Abstract
In Papua, Indonesia, the antimalarial susceptibility of Plasmodium vivax (n = 216) and P. falciparum (n = 277) was assessed using a modified schizont maturation assay for chloroquine, amodiaquine, artesunate, lumefantrine, mefloquine, and piperaquine. The most effective antimalarial against P. vivax and P. falciparum was artesunate, with geometric mean 50% inhibitory concentrations (IC50s) (95% confidence intervals [CI]) of 1.31 nM (1.07 to 1.59) and 0.64 nM (0.53 to 0.79), respectively. In contrast, the geometric mean chloroquine IC50 for P. vivax was 295 nM (227 to 384) compared to only 47.4 nM (42.2 to 53.3) for P. falciparum. Two factors were found to significantly influence the in vitro drug response of P. vivax: the initial stage of the parasite and the duration of the assay. Isolates of P. vivax initially at the trophozoite stage had significantly higher chloroquine IC50s (478 nM [95% CI, 316 to 722]) than those initially at the ring stage (84.7 nM [95% CI, 45.7 to 157]; P < 0.001). Synchronous isolates of P. vivax and P. falciparum which reached the target of 40% schizonts in the control wells within 30 h had significantly higher geometric mean chloroquine IC50s (435 nM [95% CI, 169 to 1,118] and 55.9 nM [95% CI, 48 to 64.9], respectively) than isolates that took more than 30 h (39.9 nM [14.6 to 110.4] and 36.9 nM [31.2 to 43.7]; P < 0.005). The results demonstrate the marked stage-specific activity of chloroquine with P. vivax and suggest that susceptibility to chloroquine may be associated with variable growth rates. These findings have important implications for the phenotypic and downstream genetic characterization of P. vivax.
In vitro drug susceptibility assays assess antimicrobial activity in the absence of the confounding effects of the host. Although such assays have become useful for monitoring the antimalarial resistance of Plasmodium falciparum, the assay has been of limited use with P. vivax. This is in part a consequence of a perception of the importance of antimalarial drug resistance with P. vivax, compounded by difficulties in standardizing a field-based assay. Over the last decade, a number of clinical studies have demonstrated the emergence of high-grade chloroquine resistance in Papua, Indonesia, and Papua, New Guinea (1, 18, 21), and its spread to other regions of Asia (6) and South America (20). However, assessment of the clinical efficacy of antimalarial drugs against P. vivax infection is confounded by the occurrence of both reinfections and relapses, making the attributable fraction of recurrent infections due to intrinsic parasite resistance difficult to gauge (2, 3, 10). To confirm the emergence of the spread of antimalarial drug resistance of P. vivax and to investigate alternative antimalarial drugs, it is critical that a standardized in vitro assay be developed and validated. The aim of this study was to define the in vitro susceptibility profiles of a range of antimalarial drugs and to investigate the confounding factors that modulate the derived estimate of drug efficacy.
MATERIALS AND METHODS
Field location and sample collection.
Between March 2004 and May 2007, Plasmodium isolates were collected from patients attending malaria clinics in Timika, located in the southern part of Papua province, Indonesia. Timika is a region of endemicity for multidrug-resistant strains of P. vivax and P. falciparum, with a risk of treatment failure of 65% within 28 days after chloroquine monotherapy for P. vivax malaria and 48% failure after multidrug therapy with chloroquine-sulfadoxine-pyrimethamine for P. falciparum malaria (16). In 2004, treatment guidelines were changed accordingly to recommend an artemisinin combination therapy for both P. falciparum and P. vivax infection, precluding further clinical studies of the use of chloroquine monotherapy in this region (15). Patients with symptomatic malaria who presented to an outpatient facility were recruited into the study if they were singly infected with P. falciparum or with P. vivax, with a parasitemia of between 2,000 μl−1 and 80,000 μl−1. Patients treated with antimalarials in the previous 3 weeks were excluded from this study. Venous blood (5 ml) was collected by venipuncture and, after the host white blood cells were removed using a CF11 column, 2 ml of packed infected red blood cells were divided as follows: 1 ml was cryopreserved in Glycerolyte 57 solution (Baxter, Deerfield, IL), 200 μl was spotted onto a filter paper, and 800 μl was used for the in vitro drug susceptibility assay. Since most patients were not enrolled in clinical studies, the therapeutic response to treatment could not be determined.
In vitro drug susceptibility assay.
The antimalarial susceptibility of P. vivax and P. falciparum isolates was measured using a protocol modified from the WHO microtest, as described previously (11, 19). Two hundred microliters of a 2% hematocrit blood medium mixture consisting of McCoy's 5A medium and 20% AB+ human serum was added to each well of predosed drug plates. The drug plates contained 11 serial concentrations of the antimalarials, with maximum concentrations of 5,910 nM for chloroquine, 557 nM for amodiaquine, 93 nM for artesunate, 489 nM for lumefantrine, 338 nM for mefloquine, and 769 nM for piperaquine. A candle jar was used to mature the parasites at 37.5°C (22 to 42 h). Incubation was stopped when >40% of ring-stage parasites had matured to mature schizonts in the drug-free control. Preliminary studies demonstrated that once the 40% schizont threshold had been reached, further incubation did not increase the final count.
