Abstract
Transforming growth factor (TGF)-β1 is an essential regulatory cytokine that has been implicated in the pathogenesis of diverse facets of the injury and repair responses in the lung. The types of responses that it elicits can be appreciated in studies from our laboratory that demonstrated that the transgenic (Tg) overexpression of TGF-β1 in the murine lung causes epithelial apoptosis followed by fibrosis, inflammation, and parenchymal destruction. Because a cyclin-dependent kinase inhibitor, p21, is a key regulator of apoptosis, we hypothesized that p21 plays an important role in the pathogenesis of TGF-β1–induced tissue responses. To test this hypothesis we evaluated the effect of TGF-β1 on the expression of p21 in the murine lung. We also characterized the effects of transgenic TGF-β1 in mice with wild-type and null mutant p21 loci. These studies demonstrate that TGF-β1 is a potent stimulator of p21 expression in the epithelial cells and macrophages in the murine lung. They also demonstrate that TGF-β1–induced lung inflammation, fibrosis, myofibroblast accumulation, and alveolar destruction are augmented in the absence of p21, and that these alterations are associated with exaggerated levels of apoptosis and caspase-3 activation. Finally, our studies further demonstrated that TGF-β1 induces p21 via a TNF-α–signaling pathway and that p21 is a negative modulator of TGF-β1–induced TNF-α expression. Collectively, our studies demonstrate that p21 regulates TGF-β1–induced apoptosis, inflammation, fibrosis, and alveolar remodeling by interacting with TNF-α–signaling pathways.
Keywords: TGF-β, p21, apoptosis, fibrosis, emphysema
CLINICAL RELEVANCE
This is the first report directly demonstrating the in vivo role of p21 in fibrosis and alveolar destruction in association with TGF-β1 expression. Intervention of p21 has therapeutic potential for the fibrotic and destructive lung disease as well.
Transforming growth factor (TGF)-β family proteins are multifunctional cytokines that have been implicated in the pathogenesis of diverse biologic processes including cell growth and survival, cell and tissue differentiation, development, inflammation, immunity, hematopoiesis, and tissue remodeling and repair (reviewed in Ref. 1). A number of studies demonstrate that, in the proper setting, TGF-β1 is essential for wound healing, stimulates matrix molecule deposition and angiogenesis, and is an essential mediator of the pathologic scarring in fibrotic disorders (2–6). On the other hand, TGF-β1 can also induce tissue injury and cellular apoptosis, decrease epithelialization, and inhibit wound healing (7–12). The “contradictory” and complex nature of these responses reflects an inadequate understanding of the mechanisms that TGF-β1 uses to induce tissue responses. This is due, in part, to the lack of experimental systems in which the acute and chronic effects of TGF-β1 can be characterized and their interrelationships can be investigated in vivo. We recently generated transgenic (Tg) mice in which bioactive TGF-β1 is specifically overexpressed in the lung (13). These TGF-β1 Tg mice manifest impressive bronchoalveolar and tissue inflammation, fibrosis, and pulmonary alveolar remodeling (13). Studies of these mice have also demonstrated that these TGF-β1–induced tissue responses are dependent on an epithelial apoptotic response that precedes fibrosis and alveolar destruction. In spite of its importance, however, the molecular mechanisms and mediators that regulate this TGF-β1–induced apoptosis response have not been adequately described.
p21Cip1/WAF1/Sdi1 (p21), the first identified cyclin-dependent kinase (CDK) inhibitor, belongs to Cip/Kip family of CDK inhibitors and is an essential regulator of cell cycle progression, DNA repair, and apoptosis (14–17). In in vitro studies p21 has been shown to be induced by TGF-β1 (18, 19) and to inhibit TGF-β1–induced apoptosis (20). p21 has been shown to have protective effects in several animal models of pulmonary injury (21, 22), and it has also been shown that down-regulation of p21 expression caused enhanced Fas-mediated epithelial apoptosis by TGF-β1 (23). However, the role(s) and the mechanism of p21 in in vivo TGF-β1–induced tissue responses have not been adequately defined.
We hypothesized that p21 plays an important role in the pathogenesis of TGF-β1–induced tissue alterations in vivo. To test this hypothesis, we characterized the expression of p21 in TGF-β1 Tg mice and defined the effects of a null mutation of p21 on the phenotypes induced by transgenic TGF-β1 in these animals. These studies demonstrated that TGF-β1 is a potent stimulator of p21 expression in epithelial cells and macrophages in the lung. They also demonstrate that a deficiency of p21 significantly augments TGF-β1–induced apoptotic tissue responses, inflammation, fibrosis, and alveolar destruction. Our studies further indicated that TGF-β1 induces p21 in vivo via a TNF-α–signaling pathway and that p21 is a negative modulator of TGF-β1–induced TNF-α expression. Collectively, our studies demonstrate that p21 significantly regulate TGF-β1-induced tissue responses by interacting with TNF-α–signaling pathways.
MATERIALS AND METHODS
Overexpression Transgenic Mice
CC10-tTS-rtTA-TGF-β1 Tg mice were generated in our laboratory, bred onto a C57BL/6 background, and used in these studies. These mice use the Clara cell 10-kD protein (CC10) promoter to specifically target TGF-β1 to the lung. The methods that were used to generate and characterize these mice were described previously (13). In this modeling system, TGF-β1 caused macrophage-dominant bronchoalveolar lavage (BAL) and tissue inflammatory responses, subepithelial and parenchymal fibrosis, and alveolar destruction (13).
Breeding to p21 and TNF-α Receptor Null Mutant Mice
TGF-β1 Tg mice were bred with mice with wild-type and null p21 or TNF-α receptor loci. p21(−/−), TNF-α Receptor I (TNFRI[−/−]), and TNF-α Receptor II (TNFRII[−/−]) null mice were purchased from Jackson Laboratory (Bar Harbor, ME). The mice with both TNF-α I and II receptors null mutant loci (TNFR[−/−]) were generated by the mating the TNFRI(−/−) mice with TNFRII(−/−) mice. All the mice had been bred for over 10 generations onto a C57BL/6 genetic background. As a result of these crosses, TGF-β1 Tg animals with (+/+) and (−/−) p21 or TNFR loci were obtained and used for analysis. Genotyping of TGF-β1 Tg, p21(−/−), and TNFR(−/−) mice was accomplished according to the protocols established in our laboratory and provided by Jackson Laboratory, respectively (13). The phenotypes of these mice were compared as described below.
Doxycycline Water Administration
Six-week-old transgene (+) mice and transgene (−) littermate controls were randomized to normal water or water containing 0.5 mg/ml of doxycycline (dox) as described previously (13, 24). Phenotypic alterations were evaluated at intervals thereafter.
BAL and Lung Inflammation
Lung inflammation was assessed by BAL as described previously (13, 25). The BAL samples from each animal were pooled and centrifuged. The number and type of cells in the cell pellet were determined with light microscopy.
