Abstract
The emergence of antimony (Sb) resistance has jeopardized the treatment of visceral leishmaniasis in various countries. Previous studies have considered the part played by leishmanial parasites in antimony resistance, but the involvement of host factors in the clinical scenario remained to be investigated. Here we show that unlike infection with Sb-sensitive (Sbs) Leishmania donovani, infection with Sb-resistant (Sbr) L. donovani induces the upregulation of multidrug resistance-associated protein 1 (MRP1) and permeability glycoprotein (P-gp) in host cells, resulting in a nonaccumulation of intracellular Sb following treatment with sodium antimony gluconate (SAG) favoring parasite replication. The inhibition of MRP1 and P-gp with resistance-modifying agents such as lovastatin allows Sb accumulation and parasite killing within macrophages and offers protection in an animal model in which infection with Sbr L. donovani is otherwise lethal. The occurrence of a similar scenario in clinical cases is supported by the findings that unlike monocytes from SAG-sensitive kala-azar (KA) patients, monocytes from SAG-unresponsive KA patients overexpress P-gp and MRP1 and fail to accumulate Sb following in vitro SAG treatment unless pretreated with inhibitors of ABC transporters. Thus, the expression status of MRP1 and P-gp in blood monocytes may be used as a diagnostic marker for Sb resistance and the treatment strategy can be designed accordingly. Our results also indicate that lovastatin, which can inhibit both P-gp and MRP1, might be beneficial for reverting Sb resistance in leishmaniasis as well as drug resistance in other clinical situations, including cancer.
The emergence of antimony-resistant (Sbr) visceral leishmaniasis (VL) in various parts of the world (1, 8, 17, 48) has severely compromised control of the disease. Among alternative drugs, pentamidine is toxic; amphotericin B is both expensive and toxic, with reported cases of resistance (8, 48); and oral miltefosine is limited by cost, contraindications, and emerging resistance (1, 8, 41, 53). Therefore, an understanding of the mode of resistance and an identification of cost-effective therapeutic combinations have become major issues.
ATP binding cassette (ABC) transporters have been widely reported to export xenobiotics (24, 55) and cause drug resistance in various diseases such as cancer (23). Earlier studies have reported the expression of analogs of ABC transporters on the surfaces of Sbr strains of Leishmania promastigotes (27, 35, 41), believed to efflux antimonials. However, the demonstration of these transporters in promastigotes may not be very relevant to clinical situations. There are a few reports available on the expression of similar transporters in laboratory isolates of in vitro-developed Sbr strains of leishmanial amastigotes (15) or on amastigotes from field isolates of Sbr L. donovani (10, 51). Although sodium antimony gluconate (SAG) kills leishmanial amastigotes directly at higher doses in vitro as reported previously (54), a much lower dose is required for killing the parasite within macrophages (Mφ) (25). Furthermore, SAG fails to act in immunocompromised hosts, such as patients who are suffering from AIDS or receiving immunosuppressive agents (19, 36) and nude (39) and severe combined immunodeficient mice (16). Several studies have shown that endogenous interleukin-2 (IL-2) (38), IL-4 (2, 40), and IL-12 (37) influence the effectiveness of chemotherapy with pentavalent antimony. Together, these findings are inclined to indicate the requirement of a somewhat functional immune system for SAG action. We also demonstrated earlier that SAG induces the Mφ to produce leishmanicidal molecules like nitric oxide (NO) and reactive oxygen species (ROS), leading to the elimination of intracellular L. donovani (33). Thus, SAG may act both directly and through activation of the host's Mφ. Moreover, SAG can also induce the generation of gamma interferon from splenic lymphocytes, the proliferation of sp lenocytes (34, 44), and even the proliferation of IL-2-dependent CTLL2 and HT2 T-cell lines in the absence of IL-2 (34) and can upregulate NF-kB activation and the expression of major histocompatibility complex I in normal as well as L. donovani-infected Mφ (J. Mookerjee Basu, P. Sen, and S. Roy, unpublished observations), indicating that SAG may activate the immune system. Therefore, it is necessary to decipher the role played by the host cell, if any, in Sb unresponsiveness.
In this study, we show that infection with Sbr L. donovani (but not Sb-sensitive [Sbs] L. donovani) upregulates the host's ABC transporters and causes Sb nonretention and that host cells also play a role in the induction of resistance. We also propose a prognostic test for early identification of SAG-unresponsive cases. Moreover, we also identify a widely used antihypercholesteremic drug as a resistance-modifying agent (RMA) and propose a new strategy for the treatment of SAG-unresponsive kala-azar (KA) cases.
MATERIALS AND METHODS
Antibodies and other reagents.
Anti-multidrug resistance-associated protein 1 (MRP1) and anti-MDR1 were obtained from Santa Cruz Biotech, Inc. Fetal bovine serum was obtained from Invitrogen Corporation (Carlsbad, CA). Inhibitors (lovastatin, verapamil, and probenecid), Giemsa stain, N-(1-naphthyl)ethylenediamine dihydrochloride, sulfanilamide, starch, penicillin, streptomycin, medium 199, RPMI 1640, doxorubicin (Dox), and trypan blue were from Sigma (St. Louis), and 2′,7′-dichlorodihydrofluorescein diacetate was from Molecular Probes (Eugene, OR). Preservative-free sodium stibogluconate/SAG was the kind gift of Albert David India Ltd. (Calcutta, India). SAG was diluted in RPMI medium 1640 (pH 7.4) just before being added to cultures.
Parasite strains.
Three strains of L. donovani, SAG-sensitive (Sbs) MHOM/IN/1983/AG83 (AG83) (45) and two SAG-resistant (Sbr) strains, GE1F8R (raised in vivo in hamsters) (4) and K39 (isolated from a SAG-unresponsive patient) (46), were used. Amastigotes obtained from the spleens of infected hamsters were cultured at 22°C to obtain promastigotes (6).
Animals.
BALB/c mice originally obtained from Jackson Laboratories, (Bar Harbor, ME) and golden Syrian hamsters (Mesocrecetus auratus) originally obtained from the National Institute of Nutrition, Hyderabad, India, both reared in the institute's animal facilities, were used for experimental purposes, with prior approval of the animal ethics committee.
Cell lines and cell culture.
Four different cell lines were used in this investigation, and they were (i) Dox-resistant MRP1-overexpressing Ehrlich ascites carcinoma (EAC/Dox) (32), (ii) EAC/S, a parental Dox-sensitive counterpart of EAC/Dox cells lacking overexpression of MRP1, (iii) Dox-resistant human acute T-lymphoblastic leukemia cell line CEM-ADR5000 overexpressing ABCB1/MDR1/permeability glycoprotein (P-gp) (14) (kind gift from T. Efferth, German Cancer Research Center, Heidelberg, Germany), and (iv) CCRF/CEM, a parental Dox-sensitive counterpart of CEM-ADR 5000, a human acute T-lymphoblastic leukemia cell line lacking overexpression of P-gp.
Peritoneal Mφ were obtained from BALB/c mice as described previously (33) and used for further experimentations. Splenic Mφ were isolated from BALB/c mice infected for 2 months with Sbs or Sbr L. donovani as described previously (45).
L. donovani infection (in vitro and in vivo) and determination of parasite burden.
Mφ were infected with either Sbs or Sbr L. donovani promastigotes as described previously (33). The number of Mφ infected with the intracellular L. donovani parasite was enumerated under Giemsa staining as described previously (33).
BALB/c mice (4 to 6 weeks old) were infected with 1 × 107 promastigotes (AG83, GE1F8R, or K39)/0.1 ml per animal via the intracardiac route (45), and mice infected for 2 months were used for experimental purposes.
Golden hamsters (4 weeks old) were infected with K39 (2 × 107 promastigotes/0.1 ml per animal) or GE1F8R (5 × 106 amastigotes/0.1 ml per animal) via the intracardiac route (on day 0) (4). For subsequent experiments, hamsters infected with GE1F8R for 30 days and hamsters infected with K39 for 60 days were used, because at these time points, the two groups of hamsters showed almost equal parasite burdens in spleens and livers. The parasite burden was determined by limiting the dilution of tissue samples (4).
Peripheral blood sample of patients.