Thick blood films made from each well were stained with 5% Giemsa stain for 30 min and examined microscopically. Differential counts of 200 asexual parasites on the preincubation and test slides were divided into ring-stage parasites (ring-shaped trophozoites and early amoebids without pigment), mature trophozoites (in which single or double chromatin dot and hemozoin pigment were visible), and schizonts. Free merozoites and gametocytes were not included in the count. To ensure optimal maturity and ease of parasite identification and to reduce the parasite classification error between microscopists, only schizonts with at least five well-defined chromatin dots were classified as schizonts at harvest.
The number of schizonts per 200 asexual-stage parasites was determined for each drug concentration and normalized to the control well. The dose-response data were analyzed using nonlinear regression analysis (WinNonLin version 4.1; Pharsight), and the 50% inhibitory concentration (IC50) value was derived using an inhibitory sigmoid Emax model. In vitro data were used only from predicted curves where the Emax and E0 values were within 15% of 100 or 0, respectively.
Data analysis.
Analysis was performed using SPSS software for Windows (version 15; SPSS, Inc., Chicago, IL). The Mann-Whitney U test or the Kruskal-Wallis method was used for nonparametric comparisons, and Student's t test (paired and unpaired) or one-way analysis of variance was used for parametric comparisons of log-transformed data. For categorical variables, percentages and corresponding 95% confidence intervals (CI) were calculated using Wilson's method. Proportions were examined using the χ2 test with Yates' correction or by Fisher's exact test.
Ethical approval.
Ethical approval for this study was obtained from the ethics committees of the National Institute of Health Research and Development, Ministry of Health, Indonesia, and Menzies School of Health Research, Darwin, Australia.
RESULTS
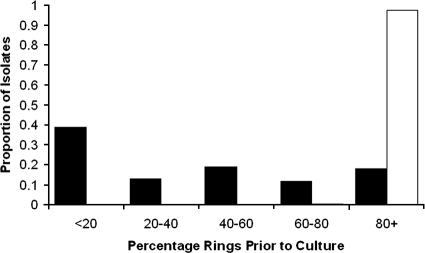
Between March 2004 and April 2007, venous blood was collected from 493 patients with single-species infections of either Plasmodium vivax (n = 216) or P. falciparum (n = 277) and assayed for drug susceptibility. Adequate growth for harvest was achieved with 81% of isolates (401/493). Baseline characteristics of the isolates processed are presented in Table 1. Whereas all P. falciparum isolates had ring-stage counts of more than 80% prior to culture, the P. vivax isolates were mostly asynchronous (median percentage of rings = 36.5% [interquartile range, 8 to 66%]) (Fig. 1). For those isolates that reached harvest, the median duration of the assay was 26 h (range, 24 to 48 h), with no differences between species. At harvest, the mean percentage of schizonts in the culture was 43.3% (95% CI, 41.7 to 44.9) and did not differ between the species of infection. The number of harvested isolates for which the susceptibility data were rejected differed between drug assays, varying from 0% (0/99) for mefloquine to 23% (51/221) for chloroquine. The overall geometric mean IC50s are presented in Table 2.
TABLE 1.
Baseline characteristics of isolates with which in vitro assay was attempted
| Isolate | Total no. of isolates assayed | Total no. of isolates harvested (% of isolates) | Median (range) delay from venipuncture to start of culture (h) | Geometric mean parasitemia (no. of parasites/μl [95% CI]) | % of patients receiving antimalarial treatment in previous 2 mo (n/total) | Median initial % of parasites at ring stage (IQR)a | % of isolates with an RT ratio of ≥1 (n/total) |
|---|---|---|---|---|---|---|---|
| P. vivax | 216 | 175 (81) | 2.00 (0.33-11.0) | 7,338 (6,665-8,079) | 25 (39/158) | 36.5 (8-66) | 37 (73/196) |
| P. falciparum | 277 | 226 (82) | 2.96 (0.17-11.3) | 10,968 (9,862-12,187) | 14 (32/224) | 100 (100-100) | 98 (222/227) |
Not available for 20 isolates. IQR, interquartile range.
FIG. 1.
Histogram of the initial percentage of rings prior to culture. Dark bars indicate P. vivax, and the light bar indicates P. falciparum.
TABLE 2.