Quantification of Lung Collagen
Animals were anesthetized, a median sternotomy was performed, and right heart perfusion was accomplished with calcium- and magnesium-free PBS. The heart and lungs were then removed en bloc. The right lung was frozen in liquid nitrogen and stored at −80°C until used. Collagen content was determined by quantifying total soluble collagen using the Sircol Collagen Assay kit (Biocolor) according to the manufacturer's instructions. The data are expressed as the collagen content of the entire right lung.
Histologic Analysis
The lungs were removed en bloc as described above, inflated at 25 cm pressure with PBS containing 0.5% low melting point agarose gel, fixd in Streck solution (Streck Laboratories, La Vista, NE), embedded in paraffin, sectioned, and stained. Hematoxylin and eosin, and Mallory's trichrome stains were performed in the Research Histology Laboratory of the Department of Pathology at the Yale University School of Medicine. The paraffin-embedded sections were also used for immunohistochemistry and TdT-mediated dUTP nick-end labeling (TUNEL) evaluations as described below.
Morphometric Analysis
Alveolar remodeling was estimated from the mean cord length of the airspace. This measurement is similar to the mean linear intercept, a standard measure of air space size, but it has the advantage of being independent of alveolar septal thickness. These evaluations were performed as described previously by our laboratory (13, 26).
TUNEL Evaluations
End labeling of exposed 3′-OH ends of DNA fragments was undertaken with the TUNEL in situ cell death detection kit AP (Roche Diagnostics, Indianapolis, IN) as described by the manufacturer. After staining, 20 fields of alveoli were randomly chosen for examination. The labeled cells were expressed as a percentage of total nuclei.
Fluorescence-Activated Cell Sorter Analysis for Apoptotic Cells
Whole lung dispersed cells were prepared using the methods developed by Rice and coworkers (27). After anesthesia, the trachea was cannulated with 20-gauge tubing, the lungs were filled with 2 ml dispase (Roche Diagnostics, Alameda, CA), followed by 0.5 ml of 1% low-melting-point agarose. The agarose was allowed to harden under crushed ice. The lungs were then placed in 2 ml dispase for 1 hour at room temperature and transferred to Dulbecco's modified Eagle's medium with 25 mM Hepes with 0.01% DNase I (Sigma-Aldrich, St. Louis, MO). After teasing apart the digested tissue, the resulting cell suspension was sequentially filtered through nylon mesh filters and collected after centrifugation for 8 minutes at 130 × g. In accord with the literature, the resulting cells were more than 97% viable as demonstrated by trypan blue dye exclusion (27). These cells were resuspended in 1× binding buffer at 106 cells/ml for subsequent fluorescence-activated cell sorter analysis. Annexin V and propidium iodide staining were undertaken with the annexin V fluorescein isothiocyanate apoptosis detection kit (BD Biosciences, San Diego, CA). Analysis was undertaken by flow cytometry (Becton Dickinson, San Jose, CA).
mRNA Analysis
mRNA levels were assessed using conventional reverse transcription PCR assays as described by our laboratories (2, 13, 25). In these assays, total cellular RNA from lungs were obtained using trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. The primer sequences used for amplification of p21 were used as previously described (28).
Immunoblot Analysis
Lung lysates were prepared and Western analysis was undertaken with antibodies that reacted selectively with p21, caspase-3, poly(ADP)ribose polymerase (PARP), Bcl-2, β-tubulin (Santa Cruz Biotechnology, Santa Cruz, CA), and ICAD (Chemicon International, Temecula, CA) as described previously (25).
Cytokine Measurements
The levels of TGF-β1 in BAL fluids were evaluated by enzyme-linked immunosorbent assay using commercial assays (R&D Systems, Minneapolis, MN) as described by the manufacturer.
Immunohistochemistry
Immunohistochemistry (IHC) was undertaken to localize p21 (Santa Cruz Biotechnology), α-smooth muscle actin (DakoCytomation, Glostrup, Denmark), and α-smooth muscle myosin heavy chain (Biomedical Technology, Inc., Stoughton, MA). These assays were undertaken as described previously by our laboratories (13). We used lungs from p21(−/−) mice as a negative control for IHC, and no significant signals were detected in this setting.
Statistics
Normally distributed data are expressed as means ± SEM and assessed for significance by Student's t test or ANOVA as appropriate. Data that were not normally distributed were assessed for significance using the Wilcoxon rank sum test.
RESULTS
TGF-β1 Regulation of p21
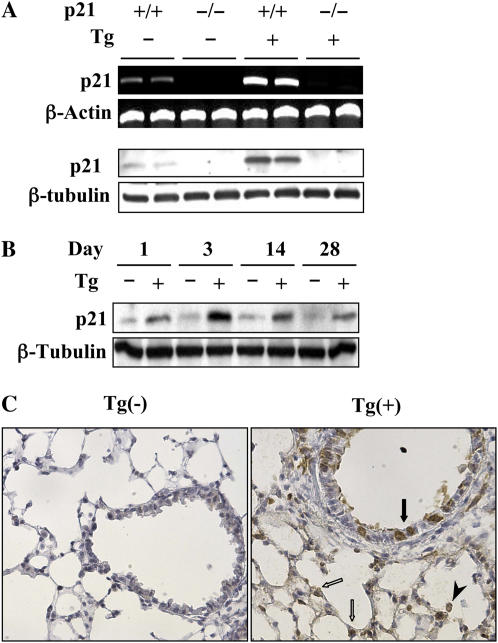
To address the possibility that p21 contributes to TGF-β1–induced responses in the murine lung, studies were first undertaken to determine if the expression of p21 was regulated by transgenic TGF-β1. This was done by comparing the levels of p21 mRNA and protein in transgene (−) and transgene (+) mice at various times after transgene activation via the administration of dox. These studies demonstrate that TGF-β1 is a potent stimulator of p21 mRNA and protein in lungs from dox-treated transgenic mice (Figure 1A). This induction was seen after as little as 1 day of dox administration and persisted throughout the 28-day study interval (Figure 1B). In these mice, immunohistochemistry demonstrated that the p21 was most prominently appreciated in airway epithelial cells, alveolar Type II cells, and macrophages (Figure 1C). Thus, TGF-β1 is a potent stimulator of p21 in epithelial cells and macrophages in the murine lung.
Figure 1.
