consistent with previous reports, although most previous reports focused on IL-1β and not IL-1α.
The present study demonstrates the role of an early cytokine response to ozone that can stimulate chemotactic chemokine release from epithelial cells. The ozone-conditioned media from macrophages was collected just after ozone exposure. This early stimulatory response suggested that biological activity of conditioned media must be due to release of preformed biologically active molecules in response to ozone exposure. In our experiments, both IL-1α and IL-1β were detected in ozone-exposed conditioned media from macrophages. Although IL-1α and IL-1β exert similar biological activity, they are encoded by two distinct genes, and their precursor polypeptides are quite different in biological activity. The IL-1α precursor is biologically active even when membrane bound, whereas IL-1β precursor exerts no biological activity until IL-1β has been processed to the mature form by caspase-1 (25). While it is commonly accepted that IL-1α lacks the classical signal sequence that regulates processing of secreted proteins and that most IL-1α remains intracellular or membrane bound (26), consensus has not yet been reached as to what regulates intracellular distribution and release of IL-1α. It has been reported that physical injury and apoptosis play a role in IL-1α and IL-1β secretion through membrane disruption, and the nature of injury determine the forms of IL-1 released (27). Niki and coworkers showed that membrane-associated IL-1 contributes to cartilage destruction in inflammatory joint diseases (28). We therefore infer that the macrophage-conditioned media after ozone exposure contain IL-1α and its membrane-bound precursor, both of which are biologically active.
Our data demonstrate that IL-1α is more potent than IL-1β, and that IL-1α stimulates alveolar type I–like cells to produce CXC chemokines. We believe that stronger stimulatory response of IL-1α on alveolar epithelial cells is due to its higher affinity to the IL-1 receptor (IL-1R). Mosley and colleagues have shown that, although IL-1α and IL-1β exert the same biological activities, their precursors have differential affinities to the common IL-1 receptor and bind differently (25). Furthermore, Nakane and coworkers reported that IL-1α is more potent than IL-1β in stimulation of A549 cells to increase expression of β adrenergic receptors (29). Hence, in our system IL-1α was the IL-1 molecule that seemed to be the isoform responsible for stimulating the epithelial cells. However, both IL-1α and IL-1β use the same receptor and IL-1β could be more effective in other situations, since the level is usually greater (30).
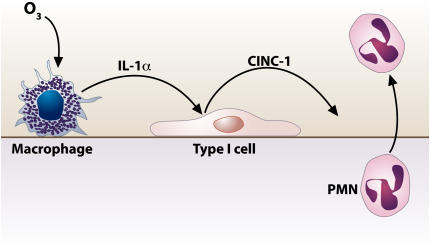
Taken together, our results indicate that CXC chemokines are not produced by direct ozone exposure to alveolar macrophages (21) and that cell-to-cell interaction between alveolar macrophages and alveolar epithelial cells are important for the CXC chemokines production in response to ozone exposure. Our model indicates that ozone causes the release of IL-1α from macrophages, which in turn stimulates alveolar epithelial cells to secrete CINC-1, a neutrophil chemoattractant (Figure 6). This concept is supported by recent studies on the sterile inflammatory response due to cell injury mediated by IL-1α and the IL-1R–Myd88 pathway (31).
Figure 6.
Schematic diagram of proposed mechanisms of ozone toxicity and cellular responses. Our hypothesis is that ozone induces release of IL-1α from alveolar macrophages, which in turn stimulates alveolar epithelial cells to secrete CXC chemokines, predominantly CINC-1 (CXCL-1). Consequently, the chemokines recruit the neutrophils to infiltrate the airspace.
Acknowledgments
The authors are thankful to Dr. Patricia Giclas, Cynthia Marschner, and Benjamin Efaw from the Clinical labs of National Jewish Medical Research Center for assistance with the Luminex Assay. The authors are grateful to Teneke Warren and Caroline Cook for their help with manuscript preparation.
This work was supported by grants from the Environmental Protection Agency (CRX-830846010), the ExxonMobil Foundation, and the National Institutes of Health (AI15614 and HL029891).
