Abstract
The objective of this study was to determine the population pharmacokinetic parameters of levofloxacin, gatifloxacin, and moxifloxacin following multiple oral doses. Twenty-nine patients with tuberculosis at the University Hospital in Vitória, Brazil, participated. Subjects received multiple doses of one drug (levofloxacin, 1,000 mg daily, or gatifloxacin or moxifloxacin, 400 mg daily) as part of a 7-day study of early bactericidal activity. Serum samples were collected over 24 h after the fifth dose and assayed using validated high-performance liquid chromatography assays. Concentration-time data were analyzed using noncompartmental, compartmental, and population methods. The three drugs were well tolerated. Levofloxacin produced the highest maximum plasma concentrations (median, 15.55 μg/ml; gatifloxacin, 4.75 μg/ml; moxifloxacin, 6.13 μg/ml), largest volume of distribution (median, 81 liters; gatifloxacin, 79 liters; moxifloxacin, 63 liters), and longest elimination half-life (median, 7.4 h; gatifloxacin, 5.0 h; moxifloxacin, 6.5 h). A one-compartment model, with or without weight as a covariate, adequately described the data. Postmodeling simulations using median population parameter estimates closely approximated the median values from the original data. Area under the concentration-time curve/MIC ratios for free drug were high. All three quinolones showed favorable pharmacokinetic and pharmacodynamic indices, with the most favorable results in this population being seen with levofloxacin at the comparative doses used.
Fluoroquinolones are bactericidal against Mycobacterium tuberculosis and are employed as second-line agents for the treatment of tuberculosis (TB) in cases of patient intolerance or mycobacterial resistance (multidrug-resistant TB) (1, 15). Increasingly, these drugs are being evaluated as first-line agents for newly diagnosed, drug-susceptible TB (2, 9). The pharmacokinetics (PK) of levofloxacin, gatifloxacin, and moxifloxacin have been characterized in healthy volunteers and in several clinical settings, including a previous PK study of ofloxacin (the racemic mixture that includes levofloxacin) in patients with TB (4, 9, 16).
In our previous report, we described the early bactericidal activity of isoniazid, levofloxacin, gatifloxacin, and moxifloxacin in patients with TB (9). In this study, we further explored the PK of these fluoroquinolones in TB patients and developed structural models of their behavior, and this report provides population PK parameter estimates that can be used in simulations of these three quinolones in TB patients.
MATERIALS AND METHODS
Study design.
HIV-uninfected 18- to 65-year-old Brazilian adults with newly diagnosed smear-positive TB who weighed more than 75% of their ideal body weight and who had relatively normal hematologic, renal, and hepatic function were eligible (9). The institutional review boards of the Universidade Federal do Espírito Santo and Case Western Reserve University approved the protocol. Patients gave written informed consent.
Patients were randomized to receive 7 days of oral isoniazid (300 mg) (standard positive comparator for early bactericidal activity studies), levofloxacin (1,000 mg), gatifloxacin (400 mg), or moxifloxacin (400 mg). Data for the fluoroquinolones are presented here. The gatifloxacin and moxifloxacin doses are doses currently being studied in clinical trials. Higher doses might be considered, but concerns about potentially exacerbating hypo- and hyperglycemia (gatifloxacin) and approaching concentrations that might be associated with QTc interval prolongation (moxifloxacin) led to the decision to test only the 400-mg doses in this study. Levofloxacin is an older quinolone, and over the years, many clinical centers have gradually increased doses from 500 to 750 to 1,000 mg for selected patients with TB. The 1,000-mg levofloxacin dose was chosen for this study based on this experience and based on studies suggesting greater activity and acceptable tolerability at this dose in patients with drug-susceptible and multidrug-resistant TB (9, 16). Drugs were purchased in the United States and manufactured under good manufacturing practice guidelines. Patients were hospitalized in a research ward for supervised drug administration and specimen collection. After the study, all patients were treated with standard chemotherapy.
Pharmacokinetic studies.