Overall in vitro sensitivity for each drug according to the species tested
| Drug |
P. vivax
|
P. falciparum
|
||
|---|---|---|---|---|
| No. of acceptable assays/ total no. of assays harvested (%)a | Geometric mean IC50 value (nM) (95% CI) | No. of acceptable assays/total no. of assays harvested (%) | Geometric mean IC50 value (nM) (95% CI) | |
| Chloroquine | 155/170 (91.2) | 295 (227-384) | 170/221 (77) | 47.4 (42.2-53.3) |
| Amodiaquine | 143/144 (99.3) | 15.4 (12.7-18.7) | 120/137 (88) | 5.59 (4.96-6.29) |
| Artesunate | 136/149 (91.3) | 1.31 (1.07-1.59) | 127/157 (81) | 0.64 (0.53-0.79) |
| Lumefantrine | 54/64 (83) | 19.3 (13.2-28.0) | 101/124 (82) | 10.2 (7.6-13.9) |
| Mefloquine | 108/108 (100) | 12.7 (10.3-15.5) | 146/166 (88) | 8.5 (6.98-10.4) |
| Piperaquine | 125/137 (91.2) | 21.9 (18.5-25.8) | 165/200 (83) | 14.5 (13.2-16.1) |
Values shown are the total number of assays with acceptable IC50 values/number of assays harvested.
The time taken for P. vivax isolates to reach 40% schizonts, and thus the duration of the assay, was correlated significantly with the ratio of rings to trophozoites (the RT ratio) prior to culture. The greater the initial proportion of trophozoites the shorter the duration of the assay (Spearman correlation coefficient, rs = 0.485; P < 0.001) (Fig. 2). Both the RT ratio and the duration of the assay were negatively correlated with the delay between venipuncture and setting the isolate up in culture (rs = −0.171 and P = 0.025; and rs = −0.209 and P = 0.006, respectively). For the P. vivax isolates with a long delay before processing (greater than 4 h), 84% (27/32) of the isolates had a majority of parasites at the trophozoite stage (RT < 1) at the time of culture, compared to 57% (80/140) of those processed within 4 h (P = 0.0077).
FIG. 2.
Scatter plot of the ratio of rings to trophozoites stages present initially in P. vivax isolates relative to the time taken to reach 40% schizonts in the control wells.
Initial stage of parasite and in vitro susceptibility.
For P. vivax, the RT ratio was correlated significantly with the IC50 values for chloroquine (rs = −0.349; P < 0.001), for amodiaquine (rs = −0.251; P = 0.003), for artesunate (rs = −0.162; p = −0.05), and for mefloquine (rs = −0.258; P = 0.007). Compared to isolates for which the assay was set up predominantly at the ring stage (RT > 4), isolates set up predominantly at the trophozoite stage (RT < 0.25) had significantly higher geometric mean IC50 results for chloroquine (478 nM versus 85.8 nM; P < 0.001), amodiaquine (22.1 nM versus 10.0 nM; P = 0.007), artesunate (1.61 nM versus 0.86 nM; P = 0.024), and mefloquine (17.0 nM versus 6.39 nM; P = 0.001) but not from lumefantrine or piperaquine.
To investigate the relationship between the stage of the isolate and in vitro susceptibility, isolates with greater than 90% rings were set up in culture in the presence of the drug directly and again after culture in the absence of the drug to achieve 90% trophozoites. Plasmodium vivax isolates added to the assay at the ring stage had significantly lower chloroquine IC50s than the same isolates added at the trophozoite stage (median IC50 of 55.2 nM versus 2,812 nM, respectively; P = 0.008). P. falciparum isolates exposed at the ring stage to chloroquine also resulted in lower IC50 values than those at the trophozoite stage, although the difference was more modest (median IC50 values of 36.1 versus 99.7 nM, respectively; P = 0.008 and P = 0.04, respectively). The effect of the initial stage of drug exposure for both P. vivax and P. falciparum was also significant for amodiaquine but did not reach significance for mefloquine (Table 3).
TABLE 3.
In vitro susceptibility for paired isolates tested at both the ring and the trophozoite stagesa
| Antimalarial |
P. falciparum
|
P. vivax
|
||||||
|---|---|---|---|---|---|---|---|---|
| No. of isolates | Median IC50 value (range) at the ring stage | Median IC50 value(range) at the trophozoite stage | P value | No. of isolates | Median IC50 value(range) at the ring stage | Median IC50 value(range) at the trophozoite stage | P value | |
| Chloroquine | 6 | 36.1 (11.9-55.0) | 99.7 (19.6-157.8) | 0.04 | 9 | 55.2 (18.3-171.8) | 2812 (384-4611) | 0.008 |
| Amodiaquine | 6 | 8.2 (2.6-16.2) | 15.2 (5.9-22.4) | 0.028 | 9 | 16.6 (6.7-37.9) | 24.2 (12.5-59.9) | 0.05 |
| Mefloquine | 6 | 4.6 (2.1-12.0) | 6.4 (2.4-34.1) | 0.075 | 9 | 7.9 (1.3-24.6) | 12.5 (1.9-25.0) | 0.123 |
In vitro susceptibility for paired isolates was tested at both the ring(>90%) stage and after culture in the absence of the drug to the trophozoite stage (>90%).