Excesses of blood drawn for routine examinations of confirmed KA patients, confirmed as either unresponsive to SAG (patients who reported to the clinic with KA within 2 months of receiving full courses of SAG) or sensitive to SAG (KA patients who showed improvement of clinical symptoms of cure-like remissions of fever, increases in hemoglobin levels and white blood cell counts, and reductions in spleen size and splenic parasites after 3 weeks of SAG treatment), or from untreated (fresh) KA cases from zones of SAG resistance (SAG-resistant zone) or SAG sensitivity (SAG-sensitive zone) were collected as samples from the Department of Clinical Biochemistry, Hospital Unit, Rajendra Memorial Research Institute of Medical Sciences, Patna, Bihar, India. All the patients included in this study were of mixed ages (ranging from 20 to 50 years), were from both sexes, were rK39 and direct agglutination test positive, and were human immunodeficiency virus (HIV) negative. The parasite burden in splenic aspirates was determined using the method described previously by Chulay and Bryceson (7). Brief profiles of the patients are presented in Tables 1 and 2. Monocytes from peripheral blood mononuclear cells from KA patients were isolated by adherence of the cells on a plastic petri dish and used for further experimentation.
TABLE 1.
Profiles of the confirmed SAG-responsive/-unresponsive KA patientsa
| Patient no. | Residence area in Bihar (district) | Age (yr) | Sex | Splenic parasitesb | Previous therapy | Remarks |
|---|---|---|---|---|---|---|
| 1 | Vaisali | 24 | M | 3+ | SAG, full coursed | SAG unresponsive |
| 2 | Nalanda | 35 | F | 2+ | SAG, full coursed | SAG unresponsive |
| 3 | Patna | 45 | F | 3+ | SAG, full coursed | SAG unresponsive |
| 4 | Patna | 35 | F | 2+ | SAG, full coursed | SAG unresponsive |
| 5 | Motihari | 52 | F | 2+ | SAG, full coursed | SAG unresponsive |
| 6 | Vaisali | 26 | M | 3+ | SAG, full coursed | SAG unresponsive |
| 7 | Vaisali | 19 | F | 3+ | SAG, full coursed | SAG unresponsive |
| 8 | Nalanda | 29 | M | 2+ | SAG, full coursed | SAG unresponsive |
| 9 | Nalanda | 34 | M | 2+ | SAG, full coursed | SAG unresponsive |
| 10 | Vaisali | 42 | F | 4+ | SAG, full coursed | SAG unresponsive |
| 11 | Nalanda | 32 | M | 3+ (before treatment) | Received SAG for 3 wk | Clinically responsive to SAGc |
| 12 | Gopalgung | 50 | F | 2+ (before treatment) | Received SAG for 25 days | Clinically responsive to SAGc |
| 13 | Siwan | 45 | F | 3+ (before treatment) | Received SAG for 18 days | Clinically responsive to SAGc |
| 14 | Siwan | 35 | M | 2+ (before treatment) | Received SAG for 22 days | Clinically responsive to SAGc |
| 15 | Gopalgung | 25 | M | 4+ (before treatment) | Received SAG for 17 days | Clinically responsive to SAGc |
| 16 | Gopalgung | 25 | M | 2+ (before treatment) | Received SAG for 25 days | Clinically responsive to SAGc |
| 17 | Gopalgung | 25 | M | 3+ (before treatment) | Received SAG for 27 days | Clinically responsive to SAGc |
| 18 | Gopalgung | 25 | M | 2+ (before treatment) | Received SAG for 23 days | Clinically responsive to SAGc |
| 19 | Siwan | 45 | F | 2+ (before treatment) | Received SAG for 24 days | Clinically responsive to SAGc |
| 20 | Siwan | 45 | F | 2+ (before treatment) | Received SAG for 25 days | Clinically responsive to SAGc |
F, female; M, male.
Determined microscopically from stained smears of splenic aspirates (7). Numbers are standard parasite burden grades determined by counting amastigotes versus host cells from Giemsa-stained slides.
Appearance of clinical symptoms of cure-like remission of fever, increase in hemoglobin level and white blood cell count and reduction in spleen size, ultimately checked for reduction of parasite in spleen after completion of treatment.
Received 20 mg/kg body weight of SAG for 28 days are indicated as full course of SAG.
TABLE 2.
Profiles of the untreated KA patients from SAG-responsive or -unresponsive zonesa
| Patient no. | Residence area in Bihar (district) | Age (yr) | Sex | Splenic parasitesb | Previous therapy | SAG zone |
|---|---|---|---|---|---|---|
| 1 | Gopalgung | 35 | F | 3+ | NT | R |
| 2 | Gopalgung | 33 | M | 2+ | NT | R |
| 3 | Gopalgung | 24 | F | 2+ | NT | R |
| 4 | Siwan | 28 | F | 3+ | NT | R |
| 5 | Siwan | 40 | M | 2+ | NT | R |
| 6 | Siwan | 45 | M | 2+ | NT | R |
| 7 | Gopalgung | 43 | M | 3+ | NT | R |
| 8 | Gopalgung | 34 | F | 2+ | NT | R |
| 9 | Siwan | 28 | F | 2+ | NT | R |
| 10 | Gopalgung | 42 | F | 2+ | NT | R |
| 11 | Vaisali | 31 | M | 2+ | NT | U |
| 12 | Vaisali | 23 | F | 3+ | NT | U |
| 13 | Vaisali | 35 | M | 2+ | NT | U |
| 14 | Vaisali | 28 | F | 2+ | NT | U |
| 15 | Vaisali | 25 | F | 3+ | NT | U |
| 16 | Vaisali | 21 | M | 4+ | NT | U |
| 17 | Samastipur | 23 | M | 3+ | NT | U |
| 18 | Vaisali | 43 | M | 2+ | NT | U |
| 19 | Vaisali | 28 | M | 3+ | NT | U |
| 20 | Vaisali | 28 | F | 2+ | NT | U |
F, female; M, male; NT, no previous treatment; R, SAG-responsive zone; U, SAG-unresponsive zone.
Determined microscopically from stained smears of splenic aspirates (7). Numbers are standard parasite burden grades determined by counting amastigotes versus host cells from Giemsa-stained slides.
Treatments.
At 22 h postinfection, the Mφ (1 × 106) were treated with SAG (10 μg/ml in terms of Sb content) (33). In some experiments, cells were treated with verapamil (2.5 μM) (50) and/or probenecid (200 μM) (21) or lovastatin (10 μM) (52) for 1 h prior to treatment with SAG. Viability was tested for each inhibitor dose by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay (data not shown) and found to be nontoxic under experimental conditions.
EAC/Dox cells and CEM-ADR5000 cells were treated with verapamil and/or probenecid or lovastatin at above-mentioned doses 1 h prior to treatment with either SAG (10 μg/ml) or Dox (15 μg/ml; equivalent to ∼26 μM of Dox hydrochloride).
GE1F8R-infected hamsters (or K39-infected hamsters) were divided into six groups (I to VI). Each group consisted of 20 hamsters. Group I of both types (GE1F8R and K39) of Sbr L. donovani-infected hamsters was the infected group that was untreated. Hamsters in groups II to VI received SAG injections ([250 mg/kg of body weight per intramuscular shot] twice a week for 4 weeks) (28). Hamsters from group II received SAG only. Group III received an oral treatment of probenecid (10 mg/kg) (47, 13), group IV received an oral treatment of verapamil (20 mg/kg) (9), group V received an oral treatment of a combination of 20 mg/kg of probenecid and verapamil and 10 mg/kg of probenecid, and group VI received an oral treatment of lovastatin (10 mg/kg) (11). Verapamil and/or probenecid or lovastatin was administered orally 3 h prior to each intramuscular administration of SAG. Ten animals from each group were sacrificed 30 days after the completion of treatment, and their hepatic and splenic parasite burdens were enumerated by the limiting dilution assay. The rest of the animals were monitored for a survival study, with adequate care as per the regulations of the institutional animal ethics committee. The experiment on survival was terminated after 240 days, and the surviving animals were sacrificed and checked for the presence of residual parasites in their bone marrow, spleens, and livers by limiting dilution (4).
Measurement of ROS.
The level of ROS (includes superoxide, hydrogen peroxide, and other reactive oxygen intermediates) was monitored using the cell-permeable, nonpolar, H2O2-sensitive probe 2′,7′-dichlorodihydrofluorescein diacetate as described before (33).
Measurement of NO.
NO generation in response to SAG treatment was monitored by Griess reaction as described previously (33).
Quantification of intracellular antimony.
Total intracellular Sb contents were measured in SAG (10 μg/ml)-treated cells by electrothermal atomic absorption spectroscopy as described previously (43) with minor modifications.
Detection of expression of MRP1 and P-gp.
The levels of expression of MRP1 and P-gp in Mφ or peripheral blood monocytes of the patients were determined by immunostaining, followed by flow cytometry (Becton Dickson) and/or confocal microscopy (LSM 510; Carl Zeiss, Jena, Germany), as described earlier (6). The appropriate isotype control was used for each individual case. Since these antibodies were found to recognize MRP1-like molecules in GE1F8R and P-gp-like molecules in K39 promastigotes, all stainings were performed 18 to 24 h after washing in cold the unpermeabilized infected cells to specifically check the expression of P-gp and MRP1 on the host cell surface. Hence, no counterstaining with DNA binding dyes like DAPI (4′,6′-diamidino-2-phenylindole) or propidium iodide was performed even after fixation of the cells. No epitopes were recognized in AG83 promastigotes.