Subjects fasted overnight for 8 h before the fifth daily dose. Plasma samples were collected immediately before and 1, 2, 4, 8, 12, 18, and 24 h after the fifth dose of study drug (steady state). Samples were kept in a −80°C freezer until shipped frozen to the United States. Samples were assayed at National Jewish Medical and Research Center, Denver, CO, using validated assays on a ThermoFinnigan P4000 HPLC pump (San Jose, CA) with a model AS1000 fixed-volume autosampler, a model FL3000 fluorescence detector, a Gateway Series e computer (Poway, CA), and a Chromquest HPLC data management system. The six-point standard curves ranged from 0.20 to 15 μg/ml, with linearity extending above and below this range. The recovery of the quinolones from human plasma was approximately 90%. The overall validation precision for levofloxacin quality control samples was 0.76 to 4.83%; that for gatifloxacin was 2.00 to 2.50%, and that for moxifloxacin was 4.19 to 6.62%. Concentrations for the quality control samples were 0.8, 8, and 14 μg/ml. Prior to the assay, we planned to reanalyze any samples that did not meet quality control criteria (per standard operating procedure) or that appeared to be PK outliers based upon visual inspection of the graphical data (6).
Safety.
A standard survey of TB symptoms and toxicity was completed daily during the inpatient study and at day 42. Complete blood count, urinalysis, and measurements of serum aspartate aminotransferase, total bilirubin, creatinine, and glucose were repeated at day 4 and day 7. Safety was assessed by comparing the incidence of adverse events and changes in laboratory parameters between arms.
Data analyses.
We planned to remove any serum concentrations that were below the lower limit of quantification (0.20 μg/ml) from the analysis and to remove any apparent outliers that could not be successfully comodeled with the remaining data for a given patient (6). Pharmacokinetic analyses were performed initially using a noncompartmental analysis (NCA) (WinNonLin 4 [WNL]; Pharsight, Mountain View, CA). Next, compartmental analyses were performed using WNL to determine the structural model and to gain initial parameter estimates for the population analyses. The Akaike information criterion was used to discriminate between one- and two-compartment models for each of the quinolones separately.
(i) Population PK analysis.
Patient data files were created using the PASTRX program in the USC*PACK software. The assay error pattern describing the reproducibility of results obtained with quality control samples for each assay for several months before and after the analysis of the study samples was used during the modeling (5). Population PK models were created using the nonparametric adaptive grid (NPAG) in the USC*PACK software (3). The iterative two-stage Bayesian population model algorithm was run initially to define the boundary ranges for the estimates used in the NPAG modeling and to assess error that was not attributable to the assay used (termed gamma within the software package). Three parameters were estimated simultaneously, including an absorption rate constant (ka [h−1]), an elimination rate constant (kel [h−1]), and the apparent volume of distribution (V/F [liters]), where F is absolute bioavailability (not determined in this study). The fraction of the dose absorbed (F) was arbitrarily fixed at 1 for purposes of data analysis. Goodness of fit was assessed by regression with an observed-predicted plot, coefficients of determination, and log-likelihood values. Predictive performance was assessed using the weighted mean error for bias and the bias-adjusted weighted mean square error for precision. The Bayesian posterior parameter joint densities of individual subjects were estimated starting from the population parameter joint density and continuing to analyze each subject's data. The ka, kel and V/F estimates for each individual were obtained from the NPAG model, and they were used to compute the remaining PK parameters, including the apparent volume of distribution (liters/kg), the total clearance (CL/F [liters/h]), and the absorption and elimination half-lives (t1/2), using standard equations. The population model-based area under the concentration-versus-time curve (AUC [μg · h/ml]) was calculated as dose/(CL/F). Individual parameter estimates from early models were placed into JMP statistical software (version 6.0.3; SAS Institute, Cary, NC), and linear and logistic regression analyses were performed to assess the associations between PK parameter estimates and demographic variables. Based on these analyses, additional models were constructed using different parameterizations, such as volume of distribution adjusted for weight (liters/kg).
Model verification was performed by using the PK estimates obtained from NPAG to perform simulations using WNL. The maximum serum concentration (Cmax [μg/ml]), the time to achieve Cmax (Tmax [h]), and the AUC from 0 h to infinity (AUC0-∞ [μg · h/ml]) for the three regimens were estimated using a one-compartment open model and compared to the median patient data from the NCA (retrodiction of the original data). We also compared the data to those obtained with other published PK models for these three drugs, including the data from a previous study of ofloxacin in TB patients (16).