Duration of assay and in vitro susceptibility.
The duration of the assay was negatively correlated with the in vitro drug susceptibility of chloroquine, amodiaquine, artesunate, and mefloquine for both P. falciparum and P. vivax; this was independent of the RT ratio and remained significant even after parasites with an RT ratio greater than 4 were selected (see Table 4). Of the 16 synchronous P. vivax isolates (RT > 10) with valid chloroquine IC50 data, the median duration of the assay was 42 h (range, 24 to 48 h), with 31% (5/16) of the isolates reaching the harvest criteria within 30 h. The geometric mean chloroquine IC50 value for fast-growing synchronous parasites was 435 nM (95% CI, 169 to 1,118) compared to 39.9 nM (14.6 to 110.4) for isolates taking longer than 30 h (P = 0.005). Although the majority of P. falciparum isolates were synchronous at the start of the assay, the duration of the assay also ranged from 24 to 48 h, with 53% (83/155) of the isolates reaching harvest within 30 h. Faster growing P. falciparum parasites also had higher geometric mean chloroquine IC50 values (55.9 nM [95% CI, 48 to 64.9]) than the slower growing parasites (36.9 nM [31.2 to 43.7]; P < 0.001).
TABLE 4.
Correlation of in vitro susceptibility (IC50) with the duration of assay
| Drug |
P. falciparum
|
All P. vivax
|
P. vivax RT > 4
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| rs value | No. of isolates | P value | rs value | No. of isolates | P value | rs value | No. of isolates | P value | |
| Chloroquine | −0.245 | 168 | 0.001 | −0.457 | 155 | 0.001 | −0.616 | 30 | <0.001 |
| Amodiaquine | −0.220 | 118 | 0.017 | −0.356 | 143 | <0.001 | −0.400 | 27 | 0.039 |
| Artesunate | −0.290 | 125 | 0.001 | −0.350 | 136 | <0.001 | −0.544 | 24 | <0.001 |
| Lumefantrine | −0.023 | 101 | 0.818 | 0.036 | 54 | 0.797 | 0.359 | 5 | 0.553 |
| Mefloquine | −0.340 | 144 | <0.001 | −0.413 | 108 | <0.001 | −0.381 | 20 | 0.097 |
| Piperaquine | −0.201 | 163 | 0.010 | −0.147 | 125 | 0.101 | −0.316 | 20 | 0.174 |
rs values indicate the Spearman rank correlation.
Stratified results of in vitro susceptibility of P. vivax.
Using criteria derived previously (23), P. vivax results were stratified into four groups according to whether the parasites were set up in culture with a majority of rings (RT ≥ 1) or a majority of trophozoites (RT < 1) and whether the duration of the assay was below or above 30 h (Fig. 2). The stratified IC50 results are presented in Table 5. For isolates which were predominantly at the ring stage prior to culture (RT ≥ 1) (Fig. 2, quadrants B plus C), the geometric mean IC50 estimates for P. vivax were 142 nM (95% CI, 90.4 to 222) for chloroquine, 11.8 nM (95% CI, 8.6 to 16.2) for amodiaquine, 1.00 nM (95% CI, 0.70 to 1.42) for artesunate, 28.5 nM (95% CI, 15.6 to 51.8) for lumefantrine, 9.99 nM (95% CI, 6.98 to 14.3) for mefloquine, and 18.6 nM (95% CI, 14.0 to 24.6) for piperaquine. The correlation coefficients for P. vivax and P. falciparum isolates are presented in Table 6.
TABLE 5.
In vitro sensitivities of P. vivax, stratified by duration of assay and RT ratio prior to culturea
| Drug | Quadrant A
|
Quadrant B
|
Quadrant C
|
Quadrant D
|
P valued | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. of isolates | IC50 value(95% CI) for trophozoite majority and duration ≥30 hb | No. of isolates | IC50 value (95% CI) for ring majority and duration ≥30 hc | No. of isolates | IC50 value (95% CI) for ring majority and duration <30 hc | No. of isolates | IC50 value (95% CI) for trophozoite majority and duration <30 hb | ||
| Chloroquine | 18 | 298 (153-583) | 35 | 59.8 (34.8-103) | 25 | 475 (296-762) | 75 | 539 (387-751) | <0.001* |
| Amodiaquine | 15 | 16.5 (9.8-27.9) | 35 | 7.85 (5.31-11.6) | 21 | 23.4 (15.6-35.0) | 71 | 18.9 (14.2-25.0) | 0.001* |
| Artesunate | 14 | 1.20 (0.71-2.02) | 32 | 0.83 (0.56-1.23) | 18 | 1.39 (0.69-2.80) | 71 | 1.60 (1.23-2.09) | 0.06 |
| Lumefantrine | 6 | 32.9 (5.59-193) | 5 | 37.6 (22.4-63.0) | 10 | 24.8 (9.8-62.6) | 33 | 14.6 (8.9-24.1) | 0.296 |
| Mefloquine | 12 | 7.27 (3.84-13.8) | 30 | 7.26 (4.89-10.8) | 12 | 22.2 (11.7-42.2) | 53 | 17.4 (13.5-22.4) | <0.001** |
| Piperaquine | 13 | 29.1 (22.3-38.1) | 30 | 14.6 (10.3-20.7) | 14 | 31.2 (20.9-46.5) | 67 | 22.9 (17.9-29.3) | 0.03*** |
In vitro sensitivity of P. vivax for each drug (geometric mean IC50 [95% CI]) stratified by the duration of assay and the RT ratio prior to culture. Quadrants are the same subgroups as represented in Fig. 2.