Dox retention assay.
The Dox retention assay was performed using EAC/Dox cells following the method described previously (52) with slight modifications. In brief, cells were treated with different concentrations of lovastatin for 1 h at 37°C in 5% CO2 in air. After that, Dox (15 μg/ml; equivalent to ∼26 μM of Dox hydrochloride) was added and incubated further for 3 h. Cells were harvested in phosphate-buffered saline and washed three times in phosphate-buffered saline to remove excess Dox. After 30 min, the amount of Dox retained was analyzed in a flow cytometer (Becton Dickson) and observed under a laser scanning microscope (LSM 510; Carl Zeiss, Jena, Germany).
Statistical analysis.
Each experiment was performed three to five times, and results are expressed as means ± standard errors. The Student t test for significance was performed, and a P value of <0.05 was considered significant. Analysis of variance with a posttest correction for multiple groups was used for statistical analysis of the data, and a P value of <0.05 was considered significant. Representative data of flow cytometry and fluorescence microscopy of at least three independent experiments are presented.
RESULTS
Clearance of Sb from Mφ.
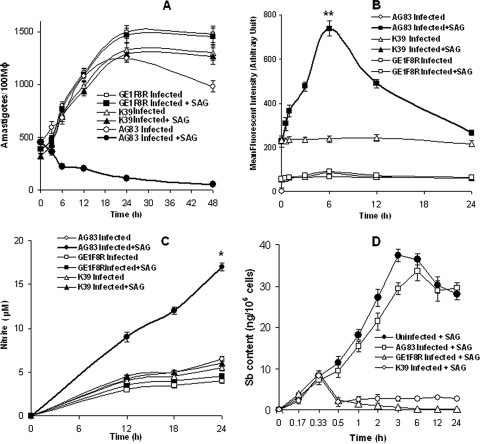
Previously we have shown that SAG induces the generation of leishmanicidal molecules like ROS through activation of the PI3K-PKC-extracellular signal-regulated kinase 1/2 pathway in early phase (6 h posttreatment) and NO through activation of the PI3K-Akt-p38 mitogen-activated protein kinase pathway in late phase (24 h posttreatment) in Sbs L. donovani-infected Mφ (Sbs-Mφ), leading to the elimination of intracellular L. donovani parasites (33). However, SAG not only failed to eliminate intracellular Sbr L. donovani (Fig. 1A) in Mφ infected with Sbr L. donovani (Sbr-Mφ) but also failed to induce the generation of ROS (Fig. 1B) and NO (Fig. 1C). Since the efflux of drug from the cell is well reported to be an important mechanism of drug resistance, especially in cancer (23), we measured the total intracellular Sb content in Mφ infected with SAG-sensitive and -resistant L. donovani strains. The Sb content in Sbs-Mφ increased sharply following SAG treatment, reaching a steady-state level at ∼6 h posttreatment. On the contrary, Sb content in Mφ infected with Sbr L. donovani strains, i.e., GE1F8R (GE1F8R-Mφ) and K39 (K39-Mφ), increased slightly until 20 min post-SAG treatment and rapidly declined thereafter, reaching the basal level (Fig. 1D). This suggested that in contrast to Sbs-Mφ, Sbr-Mφ were unable to retain Sb intracellularly upon SAG treatment.
FIG. 1.
Infection of Mφ with Sbs L. donovani (AG83) and Sbr L. donovani (K39/GE1F8R) differentially affect SAG-mediated elimination of intracellular parasites, the generation of ROS and NO, and the accumulation of Sb. Mφ from normal BALB/c were infected in vitro with L. donovani promastigotes and kept either untreated or treated with SAG (10 μg/ml). (A) Intracellular parasite number was determined for Giemsa-stained preparations. The replication of Sbr L. donovani (but not Sbs L. donovani) continued. Measurement of ROS (B) and NO (C) generations showed that SAG induced a significant (*, P < 0.001; **, P < 0.005) increase in ROS generation at 6 h and NO generation at 24 h in Sbs-Mφ but not in Sbr-Mφ. (D) Intracellular total Sb content was measured by atomic absorption spectroscopy; total Sb content was also measured in uninfected Mφ kept as controls following SAG treatment. Significant amounts of Sb accumulated in Sbs-Mφ but not in Sbr-Mφ (P < 0.0001) by 6 h posttreatment. Error bars indicate standard deviations.
Expression of MRP1 and P-gp in Mφ following infection.
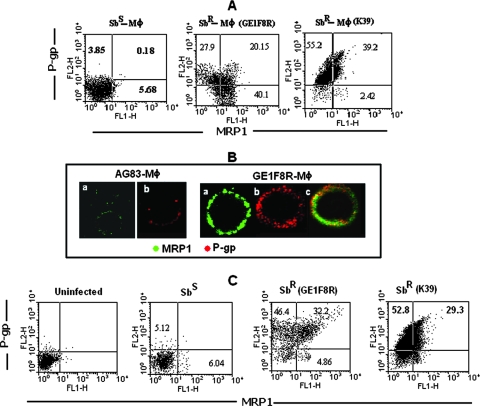
Overexpression of ABC transporters like P-gp or MRP1 causes multiple drug resistance in different diseases (5) like cancers (23, 30), HIV infection, or mycobacterium-HIV coinfection (22, 3). Therefore, we analyzed the expression status of MRP1 and P-gp in host cells, i.e., in Sbr-Mφ and Sbs-Mφ. We observed an increased number of MRP1-positive and P-gp-positive cells in the cases of both GE1F8R-Mφ and K39-Mφ, with frequent coexpression of both transporters compared to the case for Sbs-Mφ (Fig. 2A). This flow cytometric observation was further confirmed by confocal microscopy of GE1F8R-Mφ, in which various degrees of P-gp and MRP1 colocalization were observed (Fig. 2B).
FIG. 2.
Levels of expression of P-gp and MRP1 in murine peritoneal Mφ following in vitro infection and splenic Mφ following in vivo infection with Sbs L. donovani (AG83) and Sbr L. donovani (K39/GE1F8R). (A) Status of expression of P-gp and/or MRP1 in peritoneal Mφ of BALB/c were analyzed by flow cytometry following in vitro infection with Sbs or Sbr L. donovani. Representative data demonstrates enhanced expression of P-gp and/or MRP1 in Sbr-Mφ compared to that in Sbs-Mφ. The status of infection was checked in parallel sets by Giemsa staining, and it was found that more than 85% cells were infected, with infection loads being 1,152, 1,418, and 1,258 amastigotes per 100 Mφ for AG83, GE1F8R, and K39 infections, respectively, (data not shown). (B) Expression statuses of MRP1 (in green) and P-gp (in red) were studied in Sbs-Mφ and Sbr-Mφ (GE1F8R) by confocal microscopy. (C) Flow cytometric analysis of the status of MRP1 and P-gp expression in splenic Mφ derived from BALB/c mice following 2 months of infection with Sbs L. donovani or Sbr L. donovani showing the frequency of P-gp and MRP1 single- and double-positive cells. Splenic Mφ derived from uninfected BALB/c mice were kept as controls. Results are representative of five independent experiments.
In order to confirm these observations in an in vivo experimental setting, the expression levels of MRP1 and P-gp were tested in splenic Mφ from BALB/c mice infected with either Sbs L. donovani or Sbr L. donovani. The splenic Mφ population derived from Sbr L. donovani-infected mice showed a remarkable increase in P-gp single-positive (Fig. 2C) and MRP1-P-gp double-positive cells (Fig. 2C) compared to the number of cells from Sbs L. donovani-infected mice.
Involvement of ABC transporters in Sb clearance.