AUCs for free drug (unbound to plasma protein) were calculated by multiplying the individual AUCs by (100% − the published percent protein binding values for levofloxacin [25%], gatifloxacin [18%], and moxifloxacin [50%]) (4). MICs for the fluoroquinolones were measured using recommended methods in the BACTEC 460 radiometric culture system and on Middlebrook 7H10 agar plates (12). Drug concentrations were tested in twofold dilutions from 0.125 to 4.0 μg/ml for levofloxacin and moxifloxacin and from 0.025 to 0.8 μg/ml for gatifloxacin. Each drug was tested separately with both patient isolates and the drug-susceptible strain H37Rv. For the BACTEC 460 method, the MIC was defined as the lowest concentration for which the change in growth index was less than that of the 1:100 control. The MIC on Middlebrook 7H10 medium was defined as the lowest concentration of drug that inhibited >99% of the bacterial population. MIC results were identical using both methods. Free-drug AUC/MIC ratios were calculated using these values. In addition, more conservative MIC90s for levofloxacin (1.0 μg/ml) and gatifloxacin (0.5 μg/ml) were taken from the published literature, and alternative AUC/MIC ratios were calculated (10, 12).
(ii) Statistical analysis.
Statistical analyses were performed with JMP statistical software (version 6.0.3; SAS Institute, Cary, NC). Creatinine clearance (CLCR) was calculated by the method of Cockroft and Gault using the day 4 serum creatinine level (3a). Frequency distributions (JMP) included plots of the data, distribution curves, and the Shapiro-Wilk W test to test for normality, as well as parametric and nonparametric measures of central tendency and dispersion. The coefficient of variation (expressed as a percentage) was calculated as (standard deviation/mean) × 100. The dependence of PK variables on subject demographics was determined using Y-by-X analysis one parameter at a time. A P value of ≤0.05 was considered statistically significant. Given the small sample sizes, P values of ≤0.10 represented associated covariates that also were further evaluated in the modeling process. The demographic variables analyzed included those shown in Table 1, and the PK parameter estimates tested included those in Table 2.
TABLE 1.
Population demographics of the study subjects
| Parameter | Value for drug group
|
||
|---|---|---|---|
| Levofloxacin (n = 10) | Gatifloxacin (n = 10) | Moxifloxacin (n = 9) | |
| No of patientsa | |||
| Male | 8 | 9 | 8 |
| Female | 2 | 1 | 1 |
| Age (yr)a | 44 (30-54) | 34 (23-58) | 35 (18-46) |
| Wt (kg)a | 56 (41-66) | 56 (45-66) | 56 (43-69) |
| SCRb (mg/dl)a | 0.9 (0.5-1.3) | 0.9 (0.6-1.0) | 0.9 (0.7-1.2) |
| CLCR (ml/min)a | 79 (51-125) | 81 (61-107) | 96 (61-117) |
| Dose (mg) | 1,000 | 400 | 400 |
| Dose (mg/kg)a | 18.0 (15.3-24.0) | 7.2 (6.1-9.0) | 7.2 (5.8-9.3) |
Median (range).
SCR, serum creatinine.
TABLE 2.
NPAG PK estimates, NCA median values for drug exposure, and simulated exposure values using median population parameter estimatesa
| Drug | Dose (mg) | PK estimates
|
WNL NCA median (range) patient values
|
WNL simulations using median NPAG parameter estimates
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ka (h−1) | kel (h−1) | t1/2 (ka, h) | t1/2 (kel, h) | V/F (liters) | V/F (liters/kg)b | CL/F (liters/h) | CL/F (liters/h/kg)b | AUC (μg · h/ml) | Bias | Precision | Cmax (μg/ml) | Tmax (h) | AUC0-24,ssc | Cmax (μg/ml) | Tmax (h) | AUC0-∞ | ||
| Levofloxacin | 1,000 | 5.96 (0.76-6.00) | 0.09 (0.04-0.17) | 0.12 (0.12-0.91) | 7.37 (4.14-16.31) | 81.21 (33.50-114.50) | 1.28 (0.81-1.83) | 7.63 (1.49-19.18) | 0.13 (0.06-0.17) | 131 (52-702) | 0.059 | 1.234 | 15.55 (8.55-42.99) | 1.0 (1.0-4.0) | 129 (103-358) | 11.55 | 0.7 | 137 |
| Gatifloxacin | 400 | 0.96 (0.37-6.00) | 0.13 (0.09-0.16) | 0.72 (0.12-1.89) | 5.04 (4.38-7.35) | 79.25 (58.02-110.54) | 1.45 (0.91-2.47) | 10.42 (5.47-17.50) | 0.18 (0.13-0.30) | 38 (23-73) | −0.152 | 0.243 | 4.61 (3.78-6.40) | 1.5 (1.0-2.0) | 43 (32-51) | 3.69 | 2.4 | 39 |
| Moxifloxacin | 400 | 5.95 (1.21-6.00) | 0.11 (0.07-0.16) | 0.12 (0.12-0.57) | 6.53 (4.25-10.57) | 62.79 (43.59-101.96) | 1.25 (0.93-1.55) | 6.66 (2.86-16.64) | 0.12 (0.10-0.20) | 60 (24-140) | −0.115 | 0.914 | 6.13 (4.47-9.00) | 1.0 (1.0-2.0) | 55 (36-79) | 5.91 | 0.7 | 58 |
ka, k-el, and V were estimated directly with NPAG; t1/2 (ka), t1/2 (kel), CL, and AUC were calculated from ka, k-el, and V using standard equations. Values are medians and ranges.