RT < 1; data on the initial parasite stage was unavailable for 20 isolates.
RT ≥ 1; data on initial RT ratios were missing for two isolates.
Overall between-group differences, by analysis of variance: *, group B versus A, C, or D, P = 0.001; **, group A or B versus C or D, P = 0.006; ***, group B versus A, C, or D, P = 0.026.
TABLE 6.
Correlation coefficients for the in vitro antimalarial susceptibilities of P. falciparum and P vivaxa
| Antimalarial | Combination |
P. Falciparum
|
P. Vivax
|
||||
|---|---|---|---|---|---|---|---|
| Correlation (rs) | P value | df | Correlation | P value | df | ||
| Chloroquine | Amodiaquine | 0.648 | <0.001 | 119 | 0.698 | <0.001 | 51 |
| Artesunate | 0.596 | <0.001 | 111 | 0.285 | 0.05 | 46 | |
| Lumefantrine | 0.427 | <0.001 | 65 | −0.104 | 0.735 | 12 | |
| Mefloquine | 0.445 | <0.001 | 136 | 0.618 | <0.001 | 38 | |
| Piperaquine | 0.347 | <0.001 | 139 | 0.620 | <0.001 | 40 | |
| Amodiaquine | Artesunate | 0.673 | <0.001 | 119 | 0.743 | <0.001 | 48 |
| Lumefantrine | 0.571 | 0.006 | 21 | −0.277 | 0.438 | 9 | |
| Mefloquine | 0.386 | <0.001 | 112 | 0.703 | <0.001 | 41 | |
| Piperaquine | 0.444 | <0.001 | 115 | 0.766 | <0.001 | 41 | |
| Artesunate | Lumefantrine | 0.030 | 0.879 | 27 | −0.112 | 0.793 | 7 |
| Mefloquine | 0.340 | <0.001 | 118 | 0.609 | <0.001 | 39 | |
| Piperaquine | 0.422 | <0.001 | 115 | 0.432 | 0.005 | 39 | |
| Lumefantrine | Mefloquine | 0.016 | 0.914 | 50 | 0.702 | 0.504 | 2 |
| Piperaquine | 0.152 | 0.215 | 67 | −0.697 | 0.124 | 5 | |
| Mefloquine | piperaquine | 0.225 | 0.011 | 125 | 0.644 | <0.001 | 40 |
Correlation coefficients (rs) are shown for the in vitro antimalarial susceptibility of P. falciparum and P vivax. P. vivax isolates shown are restricted to those with a RT ≥ 1.
DISCUSSION
Unlike Plasmodium falciparum, P. vivax has a selective preference for invading young red cells (8), which has confounded the development of a standardized in vitro drug susceptibility assay. Susceptibility testing for P. vivax continues to rely on the modifications of the schizont maturation test (4, 7, 9, 14, 19, 23), whereas assays used for P. falciparum are increasingly incorporating red cell reinvasion and adopting alternative methods of growth quantification. In the present study, we compared the schizont maturation test for P. falciparum and P. vivax isolates in Papua, Indonesia, where clinical multidrug resistance of both species has emerged (16).
Whereas the isolates of P. falciparum were mostly synchronous, those of P. vivax were predominantly asynchronous, with all stages of the parasite present in the peripheral blood. The asynchronicity of P. vivax is well documented but, importantly, varies between areas of differing endemicity, a factor recently found to confound the in vitro susceptibility of chloroquine in both Thai and Indonesian isolates (22). In view of the high proportion of patients with asynchronous P. vivax infections in Papua, Indonesia, we adopted an inclusive approach for parasite testing based on the total parasitemia rather than the staging. As a consequence, more than 60% of the isolates that were tested had a majority of trophozoites at the start of the assay. A major finding of our analysis is that in vitro susceptibility was correlated with the initial stage of the parasite, with isolates predominantly at the trophozoite stage having a twofold increase in IC50s compared to those of parasites predominantly at the ring stage. To assess the stage specificity of drug activity further, synchronous isolates presenting with more than 90% ring stage were exposed to the drug immediately and after culturing to trophozoites in the absence of the drug. Exposure at the trophozoite stage resulted in higher IC50s to amodiaquine and chloroquine, although the value did not reach statistical significance for mefloquine. Our findings confirmed the absence of chloroquine activity on P. vivax trophozoites, a developmental stage usually considered to be the specific target of this drug for P. falciparum (25), and concur with those made by Powell and Berglund some 30 years ago, using an in vitro MacroTest (13). Potentially, these observations have important biological implications, highlighting a fundamental difference between the activity of chloroquine with P. vivax and that with P. falciparum.