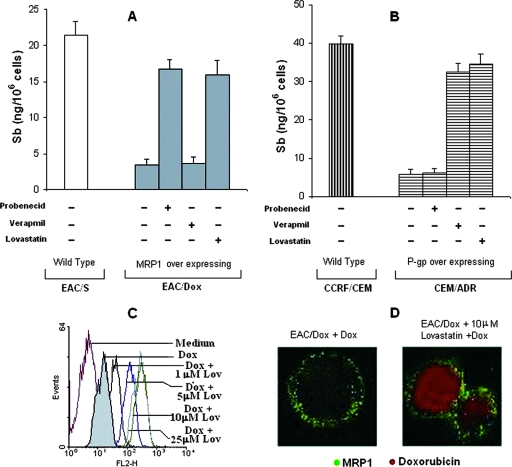
To determine whether higher expression levels of P-gp and MRP1 are indeed involved in the nonretention of Sb, four cell lines, viz., MRP1 overexpressing EAC/Dox (31, 32), P-gp overexpressing CEM-ADR5000 (14), and their respective parental Dox-sensitive counterparts EAC/S and CCRF/CEM, were treated with SAG for 6 h and total intracellular Sb contents were measured. Unlike their parental counterparts, both EAC/Dox and CEM/ADR5000 failed to retain Sb (Fig. 3A and B). Furthermore, EAC/Dox retained Sb following SAG treatment in the presence of the MRP1 inhibitor probenecid (18, 21) but not in the presence of verapamil (Fig. 3A), while CEM/ADR5000 retained Sb in the presence of the P-gp-inhibitory isoform of verapamil (26, 49, 50) but not in the presence of probenecid (Fig. 3B). Thus, drug sensitivity correlates with ABC transporter expression and strongly suggests a selective involvement of P-gp and MRP1 in Sb clearance from EAC/Dox and CEM/ADR5000 lines, respectively. Interestingly, both EAC/Dox and CEM/ADR5000 cell lines retained Sb when treated with SAG in the presence of lovastatin (Fig. 3A and B). These results indicate that in addition to its previously reported P-gp-inhibitory effect (52), lovastatin may also inhibit MRP1. To confirm this possibility, we performed a Dox retention assay in EAC/Dox in the presence of lovastatin. Pretreatment of EAC/Dox with lovastatin for 60 min before being loaded with Dox resulted in a dose-dependent increase in the retention of Dox, which reached a plateau at a 10-μM lovastatin dose (Fig. 3C). Confocal microscopy further confirmed that Dox is retained in EAC/Dox cells in the presence of lovastatin without an observable change in MRP1 expression (Fig. 3D). Thus, lovastatin exerts an inhibitory effect on both MRP1-mediated and P-gp-mediated transport. Therefore lovastatin stands out as an excellent candidate as RMA that could induce not only the retention of Sb but also that of an unrelated chemotherapeutic agent such as Dox in cancer cells.
FIG. 3.
Effects of verapamil and/or probenecid or of lovastatin on the drug retention ability of MRP1-overexpressing murine breast carcinoma cell line EAC/Dox and P-gp-overexpressing human lymphoblastic leukemia cell line CEM-ADR 5000. Ability of Sb retention in (A) MRP1-overexpressing EAC/Dox and (B) P-gp-overexpressing CEM-ADR5000 cell lines and their parental counterparts, i.e., EAC/S (lacking overexpression of MRP1) and CCRF/CEM (lacking overexpression of P-gp), respectively, was estimated at 6 h post-SAG treatment (10 μg/ml) in the presence or the absence of 200 μM of probenecid (inhibitor of MRP1) or 2 μM of verapamil (inhibitor of P-gp) or 10 μM of lovastatin. (C) Inhibitory effect of lovastatin (lov) on MRP1-mediated transport was assessed by the Dox retention assay. An increase in retention of Dox by EAC/Dox cells with increasing dose of lovastatin treatment (1 h prior to Dox treatment) was evident from flow cytometry. (D) Retention of Dox (as shown by red fluorescence) by MRP1 overexpressing (as shown by green fluorescence) EAC/Dox cells in the presence of an optimum dose of lovastatin (10 μM) was observed by confocal microscopy. Results are presented as means ± standard errors of the means of five independent experiments (A and B) or as representative of four independent experiments (C and D). Error bars indicate standard deviations. −, absence of; +, presence of.
Effect of inhibition of MRP1 and P-gp in Sbr-Mφ.
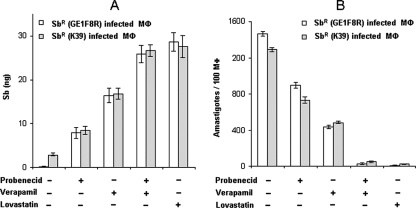
As pretreatment with inhibitors of ABC transporters resulted in the accumulation of Sb, these RMAs were tested for their abilities to restore Sb retention in Sbr-Mφ upon SAG treatment. Pretreatment of Sbr-Mφ with a combination of verapamil and probenecid or with lovastatin significantly allowed Sb retention (P < 0.001) even by 24 h after SAG treatment (Fig. 4A). The treatment induced ROS generation at 6 h and NO at 24 h (data not shown) and resulted in SAG-mediated killing of intracellular Sbr L. donovani (Fig. 4B). The combination of verapamil and probenecid was more effective than single inhibitors alone were, allowing clearance of more than 90% of the parasite by 24 h of SAG treatment. Pretreatment with lovastatin alone resulted in a more than 95% elimination of intracellular Sbr L. donovani following SAG treatment (Fig. 4B). This result confirms that the inhibitions of both P-gp and MRP1 are necessary to prevent Sb efflux and promote SAG-mediated parasite clearance.
FIG. 4.
Effects of verapamil and/or probenecid or of lovastatin on the Sb retention ability of Sbr-Mφ and SAG-mediated killing of intracellular parasite. (A) Effect of verapamil (2 μM) and/or probenecid (200 μM) or lovastatin (10 μM) on the ability of intracellular Sb retention in Sbr-Mφ. (B) Intracellular parasite number was determined for Sbr-Mφ in the presence and the absence of verapamil (2 μM) and/or probenecid (200 μM) or of lovastatin (10 μM) at 24 h following SAG treatment. An inverse relationship was obtained between Sb retention and killing of intracellular parasites upon pretreatment with a combination of verapamil and probenecid or with lovastatin. Error bars indicate standard deviations. −, absence of; +, presence of.
Inhibitors of ABC transporters as RMAs.
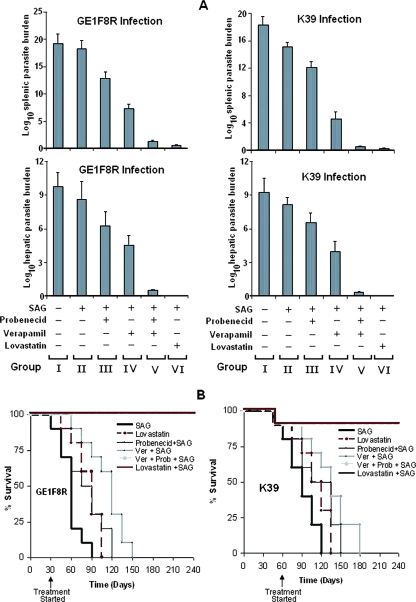
Since experimental VL in hamsters closely mimics KA in humans, inhibitors of P-gp and MRP1 as potential RMAs were evaluated in hamsters infected with Sbr L. donovani. GE1F8R-infected or K39-infected hamsters were divided into six groups (I to VI) and received treatments as described in Materials and Methods. GE1F8R-infected hamsters showed hardly any reduction of parasite burden upon SAG treatment (Fig. 5A), whereas similar treatment of K39-infected hamsters caused an approximately 15% reduction in parasite burdens in spleens, confirming that these two L. donovani parasite strains are indeed SAG resistant in an in vivo setting. Treatment with probenecid (group III) led to SAG-mediated reductions in spleen and liver parasite burdens by ∼27% and ∼35%, respectively, in GE1F8R-infected hamsters and by 33% and 23%, respectively, in K39-infected hamsters compared to values for the untreated infected group (group I). Interestingly, group IV hamsters, which received verapamil plus SAG, showed significantly more reduced parasite burdens in both spleens and livers compared to the burdens for hamsters treated with SAG only (P < 0.001). Oral treatment with a combination of probenecid and verapamil (group V) or with lovastatin (group VI) prior to SAG treatment showed reductions of more than 90% (about 1017-fold) and more than 95% (about 1018-fold), respectively, in both hepatic and splenic parasite burdens (Fig. 5A) and offered sterile protection in spleens and livers in the majority of GE1F8R-infected and K39-infected hamsters (Table 3).
FIG. 5.
Oral administration of probenecid and/or verapamil or of lovastatin prior to SAG treatment in Sbr L. donovani-infected hamsters offered protection. GE1F8R (30 day)- and K39 (60 day)-infected hamsters were used because of their essentially equal parasite burdens in spleens at the two time points. Both GE1F8R-infected and K39-infected hamsters were divided into six groups (I to VI). All the groups (20 in each group) except group I (the untreated infected group) received SAG injections (two injections [250 mg/kg per shot] per week for 4 weeks). Groups III to VI also received other treatments, together with SAG. Groups III, IV, V, and VI received oral treatments of probenecid, verapamil, verapamil plus probenecid, and lovastatin, respectively, 3 h prior to each SAG injection. Ten animals from each group were sacrificed 30 days after the last SAG treatment. Parasite burdens of spleens and livers were enumerated by limiting dilution and were expressed as log10 parasite burden (A). In each of these experiments, additional groups of animals were taken to test the efficacies of treatments with inhibitors alone. In these groups, the splenic parasite burdens were ∼2 × 1015 (treated with only probenecid), ∼8 × 1014 (treated with only verapamil), ∼7 × 1014 (treated with only probenecid plus verapamil), and ∼2 × 1014 (treated with only lovastatin). Ten animals from each group (I to VI) were kept for a study of survival kinetics up to 240 days (B). Groups I (untreated infected) and II (treated with only SAG) showed similar survival kinetics; therefore, the result for group I is not presented. Asterisks indicate the termination of experiment. Error bars indicate standard deviations. −, absence of; +, presence of.