TGF-β1 regulation of p21. TGF-β1 Tg (+) and Tg (−) mice with (+/+) and without (−/−) p21 loci were given normal or doxycycline (dox) water for 1 to 28 days. RNA (upper two lanes) and protein (lower two lanes) expression levels at 2 weeks of dox incubation (A) and time kinetics of p21 protein expression (B) were detected by RT-PCR and Western blot analysis. The p21 expressions of the lung from mice given dox water for 3 days were localized using immunohistochemistry (C) (original magnification: ×20). Solid and open arrows highlight epithelial and type II cells, respectively. Arrowhead denotes the alveolar macrophage. Each panel is illustrative of a minimum of three similar experiments.
p21 Regulation of TGF-β1–Induced Inflammation
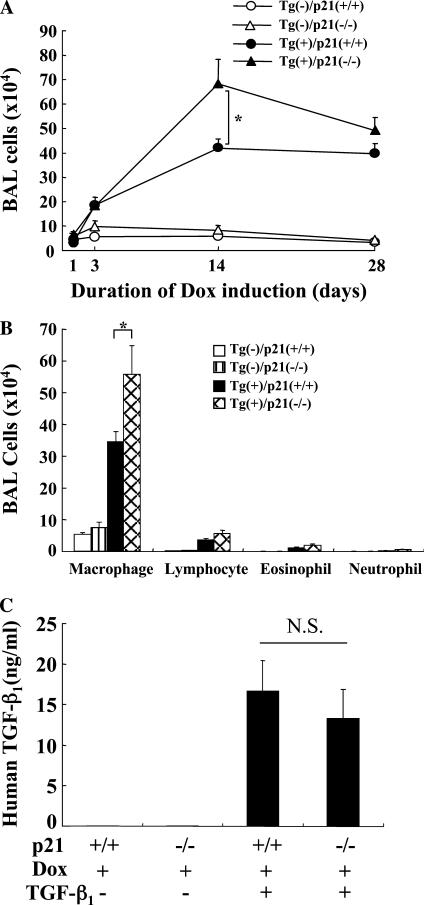
Studies were next undertaken to determine if p21 regulated TGF-β1–induced tissue inflammation. This was done by comparing the inflammation in BAL and tissues from transgene (+) mice with wild-type and null p21 loci. These studies demonstrate that, in the absence of p21, the total cell recovery in BAL fluids was increased when compared with transgenic mice that produce p21 normally (Figure 2A). This effect was most prominent after 14 days of dox administration and could still be appreciated after 28 days of transgene activation (Figure 2A). At the 14-day time point, this effect was due to an increase in the recovery of macrophages, lymphocytes, and to a lesser degree, eosinophils and neutrophils in BAL fluids from p21 null animals (Figure 2B, and Figure E1 in the online supplement). Similar increases in inflammation were seen in tissue histologic evaluations (data not shown). Thus, these studies demonstrate that p21 inhibits TGF-β1–induced inflammation in the murine lung. The expression level of transgene (human TGF-β1) in the BALF of the Tg(+)/p21(−/−) was not significantly different from Tg(+)/p21(+/+) mice (Figure 2C).
Figure 2.
p21 regulation of TGF-β1–induced inflammation. TGF-β1 Tg (+) and Tg (−) mice with (+/+) and without (−/−) p21 loci were given normal or dox water for 1 to 28 days. After dox incubation for the indicated period, time kinetic changes of bronchoalveolar lavage (BAL) cell recovery were evaluated (A) and a differential count on BAL cells after 2 weeks of dox induction was illustrated (B). The transgenic expression of TGF-β1 in the BAL fluid was evaluated by enzyme-linked immunosorbent assay (C). The values represent the mean ± SEM of evaluations in a minimum of five animals (*P < 0.05; N.S., nonsignificant).
Role of p21 in TGF-β1–Induced Fibrosis
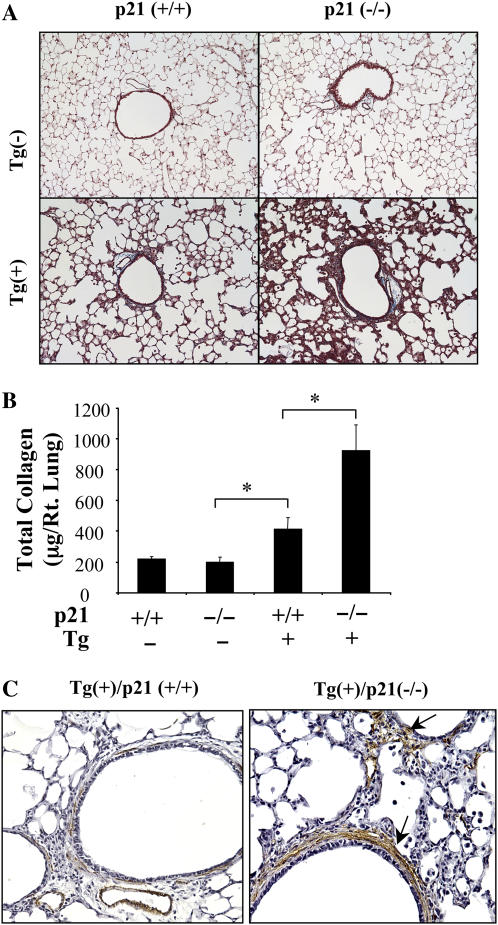
To determine if fibrotic effects of TGF-β1 were altered in the absence of p21, we used biochemical (Sircol) and histologic approaches to quantitate the collagen in lungs from transgenic mice with wild-type and null p21 loci. In accord with previous studies from our laboratory (13), transgenic TGF-β1 caused a significant increase in lung collagen content (Figures 3A and 3B). Interestingly, this fibrotic response was augmented in the absence of p21. This increase was most prominent after 2 to 4 weeks of dox administration. It was associated with a prominent increase in the accumulation of myofibroblasts based on an increase in α-smooth muscle actin (+) cells (Figure 3C). α-smooth muscle myosin containing myocytes were not similarly altered (data not shown). These studies demonstrate that p21 is an important inhibitor of TGF-β1–induced fibrosis and myofibroblast accumulation in the murine lung.
Figure 3.
Role of p21 in the TGF-β1–induced fibrosis. The collagen content of lungs from Tg (−) and Tg (+) mice with (+/+) and (−/−) p21 loci were compared using Mallory's trichrome (A; original magnification: ×10) and Sirchol collagen evaluations (B) after 2 weeks of dox induction. (C) Immunohistochemical localization of α-smooth muscle actin–positive cells (arrows) after 2 weeks of dox induction (original magnification: ×15). A and C are representative of a minimum of three similar evaluations. In B, each value represents the mean ± SEM of evaluations in a minimum of six mice (*P < 0.05).
Role of p21 in Alveolar Remodeling
Previous studies from our laboratory demonstrated that TGF-β1, in addition to inducing tissue fibrosis, also induced alveolar remodeling with septal destruction and an increase in alveolar chord length (13). To define the role of p21 in these responses, we compared the alveoli from transgenic mice with (+/+) and (−/−) p21 loci. In accord with our prior report (13), an increase in lung destruction was readily apparent in transgenic mice with normal p21 loci after 2 weeks of dox administration. At these time points, alveolar remodeling was heightened in the absence of p21. This is readily apparent in histologic evaluations (Figure 4A) and morphometric chord length evaluations (Figure 4B). Thus, p21 is a potent inhibitor of TGF-β1–induced alveolar rupture and remodeling in the murine lung.