For convenience, most schizont maturation assays adopt a fixed duration of culture (usually 30 to 42 h) prior to the quantification of parasite growth (4, 12, 24). However, the time at which the assay is “harvested” is critical. Stopping the culture early reduces the proportion of schizonts present, thus decreasing the accuracy of quantification, whereas extending the culture beyond schizogony results in a significant reduction in the overall parasitemia as the mature schizonts start to rupture. In contrast to other methods, we report an assay in which in vitro culture was maintained until a threshold of 40% schizonts had been reached in the control wells. The time taken for isolates to reach this target varied considerably for both P. vivax and P. falciparum, from 22 to 48 h, even when synchronous ring-stage isolates were put into culture. The importance of this became apparent in the significant correlation of the in vitro susceptibility with the duration of the assay (Tables 4 and 5). Assays of shorter duration had significantly higher chloroquine, amodiaquine, artesunate, and mefloquine IC50s than assays of longer duration. Predictably, the initial stage of the parasite was an important determinant of the time to harvest (rs = 0.489; P < 0.001); isolates at the trophozoite stage had a shorter duration of assay (Fig. 2). However, the relation between the IC50s and the duration of the assay remained after controlling for the initial stage of the parasite (Table 4). Moreover, after highly synchronous cultures (RT ratio > 10) were selected, 31% of P. vivax assays and 53% of P. falciparum assays were harvested within 30 h, and the isolates with a short assay duration had a 10-fold and a 1.5-fold increase in chloroquine IC50, respectively, compared to isolates with longer assay durations.
Our findings raise the possibility that chloroquine resistant parasites grow faster than chloroquine susceptible parasites as has recently been reported in laboratory adapted strains of P. falciparum (17). Alternatively isolates rapidly reaching the threshold for harvest may represent parasites initially at a more mature ring stage. These findings have practical implications for interpreting in vitro assays and relevance for resent attempts to develop a nonmicroscopy assay. In assays that advocate culture for at least 36 h, faster growing isolates will rupture prior to quantification and thus will be recorded as unsuccessful. If these faster growing parasites are intrinsically more resistant then this will systematically bias the assessment of in vitro susceptibility toward a predominantly sensitive population. A recent comparison between Thai and Papuan isolates using the same assay highlighted the problem. In Thailand, where P. vivax is predominantly sensitive to chloroquine, the geometric mean IC50 was reported as 46.8 nM (22). In Papua, chemotherapeutic studies have confirmed high levels of clinical resistance to chloroquine (16). If we had adopted a criterion based on the duration of the assay and initial parasite staging, then the derived estimate of IC50 would not have been significantly different (59.8 nM) (Table 5). Applying a criterion of selecting isolates with a majority of rings irrespective of the duration of the assay (i.e., quadrants B and C in Table 5) yielded a significantly higher population mean (142 nM).
These data have relevance for the development of nonmicroscopic methods for determining the in vitro susceptibility of P. vivax, such as enzyme-linked immunosorbent sensitivity or DNA detection (5). The absence of chloroquine sensitivity at the trophozoite stage combined with minimal reinvasion and parasitemia, generally less than 0.5%, will both contribute to a poor signal-to-background ratio, which are likely to undermine the sensitivity and reproducibility of such assays (11).
Although population estimates of drug susceptibility would best be based on isolates which are synchronous and are initially exposed at the tiny ring stage, in practice, such strict criteria are likely to exclude almost 90% of P. vivax isolates, which in itself may constitute an appreciable bias. Hence, to accommodate both the stage specificity of the drug action and the possible confounding effects of the speed of parasite growth, we recommend selecting parasites with ring stage predominance (RT > 1). Ensuring that isolates are set up in culture within 2 h of venipuncture will help to increase the proportion fulfilling this criterion. Although the trophozoite minority will still contribute to the schizont quantification, by 30 h of culture, most will have ruptured, leaving those present at harvest to represent parasites exposed to the drug at all stages. Cultures should be stopped upon reaching a certain threshold rather than used in a fixed-duration assay, which has the potential to bias toward sensitive isolates.
In conclusion, the primary objective of this study was to determine the major factors underlying P. vivax drug susceptibility, using a schizont maturation assay. Although we have demonstrated that this method can be used to discriminate between resistant and sensitive populations of P. vivax (22), the derived IC50 value needs to be interpreted in conjunction with the initial stage of the parasite as well as the duration of the assay.
