Abstract
Dapivirine is a nonnucleoside reverse transcriptase inhibitor being developed as a topical microbicide for the prevention of human immunodeficiency virus infection. The distribution of radioactivity and drug in plasma and in vaginal, cervical, and draining lymph node tissues was investigated after daily application of a vaginal gel formulation of [14C]dapivirine to rhesus macaques. This was preceded by a preliminary study with rabbits. Following the intravaginal administration of [14C]dapivirine (∼0.1 mg/ml [15 μCi/ml]) to rabbits (0.5 ml/day) and macaques (1 ml/day) for 7 days, the dapivirine levels associated with vaginal and cervical tissue samples 1 h after the final dose were high (quantities of μg/g of tissue) and remained detectable at 24 h (mean, ≥2.5 ng/g in rabbits) and 48 h (mean, >80 ng/g in macaques). Radioactivity levels were low in the plasma and very low or unquantifiable in the draining lymph nodes of the macaques. Microautoradiography identified drug-related material (DRM) on the surfaces of the vaginal and cervical tissues of the rabbits and macaques. Although DRM was primarily associated with the outermost layer of shedding cells in rabbits, two animals showed some evidence of small quantities in the mucosal epithelium of the cervix. In macaques, DRM was seen within the keratinized layer of the vaginal epithelium and and was found to extend into the superficial cellular layers, and in at least one animal it appeared to be present in the deepest (germinal) layer of the epithelium and in submucosal tissues. The persistence of biologically significant concentrations of dapivirine in vaginal and cervical tissues for >24 h supports the development of dapivirine as a microbicide for once daily application.
With women in developing countries increasingly bearing the brunt of the human immunodeficiency virus (HIV)/AIDS epidemic (10) and in the absence of an effective vaccine, women-initiated methods of HIV prevention are greatly needed. Topical microbicides are self-administered prophylactic agents applied to the vagina to impede the transmission of HIV and/or other sexually transmitted pathogens.
Dapivirine (4-[[4-[(2,4,6-trimethylphenyl)amino]-2-pyrimidinyl]amino] benzonitrile), also known as TMC120, is a substituted diarylpyrimidine derivative and a nonnucleoside reverse transcriptase (RT) inhibitor (NNRTI) (11). NNRTIs comprise a range of structurally diverse hydrophobic compounds that bind to the RT enzyme of HIV type 1 (HIV-1), and since RT is essential to replication, this prevents further production of the virus (1, 5). Although it was first conceived as an oral therapeutic agent (9), dapivirine is an ideal candidate for topical microbicide development due to its proven in vitro (12) and in vivo (6, 9) efficacy and safety profiles, as well as its physical and chemical properties. In particular, dapivirine is one of a new class of tightly binding lipophilic NNRTIs that are active against cell-free and cell-associated HIV-1 and may have direct virucidal activity (5). Dapivirine has been demonstrated to have potent activity against wild-type virus strains and strains harboring different NNRTI resistance-inducing mutations (3). Dapivirine has also shown an antiviral profile superior to that of the existing NNRTI class of compounds, such as nevirapine, delavirdine, and efavirenz (11). Dapivirine is not active against HIV-2.
A topical vaginal gel formulation of dapivirine is being developed for the prevention of the male-to-female transmission of HIV-1 infection in developing countries. The intention is that a dapivirine microbicide gel may be applied once daily, with each application conferring protection against HIV infection over a period of at least 24 h. In order to achieve this, sufficient levels of the drug must remain at the target sites for infection during this period. A study was therefore performed to determine the levels of drug in cervical and vaginal tissues and in the draining lymph nodes in rhesus macaques following the once daily application of a gel containing [14C]dapivirine. This was preceded by a preliminary study with rabbits.
MATERIALS AND METHODS
Animal facilities.
The in-life phases of the studies were performed by BioQual, Inc. (Rockville, MD), in accordance with the regulations outlined in the USDA Animal Welfare Act (9 CFR Parts 1, 2, and 3) and/or the conditions specified in the Guide for the Care and Use of Laboratory Animals (8). The protocols and any amendments or procedures involving the care or use of animals in these studies were reviewed and approved by BioQual's Animal Care and Use Committee prior to the initiation of such procedures. BioQual is also accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
Test materials.
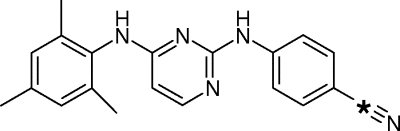
The [14C]dapivirine was manufactured by Moravek Biochemcials, Inc. (Brea, CA), and the molecular structure and position of the radiolabel are illustrated in Fig. 1.