Based on median of individual Bayesian parameters estimates, rather than the original population estimate.
AUC0-24,ss is the equivalent of AUC0-∞.
RESULTS
Twenty-nine subjects completed the study, including 25 males and 4 females with a median age of 35 years (Table 1). All had newly diagnosed initial episodes of sputum smear-positive, cavitary pulmonary TB. The PK parameter estimates are provided in Table 2. No serum concentrations were less than the lower limit of quantification. One high gatifloxacin concentration 8 h postdose was considered an outlier and removed from the data analysis. Because these drugs were rapidly absorbed in many patients, and because of the lack of samples prior to 1 h, initial estimates for ka were unrealistically high. Therefore, ka was capped at 6 for all subsequent models across the three drugs, equivalent to an absorption half-life of 7 min. Although some statistical association was seen between volume estimates and weight, or between elimination rate constant estimates and the calculated CLCR, inclusion of these parameters produced only minor improvements in log-likelihood or precision, while producing minor degradations in bias. Further, no single model parameterization proved to be best for all three drugs. Therefore, in the interest of clarity, the parameter estimates for the simplest model (ka, V/F, kel) are presented for all three drugs.
Levofloxacin.
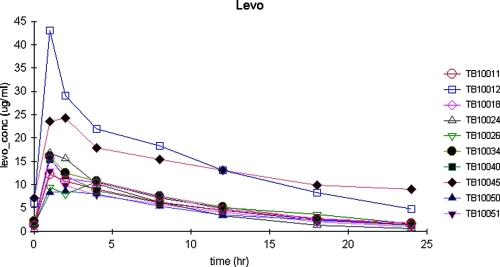
Figure 1 displays the serum concentrations versus time for the 10 patients. Two patients had unusually high levofloxacin serum concentrations, which were verified with repeat analyses. Levofloxacin Cmaxs ranged from just under 9 to 43 μg/ml, higher than described for other populations. One of these patients, a 46-year-old male with a Cmax of 24.27 μg/ml, had a serum creatinine level of 1.3 mg/dl, a CLCR of 51 ml/min, and a Bayesian post-hoc estimated t1/2 of 15.4 h. The other patient, a 40-year-old female with a Cmax of 42.99 μg/ml, had a serum creatinine level of 0.9 mg%, a CLCR of 55 ml/min, and a Bayesian post hoc estimated t1/2 of 9.2 h. The distinguishing feature for this patient was a Bayesian post hoc estimated volume of only 33.8 liters (0.81 liters/kg), the smallest in the group. For the remaining eight patients, the minimum CLCR was 74 ml/min.
FIG. 1.
Plasma concentrations of levofloxacin versus time after dosing in 10 adults with pulmonary tuberculosis.
Seven of 10 levofloxacin patients had an apparent Tmax of 1 h, and all seven had an estimated ka near the capped value of 6. One patient showed a Tmax of 4 h. On regression analysis, serum creatinine explained 66% of the variability in kel (P < 0.01), and calculated CLCR explained 48% (P < 0.03). In contrast, serum creatinine explained only 6% of the variability in CL/F (P = 0.49), while CLCR explained 34% (P < 0.08). Levofloxacin displayed the longest median population t1/2 estimate, at 7.37 h. The model displayed limited bias and good precision. Model-based and simulated estimates of AUC were consistent with those calculated using the WNL NCA.
Free-levofloxacin AUC/MIC ratios are shown in Table 3. The BACTEC-generated MICs for these isolates were generally 1 doubling dilution lower than previously published MIC90s, and both sets of values are presented (10, 12). The target AUC/MIC ratio for levofloxacin against M. tuberculosis has not been prospectively established. Compared to established target ratios of gram-positive organisms (AUC/MIC of 40), the calculated ratios for this study were far in excess of the target for all patients using either MIC. Compared to established target ratios of gram-negative organisms (AUC/MIC of 125), the calculated ratios for this study were in excess of the target for all but one patient with the study MICs and approached or exceeded the target value using the published MICs.
