Abstract
Tubulin is essential to eukaryotic cells and is targeted by several antineoplastics, herbicides, and antimicrobials. We demonstrate that Acanthamoeba spp. are resistant to five antimicrotubule compounds, unlike any other eukaryote studied so far. Resistance correlates with critical amino acid differences within the inhibitor binding sites of the tubulin heterodimers.
Tubulin is an essential structural element of the cytoskeleton of eukaryotic cells, where it plays a central role in chromosomal segregation, organelle movement, and cellular motility (7, 21). Tubulin has been exploited as a target for antineoplastics (8, 25), herbicides (18), and antihelminthic (9, 23), antifungal (14), and antiprotozoal (27, 28, 29) compounds. In addition, colchicine has been used for the treatment of gout in humans (2). Despite the highly conserved nature of α-tubulin and β-tubulin across the phyla, organisms present diverse degrees of susceptibility and resistance to the different groups of antimicrotubule agents. The success of benzimidazoles and dinitroanilines is due to their selectivity for helminths and plants, respectively, and their low toxicity in mammals (6, 23). However, even within these broad classifications of organisms there are many important differences. Some protozoans, including apicomplexans, are susceptible to dinitroanilines (e.g., Toxoplasma gondii, with a 50% inhibitory concentration [IC50] of 0.3 μM [19]), while others, such as Trypanosoma cruzi (IC50 of 17.6 μM), are resistant (26, 27). Similarly, there is considerable variation in susceptibility of protozoans to paclitaxel, as exemplified by Leishmania spp. (IC50 of 35 nM) (10) and T. gondii (IC50 of 1 μM) (4). A few protozoa, such as Giardia lamblia, are susceptible to benzimidazoles, a class of drug normally used to treat helminth infections (14). Studies have demonstrated that amino acid differences that influence tertiary structure or alter inhibitor-docking regions are responsible for determining resistance to antitubulins. For example, site-directed mutagenesis in the oryzalin-docking site on α-tubulin in T. gondii and Eleusine indica has been successful in altering the phenotype to oryzalin resistant (6, 19, 24).
Using the previously described alamar blue assay (17), we demonstrated that the two species of Acanthamoeba most commonly reported as causing Acanthamoeba keratitis in humans (15, 16, 20), A. castellanii and A. polyphaga, are resistant to five classes of tubulin inhibitor represented by oryzalin, paclitaxel, vinblastine, albendazole, and colchicine (Table 1).
TABLE 1.
Relative IC50s of Acanthamoeba species and rabbit corneal cells (RCE) to antitubulin compoundsa
| Compound | IC50
|
||
|---|---|---|---|
| A. polyphaga | A. castellanii | RCE | |
| Oryzalin | >100 μM | >100 μM | >500 μM |
| Paclitaxel | >10 μM | >10 μM | 0.04-0.08 μM |
| Vinblastine | 0.68-1.375 μM | 0.68-1.375 μM | 17 nM |
| Albendazole | >47 μM | >47 μM | 0.7-1.469 μM |
| Colchicine | 2.5-5 mM | 2.5 mM | 2.4 μM |
Both A. castellanii and A. polyphaga were susceptible to chlorhexidine (IC50s were 1.5625 to 3.125 μM and 3.125 to 6.25 μM, respectively).
To explore the potential basis for these observations, both α- and β-tubulin genes were cloned and sequenced from A. castellanii (neff strain) and A. polyphaga (strain 1501/18) (GenBank accession numbers DQ099493, DQ099491, DQ0994494, and DQ099492). The sequence identity on the amino acid level between the two species is 67% for α-tubulin and 99% for β-tubulin (see the table in the supplemental material). By using previously solved tubulin structures and their known inhibitor binding sites, it has been possible to model the tubulins from both species of Acanthamoeba and predicted inhibitor interactions.