TABLE 3.
Organ sterile protection in hamsters receiving verapamil plus probenecid or lovastatin prior to SAG injection
| Group | L. donovani strain used for infection | No. of hamsters with sterile protection of organa
|
|
|---|---|---|---|
| Spleen | Liver | ||
| V | GE1F8R | 8 | 9 |
| K39 | 9 | 9 | |
| VI | GE1F8R | 9 | 10 |
| K39 | 10 | 10 | |
Ten hamsters were used to determine the level of organ sterile protection. Protection was determined by the limiting dilution method.
The survival rate was studied for each experimental group until day 240 was reached (Fig. 5B). Data from group I was similar to that from group II and is not shown. All GE1F8R-infected and K39-infected hamsters belonging to groups I and II died by 90 days and 120 days, respectively, after infection. Survival of both GE1F8R-infected and K39-infected hamsters could be improved only to some extent with either probenecid or verapamil treatment prior to each SAG treatment, while all animals belonging to groups V (probenecid plus verapamil plus SAG) and VI (lovastatin plus SAG) survived until the termination of the experiment (at 240 days) (Fig. 5B). After sacrifice, no residual parasites could be detected in livers, spleens, and bone marrow by the limiting dilution assay. Thus, oral treatment with a combination of verapamil and probenecid or with lovastatin allowed SAG-mediated cure of VL in animals infected with Sbr L. donovani.
Animals treated with only inhibitors did not show major changes in the course of infection, although the survival of the group treated with only verapamil plus probenecid increased slightly (approximately 70% of the animals treated with only verapamil plus probenecid died within 90 days postinfection in the case of GE1F8R infection and within 120 days postinfection in the case of K39 infection [data not shown]). Interestingly, about 60% of the animals treated with only lovastatin survived until 75 days postinfection in the case of GE1F8R infection and 105 days postinfection in the case of K39 infection (Fig. 5), indicating that only lovastatin treatment slows down the progression of the disease.
Expression of ABC transporters in monocytes of KA patients.
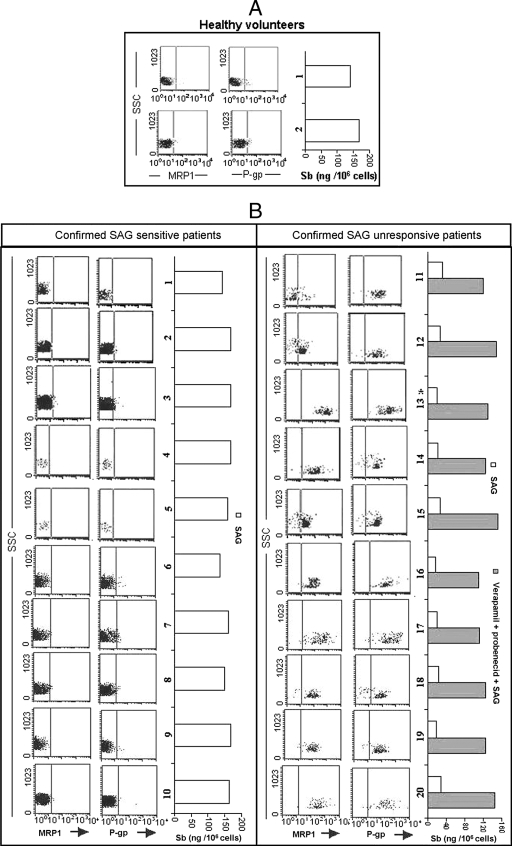
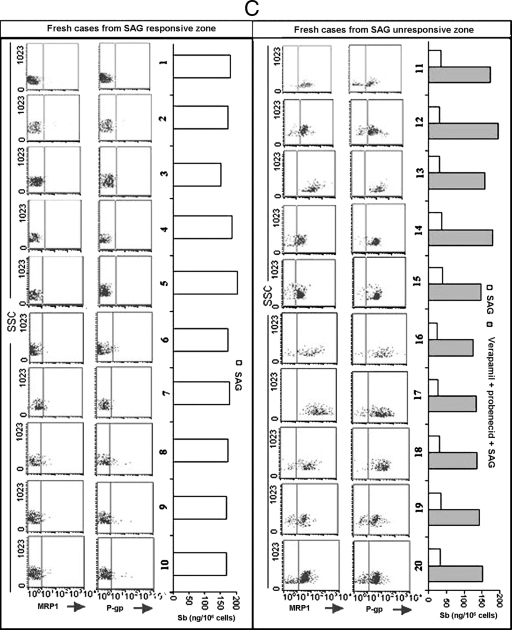
In order to evaluate the role of ABC transporters in KA patients, we analyzed the status of P-gp and MRP1 expression and Sb accumulation following in vitro SAG treatment of peripheral blood monocytes from 10 confirmed SAG-responsive KA (Sbs-KA) and 10 confirmed SAG-unresponsive KA (Sbr-KA) patients. Monocytes of all Sbr-KA patients not only had much higher glutathione contents (data not shown) but also expressed significantly higher levels of P-gp and MRP1 compared to those of Sbs-KA patients (P < 0.01), although the levels of expression of MRP1 and P-gp varied from patient to patient (Fig. 6). When the monocytes from these patients were treated with SAG in vitro, the cells from confirmed Sbs-KA patients showed significantly higher Sb retention levels (149 ± 9 ng/106 cells) than those of cells from confirmed Sbr-KA patients (22.8 ± 10 ng/106 cells) (P < 0.005). Pretreatment with verapamil plus probenecid restored Sb retention by Sbr-KA monocytes upon SAG treatment (Fig. 6). We further extended our studies with peripheral blood monocytes of fresh KA patients from areas of India where SAG responsiveness (SAG-responsive area) and unresponsiveness (SAG-unresponsive area) are found. Interestingly, though the levels of expression of MRP1 and P-gp and the frequencies of coexpressing cells varied, monocytes of fresh KA patients from SAG-unresponsive areas showed significantly higher (P < 0.01) levels of expression of P-gp and MRP1 compared to those of fresh KA patients from SAG-responsive zones. Again, monocytes of fresh KA patients from SAG-responsive zones showed significantly higher Sb retention levels (173 ± 10 ng/106 cells) (P < 0.005) compared to those of patients from SAG-unresponsive areas (30.8 ± 8 ng/106 cells) (Fig. 6).
FIG. 6.
Status of MRP1 and P-gp expression and the Sb retention ability of monocytes of confirmed SAG-sensitive and SAG-unresponsive KA patients as well as of fresh KA patients from SAG-unresponsive and -sensitive zones. Expression status of MRP1 and P-gp and Sb retention in monocytes of (A) healthy volunteers, (B) 10 confirmed SAG-sensitive KA patients (Sbs-KA), and 10 unresponsive KA patients (Sbr-KA) were studied by flow cytometry. Intracellular Sb retention was analyzed by atomic absorption spectroscopy 6 h post-SAG treatment. Monocytes of Sbs-KA patients showed much lower frequencies of MRP1-positive and P-gp-positive cells and retained much higher amounts of Sb 6 h following SAG treatment compared to results for Sbr-KA monocytes. Treatment of all Sbr-KA monocytes with a combination of verapamil (2 μM) and probenecid (200 μM) 1 h prior to SAG treatment allowed substantial Sb retention. Details of the patients are described in Table 1. (C) Expression status of MRP1 and P-gp and Sb retention ability of monocytes of 20 fresh KA patients, of which 10 came from the SAG-sensitive zone and the rest from the SAG-resistant zone. Identical studies were performed as with Sbr-KA and Sbr-KA monocytes. Details of the patients are described in Table 2. For SSC, 1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate.