TABLE 3.
AUCs of free drug (unbound to plasma protein) compared to measured and previously published MICs
| Drug | Free AUC (μg · h/ml) | Actual MIC (μg/ml) | Published MIC90 (μg/ml) | Free AUC/actual MIC | Free AUC/ published MIC90 |
|---|---|---|---|---|---|
| Levofloxacin | 96.51 | 0.5 | 1.0 | 193.02 | 96.51 |
| 295.86 | 0.5 | 1.0 | 591.72 | 295.86 | |
| 109.33 | 0.5 | 1.0 | 218.67 | 109.33 | |
| 89.83 | 0.5 | 1.0 | 179.66 | 89.83 | |
| 91.16 | 0.5 | 1.0 | 182.33 | 91.16 | |
| 109.35 | 0.5 | 1.0 | 218.69 | 109.35 | |
| 96.51 | 1.0 | 1.0 | 96.51 | 96.51 | |
| 266.24 | 0.5 | 1.0 | 532.48 | 266.24 | |
| 77.26 | 0.5 | 1.0 | 154.53 | 77.26 | |
| 82.48 | 0.5 | 1.0 | 164.96 | 82.48 | |
| Median | 0.5 | 1.0 | 187.67 | 96.51 | |
| Mediana | 0.5 | 1.0 | 180.99 | 93.84 | |
| Gatifloxacin | 35.72 | 0.1 | 0.5 | 357.17 | 71.43 |
| 30.46 | 0.2 | 0.5 | 152.31 | 60.92 | |
| 28.88 | 0.2 | 0.5 | 144.39 | 57.75 | |
| 44.01 | 0.2 | 0.5 | 220.07 | 88.03 | |
| 26.89 | 0.2 | 0.5 | 134.44 | 53.77 | |
| 36.05 | 0.2 | 0.5 | 180.25 | 72.10 | |
| 35.86 | 0.2 | 0.5 | 179.29 | 71.71 | |
| 36.98 | 0.2 | 0.5 | 184.92 | 73.97 | |
| 26.89 | 0.2 | 0.5 | 134.44 | 53.78 | |
| 24.62 | 0.1 | 0.5 | 246.23 | 49.25 | |
| Median | 0.2 | 0.5 | 179.77 | 66.18 | |
| Moxifloxacin | 25.67 | 0.5 | 0.5 | 51.33 | 51.33 |
| 39.13 | 0.5 | 0.5 | 78.27 | 78.27 | |
| 27.17 | 0.5 | 0.5 | 54.33 | 54.33 | |
| 18.32 | 0.5 | 0.5 | 36.64 | 36.64 | |
| 21.08 | 0.5 | 0.5 | 42.16 | 42.16 | |
| 29.68 | 0.5 | 0.5 | 59.35 | 59.35 | |
| 31.51 | 0.5 | 0.5 | 63.02 | 63.02 | |
| 30.96 | 0.5 | 0.5 | 61.93 | 61.93 | |
| 30.78 | 0.5 | 0.5 | 61.57 | 61.57 | |
| Median | 0.5 | 0.5 | 59.35 | 59.35 |
Excluding the two patients with the highest values.
Gatifloxacin.
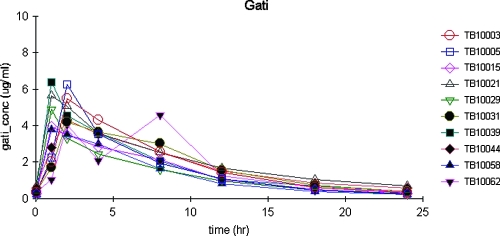
Figure 2 displays the serum concentrations versus time for the 10 patients. Gatifloxacin Cmaxs ranged from just under 4 to 6.40 μg/ml. The apparent Tmax for gatifloxacin occurred by 2 h. One concentration (4.59 μg/ml) that produced an apparent Tmax of 8 h was excluded as an outlier, since this point was inconsistent with the rest of the patient's data and inconsistent with the data for the remaining patients. The preceding and subsequent concentrations for this patient were 2.09 and 1.24 μg/ml.
FIG. 2.
Plasma concentrations of gatifloxacin versus time after dosing in 10 adults with pulmonary tuberculosis.