FIG. 1.
Structure of [14C]dapivirine. The position of the 14C radiolabel is denoted by an asterisk.
[14C]dapivirine was formulated by Particle Sciences Inc. (Bethlehem, PA) as a gel containing polyethylene glycol, hydroxyethylcellulose, polycarbophil, methylparaben, propylparaben, sodium hydroxide, and water with a nominal dapivirine concentration of 0.009% (0.09 mg/ml; 0.273 mM) and activity of 15 μCi/ml. This concentration was selected to approximate the anticipated clinical concentration. Analysis of the gel by high-pressure liquid chromatography with photodiode array detection (286 nm) (Analytisch Biochemisch Laboratorium BV [ABL], Assen, The Netherlands) indicated that the gel was homogeneous and that the actual concentration achieved was 0.00855% (95% of the nominal concentration). The formulated material was stored at ambient temperature.
Dose administration.
The [14C]dapivirine gel was administered intravaginally once daily to six female rabbits (0.5 ml/day) for 7 days. The formulation was administered by using a non-leuer-lock syringe fitted with an 8-cm French feeding tube inserted approximately 6 cm into the vagina. In the macaque study, the [14C]dapivirine gel was administered intravaginally once daily (1 ml/day) to nine females for 7 days. Before they were dosed, the macaques were sedated with ketamine (5 to 20 mg/kg of body weight) and were maintained under sedation for approximately 15 to 30 min. The formulation was administered by using a 3 ml leuer-lock syringe with a bent 10-gauge stainless steel feeding tube attached to a bulbous end (Popper and Sons, New York, NY). An Elizabethan-type collar was fitted to the rabbits and macaques for approximately 8 h following dosing in order to minimize the possibility of oral ingestion of the dose material during this period.
Plasma analysis.
Blood samples (approximately 4 ml) were collected in tubes containing EDTA from three rabbits at 6 h after each dose and from two rabbits at 1, 8, and 24 h after the final dose. In the macaque study, blood samples (approximately 3 ml) were collected in tubes containing heparin from all animals before dosing on day 1 and from three or six animals at 24 h after each dose and at 1, 2, 4, 12, 16, 30, 36, and 48 h after the final (seventh) dose. The plasma was separated, and the levels of radioactivity (in disintegrations per minute) were determined by liquid scintillation counting (BioQual). The lower limit of quantitation (LLOQ) was set at twice the background level of radiation. For macaques, additional blood samples (approximately 3 ml) were collected in tubes containing heparin, the plasma was separated, and the dapivirine concentrations were measured by liquid chromatography tandem mass spectrometry (LC-MS/MS; ABL) with an analytical range of 0.100 to 50.0 ng/ml. Dapivirine and the internal standard were extracted from plasma samples by liquid-liquid extraction with t-butylmethylether. The chromatographic separation was performed on a reversed-phase 3-μm C18 column with a mobile phase of approximately 0.01 M ammonium acetate in 40:60 H2O-acetonitrile (ACN). Dapivirine was quantified in the negative ionization mode by multiple-reaction monitoring. The mass transitions m/z (mass-to-charge ratio) 328→142 and m/z 435→325 were used to measure dapivirine and the internal standard, respectively, with a run time of 5 min per analysis. Individual values below the LLOQ were assigned a value of 0 for the calculation of the means.
Tissue sample analysis.
