Abstract
Invasive pulmonary aspergillosis (IPA) has significantly increased over the last decade. Here, a fusion protein consisting of the Dectin-1 extracellular domain linked to the Fc portion of murine immunoglobulin G1 augmented alveolar macrophage killing of Aspergillus fumigatus and shifted mortality associated with IPA via attenuation of A. fumigatus growth in the lung.
Immunosuppressed individuals undergoing solid organ or hematopoietic cell transplantation are at high risk for developing invasive fungal infections (3, 4). Among these, invasive pulmonary aspergillosis (IPA) caused by the fungal pathogen Aspergillus fumigatus is associated with an extraordinary mortality rate. A recent analysis of invasive fungal infections in patients with hematologic malignancies has reported an increase in infections caused by A. fumigatus from 0.9% to 2.9% between 1989 and 2003 (6).
We have previously reported that (i) interruption of A. fumigatus recognition by the beta-glucan receptor Dectin-1 attenuated alveolar macrophage (AM) inflammatory responses to A. fumigatus (11), (ii) beta-glucans were exposed at the highest levels in A. fumigatus swollen conidia (SC) (11), and (iii) a fusion protein consisting of the extracellular domain of Dectin-1 linked to the Fc portion of murine immunoglobulin G1 (Dectin-Fc) augmented innate killing of Pneumocystis carinii and attenuated the growth of P. carinii in the lungs of SCID mice (7). Although the role of antibody-mediated immunity in the host defense against A. fumigatus is poorly understood, it is recognized that antibodies may contribute to host cell effector functions (2). To this end, we hypothesize that Dectin-Fc will promote opsonic killing of A. fumigatus and augment its clearance from the lung during immunosuppression.
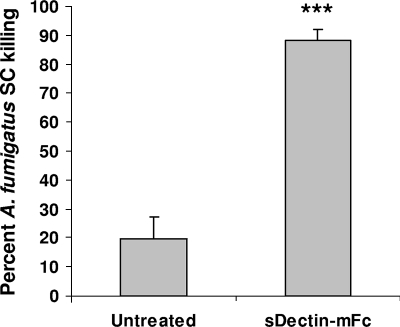
AMs were isolated from male C57BL/6 mice by bronchoalveolar lavage as previously described (10, 11). Animal studies were approved by the Children's Hospital of Pittsburgh Animal Research and Care Committee. AMs were cocultured with A. fumigatus (isolate 13073; ATCC) (5) SC (generated by incubation at 37°C for 6 h) (11) at a ratio of AM to conidia of 1:2 in the presence or absence of Dectin-Fc-conditioned supernatant (7). The development of Dectin-Fc and the adenoviral vector has been previously described (7). A viability control of A. fumigatus SC incubated with medium alone or Dectin-Fc was included. After 6 h at 37°C, total A. fumigatus RNA was isolated with the MasterPure yeast RNA kit (Epicenter, Madison, WI) (9) and reverse transcribed and the viability of A. fumigatus was quantified against a standard curve of diluted live A. fumigatus SC by real-time PCR measurement of the A. fumigatus 18S rRNA (GenBank accession no. AB008401) (1). As a validation of the real-time PCR method, heat-killed A. fumigatus SC did not yield a signal by real-time PCR and were unable to grow on potato dextrose agar plates (data not shown). Figure 1 shows that AMs were relatively ineffective at killing A. fumigatus SC after 6 h of coculture. However, addition of Dectin-Fc to the coculture dramatically enhanced killing by more than fourfold (P < 0.001; analyzed by the Student t test with GraphPad Prism version 5 statistical software). Thus, killing of A. fumigatus by AMs is enhanced when targeting A. fumigatus for opsonic elimination via beta-glucan recognition.
FIG. 1.
Dectin-Fc enhances AM killing of A. fumigatus SC. AMs were isolated from 8- to 12-week-old male C57BL/6 mice and cocultured for 6 h with A. fumigatus SC at a ratio of macrophage to conidia of 1:2 in the presence or absence of Dectin-Fc (7). Controls included A. fumigatus cultured in medium alone or in the presence of Dectin-Fc. Thereafter, RNA was isolated from the contents of each well and a quantitative real-time PCR for A. fumigatus 18S rRNA was performed. Shown are cumulative results of three experiments. ***, P < 0.001. Data are expressed as mean percent killing plus the standard error of the mean.