Originally Published in Press as DOI: 10.1165/rcmb.2007-0250OC on September 27, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Uysal N, Schapira RM. Effects of ozone on lung function and lung diseases. Curr Opin Pulm Med 2003;9:144–150. [DOI] [PubMed] [Google Scholar]
- 2.Harkema JR, Wagner JG. Epithelial and inflammatory responses in the airways of laboratory rats coexposed to ozone and biogenic substances: enhancement of toxicant-induced airway injury. Exp Toxicol Pathol 2005;57:129–141. [DOI] [PubMed] [Google Scholar]
- 3.Pino MV, Levin JR, Stovall MY, Hyde DM. Pulmonary inflammation and epithelial injury in response to acute ozone exposure in the rat. Toxicol Appl Pharmacol 1992;112:64–72. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi N, Yu XY, Schofield BH, Kleeberger SR, Scott AL, Hasegawa S, Spannhake EW. Expression of ICAM-1 in airway epithelium after acute ozone exposure in the mouse. J Appl Physiol 1995;79:1753–1761. [DOI] [PubMed] [Google Scholar]
- 5.Haddad EB, Salmon M, Koto H, Barnes PJ, Adcock I, Chung KF. Ozone induction of cytokine-induced neutrophil chemoattractant (CINC) and nuclear factor-kappa b in rat lung: inhibition by corticosteroids. FEBS Lett 1996;379:265–268. [DOI] [PubMed] [Google Scholar]
- 6.Koto H, Salmon M, Haddad EB, Huang TJ, Zagorski J, Chung KF. Role of cytokine-induced neutrophil chemoattractant (CINC) in ozone-induced airway inflammation and hyperresponsiveness. Am J Respir Crit Care Med 1997;156:234–239. [DOI] [PubMed] [Google Scholar]
- 7.Pryor WA. How far does ozone penetrate into the pulmonary air/tissue boundary before it reacts? Free Radic Biol Med 1992;12:83–88. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Wang S, Manzer R, McConville G, Mason RJ. Ozone induces oxidative stress in rat alveolar type II and type I-like cells. Free Radic Biol Med 2006;40:1914–1928. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Q, Simpson LG, Driscoll KE, Leikauf GD. Chemokine regulation of ozone-induced neutrophil and monocyte inflammation. Am J Physiol 1998;274:L39–L46. [DOI] [PubMed] [Google Scholar]
- 10.Manzer R, Wang J, Nishina K, McConville G, Mason RJ. Alveolar epithelial cells secrete chemokines in response to IL-1beta and lipopolysaccharide but not to ozone. Am J Respir Cell Mol Biol 2006;34:158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janic B, Umstead TM, Phelps DS, Floros J. Modulatory effects of ozone on THP-1 cells in response to SP-A stimulation. Am J Physiol Lung Cell Mol Physiol 2005;288:L317–L325. [DOI] [PubMed] [Google Scholar]
- 12.Devlin RB, McKinnon KP, Noah T, Becker S, Koren HS. Ozone-induced release of cytokines and fibronectin by alveolar macrophages and airway epithelial cells. Am J Physiol 1994;266:L612–L619. [DOI] [PubMed] [Google Scholar]
- 13.Jaspers I, Chen LC, Flescher E. Induction of interleukin-8 by ozone is mediated by tyrosine kinase and protein kinase A, but not by protein kinase C. J Cell Physiol 1998;177:313–323. [DOI] [PubMed] [Google Scholar]
- 14.Nichols BG, Woods JS, Luchtel DL, Corral J, Koenig JQ. Effects of ozone exposure on nuclear factor-kappaB activation and tumor necrosis factor-alpha expression in human nasal epithelial cells. Toxicol Sci 2001;60:356–362. [DOI] [PubMed] [Google Scholar]
- 15.Rusznak C, Devalia JL, Sapsford RJ, Davies RJ. Ozone-induced mediator release from human bronchial epithelial cells in vitro and the influence of nedocromil sodium. Eur Respir J 1996;9:2298–2305. [DOI] [PubMed] [Google Scholar]