Gatifloxacin also displayed the shortest median population t1/2 estimate, at 5 h. Serum creatinine alone explained 25% of the variability in kel (P = 0.14) and less than 6% of the variability in CL/F (P = 0.50). Similarly, CLCR explained only 3% of the variability in CL/F (P = 0.65), but CLCR did explain 85% of the variability in kel (P < 0.01). Again, the model displayed limited bias and good precision, and the model-based and simulated estimates of AUC were consistent with those calculated using the WNL NCA.
Free-gatifloxacin pharmacodynamic data are shown in Table 3. The BACTEC-generated MICs for these isolates were generally at least 2 doubling dilutions lower than previously published MIC90s (10). Compared to established target ratios for gram-positive organisms (AUC/MIC of 40), the calculated ratios for this study were far in excess of the target for all patients using either MIC. Compared to established target ratios of gram-negative organisms (AUC/MIC of 125), the calculated ratios for this study were in excess of the target ratios for all patients with the study MICs but were below the target ratios using the published MIC90s.
Moxifloxacin.
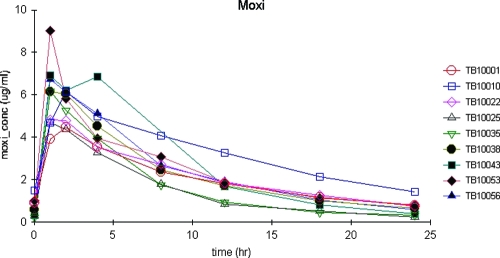
Figure 3 displays the serum concentrations versus time for the nine patients. Moxifloxacin Cmaxs ranged from 4.47 to 9.00 μg/ml. The highest Cmax was seen in a 46-year-old male patient with a calculated CLCR of 96 ml/min and the smallest volume when corrected for body weight (0.93 liters/kg). All patients had apparent moxifloxacin Tmax by 2 h, with seven of nine reaching Tmax by 1 h. Five of those seven had an estimated ka near the capped value of 6. Moxifloxacin also displayed a median population t1/2 estimate of 6.66 h. Serum creatinine explained 21% of the variability in kel (P = 0.22), and CLCR explained <1% (P = 0.97). Serum creatinine and CLCR explained <1% of the variability in CL/F (P = 0.81 and 0.89, respectively). Once again, the model displayed limited bias and good precision, and the model-based and simulated estimates of AUC were consistent with those calculated using the WNL NCA.
FIG. 3.
Plasma concentrations of moxifloxacin versus time after dosing in nine adults with pulmonary tuberculosis.
The BACTEC-generated moxifloxacin MICs for these isolates generally were the same as previously published MIC90s (Table 3). In this study, the calculated AUC/MIC ratios for gram-positive organisms were generally at or above the established target ratio of 40 in all patients except one. The calculated AUC/MIC ratios for gram-negative organisms were below the target ratio of 125 in all patients with the study MICs.
Safety.
The study drugs were well tolerated. No serious adverse events occurred, including in the two subjects with high plasma concentrations of levofloxacin and the patient with the high moxifloxacin concentration, over the 7 days of treatment. All subjects successfully completed standard short-course anti-TB treatment after the study.
DISCUSSION
Levofloxacin.
Two patients had very high levofloxacin serum concentrations compared to published values for patients with bacterial infections, and compared to our experience using relatively high doses of ofloxacin or levofloxacin for patients with TB (9, 11, 16). While it is common for our clinical laboratory service to measure levofloxacin concentrations up to 15 μg/ml, it is rare to see concentrations above 20 μg/ml under any circumstances. Despite these high concentrations, there were no adverse drug reactions attributable to levofloxacin in these study patients. Since treatment lasted only 7 days, we do not know if such high levofloxacin concentrations would be tolerated for the long duration of TB treatment.
This patient population showed rapid absorption of levofloxacin, consistent with published experience (4, 16). Blood sampling earlier than 1 h should be used for future studies looking to better define ka in TB patients. Parameter estimates for V/F and CL/F were comparable to data in our earlier report on ofloxacin, despite the fact that patients were recruited on two different continents.
The reason for the lower MICs in this study are not known, but they are within 1 doubling dilution of MIC90s published previously (10, 12). It is possible that the isolates of M. tuberculosis found in Brazil differ from those studied elsewhere and have lower MIC. Free-levofloxacin AUC/MIC ratios were favorable at the comparative doses used, regardless of which target value one selected using the study MICs, and remained good even when the higher published MIC90s and the higher target ratio for gram-negative organisms were used.