Two rabbits per time point were killed by intravenous pentobarbital overdose at 1, 8, and 24 h after the final dose and samples of vaginal wall and cervical (ectocervical) tissues were collected and rinsed in phosphate-buffered saline. The levels of radioactivity in the tissue samples were analyzed in triplicate by liquid scintillation counting (LLOQ, two times the background radiation; BioQual), and the dapivirine concentrations were measured by LC-MS/MS (analytical range, 0.250 to 250 ng/g of tissue; ABL), as follows: the tissues were homogenized in a 2:1 H2O-ACN mixture and treated with methanol, the internal standard (TMC125), 25% NH4OH, and t-butylmethylether. After the components were mixed, the samples were centrifuged at 4,700 rpm for 2 min and then frozen. The organic layer was dried under nitrogen and was reconstituted in LC solvent for analysis. The chromatographic separation was performed on a reversed-phase 3-μm C8 column with a gradient mobile phase system consisting of 100 mM ammonium formate, water, and ACN. Dapivirine was quantified in the positive ionization mode by multiple-reaction monitoring. The mass transitions m/z 330.2→158.2 and m/z 435.1→163.1 were used to measure dapivirine and the internal standard, respectively, with a run time of 6 min per analysis. Data acquisition was performed with Analyst software (Applied Biosystems, Foster City, CA). A 10-point calibration curve was fitted to the model y = A + B·x + Cx2, by using 1/x2 as the weighting factor, where y is the peak area ratio of the analyte and the internal standard, x is the nominal calibration level (in ng/g), A is the intercept, B is the slope, and C is a description of the curvature. Unknowns were calculated by using the inverse of the response model, as follows:
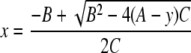 |
Three macaques per time point were killed at 1, 24, and 48 h after the final dose by intravenous sodium pentobarbital overdose (65 mg/kg) while they were under deep anesthesia induced with ketamine. Samples of vaginal wall, cervical (ectocervical), and draining lymph node tissues and plasma were collected. Additional vaginal tissue samples (three or four samples per time point) were collected by biopsy at 2, 4, 8, 12, 16, 30, and 36 h; and cervical biopsy samples (three samples per time point) were collected at 4, 8, 12, and 30 h after the final dose while the animals were under anesthesia with tiletamine-zolazepam (Telazol; 4 to 6 mg/kg). The tissue and plasma samples were analyzed for the concentrations of radioactivity by liquid scintillation counting (LLOQ, two times the background radiation; BioQual) and for the dapivirine concentration by LC-MS/MS (analytical range, 0.250 to 250 ng/g; ABL), as described above. Both methods were used in order to establish whether there were any significant differences between the dapivirine concentrations and the total levels of drug-related material (DRM) that might indicate metabolism of the parent drug. All samples collected at necropsy were rinsed in phosphate-buffered saline at the time of collection to remove residual gel, whereas the biopsy samples were inadvertently not rinsed after collection, so a rinsing step was performed by ABL prior to analysis. However, the data for the biopsy samples were highly variable, which was most likely due to the difference in sample preparation and the quantity of residual gel associated with these samples. Therefore, the data for the biopsy samples are not presented. Individual values below the LLOQ were assigned a value of 0 for calculation of the means.
Histology.
Samples of vaginal and cervical tissues from all rabbits were embedded in paraffin, sectioned at approximately 4 μm, and stained with hematoxylin-eosin (Seventh Wave Laboratories, LLC, Chesterfield, MO). Samples of these tissues were also processed for autoradiographic exposure (NeuroScience Associates, Knoxville, TN). Separate sets of slides of the vaginal tissue were exposed for 2, 4, or 8 weeks to determine the optimal exposure time; and a single set of slides of the cervical tissue was exposed for 8 weeks. The slides were then returned to Seventh Wave Laboratories for histopathological examination and to evaluate whether the presence of DRM could be seen microscopically.
In the macaque study, one slide of the vaginal, cervical, and iliac and inguinal lymph node tissue samples from each animal was stained with hematoxylin-eosin (Seventh Wave Laboratories). Additional slides were prepared for immunohistochemical staining to identify cells of histiocytic lineage (CD68), Langerhans' cells (CD1a), and T helper cells (CD4). Different slide sets were used for each target antigen, but the staining methods were the same for each antigen. The primary monoclonal mouse antibodies used were clone KP1 (Lab Vision, Fremont, CA) for CD68, clone 010 (Dako, Carpinteria, CA) for CD1a, and clone 1F6 (Lab Vision) for CD4. The slides were cleared in xylene and rehydrated in graded alcohols. Antigen retrieval was accomplished by treating the slides with a Dako target retrieval kit (Tris-EDTA buffer, pH 9) in a pressure cooker. Avidin and biotin blocking solutions (Vector, Burlingame, CA) were applied prior to application of the primary antibody. The secondary antibody was biotinylated polyclonal rabbit anti-mouse immunoglobulins (Dako). Streptavidin-alkaline phosphatase was then applied, followed by the addition of permanent red chromogen (Dako). Mayers hematoxylin was used as the counterstain. Specificity was evaluated by the use of a negative mouse control antibody (Dako) in place of the primary antibody. Positive control slides (human skin for CD1a and human tonsil for CD68 and CD4) were used to assess functionality. The slides were dehydrated in graded alcohols and cleared in xylene prior to autoradiographic exposure. Different slide sets were used for each target antigen.
After the completion of immunohistochemical staining, one set of stained slides and one set of unstained slides were processed for autoradiographic exposure (NeuroScience Associates). Following autoradiographic exposure for 4 weeks, the slides were returned to Seventh Wave Laboratories for evaluation and interpretation. The combined immunohistochemical and microautoradiographic evaluations were primarily intended to determine whether dapivirine was present in or on the target cell populations and to more generally identify the presence and the location of the test article at the tissue level.