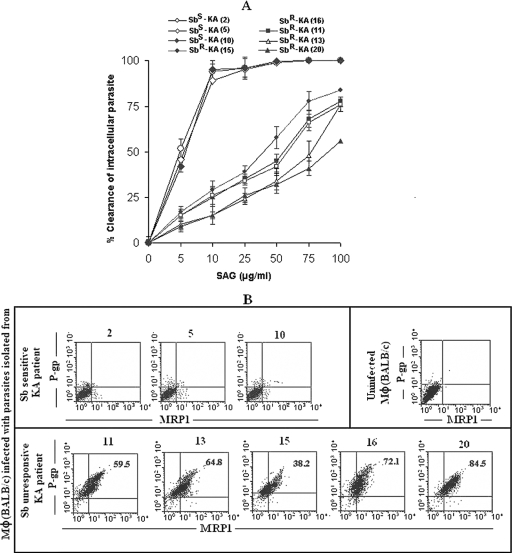
To examine the Sb sensitivity, parasites were isolated from splenic aspirates of fresh (i.e., untreated) KA patients, propagated in culture, and used to infect Mφ from normal BALB/c mice. More than 90% of intracellular parasites derived from KA patients from Sb-sensitive areas were eliminated by 10 μg/ml SAG in vitro, whereas a similar dose of SAG eliminated less than 50% of the intracellular parasites isolated from KA patients from Sb-resistant areas (Fig. 7A). Moreover, in contrast to the case for parasites derived from a KA patient from a Sb-sensitive area, infection with parasites from Sb-unresponsive areas could upregulate P-gp and MRP1 of Mφ derived from BALB/c mice (Fig. 7B).
FIG. 7.
Status of SAG sensitivity and ability of induction of MRP1 and P-gp expression of parasites isolated from splenic aspirates of KA patients from SAG-sensitive and SAG-unresponsive zones. (A) Normal BALB/c Mφ were infected with promastigotes (the ratio of Mφ versus promastigote was 1:10) cultured from splenic aspirates of KA patients from SAG-sensitive (Sbs) or -unresponsive (Sbr) areas (patient number as per Table 2 is given). After 22 h, more than 85% cells were infected (1,288 ± 167 Sbr parasites/100 Mφ and 1,056 ± 124 Sbs parasites/100 Mφ). Following infection in Mφ, parasites isolated from patients belonging to Sbr areas were much less sensitive to SAG (50% inhibitory concentration, 63.6 ± 17.9 μg/ml) compared to parasites isolated from patients belonging to Sbs areas (50% inhibitory concentration, 5.5 ± 0.5 μg/ml). (B) Parasites of patients from Sbr areas could induce high levels of expression of MRP1 and/or P-gp in host Mφ in contrast to those induced by parasites of patients from Sbs areas. Results are presented as means ± standard errors (error bars) of the means of five independent experiments (A) or are representative of five independent experiments (B).
Role of parasite antigen in upregulation of expression of host ABC transporters.
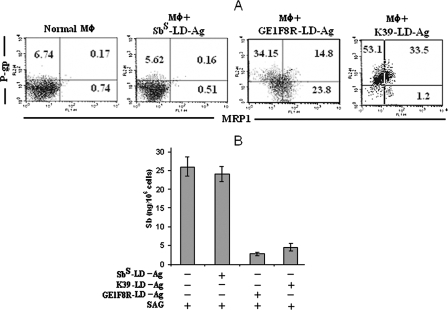
Since peripheral blood monocytes of the Sbr-KA patients do not harbor demonstrable parasites at the active stage of the disease but still possess upregulated P-gp and MRP1 expression, it is likely that soluble and circulating parasite antigens cause an upregulation of the expression of these transporters. To test this hypothesis, we conducted an overnight incubation of uninfected Mφ from normal BALB/c mice with crude antigens from sonicated Sbr L. donovani strains (GE1F8R or K39). We observed that Sbr L. donovani parasite antigens could induce the upregulation of MRP1 and P-gp in uninfected Mφ (Fig. 8A). Moreover, the incubation of uninfected Mφ with Sbr L. donovani antigens (but not with Sbs L. donovani antigens) resulted in decreased intracellular Sb (total) accumulation following SAG treatment (Fig. 8B).
FIG. 8.
Effect of incubation of normal murine Mφ with L. donovani parasite antigen. Normal BALB/c Mφ (106) were incubated overnight with crude antigens derived by sonication of 107 Sbs or Sbr L. donovani (K39 or GE1F8R), excess antigen was washed with RPMI, (A) the status of MRP1 and P-gp was checked, (B) mice were treated with SAG (10 μg/ml), and Sb content was measured at 24 h post-SAG treatment. Antigen (Ag) prepared from Sbs L. donovani (AG83) is presented as Sbs L. donovani-Ag and those from GE1F8R (Sbr L. donovani) and K39 (Sbr L. donovani) parasites are presened as GE1F8R-L. donovani-Ag and K39-L. donovani-Ag, respectively. Results are presented as representative of five independent experiments (A) or as means ± standard errors (error bars) of the means of five independent experiments (B). −, absence of; +, presence of.
DISCUSSION
Drug resistance is the major impedance to successful chemotherapy in several diseases like cancer and AIDS and in different parasitic diseases, including leishmaniasis. In the case of leishmaniasis, resistance to antimonials has emerged as the major pitfall in treatment in view of limited alternative treatments, reports of multiple drug resistance (8, 41, 42, 48), and lack of effective vaccination. Previous studies emphasizing the expression of analogs of ABC transporters by Leishmania promastigotes, however, do not fully explain the resistance displayed by amastigote forms within human hosts, especially when SAG also activates the host's immune system that helps to eliminate intracellular parasite. We show here that resistant parasites strongly increase expression of the host's P-gp and MRP1 transporters on the surfaces of infected Mφ, resulting in Sb clearance from the host cells in the course of in vitro as well as in vivo experimental infection. Moreover, studies performed on patient samples from Sb-resistant infection areas unequivocally indicate that a similar phenomenon occurs during natural human infection. Of course, this does not exclude the possibilities that analogs of mammalian ABC transporters on amastigotes also play some role in drug resistance and inhibitors of mammalian ABC transporters may also inhibit these molecules of the parasite to allow better action of SAG. However, upregulation of ABC transporters in host cells may act as the foremost barrier for drugs and, thus, the transporters are of considerable interest.
As parasites replicate in Mφ, we focused our study on these cells. However, this focus does not exclude an upregulation of these transporters in other cell types like lymphocytes. The ability of a leishmanial antigen to upregulate expression of MRP1 has been reported earlier for Th1 clones (29). Indeed, it is well established that monocytes do not harbor parasites at the active stage of the disease. In spite of this finding, peripheral blood monocytes from Sbr-KA patients upregulate P-gp and MRP1. Therefore, it is likely that soluble and circulating parasite antigens can cause an upregulation of expression in these transporters. This result is supported by our findings that formalin-fixed Sbr L. donovani or even extracts from Sbr L. donovani strains can induce the upregulation of MRP1 and P-gp in uninfected murine Mφ and reduce Sb accumulation following SAG treatment. Thus, the resistance mechanism may operate in different cells of parasite reservoirs, even in the absence of parasite replication in situ. The identification of L. donovani parasite antigens through proteome mapping (20) and an analysis of their glycosylinositol phospholipids responsible for this upregulation are currently under investigation. It also will be important to determine whether such antigens are displayed on the surfaces of infected Mφ and sensed by adjacent Mφ or whether they are released from infected Mφ phagolysosomes to trigger the overexpression of ABC transporters. However, the importance of Sbr L. donovani antigens in SAG resistance through an upregulation of expression in ABC transporters is further demonstrated by our observation that SAG fails to eliminate intracellular Sbs L. donovani from Mφ that have been preincubated with Sbr L. donovani antigens and then infected in vitro with Sbs L. donovani (data not shown).
Our results show that inhibitors of P-gp and MRP1 could restore sensitivity toward Sb not only in vitro but also in vivo. Sbr L. donovani infection in golden hamsters is fatal, even upon SAG treatment. The survival of animals infected with Sbr L. donovani and the clearance of SAG-resistant parasites when treated with SAG plus either a combination of verapamil plus probenecid or lovastatin suggest major roles for P-gp and MRP1 in drug efflux. Thus, our results clearly show that inhibitors of P-gp and MRP1 represent important new therapeutic tools to combat Sb resistance. Lovastatin is a well-tolerated drug (12), and it will be important to evaluate its beneficial effect as an RMA in clinical trials dealing with SAG treatment of VL patients. Finally, lovastatin alone could inhibit both P-gp-mediated and MRP1-mediated transport to allow the retention of antimonials as well as Dox in drug-resistant cancer cells, thereby opening up new opportunities for the treatment of other infectious diseases and cancers where drug resistance is a major challenge and often involves the overexpression of P-gp and MRP1.
Acknowledgments
This work was supported by the CSIR grant CMM002. J.M.B. was a recipient of a CSIR fellowship.