Acknowledgments
We thank Lembaga Pengembangan Masyarakat Amungme Kamoro. We also thank Karl Rieckmann for informative discussions on antimalarial in vitro susceptibility testing and the Australian Red Cross blood transfusion service for the supply of human sera.
The study was funded by the Wellcome Trust-NHRMC (Wellcome Trust ICRG GR071614MA-NHMRC ICRG ID 283321). N.A. is supported by an NHMRC practitioner fellowship. B.R. is funded by a Howard Florey fellowship, and R.P. is funded by a Wellcome Trust career development award, affiliated with the Wellcome Trust-Mahidol University-Oxford Tropical Medicine Research Programme (074637).
Footnotes
Published ahead of print on 7 January 2008.
REFERENCES
- 1.Baird, J. K., H. Basri, Purnomo, M. J. Bangs, B. Subianto, L. C. Patchen, and S. L. Hoffman. 1991. Resistance to chloroquine by Plasmodium vivax in Irian Jaya, Indonesia. Am. J. Trop. Med. Hyg. 44:547-552. [DOI] [PubMed] [Google Scholar]
- 2.Baird, J. K., B. Leksana, S. Masbar, D. J. Fryauff, M. A. Sutanihardja, Suradi, F. S. Wignall, and S. L. Hoffman. 1997. Diagnosis of resistance to chloroquine by Plasmodium vivax: timing of recurrence and whole blood chloroquine levels. Am. J. Trop. Med. Hyg. 56:621-626. [DOI] [PubMed] [Google Scholar]
- 3.Chen, N., A. Auliff, K. Rieckmann, M. Gatton, and Q. Cheng. 2007. Relapses of Plasmodium vivax infection result from clonal hypnozoites activated at predetermined intervals. J. Infect. Dis. 195:934-941. [DOI] [PubMed] [Google Scholar]
- 4.Chotivanich, K., R. Udomsangpetch, W. Chierakul, P. N. Newton, R. Ruangveerayuth, S. Pukrittayakamee, S. Looareesuwan, and N. J. White. 2004. In vitro efficacy of antimalarial drugs against Plasmodium vivax on the western border of Thailand. Am. J. Trop. Med. Hyg. 70:395-397. [PubMed] [Google Scholar]
- 5.Druilhe, P., P. Brasseur, C. Blanc, and M. Makler. 2007. Improved assessment of Plasmodium vivax response to antimalarial drugs by a colorimetric double-site plasmodium lactate dehydrogenase antigen capture enzyme-linked immunosorbent assay. Antimicrob. Agents Chemother. 51:2112-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fryauff, D. J., S. Tuti, A. Mardi, S. Masbar, R. Patipelohi, B. Leksana, K. C. Kain, M. J. Bangs, T. L. Richie, and J. K. Baird. 1998. Chloroquine-resistant Plasmodium vivax in transmigration settlements of West Kalimantan, Indonesia. Am. J. Trop. Med. Hyg. 59:513-518. [DOI] [PubMed] [Google Scholar]
- 7.Gajanana, A., and A. N. Raichowdhuri. 1984. Plasmodium vivax: micro in vitro test for assaying chloroquine susceptibility. Trans. R. Soc. Trop. Med. Hyg. 78:416-417. [DOI] [PubMed] [Google Scholar]
- 8.Galinski, M. R., C. C. Medina, P. Ingravallo, and J. W. Barnwell. 1992. A reticulocyte-binding protein complex of Plasmodium vivax merozoites. Cell 69:1213-1226. [DOI] [PubMed] [Google Scholar]
- 9.Hamedi, Y., M. Nateghpour, B. Soonthornsata, P. Tan-ariya, S. Kojima, D. Chindanond, and S. Looareesuwan. 2003. Monitoring of Plasmodium vivax sensitivity to chloroquine in vitro in Thailand. Trans. R. Soc. Trop. Med. Hyg. 97:435-437. [DOI] [PubMed] [Google Scholar]
- 10.Imwong, M., G. Snounou, S. Pukrittayakamee, N. Tanomsing, J. R. Kim, A. Nandy, J. P. Guthmann, F. Nosten, J. Carlton, S. Looareesuwan, S. Nair, D. Sudimack, N. P. Day, T. J. Anderson, and N. J. White. 2007. Relapses of Plasmodium vivax infection usually result from activation of heterologous hypnozoites. J. Infect. Dis. 195:927-933. [DOI] [PubMed] [Google Scholar]
- 11.Kosaisavee, V., R. Suwanarusk, F. Nosten, D. E. Kyle, M. Barrends, J. Jones, R. Price, B. Russell, and U. Lek-Uthai. 2006. Plasmodium vivax: isotopic, PicoGreen, and microscopic assays for measuring chloroquine sensitivity in fresh and cryopreserved isolates. Exp. Parasitol. 114:34-39. [DOI] [PubMed] [Google Scholar]