RESULTS
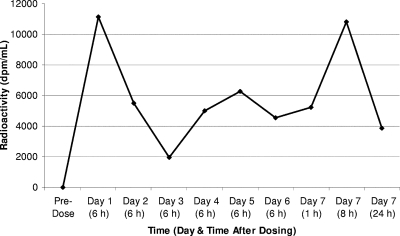
In rabbits, relatively high radioactivity levels (246 to 16,241 dpm/ml) were measured in plasma at all time points (Fig. 2). In vaginal and cervical tissue samples, dapivirine levels were high 1 h after the final dose (>3,000 ng/g) and remained detectable (≥2.5 ng/g) 24 h after the final dose (Table 1). Compared with the dapivirine levels, the values for radioactivity were lower at 1 h after dosing but were higher at 8 and 24 h, with the exception of the values at 8 h in the vaginal tissue, which were markedly higher for dapivirine than for radioactivity. This variability between dapivirine and radioactivity levels was most likely due to differences in the amounts of residual gel associated with the tissue samples. Had the variations been due to the presence of metabolites or the breakdown products of dapivirine, the radioactivity levels would be expected to have been consistently higher than the dapivirine levels, which was not the case.
FIG. 2.
Plasma radioactivity levels in rabbits treated intravaginally with [14C]dapivirine for 7 days. The results represent the means for three animals up to day 6 and two animals for day 7.
TABLE 1.
Mean total DRM and dapivirine levels in rabbits treated intravaginally with [14C]dapivirine for 7 days
| Time (h) after final dose | Animal no. | Tissue contenta
|
|||
|---|---|---|---|---|---|
| Vagina
|
Cervix
|
||||
| Total DRM | Dapivirine | Total DRM | Dapivirine | ||
| 1 | 1 | 1,421,759 | 3,202 | 564,831 | 1,021 |
| 2 | 1,035,310 | 5,732 | 836,256 | 5,264 | |
| 8 | 3 | 17,799 | 11.7 | 19,607 | 3.01 |
| 4 | 115,060 | 2,969 | 46,257 | 80.5 | |
| 24 | 5 | 26,356 | 5.00 | 2,550 | 2.95 |
| 6 | 41,499 | 8.76 | 32,019 | 2.14 | |
The data for the DRM contents are in dpm/g, and those for the dapivirine contents are in ng/g. Tissues were analyzed for radioactivity in triplicate for all animals. Radioactivity was determined in triplicate by liquid scintillation counting. Dapivirine was determined in all tissues by using a single LC-MS/MS analysis. A value of 50 dpm corresponds to 0.135 ng of [14C]dapivirine equivalents.
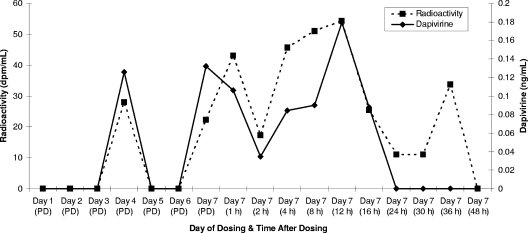
In macaques, plasma radioactivity and dapivirine levels were low (≤83 dpm/ml and ≤0.226 ng/ml, respectively) and often below the LLOQ (two times the background radioactivity or 0.100 ng/ml) (Fig. 3). Given the highly hydrophobic nature of dapivirine (7), it is plausible that the drug may partition into the cellular compartment, resulting in only a small fraction of the drug in whole blood being present in the plasma fraction. However, a blood partitioning study was performed with human blood in which whole blood from four female volunteers was spiked with dapivirine in 70% dimethyl sulfoxide at 0.0100, 1.00, 50.0, and 5,000 ng/ml for 10 min at room temperature. The samples were divided into four 0.5-ml aliquots and the plasma was separated by centrifugation. The plasma was then analyzed by LC-MS/MS (analytical range, 0.0100 to 1.00 ng/ml; ABL) in a manner similar to that described above for macaque plasma. The hematocrit value for each subject was also determined, and this was used along with the plasma concentrations to calculate the concentration of dapivirine in the cell fraction. The mean values for the proportion of drug in the cellular fraction ranged from 12 to 16%, indicating that dapivirine did not partition to this fraction. Although these values were obtained with human blood, it is highly unlikely that the extent of partitioning would be substantially different in rabbit or macaque blood.
FIG. 3.
Plasma radioactivity and dapivirine levels in macaques treated intravaginally with [14C]dapivirine for 7 days. The values represent the means for three macaques, except for the day 1 and day 7 (16-h) time points, for which the values are the means for six macaques. PD, predosing.