We are extremely grateful to R. Basu, E. Champagne, S. L. Croft, C. Davrinche, S. Dey, C. Jaffe, F. Modabber, B. Saha, G. Schönian, and P. Walden for critically reviewing the manuscript and for their helpful suggestions. We also acknowledge the technical support provided by S. Choubey, A. Prasad, and S. Burman. We also gratefully acknowledge the help extended by N. Verma and I. Sinha to obtain postdiagnosis leftover blood from patients of SAG-sensitive zones.
Footnotes
Published ahead of print on 3 December 2007.
REFERENCES
- 1.Abdo, M. G., W. M. Elamin, E. A. Khalil, and M. M. Mukhtar. 2003. Antimony-resistant Leishmania donovani in eastern Sudan: incidence and in vitro correlation. East. Mediterr. Health J. 9:837-843. [PubMed] [Google Scholar]
- 2.Alexander, J., K. C. Carter, N. Al-Fasi, A. Satoskar, and F. Brombacher. 2000. Endogenous IL-4 is necessary for effective drug therapy against visceral leishmaniasis. Eur. J. Immunol. 30:2935-2943. [DOI] [PubMed] [Google Scholar]
- 3.Back, D. J. 1999. Pharmacological issues relating to viral resistance. Infection 27(Suppl. 2):S42-S44. [DOI] [PubMed] [Google Scholar]
- 4.Basu, R., S. Bhaumik, J. Mookerjee Basu, K. Naskar, T. De, and S. Roy. 2005. Kinetoplastid membrane protein-11 DNA vaccination induces complete protection against both pentavalent antimonial-sensitive and -resistant strains of Leishmania donovani that correlates with inducible nitric oxide synthase activity and IL-4 generation: evidence for mixed Th1- and Th2-like responses in visceral leishmaniasis. J. Immunol. 174:7160-7171. [DOI] [PubMed] [Google Scholar]
- 5.Borst, P., and R. O. Elferink. 2002. Mammalian ABC transporters in health and disease. Annu. Rev. Biochem. 71:537-592. [DOI] [PubMed] [Google Scholar]
- 6.Chakraborty, D., S. Banerjee, A. Sen, K. K. Banerjee, P. Das, and S. Roy. 2005. Leishmania donovani affects antigen presentation of macrophage by disrupting lipid rafts. J. Immunol. 175:3214-3224. [DOI] [PubMed] [Google Scholar]
- 7.Chulay, J. D., and A. D. Bryceson. 1983. Quantitation of amastigotes of Leishmania donovani in smears of splenic aspirates from patients with visceral leishmaniasis. Am. J. Trop. Med. Hyg. 32:475-479. [DOI] [PubMed] [Google Scholar]
- 8.Croft, S. L., S. Sundar, and A. H. Fairlamb. 2006. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 19:111-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dal Ponte, D. B., D. L. Fogt, S. Jacob, and E. J. Henriksen. 1998. Interactions of captopril and verapamil on glucose tolerance and insulin action in an animal model of insulin resistance. Metabolism 47:982-987. [DOI] [PubMed] [Google Scholar]
- 10.Decuypere, S., S. Rijal, V. Yardley, S. De Doncker, T. Laurent, B. Khanal, F. Chappuis, and J. C. Dujardin. 2005. Gene expression analysis of the mechanism of natural Sb(V) resistance in Leishmania donovani isolates from Nepal. Antimicrob. Agents Chemother. 49:4616-4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diomede, L., D. Albani, M. Sottocorno, M. B. Donati, M. Bianchi, P. Fruscella, and M. Salmona. 2001. In vivo anti-inflammatory effect of statins is mediated by nonsterol mevalonate products. Arterioscler. Thromb. Vasc. Biol. 21:1327-1332. [DOI] [PubMed] [Google Scholar]
- 12.DiPalma, J. R. 1987. Lovastatin: cholesterol-lowering agent. Am. Fam. Physician 36:189-192. [PubMed] [Google Scholar]
- 13.Donecker, J. M., R. A. Sams, and S. M. Ashcraft. 1986. Pharmacokinetics of probenecid and the effect of oral probenecid administration on the pharmacokinetics of cefazolin in mares. Am. J. Vet. Res. 47:89-95. [PubMed] [Google Scholar]
- 14.Efferth, T., I. Verdorfer, H. Miyachi, A. Sauerbrey, H. G. Drexler, C. R. Chitambar, M. Haber, and E. Gebhart. 2002. Genomic imbalances in drug resistant T-cell acute lymphoblastic CEM leukemia cell lines. Blood Cells Mol. Dis. 29N:1-13. [DOI] [PubMed] [Google Scholar]
- 15.El Fadili, K., N. Messier, P. Leprohon, G. Roy, C. Guimond, N. Trudel, N. G. Saravia, B. Papadopoulou, D. Legare, and M. Ouellette. 2005. Role of the ABC transporter MRPA (PGPA) in antimony resistance in Leishmania infantum axenic and intracellular amastigotes. Antimicrob. Agents Chemother. 49:1988-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Escobar, P., V. Yardley, and S. L. Croft. 2001. Activities of hexadecylphosphocholine (miltefosine), AmBisome, and sodium stibogluconate (Pentostam) against Leishmania donovani in immunodeficient scid mice. Antimicrob. Agents Chemother. 45:1872-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faraut-Gambarelli, F., R. Piarroux, M. Deniau, B. Giusiano, P. Marty, G. Michel, B. Faugere, and H. Dumon. 1997. In vitro and in vivo resistance of Leishmania infantum to meglumine antimoniate: a study of 37 strains collected from patients with visceral leishmaniasis. Antimicrob. Agents Chemother. 41:827-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feller, N., H. J. Broxterman, D. C. Wahrer, and H. M. Pinedo. 1995. ATP-dependent efflux of calcein by the multidrug resistance protein (MRP): no inhibition by intracellular glutathione depletion. FEBS Lett. 368:385-388. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-Guerrero, M. L., J. M. Aguado, L. Buzon, C. Barros, C. Montalban, T. Martin, and E. Bouza. 1987. Visceral leishmaniasis in immunocompromised hosts. Am. J. Med. 83:1098-1102. [DOI] [PubMed] [Google Scholar]
- 20.Forgber, M., R. Basu, K. Roychoudhury, S. Theinert, S. Roy, S. Sundar, P. Walden. 2006. Mapping the antigenicity of the parasites in Leishmania donovani infection by proteome serology. PLoS ONE 1:e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gollapudi, S., C. H. Kim, B. N. Tran, S. Sangha, and S. Gupta. 1997. Probenecid reverses multidrug resistance in multidrug resistance-associated protein-overexpressing HL60/AR and H69/AR cells but not in P-glycoprotein-overexpressing HL60/Tax and P388/ADR cells. Cancer Chemother. Pharmacol. 40:150-158. [DOI] [PubMed] [Google Scholar]
- 22.Gollapudi, S., M. Reddy, P. Gangadharam, T. Tsuruo, and S. Gupta. 1994. Mycobacterium tuberculosis induces expression of P-glycoprotein in promonocytic U1 cells chronically infected with HIV type 1. Biochem. Biophys. Res. Commun. 199:1181-1187. [DOI] [PubMed] [Google Scholar]
- 23.Gottesman, M. M., T. Fojo, and S. E. Bates. 2002. Multidrug resistance in cancer: role of ATP dependent transporters. Nat. Rev. Cancer 2:48-58. [DOI] [PubMed] [Google Scholar]
- 24.Homolya, L., A. Varadi, and B. Sarkadi. 2003. Multidrug resistance-associated proteins: export pumps for conjugates with glutathione, glucuronate or sulfate. Biofactors 17:103-114. [DOI] [PubMed] [Google Scholar]
- 25.Ibrahim, M. E., M. Hag-Ali, A. M. el-Hassan, T. G. Theander, and A. Kharazmi. 1994. Leishmania resistant to sodium stibogluconate: drug-associated macrophage-dependent killing. Parasitol. Res. 80:569-574. [DOI] [PubMed] [Google Scholar]
- 26.Jamerson, K., A. Champion, Q. Zhou, and C. Pepine. 2005. Verapamil- and atenolol-based strategies are equally effective in black patients with hypertension and coronary artery disease: a subanalysis of the international verapamil SR-trandolapril study (INVEST). Am. J. Hypertens. 18:A109. [Google Scholar]
- 27.Legare, D., D. Richard, R. Mukhopadhyay, Y. D. Stierhof, B. P. Rosen, A. Haimeur, B. Papadopoulou, and M. Ouellette. 2001. The Leishmania ATP-binding cassette protein PGPA is an intracellular metal-thiol transporter ATPase. J. Biol. Chem. 276:26301-26307. [DOI] [PubMed] [Google Scholar]