- 16.Pearson AC, Bhalla DK. Effects of ozone on macrophage adhesion in vitro and epithelial and inflammatory responses in vivo: the role of cytokines. J Toxicol Environ Health 1997;50:143–157. [DOI] [PubMed] [Google Scholar]
- 17.Dobbs LG, Mason RJ. Pulmonary alveolar type II cells isolated from rats. Release of phosphatidylcholine in response to beta-adrenergic stimulation. J Clin Invest 1979;63:378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen CB. An automated system for exposure of cultured cells and other materials to ozone. Inhal Toxicol 2003;15:1039–1052. [DOI] [PubMed] [Google Scholar]
- 19.Portnoy J, Pan T, Dinarello CA, Shannon JM, Westcott JY, Zhang L, Mason RJ. Alveolar type II cells inhibit fibroblast proliferation: role of IL-1alpha. Am J Physiol Lung Cell Mol Physiol 2006;290:L307–L316. [DOI] [PubMed] [Google Scholar]
- 20.Chang MM, Wu R, Plopper CG, Hyde DM. IL-8 is one of the major chemokines produced by monkey airway epithelium after ozone-induced injury. Am J Physiol 1998;275:L524–L532. [DOI] [PubMed] [Google Scholar]
- 21.Michalec L, Choudhury BK, Postlethwait E, Wild JS, Alam R, Lett-Brown M, Sur S. CCL7 and CXCL10 orchestrate oxidative stress-induced neutrophilic lung inflammation. J Immunol 2002;168:846–852. [DOI] [PubMed] [Google Scholar]
- 22.Park JW, Taube C, Swasey C, Kodama T, Joetham A, Balhorn A, Takeda K, Miyahara N, Allen CB, Dakhama A, et al. Interleukin-1 receptor antagonist attenuates airway hyperresponsiveness following exposure to ozone. Am J Respir Cell Mol Biol 2004;30:830–836. [DOI] [PubMed] [Google Scholar]
- 23.Johnston CJ, Stripp BR, Reynolds SD, Avissar NE, Reed CK, Finkelstein JN. Inflammatory and antioxidant gene expression in C57BL/6J mice after lethal and sublethal ozone exposures. Exp Lung Res 1999;25:81–97. [DOI] [PubMed] [Google Scholar]
- 24.Ishii Y, Yang H, Sakamoto T, Nomura A, Hasegawa S, Hirata F, Bassett DJ. Rat alveolar macrophage cytokine production and regulation of neutrophil recruitment following acute ozone exposure. Toxicol Appl Pharmacol 1997;147:214–223. [DOI] [PubMed] [Google Scholar]
- 25.Mosley B, Urdal DL, Prickett KS, Larsen A, Cosman D, Conlon PJ, Gillis S, Dower SK. The interleukin-1 receptor binds the human interleukin-1 alpha precursor but not the interleukin-1 beta precursor. J Biol Chem 1987;262:2941–2944. [PubMed] [Google Scholar]
- 26.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood 1996;87:2095–2147. [PubMed] [Google Scholar]
- 27.Hogquist KA, Nett MA, Unanue ER, Chaplin DD. Interleukin 1 is processed and released during apoptosis. Proc Natl Acad Sci USA 1991;88:8485–8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niki Y, Yamada H, Kikuchi T, Toyama Y, Matsumoto H, Fujikawa K, Tada N. Membrane-associated IL-1 contributes to chronic synovitis and cartilage destruction in human IL-1 alpha transgenic mice. J Immunol 2004;172:577–584. [DOI] [PubMed] [Google Scholar]
- 29.Nakane T, Szentendrei T, Stern L, Virmani M, Seely J, Kunos G. Effects of IL-1 and cortisol on beta-adrenergic receptors, cell proliferation, and differentiation in cultured human A549 lung tumor cells. J Immunol 1990;145:260–266. [PubMed] [Google Scholar]
- 30.Matta SG, Linner KM, Sharp BM. Interleukin-1 alpha and interleukin-1 beta stimulate adrenocorticotropin secretion in the rat through a similar hypothalamic receptor(s): effects of interleukin-1 receptor antagonist protein. Neuroendocrinology 1993;57:14–22. [DOI] [PubMed] [Google Scholar]
- 31.Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med 2007;13:851–856. [DOI] [PubMed] [Google Scholar]