In the vaginal and cervical tissue samples, the levels of dapivirine and radioactivity were highly variable. The mean values were generally high in samples collected 1 h after the final dose (≥4,233 ng/g in vaginal tissue and ≥1,421 ng/g in cervical tissue), and the levels remained detectable in all animals at 48 h (means, ≥515 ng/g in vaginal tissue and ≥66 ng/g in cervical tissue) (Table 2). Very low levels were also present in the iliac and inguinal lymph nodes at 1 and 24 h after dosing (means, ≥3 ng/g in the inguinal lymph node tissue and ≥15 ng/g in the iliac lymph node tissue), but dapivirine and radioactivity were generally absent by 48 h.
TABLE 2.
Mean total DRM and dapivirine levels in macaques treated intravaginally with [14C]dapivirine for 7 days
| Time (h) after final dose | Animal no. | Tissue contenta
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Vagina
|
Cervix
|
Inguinal lymph node
|
Ileac lymph node
|
||||||
| Total DRM | Dapivirine | Total DRM | Dapivirine | Total DRM | Dapivirine | Total DRM | Dapivirine | ||
| 1 | 4199 | 60,260 | 1,280 | 73,842 | 604 | 586 | 3.13 | 366 | 3.13 |
| 4200 | 147,114 | 413 | 27,091 | 102 | 1,947 | 17.4 | 16,627 | 35.6 | |
| 4201 | 4,414,620 | 11,100 | 1,450,964 | 6,500 | 598 | 11.3 | 3,644 | 5.83 | |
| Mean | 1,540,664 | 4,264 | 517,229 | 2,402 | 1,044 | 10.61 | 6,879 | 14.9 | |
| SDb | 2,228,930.1 | 5,935.7 | 812,106.0 | 3,557.8 | 1,134.6 | 7.160 | 8,382.9 | 18.02 | |
| 24 | 4202 | 728,086 | 2,110 | 75,587 | 735 | 0 | 1.80 | 0 | 1.04 |
| 4203 | 230,934 | 1,350 | 44,374 | 73 | 0 | 1.82 | 0 | 1.17 | |
| 4204 | 20,573 | 4.78 | 59,455 | 5.85 | 1,600 | 1.70 | 9,861 | 32.6 | |
| Mean | 326,531 | 1,155 | 59,805 | 271 | 533 | 1.77 | 3,287 | 11.6 | |
| SD | 343,889.5 | 1,044.6 | 69,929.5 | 355.9 | 1,182.6 | 0.888 | 7,009.2 | 15.94 | |
| 48 | 4205 | 149,359 | 123 | 34,065 | NSc | 0 | 0.376 | 0 | 0.683 |
| 4206 | 122,943 | 41.4 | 26,561 | 4.73 | 0 | 0 | 0 | 2.07 | |
| 4207 | 604,148 | 1,380 | 11,514 | 158 | 0 | 1.07 | 0 | 0.733 | |
| Mean | 292,150 | 515 | 24,047 | 81 | 0 | 0.48 | 0 | 1.16 | |
| SD | 289,138.1 | 750.4 | 16,365.1 | 0.0 | 0.543 | 0.0 | 0.787 | ||
The data for the DRM contents are in dpm/g, and those for the dapivirine contents are in ng/g. Radioactivity was determined in triplicate by liquid scintillation counting. Dapivirine was determined in all tissues by using a single LC-MS/MS analysis.
SD, standard deviation.
NS, no sample available.