- 12.Lux, D., S. Prajakwong, H. Kollaritsch, G. Wernsdorfer, and W. H. Wernsdorfer. 2003. In-vitro sensitivity testing of Plasmodium vivax: response to lumefantrine and chloroquine in northwestern Thailand. Wien. Klin. Wochenschr. 115(Suppl. 3):50-54. [PubMed] [Google Scholar]
- 13.Powell, R. D., and E. M. Berglund. 1974. Effects of chloroquine upon the maturation of asexual erythrocytic forms of Plasmodium vivax in vitro. Am. J. Trop. Med. Hyg. 23:1007-1014. [DOI] [PubMed] [Google Scholar]
- 14.Pukrittayakamee, S., A. Chantra, J. A. Simpson, S. Vanijanonta, R. Clemens, S. Looareesuwan, and N. J. White. 2000. Therapeutic responses to different antimalarial drugs in vivax malaria. Antimicrob. Agents Chemother. 44:1680-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ratcliff, A., H. Siswantoro, E. Kenangalem, R. Maristela, R. M. Wuwung, F. Laihad, E. P. Ebsworth, N. M. Anstey, E. Tjitra, and R. N. Price. 2007. Two fixed-dose artemisinin combinations for drug-resistant falciparum and vivax malaria in Papua, Indonesia: an open-label randomised comparison. Lancet 369:757-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ratcliff, A., H. Siswantoro, E. Kenangalem, M. Wuwung, A. Brockman, M. D. Edstein, F. Laihad, E. P. Ebsworth, N. M. Anstey, E. Tjitra, and R. N. Price. 2007. Therapeutic response of multidrug-resistant Plasmodium falciparum and P. vivax to chloroquine and sulfadoxine-pyrimethamine in southern Papua, Indonesia. Trans. R. Soc. Trop. Med. Hyg. 101:351-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reilly, H. B., H. Wang, J. A. Steuter, A. M. Marx, and M. T. Ferdig. 2007. Quantitative dissection of clone-specific growth rates in cultured malaria parasites. Int. J. Parasitol. 37:1599-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rieckmann, K. H., D. R. Davis, and D. C. Hutton. 1989. Plasmodium vivax resistance to chloroquine? Lancet ii:1183-1184. [DOI] [PubMed] [Google Scholar]
- 19.Russell, B. M., R. Udomsangpetch, K. H. Rieckmann, B. M. Kotecka, R. E. Coleman, and J. Sattabongkot. 2003. Simple in vitro assay for determining the sensitivity of Plasmodium vivax isolates from fresh human blood to antimalarials in areas where P. vivax is endemic. Antimicrob. Agents Chemother. 47:170-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soto, J., J. Toledo, P. Gutierrez, M. Luzz, N. Llinas, N. Cedeno, M. Dunne, and J. Berman. 2001. Plasmodium vivax clinically resistant to chloroquine in Colombia. Am. J. Trop. Med. Hyg. 65:90-93. [DOI] [PubMed] [Google Scholar]
- 21.Sumawinata, I. W., Bernadeta, B. Leksana, A. Sutamihardja, Purnomo, B. Subianto, Sekartuti, D. J. Fryauff, and J. K. Baird. 2003. Very high risk of therapeutic failure with chloroquine for uncomplicated Plasmodium falciparum and P. vivax malaria in Indonesian Papua. Am. J. Trop. Med. Hyg. 68:416-420. [PubMed] [Google Scholar]
- 22.Suwanarusk, R., B. Russell, M. Chavchich, F. Chalfein, E. Kenangalem, V. Kosaisavee, B. Prasetyorini, K. A. Piera, M. Barends, A. Brockman, U. Lek-Uthai, N. M. Anstey, E. Tjitra, F. Nosten, Q. Cheng, and R. N. Price. 2007. Chloroquine resistant Plasmodium vivax: in vitro characterisation and association with molecular polymorphisms. PLoS ONE 2:e1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tasanor, O., H. Noedl, K. Na-Bangchang, K. Congpuong, J. Sirichaisinthop, and W. H. Wernsdorfer. 2002. An in vitro system for assessing the sensitivity of Plasmodium vivax to chloroquine. Acta Trop. 83:49-61. [DOI] [PubMed] [Google Scholar]
- 24.Tasanor, O., R. Ruengweerayut, J. Sirichaisinthop, K. Congpuong, W. H. Wernsdorfer, and K. Na-Bangchang. 2006. Clinical-parasitological response and in-vitro sensitivity of Plasmodium vivax to chloroquine and quinine on the western border of Thailand. Trans. R. Soc. Trop. Med. Hyg. 100:410-418. [DOI] [PubMed] [Google Scholar]
- 25.Yayon, A., J. A. Vande Waa, M. Yayon, T. G. Geary, and J. B. Jensen. 1983. Stage-dependent effects of chloroquine on Plasmodium falciparum in vitro. J. Protozool. 30:642-647. [DOI] [PubMed] [Google Scholar]