Gatifloxacin.
Gatifloxacin absorption in the study population, as evidenced by the median Tmax and median ka, was somewhat slower than that of the other two quinolones. Since subjects fasted overnight, food was not the reason for this slower absorption. Consistent with this slower absorption, and with the higher V/F, gatifloxacin had a lower Cmax than levofloxacin and moxifloxacin. Nevertheless, the Cmax matched or exceed published values (4). Gatifloxacin also showed the shortest elimination t1/2 of the three drugs, although the differences in t1/2 were not large. The t1/2 for these TB patients was shorter than those published for other populations (4).
The MIC determinations in this study are 2 to 3 doubling dilutions lower than those published previously. The resulting free-gatifloxacin AUC/MIC ratios were favorable, regardless of which target value one selected using the study MICs. These ratios were comparable to those calculated for levofloxacin. Less favorable ratios were calculated using the published MIC90, particularly if the gram-negative target should be the required target for M. tuberculosis.
Moxifloxacin.
Moxifloxacin absorption in the study population was rapid, similar to that of levofloxacin. The Cmaxs were somewhat higher than published values and somewhat higher than those typically seen with our clinical laboratory service (4). The moxifloxacin t1/2 for these TB patients was shorter than those published for other populations (4).
The MICs determined in this study were the same as previously published MIC90s (10). The free-moxifloxacin AUC/MIC ratios were lower than those for levofloxacin or gatifloxacin, although they approached the values for the latter when the published MIC90s were used to calculate the ratios. These ratios should be adequate if the gram-positive target should be the required target for M. tuberculosis but may be less favorable should the higher gram-negative target be applicable.
Recent investigations of M. tuberculosis suggest that, consistent with other bacterial infections, quinolone exposure (AUC) correlates with the efficacy of these drugs against M. tuberculosis (7, 8, 13). Our data suggest that quinolones can contribute to the treatment of TB, since the pharmacodynamic variables are consistent with those shown to be effective for other types of infections, and because the quinolones did produce an extended early bactericidal effect (4, 9). However, these concentrations may not be sufficient to prevent the selection of resistance as monotherapy (7). Not studied here was the importance of achieving a given AUC/MIC ratio in the context of the multidrug treatment used clinically for the entire course of treatment for TB. One may posit that, ideally, each drug within the regimen should be pharmacodynamically optimized, so that each drug makes its maximum contribution to the regimen. Given the recent findings of an interaction between rifampin and moxifloxacin in which the moxifloxacin AUC is reduced by 27%, this topic clearly merits further study (14).
Acknowledgments
We thank the patients and staff of the Tuberculosis Clinic and Clinical Research Center of the Hospital Universitário Cassiano Antônio de Moraes and the Núcleo de Doenças Infecciosas (NDI) of the UFES for their assistance with the study. Waleska Ribeiro Meireles, Karina de Souza Fiorotti, Plinio Meira Wetter, and Ana Paula da Silva provided outstanding nursing care during the inpatient phase of the study.
This work was supported by contracts NO1-AI95383 and NO1-AI-70022 (Tuberculosis Prevention and Control Research Unit) of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD.
Footnotes
Published ahead of print on 10 December 2007.
REFERENCES
- 1.American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America. 2003. Treatment of tuberculosis. Am. J. Respir. Crit. Care Med. 167:603-662. [DOI] [PubMed] [Google Scholar]
- 2.Burman, W. J., S. Goldberg, J. L. Johnson, G. Muzanye, M. Engle, A. W. Mosher, S. Choudhri, C. L. Daley, S. S. Munsiff, Z. Zhao, A. Vernon, and R. E. Chaisson. 2006. Moxifloxacin versus ethambutol in the first 2 months of treatment for pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 174:331-338. [DOI] [PubMed] [Google Scholar]
- 3.Bustad, A., D. Terziivanov, R. Leary, R. Port, A. Schumitzky, and R. Jelliffe. 2006. Parametric and nonparametric population methods: their comparative performance in analysing a clinical dataset and two Monte Carlo simulation studies. Clin. Pharmacokinet. 45:365-383. [DOI] [PubMed] [Google Scholar]
- 3a.Cockroft, D. W., and M. H. Gault. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31-41. [DOI] [PubMed] [Google Scholar]
- 4.Davis, S. L., M. M. Neuhauser, and P. S. McKinnon. 2005. Quinolones, p. 337-366. In V. L. Yu, G. Edwards, P. S. McKinnon, C. Peloquin, and G. D. Morse (ed.), Antimicrobial chemotherapy and vaccines, 2nd ed., vol. II. Antimicrobial agents. Esun Technologies, LLC, Pittsburgh, PA. [Google Scholar]
- 5.Dodge, W. F., R. W. Jelliffe, J. B. Zwischenberger, R. A. Bellanger, J. A. Hokanson, and W. R. Snodgrass. 1994. Population pharmacokinetic models: effect of explicit versus assumed constant serum concentration assay error patterns upon parameter values of gentamicin in infants on and off extracorporeal membrane oxygenation. Ther. Drug Monit. 16:552-559. [PubMed] [Google Scholar]
- 6.Gabrielsson, J., and D. Weiner. 2001. Pharmacokinetic and pharmacodynamic data analysis, concepts and applications, 3rd ed. Swedish Pharmaceutical Press, Stockholm, Sweden.