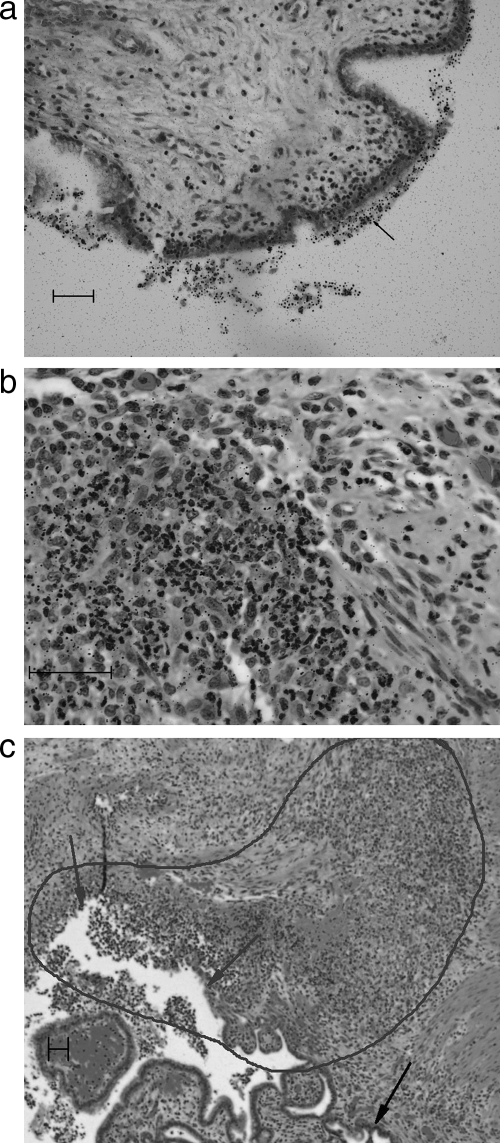
In rabbits, an increased concentration of silver grains on the surfaces of vaginal and cervical tissues from several rabbits indicated the presence of DRM at those sites (Fig. 4a). There was some evidence of small amounts of test material in the mucosal epithelium of the cervical tissue in two rabbits, suggesting that small amounts of the test material may have been taken up by the mucosal epithelium. However, the silver grains were primarily associated with the outermost layer of extruding cells. Generally, the density of the silver grains in the epithelium was at the background level. Therefore, the presence of DRM in viable mucosal epithelium was not convincingly demonstrated in this study. In one rabbit in which ulceration was present in the vaginal epithelium (considered to be unrelated to treatment, since the intravaginal administration of the gel for up to 39 weeks in rabbits produced no evidence of toxicity [unpublished data]), the density of silver grains was increased in the inflammatory tissue at the site of ulceration, indicating the presence of DRM (Fig. 4b and c). The presence of DRM in the inflammatory tissue at the site of ulceration suggests that it may accumulate at sites where the integrity of the epithelium has been compromised. There were no further lesions or changes in vaginal tissues in any rabbits. The only lesion observed in the cervical tissue was a submucosal hemorrhage in one rabbit, which, again, was considered to be unrelated to treatment, given the absence of toxicity in intravaginal studies with rabbits of up to 39 weeks' duration.
FIG. 4.
Rabbit, 1 h after seventh dose. (a) Test material on the surface of the cervical epithelium (arrow), as indicated by the increased density and size of the silver grains. (b) Mixed inflammatory cells in the area of ulceration; includes both cells of lymphohistiocytic origin and neutrophils. Note the density of the grains in the area of inflammation on the left compared to the area to the right of the slide with few inflammatory cells for comparison of the grain density with the background density. (c) Lower magnification of the same field in panel b showing the area of ulceration in the vaginal mucosa as shown by gray arrows. The intact mucosal epithelium is indicated by the black arrow. The area outlined demonstrates the most intense area of inflammation. Bars, 50 μm.
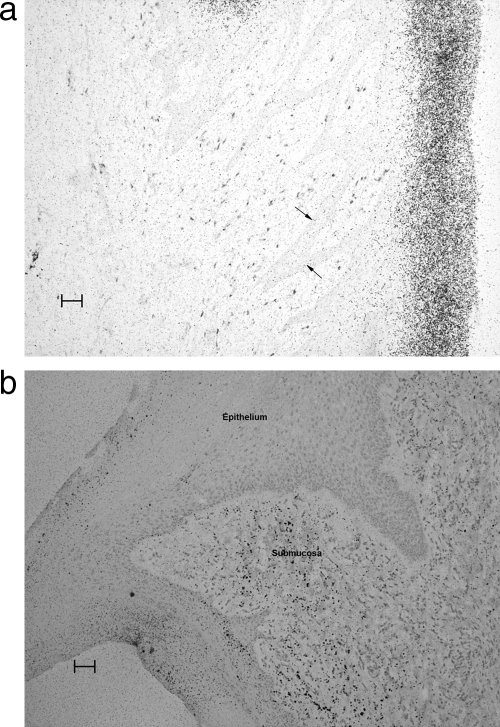
In macaques, histopathological examination revealed that DRM was variably present in sections of the vaginal and cervical tissues. In most instances, the DRM was present on the surface or within the keratinized layer of the vaginal epithelium (Fig. 5a) and extended into the superficial cellular layers. In at least one animal it appeared to be present in the deepest (germinal) layer of the epithelium and in submucosal tissues (Fig. 5b).
FIG. 5.