Figure 4.
Role of p21 in TGF-β1–induced alveolar remodeling. After 2 weeks of dox induction, lungs were obtained from Tg (−) and Tg (+) mice with (+/+) and (−/−) p21 loci, fixed to pressure, and hematoxylin and eosin histologic stains (A; original magnification: ×10) and chord length (B) assessments were undertaken. A is representative of a minimum of five similar experiments. In B, the values represent the mean ± SEM of evaluations in a minimum of five mice (*P < 0.05, **P < 0.01).
p21 Regulation of TGF-β1–Induced Apoptosis
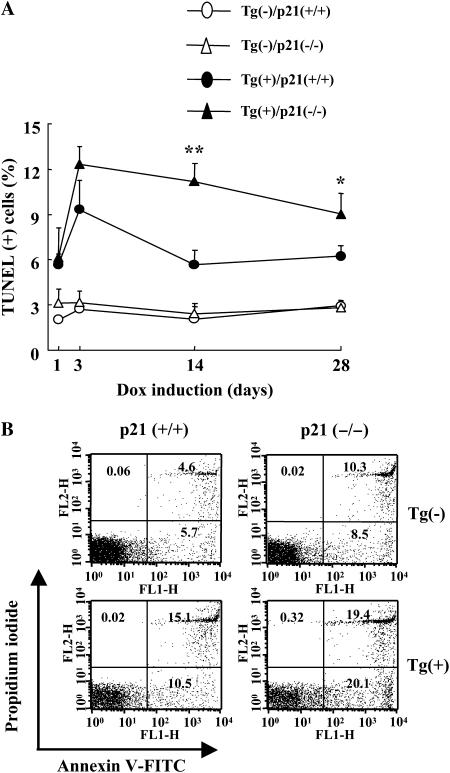
To test the hypothesis that p21 regulates TGF-β1–induced apoptosis, we compared the magnitude and kinetics of TGF-β1–induced apoptosis in transgenic mice with wild-type and null p21 loci. In accord with our prior report (13), transgenic TGF-β1 caused an impressive increase in TUNEL staining. These TUNEL (+) cells were largely epithelial cells, as evidenced by their histologic location and morphology (data not shown). This response was readily appreciated after 3 days of dox administration and decreased with longer periods of transgene activation (Figure 5A). PI/annexin V evaluations of total lung cells confirmed these findings and demonstrated that the majority of these cells were undergoing apoptosis and combined apoptosis and necrosis (Figure 5B). In all cases, p21 appeared to be an important regulator of this response, with the levels and chronicity of TUNEL staining and apoptosis being increased in transgene (+) mice with null p21 loci. Collectively, these studies demonstrate that p21 is an important inhibitor of TGF-β1–induced epithelial apoptosis that contributes to the transient nature of this response in lungs from C57BL/6 TGF-β1 transgenic mice. Western analyses demonstrated that TGF-β1 caused a significant increase in caspase-3, -8, -9, and PARP cleavage (Figure E2). p21 appeared to be an important regulator of this response because Tg (+) mice with null p21 loci manifest a significant increase in caspase-3, -8, -9 activation and PARP cleavage compared with transgenic animals with wild-type p21 loci (Figure E2).
Figure 5.
p21 regulation of TGF-β1–induced apoptosis. After dox induction, lungs were obtained from Tg (−) and Tg (+) mice with (+/+) and (−/−) p21 loci. DNA injury and cell death were evaluated with TUNEL stains and time kinetic changes for the indicated dox induction period were illustrated (A). After 2 weeks of dox induction, cells were isolated from both lungs and fluorescence-activated cell sorter analysis was undertaken using annexin V and propidium iodide staining (B). In B, the values represent the mean ± SEM of evaluations in a minimum of six mice (*P < 0.05, **P < 0.01).
P21 Regulation of TGF-β1–Induced TNF-α Expression and Signaling Pathway of p21 Induction
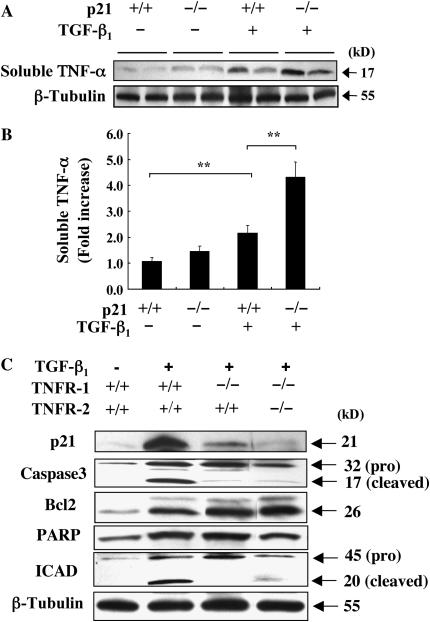
To understand the underlying mechanism of p21 in the TGF-β1–induced tissue responses, first we have investigated the mRNA expression of selected chemokines, their receptors, and cytokines that possibly associated with BAL and tissue inflammation in the Tg (+) and Tg (−) mice with and without p21 null mutation. The mRNA expression of MCP-1, MCP-2, MIP-1α, CCR2, CCR5, and TARC were increased in the Tg(+)/p21(+/+) mice, but significant changes were not detected between Tg(+)/p21(+/+) and Tg(+)/p21(−/−) mice (Figure E3). However, the expression of soluble TNF-α was significantly increased in the lung lysate of Tg(+)/p21(+/+) mice and further increased in the Tg(+)/p21(−/−) mice (Figures 6A and 6B). The significant role of TNF-α in the generation of pulmonary fibrosis and/or alveolar remodeling has been described in a number of papers (29–31). Thus, we investigated the interaction of TNF-α and p21 in our modeling system. Interestingly, as shown in Figure 6C, the increased expression of p21 in the Tg(+)/TNFR(+/+) mice was significantly reduced to the comparable level of the wild-type mice in Tg(+)/TNFR(−/−) mice. It is also interesting to note that expression of another anti-apoptotic molecule, Bcl-2, was not altered, while TGF-β1–induced caspase 3 activation or ICAD cleavage were impressively decreased in the absence of TNF-α receptors. These studies demonstrated that reciprocal interaction between TNF-α and p21: induction of p21 is largely regulated by TNF-α signaling and also p21 negatively regulate TNF-α expression in the lung of TGF-β1 transgenic mice. These studies also demonstrate that TNF-α signaling mediate anti-apoptotic role of p21 in TGF-β1–induced apoptosis and remodeling tissue responses.
Figure 6.