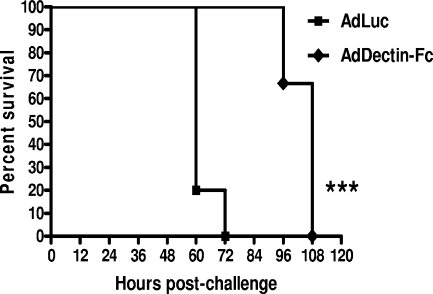
We next subjected mice to a level of immunosuppression that is permissive for the development of IPA (8, 9, 12). Four days and 1 day prior to A. fumigatus challenge, mice received 200 mg/kg and 150 mg/kg, respectively, of cyclophosphamide (Sigma) intraperitoneally. Mice further received 10 mg of cortisone (Sigma) subcutaneously 3 days prior to A. fumigatus challenge and again at the time of challenge. Forty-eight hours after immunosuppression was initiated, mice received adenoviral vectors encoding either Dectin-Fc (AdDectin-Fc) or firefly luciferase (AdLuc; control) intravenously. Forty-eight hours thereafter, mice (six per adenoviral group) were challenged intratracheally with 5 × 105, 5 × 104, or 1 × 104 A. fumigatus conidia. Four days after adenovirus administration, Dectin-Fc protein was detected at high levels in the lungs of immunosuppressed mice receiving AdDectin-Fc, but not AdLuc, as previously reported (7). The protective effects of Dectin-Fc at the higher two inoculum concentrations were significant but subtle and showed a shift in median survival time (MST) (5 × 105 conidia, 48 h versus 66 h for AdLuc and AdDectin-Fc, respectively; 5 × 104 conidia, 60 h versus 72 h for AdLuc and AdDectin-Fc, respectively; P < 0.05 for both inocula; survival analysis was performed with an asymmetrical 95% confidence interval and the Mantel-Cox log-rank test). Figure 2 shows that despite being challenged with a much lower dose of A. fumigatus, 1 × 104 conidia, the MST of AdLuc-treated mice was similarly 60 h. In contrast, mice that received AdDectin-Fc were significantly more protected from death and had an MST of 108 h (P < 0.001). Thus, Dectin-Fc can preserve antifungal immunity in mice that were pharmacologically targeted to have severe suppression of innate immune responses.
FIG. 2.
Systemic administration of Dectin-Fc shifts mortality in an immunosuppressive model of IPA. Male 8- to 12-week-old C57BL/6 mice were immunosuppressed as described in the text and administered either AdDectin-Fc (7) or AdLuciferase (1 × 109 PFU intravenously in 100 μl). Forty-eight hours after adenoviral vector treatment, mice were intratracheally challenged with 1 × 104 A. fumigatus conidia in a volume of 50 μl and monitored for 5 days. Shown are results for six mice per adenoviral group. ***, P < 0.001. Data are expressed as percent survival.
Data presented in Fig. 2 indicated that Dectin-Fc shifted A. fumigatus-associated mortality in immunosuppressed mice. Immunosuppressed mice were therefore administered AdLuc and AdDectin-Fc as before and subsequently challenged with 1 × 104 conidia. At 24 and 48 h postinoculation, A. fumigatus lung burdens were analyzed by real-time PCR, a detection method reported to be superior to quantitative cultures or galactomannan enzyme immunoassay (9). To quantify the A. fumigatus lung burden, RNA was simultaneously isolated from 10-fold dilutions of live A. fumigatus conidia (beginning at 109), as well as the lung samples, with the MasterPure kit (9). Results showed that mice receiving either AdLuc or AdDectin-Fc had low levels of A. fumigatus organisms 24 h after receiving 1 × 104 conidia (AdLuc, 2.24 × 102 ± 1.39 × 102; AdDectin-Fc, 5.50 × 102 ± 2.90 × 102; data are expressed as the mean number of A. fumigatus 18S rRNA units per lung ± the standard error of the mean and are from one representative experiment of two, with five mice per group). However, A. fumigatus lung burdens in immunosuppressed mice receiving AdLuc dramatically increased by 48 h postinoculation (1.41 × 106 ± 5.33 × 105). In contrast, mice receiving AdDectin-Fc had significantly lower A. fumigatus lung burdens by 48 h (1.1 × 104 ± 6.7 × 103, P < 0.05; analyzed by the Student t test). Thus, the presence of Dectin-Fc in the lungs of immunosuppressed mice allows for better control of A. fumigatus overgrowth.