Macaque, 48 h after seventh dose. (a) Autoradiographically exposed section of vaginal tissue showing the accumulation of DRM (a dense accumulation of silver grains) primarily in the keratin layer. A scattering of CD4 cells (red staining cells) is present in the submucosal area (shown by arrows). (b) Autoradiographically exposed section of vaginal tissue stained for CD68 showing more dense accumulations of the test article in the submucosa as well as in some areas of the epithelium. Indications of the presence of DRM in submucosal areas were uncommon. Bars, 50 μm.
Foci of ulceration, presumably sites of previous biopsies, were observed in the vaginal epithelium from all monkeys terminated at 24 and 48 h following the final treatment and in one monkey terminated at 1 h following the final treatment. Inflammatory cells were present in those areas, some of which stained for CD68. However, there was no evidence of accumulation of the test article at the sites of ulceration, indicating that there was no preferential adherence of the product at those sites versus the intact mucosa and no evidence of localization of radioactive material in the CD68 cells that were present at those sites.
DISCUSSION
Following once daily intravaginal administration of a 1-ml dose of [14C]dapivirine (∼0.1 mg/ml [15 μCi/ml]) to female macaques, dapivirine remained associated with the vaginal and cervical epithelia for at least 48 h. In a study with human ectocervical tissue explants in vitro, it was shown that treatment with dapivirine for 1 h prior to exposure to HIV-1BaL potently inhibited viral replication, with a 50% inhibitory concentration of 0.49 ng/ml (1.5 nM), and greater than 99% inhibition of integrated provirus was observed at 3.3 ng/ml (10 nM; R. Shattock, Virology Summary Report, Cellular and Molecular Medicine, St. George's, University of London, 2005, personal communication). Therefore, the concentrations of dapivirine associated with macaque vaginal and cervical tissues in vivo were markedly higher than the concentration required for 99% proviral integration in vitro.
Histopathological evaluation revealed that, in general, DRM in rabbits and macaques was present on the surface or within the keratinized layer of the epithelium and extended into the superficial cellular layers. In one macaque, it was present in the deepest (germinal) layer of the epithelium and in submucosal tissues. The observation of DRM primarily in the surface epithelium suggests that the mucosal epithelium acts as a barrier to its absorption. The mode of action for NNRTIs is through irreversible inhibition of the viral RT, thus preventing the production of proviral DNA within the host cell (5). In HIV-infected individuals, it is likely that the primary site of action is within the host cell. However, there is evidence that membrane-permeable tightly binding NNRTIs such as dapivirine may have the ability to inactivate cell-free HIV-1 as well as cell-associated HIV-1 (5). This activity has been investigated by preincubating immobilized HIV-1 (RF strain) with dapivirine prior to culture with indicator cells (R. Shattock, 2005, personal communication). When virus was pretreated and the compound was removed prior to addition of the indicator cells, protection against infection was achieved at 32.9 ng/ml; and when dapivirine was not removed prior to addition of indicator T cells, protection was achieved at 3.3 ng/ml. Therefore, dapivirine may have the potential to inactivate both cell-free and cell-associated HIV-1 in the semen, before the virus reaches the tissues of the female reproductive tract. Thus, the absence of consistently observable DRM within the germinal layer of the epithelium and in submucosal tissues is of unknown significance for the use of dapivirine as a microbicide, since it is possible that the presence of drug on the surface or within the keratinized layer of the epithelium may be sufficient to provide protection against infection. In addition, although it was not seen in macaques, there was evidence in rabbits that the drug may accumulate at sites where the integrity of the vaginal epithelium has been compromised. Since disruption of the epithelium presents an increased risk for infection (2, 4), the accumulation of drug at such sites may be significant in the prevention of infection.
The plasma concentration data for macaques demonstrated low systemic exposure, and the dapivirine concentration was often below the LLOQ of the assay. The maximum plasma concentration of dapivirine observed was 0.226 ng/ml. Although it is difficult to draw any conclusions on the pharmacokinetics of dapivirine, the data do demonstrate that systemic absorption did occur. In a randomized, controlled, double-blind, phase I trial with women, 2.4 ml of a microbicide gel consisting of 25, 50, or 150 μM dapivirine (equivalent to 0.001, 0.002, and 0.005%, respectively, or 8.2, 16.5, and 49.4 μg/ml, respectively) with hydroxyethylcellulose and glycerol as a base, ethanol as a solvent, lactic acid and sodium hydroxide for pH adjustment, and methyl-para-hydroxybenzoate and propyl-para-hydroxybenzoate as preservatives was applied intravaginally twice daily for 7 days (9). The maximum plasma concentration detected was 0.16 ng/ml, suggesting that the absorption of dapivirine following intravaginal administration is comparable in women and macaques.