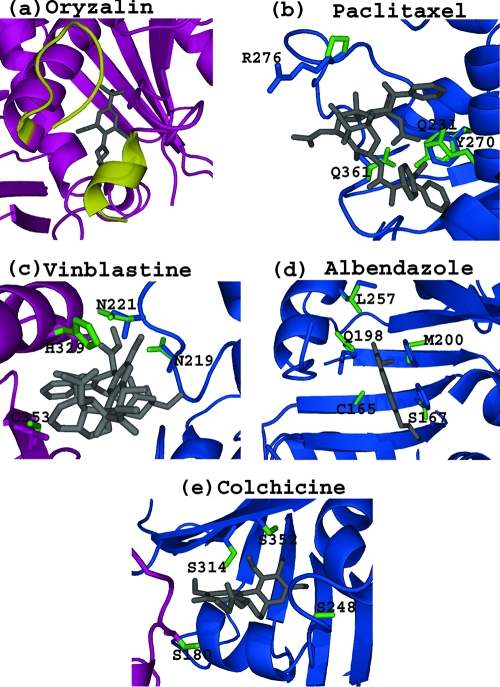
Structure-based mutagenesis studies of T. gondii α-tubulin have suggested that oryzalin binds in a pocket formed by 13 residues (19), of which 8 are identical in the Acanthamoeba family. Two of the residues which display sequence variation (Ile42 and Asp47) lie within the N loop, implicated in inhibitor binding (Fig. 1; also see the figure in the supplemental material), which is also shorter than other α-tubulin homologues by 2 residues, contributing to the loss of potency. Val4Ile (i.e., valine at position 4 in the oryzalin-sensitive T. gondii α-tubulin is replaced by an isoleucine at position 4 in Acanthamoeba α-tubulin), Phe24Tyr, and Cys65Ala replacements are predicted to have a more subtle effect on the inhibitor pocket shape.
FIG. 1.
Structural representation of the predicted Acanthamoeba tubulin inhibitor binding pocket for the five tubulin inhibitors oryzalin, paclitaxel, vinblastine, albendazole, and colchicine. For all panels, α- and β-tubulin are colored magenta and blue, respectively, and each inhibitor is colored gray. The residues that bind the inhibitor are represented in a stick format, with those that are divergent within the Acanthamoeba family colored green and labeled. In panel a, the N-loop of α-tubulin, which plays a role in forming close interactions with oryzalin, is shown in yellow. All structures are based on the B. taurus tubulin structure and were produced using the graphics program PyMOL (3).
Paclitaxel binds to the β-tubulin subunit (13), and the structure of the mammalian (Bos taurus) β-tubulin/paclitaxel complex reveals that 22 residues form the inhibitor binding pocket; 7 of these residues show sequence variation relative to the Acanthamoeba proteins (Ala231Gln, Phe270Tyr, Ser275Ala, Arg276Pro, Gln279Thr, Arg359Ala, and Leu361Gln replacements). Significantly, Ala231, which is in the heart of the inhibitor binding pocket, is replaced by Gln, producing a severe steric clash to the inhibitor (Fig. 1). An additional steric clash may be formed by the replacement of Leu361 with Gln, with Phe270Tyr and Arg276Pro (Table 2) changing the packing interactions to the inhibitor. The remaining changes are solvent exposed and predicted to make little difference to inhibitor binding.
TABLE 2.
Key amino acid residue changes in inhibitor binding sites between susceptible organisms and resistant Acanthamoeba spp.
| Compound | Organism | Phenotype | Residue in:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α-Tubulin | β-Tubulin | |||||||||||||
| Oryzalin | E. indica | Susceptible | Ile42 | Asp47 | Val4 | Phe24 | Cys65 | |||||||
| Acanthamoeba | Resistant | Ile4 | Tyr24 | Ala65 | ||||||||||
| Paclitaxel | B. taurus | Susceptible | Ala231 | Phe270 | Ser275 | Arg276 | Gln279 | Arg359 | Leu361 | |||||
| Acanthamoeba | Resistant | Gln231 | Tyr270 | Ala275 | Pro276 | Thr279 | Ala359 | Gln361 | ||||||
| Vinblastine | Homo sapiens | Susceptible | Val353 | Asn329 | Ile355 | Pro325 | Phe351 | Thr219 | Thr221 | |||||
| Acanthamoeba | Resistant | Cys353 | His329 (c)a | Val355 (c) | Thr325 (c) | Pro351 (c) | Asn219 | Asn221 | ||||||
| Ser329 (p) | ||||||||||||||
| Albendazole | A. nidulans | Susceptible | Ala165 | Phe167 | Glu198 | Phe200 | ||||||||
| Acanthamoeba | Resistant | Cys165 | Ser167 | Gln198 | Met200 | |||||||||
| Colchicine | Homo sapiens | Susceptible | Val313 | Ala314 | Ile316 | |||||||||
| Acanthamoeba | Resistant | Ala313 | Ser314 | Val316 | ||||||||||
c, A. castellanii; p, A. polyphaga.
Vinblastine binds at the interface between α- and β-tubulin subunits (5). Of the 23 residues that have been implicated in inhibitor binding, 4 show sequence variation relative to mammalian tubulin within A. castellanii and A. polyphaga proteins: Val353Cys and Asn329His (for A. castellanii) or Asn329Ser (for A. polyphaga) in α-tubulin and Thr219Asn and Thr221Asn in β-tubulin (22) (Table 2). The Thr221Asn replacement in β-tubulin may result in a steric clash with the inhibitor. In addition, Asn329 in α-tubulin makes close interactions with the inhibitor and its replacement by His (in A. castellanii) may result in a steric clash whereas its replacement by Ser (in A. polyphaga) results in a loss of the packing interactions. For A. castellanii, there are three additional changes not found in A. polyphaga, which can affect inhibitor binding (Ile355Val, Phe351Pro, and Pro325Thr replacements) (Fig. 1).