Regulation of TGF-β1–induced TNF-α expression and signaling pathway of p21 induction. After 2 weeks of dox induction, TNF-α expression in the lung lysate (soluble TNF-α) obtained from Tg (−) and Tg (+) mice with (+/+) and (−/−) p21 loci was evaluated by Western analysis (A) and densitometric quantization (B). Expression of p21 and other apoptosis-related molecules in Tg (+) and Tg (−) mice were evaluated in the mice with wild-type and TNFR(−/−) null mutant loci. A and C are representative of a minimum of three similar experiments. In B, the values represent the mean ± SEM of evaluations in a minimum of six mice (**P < 0.01).
DISCUSSION
To further understand the molecular events involved in TGF-β1–induced phenotype generation, we used a transgenic system developed in our laboratory to characterize the role(s) of p21 in the pathogenesis of TGF-β1–induced alterations in the lung. These studies demonstrate that TGF-β1 is a potent stimulator of p21 expression. They also demonstrate that p21 plays a protective role in the pathogenesis of TGF-β1–induced tissue responses because TGF-β1–induced inflammation, fibrosis, myofibroblast accumulation, alveolar destruction, and cell death were markedly augmented by p21 ablation.
TGF-β1 is a multifunctional cytokine that has the ability to regulate cell differentiation, survival, and death. In many cell types, TGF-β1 inhibits cellular proliferation by causing growth arrest in the G1 phase of the cell cycle (reviewed in Ref. 32). This TGF-β1–induced G1 cell cycle arrest has been attributed to the regulatory effects of TGF-β1 on both the levels and activities of G1 cyclins and CDKs, which include p21, p27kip1, p18, p16, and p15 (reviewed in Refs. 33 and 34). In vitro, TGF-β1 stimulates p21 via a mechanism that is, at least in part, transcriptional and independent of p53 (19, 35). Consistent with these cell cycle and in vitro observations, our studies demonstrate that TGF-β1 induces prominent increases in p21 mRNA and protein in vivo. Interestingly, in these studies p21 was most prominent in airway and alveolar epithelial cells and macrophages, the cells that have the highest levels of apoptosis in this modeling system. Our studies also demonstrate that p21 is a potent inhibitor of TGF-β1–induced apoptosis with increased numbers of annexin V– and TUNEL-positive cells being seen in lungs from transgenic mice with null mutations of p21. This demonstration that p21 has in vivo cytoprotective effects is in accord with prior studies in other modeling systems. Specifically, O'Reilly and colleagues (36) demonstrated that bronchiolar and alveolar epithelial cells damaged by hyperoxia impressively express p21. They suggested that p21 protects the lung from oxidative stress by inhibiting DNA replication and thereby allowing additional time to repair damaged DNA (21). Similarly, epithelial p21 expression is also up-regulated during bleomycin-induced lung injury (28, 37, 38), adenoviral overexpression of p21 decreases bleomycin-induced pulmonary fibrosis (22), and p21 can directly regulate the activity of caspase-3 by formation of a pro–caspase 3–p21 complex in vitro (16, 17). In humans, up-regulation of p21 in fibrotic lung diseases has also been noted (38). Idiopathic pulmonary fibrosis is characterized by alternating zones of fibrosis, fibroplasia, and normal lung. The interface between these zones shows so-called fibroblastic foci in which epithelial cells have been shown to undergo apoptosis. These cells also show up-regulation of p21, while normal lung and lung away from these foci are negative (38). Nakashima and coworkers (39) showed that nonspecific interstitial pneumonia, considered a better prognosis variant of pulmonary fibrosis, as similar levels of p21 as IPF. However, not commented on in their text was that the figures illustrating the staining showed diffuse p21 staining rather than focal, consistent with the diffuse nature of the pathologic process in nonspecific interstitial pneumonia (NSIP). Diffuse alveolar damage, in which TGF-β has also been reported to play a role, also shows diffuse high level staining with p21 (40). Good prognostic variants of chronic interstitial lung disease other than NSIP have not been reported. We can also detect increased and localized p21 expression in the fibrotic foci from patients with pulmonary fibrosis (Figure E4 and data not shown). Thus, the findings in humans are consistent with the idea that p21 expression reflects the degree and pattern of TGF-β expression. When viewed in combination, our studies suggest that p21 overexpression during tissue injury plays a protective role, at least in part, by regulating TGF-β1–induced apoptosis.
Previous studies from our laboratory demonstrated that TGF-β1 overexpression generates a macrophage-dominant inflammatory response in the lung (13). In the present studies, the absence of p21 significantly augmented this TGF-β1–induced BAL and tissue inflammatory responses. p21 can exert its anti-inflammatory effects by modulating the expression of chemokines or chemokine receptors. However, we do not think this plays an important role in this modeling system because we did not detect significant alterations in the levels of mRNA encoding a number of TGF-β1–induced chemokines and their receptors in comparisons of transgenic mice with (+/+) and null p21 loci. On the other hand, tissue and BAL expressions of a potent proinflammatory cytokine TNF-α were significantly increased in the absence of p21. Because the significant role of TNF-α in the pulmonary fibrosis and alveolar remodeling as well as in the inflammation has been described in a number of studies (29, 30), we sought the possible interactions between p21 and TNF-α in modulating TGF-β1–induced tissue phenotypes. Interestingly, we have found that induction of p21 by TGF-β1 is largely dependent on the TNF-α–signaling pathways. Also, our studies demonstrate that p21 negatively regulates the induction of TNF-α by TGF-β1 in vivo, because absence of p21 augments TNF-α expression. This observation leads us to conclude that p21 is a negative regulator of inflammation and tissue remodeling responses, at least in part, by interacting with TNF-α signaling. Another interesting finding on the role of TNF-α in the TGF-β1 Tg mice is that TGF-β1–induced tissue apoptosis, fibrosis, and alveolar destructions were significantly reduced in the absence of TNF-α signaling (H.-R. Kang and C. G. Lee, unpublished data). Our studies suggest that there is an interacting feedback regulatory loop between TNF-α and p21 in the regulation of tissue phenotypes. However, the mechanisms of TNF-α regulation of p21 expression and their interaction with caspase 3 activation are largely unknown because in the absence of TNF-α signaling, both caspase 3 activation and p21 expression were greatly reduced (Figure 6). It is intriguing to speculate that up-regulation of another anti-apoptotic molecule like Bcl-2 can suppress the caspase 3 activation in the absence of p21 in this setting, but the exact mechanism(s) remains to be determined. When viewed in combination, these studies strongly suggest that regulatory effects of p21 in TGF-β1–induced inflammatory and tissue responses are, at least in part, dependent, on TNF-α signaling. In the absence of p21, heightened expression of TNF-α increased the BAL and tissue inflammation and apoptosis of airway and alveolar epithelial cells. Increased alveolar type II cell apoptosis, as we can see in the Tg(+)/p21(−/−) mice, can further drive tissue inflammation (41). In addition, enhanced apoptotic responses of epithelial cells can be a primary cause of prominent pulmonary fibrosis and alveolar remodeling (13). As far as we know, this is the first report that demonstrate significant interactions between p21 and TNF-α in the in vivo regulation of TGF-β1–induced tissue responses. However, the exact reciprocal regulatory mechanisms between p21 and TNF-α remain to be determined.