The association of dapivirine with vaginal and cervical tissues for >24 h at levels markedly higher than those required for 99% inhibition of integrated provirus in a cervical explant model suggests that a dapivirine microbicide gel has potential as a product for once daily application. However, the concentrations required to prevent infection in vivo have yet to be established.
Acknowledgments
We thank Garry Gwozdz of Particle Sciences, Inc., for formulating the dapivirine gel and the staff of BioQual, Inc.; Analytisch Biochemisch Laboratorium BV; Neuroscience Associates, Inc.; and Seventh Wave Pathology and Biotechnical Solutions, LLC, for their technical contributions to this work.
Footnotes
Published ahead of print on 17 December 2007.
REFERENCES
- 1.Borkow, G., J. Barnard, T.-M. Nguyen, A. Belmonte, M. A. Wainberg, and M. A. Parniak. 1997. Chemical barriers to human immunodeficiency virus type 1 (HIV-1) infection: retrovirucidal activity of UC781, a thiocarboxanilide nonnucleoside inhibitor of HIV-1 reverse transcriptase. J. Virol. 71:3023-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen, M. S. 2004. HIV and sexually transmitted diseases: lethal synergy. Top. HIV Med. 12:104-107. [PubMed] [Google Scholar]
- 3.Das, K., A. D. Clark, Jr., P. J. Lewi, J. Heeres, M. R. De Jonge, L. M. Koymans, H. M. Vinkers, F. Daeyaert, D. W. Ludovici, M. J. Kukla, B. De Corte, R. W. Kavash, C. Y. Ho, H. Ye, M. A. Lichtenstein, K. Andries, R. Pauwels, M. P. De Bethune, P. L. Boyer, P. Clark, S. H. Hughes, P. A. Janssen, and E. Arnold. 2004. Roles of conformational and positional adaptability in structure-based design of TMC125-R165335 (etravirine) and related non-nucleoside reverse transcriptase inhibitors that are highly potent and effective against wild-type and drug-resistant HIV-1 variants. J. Med. Chem. 47:2550-2560. [DOI] [PubMed] [Google Scholar]
- 4.Dayal, M. B., J. Wheeler, C. Williams, J., and K. T. Barnhart. 2003. Disruption of the upper female reproductive tract epithelium by nonoxynol-9. Contraception 68:273-279. [DOI] [PubMed] [Google Scholar]
- 5.D'Cruz, O. J., and F. M. Uckun. 2006. Dawn of non-nucleoside inhibitor-based anti-HIV microbicides. J. Antimicrob. Chemother. 57:411-423. [DOI] [PubMed] [Google Scholar]
- 6.Di Fabio, S., J. Van Roey, G. Giannini, G. van den Mooter, M. Spada, A. Binelli, M. Pirillo, E. Germinario, F. Belardelli, M.-P. de Bethune, and S. Vella. 2003. Inhibition of vaginal transmission of HIV-1 in hu-SCID mice by the non-nucleoside reverse transcriptase inhibitor TMC120 in a gel formulation. AIDS 17:1597-1604. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher, P., Y. Kiselyeva, G. Wallace, J. Romano, G. Griffin, L. Margolis, and R. Shattock. 2005. The nonnucleoside reverse transcriptase inhibitor UC-781 inhibits human immunodeficiency virus type 1 infection of human cervical tissue and dissemination by migratory cells. J. Virol. 79:11179-11186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC.
- 9.Jespers, V. A., J. M. Van Roey, G. I. Beets, and A. M. Buva. 2007. Dose-ranging phase 1 study of TMC120, a promising vaginal microbicide, in HIV-negative and HIV-positive female volunteers. J. Acquir. Immune Defic. Syndr. 44:154-158. [DOI] [PubMed] [Google Scholar]
- 10.National Institute of Allergy and Infectious Diseases. May 2006. HIV infection in women. http://www.niaid.nih.gov/factsheets/womenhiv.htm.
- 11.Tarby, C. M. 2004. Recent advances in the development of next generation non-nucleoside reverse transcriptase inhibitors. Curr. Top. Med. Chem. 4:1045-1057. [DOI] [PubMed] [Google Scholar]
- 12.Van Herrewege, Y., J. Michiels, J. Van Roey, K. Fransen, L. Kestens, J. Balzarini, P. Lewi, G. Vanham, and P. Janssen. 2004. In vitro evaluation of nonnucleoside reverse transcriptase inhibitors UC-781 and TMC120-R147681 as human immunodeficiency virus microbicides. Antimicrob. Agents Chemother. 48:337-339. [DOI] [PMC free article] [PubMed] [Google Scholar]