In conclusion, Dectin-Fc effectively targeted A. fumigatus via beta-glucan recognition and opsonic elimination without having to rely on the immune system to respond to a currently uncharacterized immunoprotective A. fumigatus antigen(s). Moreover, Dectin-Fc was effective during immunosuppression and therefore lays the foundation for Dectin-Fc prophylaxis for the treatment of IPA.
Acknowledgments
We thank James Kreindler, University of Pennsylvania, for critical reading of the manuscript.
This work was supported by grants from the Parker B. Francis Foundation and the American Lung Association and Public Health Service grant HL080317 (all to C.S.).
We have no conflicts of interest to disclose.
Footnotes
Published ahead of print on 17 December 2007.
REFERENCES
- 1.Bowman, J. C., G. K. Abruzzo, J. W. Anderson, A. M. Flattery, C. J. Gill, V. B. Pikounis, D. M. Schmatz, P. A. Liberator, and C. M. Douglas. 2001. Quantitative PCR assay to measure Aspergillus fumigatus burden in a murine model of disseminated aspergillosis: demonstration of efficacy of caspofungin acetate. Antimicrob. Agents Chemother. 45:3474-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clemons, K. V., and D. A. Stevens. 2001. Overview of host defense mechanisms in systemic mycoses and the basis for immunotherapy. Semin. Respir. Infect. 16:60-66. [DOI] [PubMed] [Google Scholar]
- 3.De Le Rosa, G. R., R. E. Champlin, and D. P. Kontoyiannis. 2002. Risk factors for the development of invasive fungal infections in allogeneic blood and marrow transplant recipients. Transplant Infect. Dis. 4:3-9. [DOI] [PubMed] [Google Scholar]
- 4.Marr, K. A., T. Patterson, and D. Denning. 2002. Aspergillosis pathogenesis, clinical manifestations, and therapy. Infect. Dis. Clin. N. Am. 16:875-894. [DOI] [PubMed] [Google Scholar]
- 5.Mondon, P., C. De Champs, P. Ambroise-Thomas, and R. Grillot. 1996. Variation in virulence of Aspergillus fumigatus strains in a murine model of invasive pulmonary aspergillosis. J. Med. Microbiol. 45:186-191. [DOI] [PubMed] [Google Scholar]
- 6.Perlroth, J., B. Choi, and B. Spellberg. 2007. Nosocomial fungal infections: epidemiology, diagnosis and treatment. Med. Mycol. 45:321-346. [DOI] [PubMed] [Google Scholar]
- 7.Rapaka, R. R., E. S. Goetzman, M. Zheng, J. Vockley, L. McKinley, J. K. Kolls, and C. Steele. 2007. Enhanced defense against Pneumocystis carinii mediated by a novel Dectin-1 receptor Fc fusion protein. J. Immunol. 178:3702-3712. [DOI] [PubMed] [Google Scholar]
- 8.Sheppard, D. C., J. R. Graybill, L. K. Najvar, L. Y. Chiang, T. Doedt, W. R. Kirkpatrick, R. Bocanegra, A. C. Vallor, T. F. Patterson, and S. G. Filler. 2006. Standardization of an experimental murine model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 50:3501-3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheppard, D. C., K. A. Marr, D. N. Fredricks, L. Y. Chiang, T. Doedt, and S. G. Filler. 2006. Comparison of three methodologies for the determination of pulmonary fungal burden in experimental murine aspergillosis. Clin. Microbiol. Infect. 12:376-380. [DOI] [PubMed] [Google Scholar]
- 10.Steele, C., L. Marrero, S. Swain, A. G. Harmsen, M. Zheng, G. D. Brown, S. Gordon, J. E. Shellito, and J. K. Kolls. 2003. Alveolar macrophage-mediated killing of Pneumocystis carinii f. sp. muris involves molecular recognition by the Dectin-1 β-glucan receptor. J. Exp. Med. 198:1677-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steele, C., R. R. Rapaka, A. Metz, S. M. Pop, D. L. Williams, S. Gordon, J. K. Kolls, and G. D. Brown. 2005. The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog. 1:e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stephens-Romero, S. D., A. J. Mednick, and M. Feldmesser. 2005. The pathogenesis of fatal outcome in murine pulmonary aspergillosis depends on the neutrophil depletion strategy. Infect. Immun. 73:114-125. [DOI] [PMC free article] [PubMed] [Google Scholar]