- 7.Gumbo, T., A. Louie, M. R. Deziel, L. M. Parsons, M. Salfinger, and G. L. Drusano. 2004. Selection of a moxifloxacin dose that suppresses drug resistance in Mycobacterium tuberculosis, by use of an in vitro pharmacodynamic infection model and mathematical modeling. J. Infect. Dis. 190:1642-1651. [Erratum, 190:2059.] [DOI] [PubMed] [Google Scholar]
- 8.Gumbo, T., A. Louie, M. R. Deziel, and G. L. Drusano. 2005. Pharmacodynamic evidence that ciprofloxacin failure against tuberculosis is not due to poor microbial kill but to rapid emergence of resistance. Antimicrob. Agents Chemother. 49:3178-3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson, J. L., D. J. Hadad, W. H. Boom, C. L. Daley, C. A. Peloquin, K. D. Eisenach, D. D. Jankus, S. M. Debanne, E. D. Charlebois, E. Maciel, M. Palaci, and R. Dietze. 2006. Early and extended early bactericidal activity of levofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int. J. Tuber. Lung Dis. 10:605-612. [PubMed] [Google Scholar]
- 10.Nuermberger, E., and J. Grosset. 2004. Pharmacokinetic and pharmacodynamic issues in the treatment of mycobacterial infections. Eur. J. Clin. Microbiol. Infect. Dis. 23:243-255. [DOI] [PubMed] [Google Scholar]
- 11.Peloquin, C. A. 2002. Therapeutic drug monitoring in the treatment of tuberculosis. Drugs 62:2169-2183. [DOI] [PubMed] [Google Scholar]
- 12.Sanders, C. A., R. R. Nieda, and E. P. Desmond. 2004. Validation of the use of Middlebrook 7H10 agar, BACTEC MGIT 960, and BACTEC 460 12B media for testing the susceptibility of Mycobacterium tuberculosis to levofloxacin. J. Clin. Microbiol. 42:5225-5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shandil, R. K., R. Jayaram, P. Kaur, S. Gaonkar, B. L. Suresh, B. N. Mahesh, R. Jayashree, V. Nandi, S. Bharath, and V. Balasubramanian. 2007. Moxifloxacin, ofloxacin, sparfloxacin, and ciprofloxacin against Mycobacterium tuberculosis: evaluation of in vitro and pharmacodynamic indices that best predict in vivo efficacy. Antimicrob Agents Chemother. 51:576-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiner, M., W. Burman, C. C. Luo, C. A. Peloquin, M. Engle, S. Goldberg, V. Agarwal, and A. Vernon. 2007. Effects of rifampin and multidrug resistance gene polymorphism on concentrations of moxifloxacin. Antimicrob Agents Chemother. 51:2861-2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yew, W. W., C. K. Chan, C. C. Leung, C. H. Chau, C. M. Tam, P. C. Wong, and J. Lee. 2003. Comparative roles of levofloxacin and ofloxacin in the treatment of multidrug-resistant tuberculosis: preliminary results of a retrospective study from Hong Kong. Chest 124:1476-1481. [DOI] [PubMed] [Google Scholar]
- 16.Zhu, M., J. J. Stambaugh, S. E. Berning, A. E. Bulpitt, E. S. Hollender, M. Narita, D. Ashkin, and C. A. Peloquin. 2002. Ofloxacin population pharmacokinetics in patients with tuberculosis. Int. J. Tuber. Lung Dis. 6:503-509. [DOI] [PubMed] [Google Scholar]