The essential role of TGF-β1 in pulmonary fibrosis and remodeling has been relatively well characterized in a number of studies. Idiopathic pulmonary fibrosis (IPF), a prototypic fibrotic disorder, is a fatal progressive lung disease characterized by epithelial damage, fibroproliferative matrix deposition, and parenchymal remodeling (42–44). For many years, it was believed that IPF was caused by chronic inflammation. Recent revisions, however, have highlighted the possibility that IPF is not an inflammatory disorder, but rather one characterized by an alteration in parenchymal homeostasis (44). TGF-β1 is believed to play an important role in this dysregulation because it is expressed in an exaggerated fashion in IPF where, in contrast to controls, a sizable percentage of this cytokine is biologically active (45–47). In fact, the apposition of apoptosis, fibrosis, and the exaggerated expression of TGF-β1 are well documented in IPF (48–51). TGF-β1 is also a critical mediator of the pulmonary fibrosis that is seen after bleomycin administration, a commonly used animal model of IPF (5, 52). Recently, studies have demonstrated that Bid, a regulator of the mitochondrial cell death pathway, is required for bleomycin-induced pulmonary fibrosis (53). Our studies add to our knowledge in this area by demonstrating that p21 inhibits apoptosis, tissue fibrosis, and alveolar remodeling by interacting with TNF-α–signaling pathways in the TGF-β1 transgenic model. It is tempting to speculate from these studies that the p21 in fibrotic foci represents a healing and/or protective response in these patients. It is also tempting to speculate that interventions that increase the expression of p21 can be used to control the destructive and fibrotic responses in the lungs from these patients.
A number of lines of evidence support the concept that TGF-β1 plays an important role in alveolar remodeling in the lung. Many of these studies suggest that TGF-β1 directly contributes to the genesis of emphysema. This includes studies that demonstrate that TGF-β1 is expressed, in an exaggerated fashion, in lungs from smokers and patients with COPD (54, 55), studies that suggest that TGF-β1 plays an important role in the emphysema that is seen in patients with Marfan syndrome (56), and studies that highlight the progressive increase in small airway wall thickness and fibrosis that are seen with COPD disease progression (57, 58). In contrast to these studies, there are studies that suggest that a deficiency of TGF-β1 bioactivity contributes to emphysema generation (59). This includes studies that demonstrate that a null mutation of the β6 integrin (which activates latent TGF-β) causes age-related emphysema via a matrix metalloproteinase-12–dependent mechanism (59) and studies that demonstrate that a null mutation of SMAD3 causes spontaneous emphysema (60). When viewed in combination, it is clear that TGF-β1 is dysregulated in COPD, but its role in this disease remains controversial. The different outcomes that have been reported, however, may be due to the ability of TGF-β1 to have simultaneous roles in injury and repair in the lung. In this regard, our studies demonstrate that TGF-β1 simultaneously induces epithelial apoptosis while inducing the anti-apoptotic molecule p21 to control this response. It is intriguing to speculate that the balance between apoptosis and anti-apoptosis directs the fate of these cells and contributes to the balance of alveolar destruction and fibrosis that are induced in these animals.
In summary, using lung-specific overexpressing TGF-β1 Tg mice and p21 null mice, we have demonstrated that p21 is an important negative regulator of TGF-β1–induced inflammation, apoptosis, fibrosis, and alveolar destruction in the lung. Our studies demonstrate that p21 regulates TGF-β1–induced tissue responses, at least in part, dependently via a TNF-α–signaling pathway. These studies suggest that interventions that enhance the expression of p21 can control tissue apoptosis and fibrosis and have therapeutic potential for the fibrotic and destructive lung diseases in which TGF-β1 plays a major role. They also suggest that genetic polymorphisms, environmental exposures, or therapies that alter p21 expression can have major effects on the ability of an individual to tolerate and the natural history of TGF-β1–mediated disorders.
Supplementary Material
Acknowledgments
The authors thank Kathleen Bertier for excellent secretarial and administrative assistance.
This work was supported by NIH Grants HL-064242 (J.A.E.) and HL-084225 (C.G.L.), and American Thoracic Society Grant C-04–016 (C.G.L).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2007-0276OC on October 11, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med 2000;342:1350–1358. [DOI] [PubMed] [Google Scholar]
- 2.Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, Shipley JM, Gotwals P, Noble P, Chen Q, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1). J Exp Med 2001;194:809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin M, Lefaix J, Delanian S. TGF-beta1 and radiation fibrosis: a master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys 2000;47:277–290. [DOI] [PubMed] [Google Scholar]
- 4.Ling E, Robinson DS. Transforming growth factor-beta1: its anti-inflammatory and pro-fibrotic effects. Clin Exp Allergy 2002;32:175–178. [DOI] [PubMed] [Google Scholar]
- 5.Nakao A, Fujii M, Matsumura R, Kumano K, Saito Y, Miyazono K, Iwamoto I. Transient gene transfer and expression of Smad7 prevents bleomycin-induced lung fibrosis in mice. J Clin Invest 1999;104:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalluri R, Sukhatme VP. Fibrosis and angiogenesis. Curr Opin Nephrol Hypertens 2000;9:413–418. [DOI] [PubMed] [Google Scholar]
- 7.Pittet JF, Griffiths MJ, Geiser T, Kaminski N, Dalton SL, Huang X, Brown LA, Gotwals PJ, Koteliansky VE, Matthay MA, et al. TGF-beta is a critical mediator of acute lung injury. J Clin Invest 2001;107:1537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts AB, Piek E, Bottinger EP, Ashcroft G, Mitchell JB, Flanders KC. Is Smad3 a major player in signal transduction pathways leading to fibrogenesis? Chest 2001;120:43S–47S. [DOI] [PubMed] [Google Scholar]
- 9.Fukuda H, Motohiro T, Nakai K, Yamamichi K, Nakane Y, Fujisawa J, Hioki K. Negative effect of transforming growth factor-beta-1 on intestinal anastomotic tissue regeneration. Eur Surg Res 2001;33:388–394. [DOI] [PubMed] [Google Scholar]
- 10.Flanders KC, Sullivan CD, Fujii M, Sowers A, Anzano MA, Arabshahi A, Major C, Deng C, Russo A, Mitchell JB, et al. Mice lacking Smad3 are protected against cutaneous injury induced by ionizing radiation. Am J Pathol 2002;160:1057–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan T, Ghahary A, Demare J, Yang L, Iwashina T, Scott PG, Tredget EE. Development, characterization, and wound healing of the keratin 14 promoted transforming growth factor-beta1 transgenic mouse. Wound Repair Regen 2002;10:177–187. [DOI] [PubMed] [Google Scholar]