Of the 13 residues which form the putative albendazole inhibitor binding site (12), 4 show sequence variation in the Acanthamoeba family (Table 2). Albendazole resistance is conferred when Phe167 and Phe200 are replaced by serine and methionine, respectively. The latter replacement is also present in Leishmania spp. (1, 11). The replacement of Ala165 in susceptible helminth tubulin with a cysteine in Giardia duodenalis and Encephalitozoon cuniculi has been shown to confer resistance to several members of the benzimidazole family when mutated to a larger residue (11).
Analysis of Acanthamoeba β-tubulin has shown that the key mammalian colchicine-sensitive residues Val313, Ala314, Ala315, and Ile316 (Table 2) are replaced in Acanthamoeba by Ala, Ser, Ala, and Val, respectively, as they are present in colchicine-resistant Leishmania spp. (28, 29). In addition, there is a significant change in the environment of the hydrophobic colchicine binding pocket due to the replacement of 4 Ala residues with bulkier Ser residues, which increases the percentage of hydrophilic residues from approximately 30% to 55%.
To verify that the sequence divergence of Acanthamoeba tubulin is responsible for the resistance of Acanthamoeba tubulin to all five compounds tested, future work should involve biochemical and structural analyses of Acanthamoeba tubulins. An important consideration is that resistance to these tubulin inhibitors may not be based upon changes in the inhibitor binding site alone; other factors, such as drug metabolism, compartmentalization, or efflux, must also be potential factors. The work presented here demonstrates that the Acanthamoeba α- and β-tubulins are both unusually divergent from tubulins of other organisms and offers plausible evidence for the unusual behavior of Acanthamoeba species in the presence of tubulin polymerizing and depolymerizing inhibitors.
Supplementary Material
Acknowledgments
The William Ross Foundation and the University of Strathclyde Research and Development Fund funded this work.
Footnotes
Published ahead of print on 10 December 2007.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Armson, A., S. W. Kamau, F. Grimm, J. A. Reynoldson, W. M. Best, L. M. MacDonald, and R. C. Thompson. 1999. A comparison of the effects of a benzimidazole and the dintroanilines against Leishmania infantum. Acta Trop. 73:303-311. [DOI] [PubMed] [Google Scholar]
- 2.Cronstein, B. N., and R. Terkeltaub. 2006. The inflammatory process of gout and its treatment. Arthritis Res. Ther. 8(Suppl. 1):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delano, W. L. 2002. The PyMOL molecular graphics system. Delano Scientific LLC, San Carlos, CA. http://www.pymol.org.
- 4.Estes, R., N. Vogel, D. Mack, and R. McLeod. 1998. Paclitaxel arrests growth of intracellular Toxoplasma gondii. Antimicrob. Agents Chemother. 42:2036-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gigant, B., C. Wang, R. B. Ravelli, F. Roussi, M. O. Steinmetz, P. A. Curmi, A. Sobel, and M. Knossow. 2005. Structural basis for the regulation of tubulin by vinblastine. Nature 435:519-522. [DOI] [PubMed] [Google Scholar]
- 6.Hugdahl, J. D., and L. C. Morejohn. 1993. Rapid and reversible high-affinity binding of the dintroaniline herbicide oryzalin to tubulin from Zea mays L. Plant Physiol. 102:725-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hyams, J. S., and C. W. Lloyd. 1993. Microtubules, p. 1-439. In J. B. Harford (ed.), Modern cell biology, vol. 13. Wiley-Liss, New York, NY. [Google Scholar]
- 8.Johnson, I. S., J. G. Armstrong, M. Gorman, and J. P. Burnett, Jr. 1963. The vinca alkaloids: a new class of oncolytic agents. Cancer Res. 23:1390-1427. [PubMed] [Google Scholar]
- 9.Jung, H., M. Hurtado, M. Sanchez, M. T. Medina, and J. Sotelo. 1992. Clinical pharmacokinetics of albendazole in patients with brain cysticercosis. J. Clin. Pharmacol. 32:28-31. [DOI] [PubMed] [Google Scholar]