- 28.Li, J., S. Sutterwala, and J. P. Farrell. 1997. Successful therapy of chronic, nonhealing murine cutaneous leishmaniasis with sodium stibogluconate and gamma interferon depends on continued interleukin-12 production. Infect. Immun. 65:3225-3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lohoff, M., S. Prechtl, F. Sommer, M. Roellinghoff, E. Schmitt, G. Gradehandt, P. Rohwer, B. D. Stride, S. P. Cole, and R. G. Deeley. 1998. A multidrug-resistance protein (MRP)-like transmembrane pump is highly expressed by resting murine T helper (Th) 2, but not Th1 cells, and is induced to equal expression levels in Th1 and Th2 cells after antigenic stimulation in vivo. J. Clin. Investig. 101:703-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luqmani, Y. A. 2005. Mechanisms of drug resistance in cancer chemotherapy. Med. Princ. Pract. 14:35-48. [DOI] [PubMed] [Google Scholar]
- 31.Mookerjee, A., J. Mookerjee Basu, P. Dutta, S. Majumder, S. Bhattacharyya, J. Biswas, S. Pal, P. Mukherjee, S. Raha, R. N. Baral, T. Das, T. Efferth, G. Sa, S. Roy, and S. K. Choudhuri. 2006. Overcoming drug-resistant cancer by a newly developed copper chelate through host-protective cytokine-mediated apoptosis. Clin. Cancer Res. 12:4339-4349. [DOI] [PubMed] [Google Scholar]
- 32.Mookerjee, A., J. Mookerjee Basu, S. Majumder, S. Chatterjee, G. S. Panda, P. Dutta, S. Pal, P. Mukherjee, T. Efferth, S. Roy, and S. K. Choudhuri. 2006. A novel copper complex induces ROS generation in doxorubicin resistant Ehrlich ascitis carcinoma cells and increases activity of antioxidant enzymes in vital organs in vivo. BMC Cancer 6:267-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mookerjee Basu, J., A. Mookerjee, P. Sen, S. Bhaumik, P. Sen, S. Banerjee, K. Naskar, S. K. Choudhuri, B. Saha, S. Raha, and S. Roy. 2006. Sodium antimony gluconate induces generation of reactive oxygen species and nitric oxide via phosphoinositide 3-kinase and mitogen-activated protein kinase activation in Leishmania donovani-infected macrophages. Antimicrob. Agents Chemother. 50:1788-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mookerjee Basu, J., and S. Roy. 2007. Sodium antimony gluconate (SAG) mediates antileishmanial effect by stimulating innate and cellular arms of the immune system. Sci. Cult. 73:138-143. [Google Scholar]
- 35.Mukhopadhyay, R., S. Dey, N. Xu, D. Gage, J. Lightbody, M. Ouellette, and B. P. Rosen. 1996. Trypanothione overproduction and resistance to antimonials and arsenicals in Leishmania. Proc. Natl. Acad. Sci. USA 93:10383-10387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murray, H. W. 1999. Kala-azar as an AIDS-related opportunistic infection. AIDS Patient Care STDs 13:459-465. [DOI] [PubMed] [Google Scholar]
- 37.Murray, H. W., C. Montelibano, R. Peterson, and J. P. Sypek. 2000. Interleukin-12 regulates the response to chemotherapy in experimental visceral leishmaniasis. J. Infect. Dis. 182:1497-1502. [DOI] [PubMed] [Google Scholar]
- 38.Murray, H. W., G. D. Miralles, M. Y. Stoeckle, and D. F. McDermott. 1993. Role and effect of IL-2 in experimental visceral leishmaniasis. J. Immunol. 151:929-938. [PubMed] [Google Scholar]
- 39.Murray, H. W., M. J. Oca, A. M. Granger, and R. D. Schreiber. 1989. Requirement for T cells and effect of lymphokines in successful chemotherapy for an intracellular infection. Experimental visceral leishmaniasis. J. Clin. Investig. 83:1253-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nabors, G. S., and J. P. Farrell. 1994. Depletion of interleukin-4 in BALB/c mice with established Leishmania major infections increases the efficacy of antimony therapy and promotes Th1-like responses. Infect. Immun. 62:5498-5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ouellette, M., D. Legare, A. Haimeur, K. Grondin, G. Roy, C. Brochu, and B. Papadopoulou. 1998. ABC transporters in Leishmania and their role in drug resistance. Drug Resist. Updat. 1:43-48. [DOI] [PubMed] [Google Scholar]
- 42.Perez-Victoria, J. M., A. Di Pietro, D. Baron, A. G. Ravelo, S. Castanys, and F. Gamarro. 2002. Multidrug resistant phenotype mediated by the P-glycoprotein-like transporter in Leishmania: a search for reversal agents. Curr. Drug Targets 3:311-333. [DOI] [PubMed] [Google Scholar]
- 43.Roberts, W. L., and P. M. Rainey. 1993. Antimony quantification in Leishmania by electrothermal atomic absorption spectroscopy. Anal. Biochem. 211:1-6. [DOI] [PubMed] [Google Scholar]
- 44.Saha, S., S. Mondal, R. Ravindran, S. Bhowmick, D. Modak, S. Mallick, M. Rahman, S. Kar, R. Goswami, S. K. Guha, N. Pramanik, B. Saha, and N. Ali. 2007. IL-10- and TGF-beta-mediated susceptibility in kala-azar and post-kala-azar dermal leishmaniasis: the significance of amphotericin B in the control of Leishmania donovani infection in India. J. Immunol. 179:5592-5603. [DOI] [PubMed] [Google Scholar]
- 45.Sen, E., S. Chattopadhyay, S. Bandopadhyay, T. De, and S. Roy. 2001. Macrophage heterogeneity, antigen presentation, and membrane fluidity: implications in visceral Leishmaniasis. Scand. J. Immunol. 53:111-120. [DOI] [PubMed] [Google Scholar]
- 46.Singh, N., R. Almeida, H. Kothari, P. Kumar, G. Mandal, M. Chatterjee, S. Venkatachalam, M. K. Govind, S. K. Mandal, and S. Sundar. 2007. Differential gene expression analysis in antimony unresponsive Indian kala azar (visceral leishmaniasis) clinical isolates by DNA microarray. Parasitology 134:777-787. [DOI] [PubMed] [Google Scholar]
- 47.Stamp, L. K., J. L. O'Donnell, and P. T. Chapman. 2007. Emerging therapies in the long-term management of hyperuricaemia and gout. Intern. Med. J. 37:258-266. [DOI] [PubMed] [Google Scholar]
- 48.Sundar, S. 2001. Drug resistance in Indian visceral leishmaniasis. Trop. Med. Int. Health 6:849-854. [DOI] [PubMed] [Google Scholar]
- 49.Tolcher, A. W., K. H. Cowan, D. Solomon, F. Ognibene, B. Goldspiel, R. Chang, M. H. Noone, A. M. Denicoff, C. S. Barnes, M. R. Gossard, P. A. Fetsch, S. L. Berg, F. M. Balis, D. J. Venzon, and J. A. O'Shaughnessy. 1996. Phase I crossover study of paclitaxel with r-verapamil in patients with metastatic breast cancer. J. Clin. Oncol. 14:1173-1184. [DOI] [PubMed] [Google Scholar]
- 50.Tsuruo, T., H. Iida, S. Tsukagoshi, and Y. Sakurai. 1981. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res. 41:1967-1972. [PubMed] [Google Scholar]
- 51.Vergnes, B., B. Gourbal, I. Girard, S. Sundar, J. Drummelsmith, and M. Ouellette. 2007. A proteomics screen implicates HSP83 and a small kineto-plastid calpain-related protein in drug resistance in Leishmania donovani clinical field isolates by modulating drug-induced programmed cell death. Mol. Cell. Proteomics 6:88-101. [DOI] [PubMed] [Google Scholar]
- 52.Wang, E., C. N. Casciano, R. P. Clement, and W. W. Johnson. 2001. HMG-CoA reductase inhibitors (statins) characterized as direct inhibitors of P-glycoprotein. Pharm. Res. 18:800-806. [DOI] [PubMed] [Google Scholar]
- 53.World Health Organization. 2006. Control of leishmaniasis. Report by the Secretariat. World Health Organization, Geneva, Switzerland. www.who.int/gb/ebwha/pdf_files/EB118/B118_4-en.pdf.
- 54.Wyllie, S., M. L. Cunningham, and A. H. J. Fairlamb. 2004. Dual action of antimonial drugs on thiol redox metabolism in the human pathogen Leishmania donovani. J. Biol. Chem. 279:39925-39932. [DOI] [PubMed] [Google Scholar]
- 55.Xu, C., C. Y. Li, and A. N. Kong. 2005. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch. Pharm. Res. 28:249-268. [DOI] [PubMed] [Google Scholar]