- 12.Amendt C, Mann A, Schirmacher P, Blessing M. Resistance of keratinocytes to TGFbeta-mediated growth restriction and apoptosis induction accelerates re-epithelialization in skin wounds. J Cell Sci 2002;115:2189–2198. [DOI] [PubMed] [Google Scholar]
- 13.Lee CG, Cho SJ, Kang MJ, Chapoval SP, Lee PJ, Noble PW, Yehualaeshet T, Lu B, Flavell RA, Milbrandt J, et al. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J Exp Med 2004;200:377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature 1993;366:701–704. [DOI] [PubMed] [Google Scholar]
- 15.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 1993;75:805–816. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki A, Tsutomi Y, Miura M, Akahane K. Caspase 3 inactivation to suppress Fas-mediated apoptosis: identification of binding domain with p21 and ILP and inactivation machinery by p21. Oncogene 1999;18:1239–1244. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki A, Tsutomi Y, Akahane K, Araki T, Miura M. Resistance to Fas-mediated apoptosis: activation of caspase 3 is regulated by cell cycle regulator p21WAF1 and IAP gene family ILP. Oncogene 1998;17:931–939. [DOI] [PubMed] [Google Scholar]
- 18.Marwick JA, Kirkham P, Gilmour PS, Donaldson K, Mac NW, Rahman I. Cigarette smoke-induced oxidative stress and TGF-beta1 increase p21waf1/cip1 expression in alveolar epithelial cells. Ann N Y Acad Sci 2002;973:278–283. [DOI] [PubMed] [Google Scholar]
- 19.Datto MB, Li Y, Panus JF, Howe DJ, Xiong Y, Wang XF. Transforming growth factor beta induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc Natl Acad Sci USA 1995;92:5545–5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan Q, Sage EH. Transforming growth factor-beta1 induces apoptotic cell death in cultured retinal endothelial cells but not pericytes: association with decreased expression of p21waf1/cip1. J Cell Biochem 1998;70:70–83. [PubMed] [Google Scholar]
- 21.O'Reilly MA, Staversky RJ, Watkins RH, Reed CK, de Mesy Jensen KL, Finkelstein JN, Keng PC. The cyclin-dependent kinase inhibitor p21 protects the lung from oxidative stress. Am J Respir Cell Mol Biol 2001;24:703–710. [DOI] [PubMed] [Google Scholar]
- 22.Inoshima I, Kuwano K, Hamada N, Yoshimi M, Maeyama T, Hagimoto N, Nakanishi Y, Hara N. Induction of CDK inhibitor p21 gene as a new therapeutic strategy against pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2004;286:L727–L733. [DOI] [PubMed] [Google Scholar]
- 23.Hagimoto N, Kuwano K, Inoshima I, Yoshimi M, Nakamura N, Fujita M, Maeyama T, Hara N. TGF-beta 1 as an enhancer of Fas-mediated apoptosis of lung epithelial cells. J Immunol 2002;168:6470–6478. [DOI] [PubMed] [Google Scholar]
- 24.Zhu Z, Ma B, Homer RJ, Zheng T, Elias JA. Use of the tetracycline-controlled transcriptional silencer (tTS) to eliminate transgene leak in inducible overexpression transgenic mice. J Biol Chem 2001;276:25222–25229. [DOI] [PubMed] [Google Scholar]
- 25.Cho SJ, Kang MJ, Homer RJ, Kang HR, Zhang X, Lee PJ, Elias JA, Lee CG. Role of early growth response-1 (Egr-1) in interleukin-13-induced inflammation and remodeling. J Biol Chem 2006;281:8161–8168. [DOI] [PubMed] [Google Scholar]
- 26.Zheng T, Zhu Z, Wang Z, Homer RJ, Ma B, Riese RJ Jr, Chapman HA Jr, Shapiro SD, Elias JA. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase- and cathepsin-dependent emphysema. J Clin Invest 2000;106:1081–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rice WR, Conkright JJ, Na CL, Ikegami M, Shannon JM, Weaver TE. Maintenance of the mouse type II cell phenotype in vitro. Am J Physiol Lung Cell Mol Physiol 2002;283:L256–L264. [DOI] [PubMed] [Google Scholar]
- 28.Kuwano K, Hagimoto N, Tanaka T, Kawasaki M, Kunitake R, Miyazaki H, Kaneko Y, Matsuba T, Maeyama T, Hara N. Expression of apoptosis-regulatory genes in epithelial cells in pulmonary fibrosis in mice. J Pathol 2000;190:221–229. [DOI] [PubMed] [Google Scholar]
- 29.Lundblad LK, Thompson-Figueroa J, Leclair T, Sullivan MJ, Poynter ME, Irvin CG, Bates JH. Tumor necrosis factor-alpha overexpression in lung disease: a single cause behind a complex phenotype. Am J Respir Crit Care Med 2005;171:1363–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oikonomou N, Harokopos V, Zalevsky J, Valavanis C, Kotanidou A, Szymkowski DE, Kollias G, Aidinis V. Soluble TNF Mediates the Transition from Pulmonary Inflammation to Fibrosis. PLoS ONE 2006;1:e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sime PJ, Marr RA, Gauldie D, Xing Z, Hewlett BR, Graham FL, Gauldie J. Transfer of tumor necrosis factor-alpha to rat lung induces severe pulmonary inflammation and patchy interstitial fibrogenesis with induction of transforming growth factor-beta1 and myofibroblasts. Am J Pathol 1998;153:825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moustakas A, Pardali K, Gaal A, Heldin CH. Mechanisms of TGF-beta signaling in regulation of cell growth and differentiation. Immunol Lett 2002;82:85–91. [DOI] [PubMed] [Google Scholar]
- 33.Peter M, Herskowitz I. Joining the complex: cyclin-dependent kinase inhibitory proteins and the cell cycle. Cell 1994;79:181–184. [DOI] [PubMed] [Google Scholar]
- 34.Hunter T, Pines J. Cyclins and cancer: II. Cyclin D and CDK inhibitors come of age. Cell 1994;79:573–582. [DOI] [PubMed] [Google Scholar]
- 35.Datto MB, Yu Y, Wang XF. Functional analysis of the transforming growth factor beta responsive elements in the WAF1/Cip1/p21 promoter. J Biol Chem 1995;270:28623–28628. [DOI] [PubMed] [Google Scholar]
- 36.O'Reilly MA, Staversky RJ, Watkins RH, Maniscalco WM. Accumulation of p21(Cip1/WAF1) during hyperoxic lung injury in mice. Am J Respir Cell Mol Biol 1998;19:777–785. [DOI] [PubMed] [Google Scholar]
- 37.Mishra A, Doyle NA, Martin WJC. Bleomycin-mediated pulmonary toxicity: evidence for a p53-mediated response. Am J Respir Cell Mol Biol 2000;22:543–549. [DOI] [PubMed] [Google Scholar]
- 38.Plataki M, Koutsopoulos AV, Darivianaki K, Delides G, Siafakas NM, Bouros D. Expression of apoptotic and antiapoptotic markers in epithelial cells in idiopathic pulmonary fibrosis. Chest 2005;127:266–274. [DOI] [PubMed] [Google Scholar]
- 39.Nakashima N, Kuwano K, Maeyama T, Hagimoto N, Yoshimi M, Hamada N, Yamada M, Nakanishi Y. The p53-Mdm2 association in epithelial cells in idiopathic pulmonary fibrosis and non-specific interstitial pneumonia. J Clin Pathol 2005;58:583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guinee D Jr, Fleming M, Hayashi T, Woodward M, Zhang J, Walls J, Koss M, Ferrans V, Travis W. Association of p53 and WAF1 expression with apoptosis in diffuse alveolar damage. Am J Pathol 1996;149:531–538. [PMC free article] [PubMed] [Google Scholar]
- 41.Matute-Bello G, Winn RK, Jonas M, Chi EY, Martin TR, Liles WC. Fas (CD95) induces alveolar epithelial cell apoptosis in vivo: implications for acute pulmonary inflammation. Am J Pathol 2001;158:153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raghu G. Interstitial lung disease: a clinical overview and general approach. In: Fishman AP, Elias JA, Fishman JA, Grippi MA, Kaiser LR, Senior RM, editors. Fishman's pulmonary diseases and disorders. New York: McGraw Hill, Inc.; 1998. pp. 1037–1053.