- 10.Kapoor, P., M. Sachdeva, and R. Madhubala. 1999. Effect of the microtubule stabilising agent taxol on leishmanial protozoan parasites in vitro. FEMS Microbiol. Lett. 176:429-435. [DOI] [PubMed] [Google Scholar]
- 11.Katiyar, S. K., V. R. Gordon, G. L. Mclauglin, and T. D. Edlind. 1994. Antiprotozoal activities of benzimidazoles and correlations with beta-tubulin sequence. Antimicrob. Agents Chemother. 38:2086-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwa, M. S., J. G. Veenstra, and M. H. Roos. 1994. Benzimidazole resistance in Haemonchus contortus is correlated with a conserved mutation at amino acid 200 in beta-tubulin isotype 1. Mol. Biochem. Parasitol. 63:299-303. [DOI] [PubMed] [Google Scholar]
- 13.Lowe, J., H. Li, K. H. Downing, and E. Nogales. 2001. Refined structure of ab-tubulin at 3.5 Å resolution. J. Mol. Biol. 313:1045-1057. [DOI] [PubMed] [Google Scholar]
- 14.MacDonald, L. M., A. Armson, A. R. Thompson, and J. A. Reynoldson. 2004. Characterisation of benzimidazole binding with recombinant tubulin from Giardia duodenalis, Encephalitozoon intestinalis, and Cryptosporidium parvum. Mol. Biochem. Parasitol. 138:89-96. [DOI] [PubMed] [Google Scholar]
- 15.Marciano-Cabral, F., and G. Cabral. 2003. Acanthamoeba spp. as agents of disease in humans. Clin. Microbiol. Rev. 16:273-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez, A. J., and G. S. Visvesvara. 1997. Free-living, amphizoic and opportunistic amoebas. Brain Pathol. 7:583-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McBride, J., P. R. Ingram, F. L. Henriquez, and C. W. Roberts. 2005. Development of colorimetric microtiter plate assay for assessment of antimicrobials against Acanthamoeba. J. Clin. Microbiol. 43:629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morejohn, L. C., and D. E. Fosket. 1991. The biochemistry of compounds with anti-microtubule activity in plant cells. Pharmacol. Ther. 51:217-230. [DOI] [PubMed] [Google Scholar]
- 19.Morrissette, N. S., A. Mitra, D. Sept, and L. D. Sibley. 2004. Dinitroanilines bind alpha-tubulin to disrupt microtubules. Mol. Biol. Cell 15:1960-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niederkorn, J. Y., H. Alizadeh, H. Leher, and J. P. McCulley. 1999. The pathogenesis of Acanthamoeba keratitis. Microbes Infect. 1:437-443. [DOI] [PubMed] [Google Scholar]
- 21.Nogales, E., S. G. Wolf, and K. H. Downing. 1998. Structure of the alpha beta tubulin dimer by electron crystallography. Nature 391:199-203. [DOI] [PubMed] [Google Scholar]
- 22.Rai, S. S., and J. Wolff. 1996. Localization of the vinblastine-binding site on beta-tubulin. J. Biol. Chem. 271:14707-14711. [DOI] [PubMed] [Google Scholar]
- 23.Rossignol, J. F., and H. Maisonneuve. 1984. Albendazole: a new concept in the control of intestinal helminthiasis. Gastroenterol. Clin. Biol. 8:569-576. [PubMed] [Google Scholar]
- 24.Roy, D., and A. Lohia. 2004. Sequence divergence of Entamoeba histolytica tubulin is responsible for its altered tertiary structure. Biochem. Biophys. Res. Commun. 319:1010-1016. [DOI] [PubMed] [Google Scholar]
- 25.Schiff, P. B., J. Fant, and S. B. Horwitz. 1979. Promotion of microtubule assembly in vitro by taxol. Nature 277:665-667. [DOI] [PubMed] [Google Scholar]
- 26.Stokkermans, T. J., J. D. Schwartzman, K. Keenan, N. S. Morrissette, L. G. Tilney, and D. S. Roos. 1996. Inhibition of Toxoplasma gondii replication by dinitroaniline herbicides. Exp. Parasitol. 84:355-370. [DOI] [PubMed] [Google Scholar]
- 27.Traub-Cseko, Y. M., J. M. Ramalho-Ortigao, A. P. Dantas, S. L. de Castro, H. S. Barbosa, and K. H. Downing. 2001. Dinitroaniline herbicides against protozoan parasites: the case of Trypanosoma cruzi. Trends Parasitol. 17:136-141. [DOI] [PubMed] [Google Scholar]
- 28.Werbovetz, K. A. 2002. Tubulin as an antiprotozoal drug target. Mini Rev. Med. Chem. 2:519-529. [DOI] [PubMed] [Google Scholar]
- 29.Werbovetz, K. A., J. J. Brendle, and D. L. Sackett. 1999. Purification, characterization, and drug susceptibility of tubulin from Leishmania. Mol. Biochem. Parasitol. 98:53-65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.