- 43.Krein PM, Winston BW. Roles for insulin-like growth factor I and transforming growth factor-beta in fibrotic lung disease. Chest 2002;122:289S–293S. [DOI] [PubMed] [Google Scholar]
- 44.Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 2001;134:136–151. [DOI] [PubMed] [Google Scholar]
- 45.Khalil N, O'Connor RN, Flanders KC, Unruh H. TGF-β1, but not TGF-β2 or TGF-β3, is differentially present in epithelial cells of advanced pulmonary fibrosis: an immunohistochemical study. Am J Respir Cell Mol Biol 1996;14:131–138. [DOI] [PubMed] [Google Scholar]
- 46.Khalil N, Parekh TV, O'Connor R, Antman N, Kepron W, Yehaulaeshet T, Xu YD, Gold LI. Regulation of the effects of TGF-beta 1 by activation of latent TGF-beta 1 and differential expression of TGF-beta receptors (T beta R-I and T beta R-II) in idiopathic pulmonary fibrosis. Thorax 2001;56:907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu YD, Hua J, Mui A, O'Connor R, Grotendorst G, Khalil N. Release of biologically active TGF-(beta)1 by alveolar epithelial cells results in pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2003;285:527–539. [DOI] [PubMed] [Google Scholar]
- 48.Kuwano K, Miyazaki H, Hagimoto N, Kawasaki M, Fujita M, Kunitake R, Kaneko Y, Hara N. The involvement of Fas-Fas ligand pathway in fibrosing lung diseases. Am J Respir Cell Mol Biol 1999;20:53–60. [DOI] [PubMed] [Google Scholar]
- 49.Kuwano K, Maeyama T, Inoshima I, Ninomiya K, Hagimoto N, Yoshimi M, Fujita M, Nakamura N, Shirakawa K, Hara N. Increased circulating levels of soluble Fas ligand are correlated with disease activity in patients with fibrosing lung diseases. Respirology 2002;7:15–21. [DOI] [PubMed] [Google Scholar]
- 50.Kuwano K, Kunitake R, Kawasaki M, Nomoto Y, Hagimoto N, Nakanishi Y, Hara N. P21Waf1/Cip1/Sdi1 and p53 expression in association with DNA strand breaks in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1996;154:477–483. [DOI] [PubMed] [Google Scholar]
- 51.Kuwano K, Hagimoto N, Kawasaki M, Yatomi T, Nakamura N, Nagata S, Suda T, Kunitake R, Maeyama T, Miyazaki H, et al. Essential roles of the Fas-Fas ligand pathway in the development of pulmonary fibrosis. J Clin Invest 1999;104:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yehualaeshet T, O'Connor R, Begleiter A, Murphy-Ullrich JE, Silverstein R, Khalil N. A CD36 synthetic peptide inhibits bleomycin-induced pulmonary inflammation and connective tissue synthesis in the rat. Am J Respir Cell Mol Biol 2000;23:204–212. [DOI] [PubMed] [Google Scholar]
- 53.Budinger GR, Mutlu GM, Eisenbart J, Fuller AC, Bellmeyer AA, Baker CM, Wilson M, Ridge K, Barrett TA, Lee VY, et al. Proapoptotic Bid is required for pulmonary fibrosis. Proc Natl Acad Sci USA 2006;103:4604–4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takizawa H, Tanaka M, Takami K, Ohtoshi T, Ito K, Satoh M, Okada Y, Yamasawa F, Nakahara K, Umeda A. Increased expression of transforming growth factor-beta1 in small airway epithelium from tobacco smokers and patients with chronic obstructive pulmonary disease (COPD). Am J Respir Crit Care Med 2001;163:1476–1483. [DOI] [PubMed] [Google Scholar]
- 55.Kokturk N, Tatlicioglu T, Memis L, Akyurek N, Akyol G. Expression of transforming growth factor beta1 in bronchial biopsies in asthma and COPD. J Asthma 2003;40:887–893. [DOI] [PubMed] [Google Scholar]
- 56.Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet 2003;33:407–411. [DOI] [PubMed] [Google Scholar]
- 57.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004;350:2645–2653. [DOI] [PubMed] [Google Scholar]
- 58.Fabbri LM, Romagnoli M, Corbetta L, Casoni G, Busljetic K, Turato G, Ligabue G, Ciaccia A, Saetta M, Papi A. Differences in airway inflammation in patients with fixed airflow obstruction due to asthma or chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003;167:418–424. [DOI] [PubMed] [Google Scholar]
- 59.Morris DG, Huang X, Kaminski N, Wang Y, Shapiro SD, Dolganov G, Glick A, Sheppard D. Loss of integrin alpha(v)beta6-mediated TGF-beta activation causes Mmp12-dependent emphysema. Nature 2003;422:169–173. [DOI] [PubMed] [Google Scholar]
- 60.Bonniaud P, Kolb M, Galt T, Robertson J, Robbins C, Stampfli M, Lavery C, Margetts PJ, Roberts AB, Gauldie J. Smad3 null mice develop airspace enlargement and are resistant to TGF-beta-mediated pulmonary fibrosis. J Immunol 2004;173:2099–2108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.