Abstract
In the absence of a fully effective herpes simplex virus (HSV) vaccine, topical microbicides represent an important strategy for preventing HSV transmission. (−)-Epigallocatechin gallate (EGCG) (molecular weight, 458.4) is the primary catechin in green tea. The present study shows that EGCG has greater anti-HSV activity than other green tea catechins and inactivates multiple clinical isolates of HSV type 1 (HSV-1) and HSV-2. EGCG reduced HSV-2 titers by ≥1,000-fold in 10 to 20 min and reduced HSV-1 titers by the same amount in 30 to 40 min. The anti-HSV activity of EGCG is due to a direct effect on the virion, and incubating Vero and CV1 cells with EGCG for 48 h prior to infection with HSV-1 and HSV-2, respectively, does not reduce HSV production. Electron microscopic (EM) studies showed that purified virions exposed to EGCG were damaged, and EM immunogold labeling of the envelope glycoproteins gB and gD was significantly reduced following EGCG treatment while capsid protein labeling was unchanged. When purified HSV-1 envelope glycoproteins gB and gD were incubated with EGCG and then examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, lower-molecular-weight gB and gD bands decreased and new higher-molecular-weight bands appeared, indicating the EGCG-dependent production of macromolecular complexes. gB and gD are essential for HSV infectivity, and these results suggest that EGCG could inactivate HSV virions by binding to gB, gD, or another envelope glycoprotein. EGCG is stable in the pH range found in the vagina and appears to be a promising candidate for use in a microbicide to reduce HSV transmission.
The prevalence of herpes simplex virus type 2 (HSV-2) infections has increased by 30% since the late 1970s, and there are at least 500,000 new genital herpes infections each year in the United States (13). HSV is the leading cause of genital ulcers in the developed parts of the world (2). In the United States alone, 40 to 60 million people are infected with HSV-2 (1). In some cities in North America and Europe, HSV-1, which is usually orally located, causes more than half of the new cases of genital herpes, particularly in women (26). There is a synergistic link between human immunodeficiency virus (HIV) and HSV (5, 28, 48, 50). A recent study (28) showed that HSV-suppressive therapy greatly reduced genital and plasma HIV type 1 RNA levels in women infected with HSV and HIV. Infection with HSV-2 was estimated to have accounted for more than 30% of the new HIV infections in four African cities (9). These studies also showed that HSV-2 infection strongly increased the likelihood of HIV transmission. This is not unexpected because the HIV viral load has been shown to be the chief predictor of risk for heterosexual HIV transmission (35). By reducing the spread of genital herpes, it may be possible to greatly decrease an individual's risk of acquiring or transmitting HIV.
The major determinants of effective immunity against HSV infection have not been identified (42). Animal efficacy has not predicted success in humans (21, and therapeutic vaccines which induce antibody-specific responses alone have failed to protect recipients from recurrences (42). In the absence of a licensed HSV vaccine (21), topical microbicides represent an important potential strategy for preventing the sexual spread of HSV (28). Since viral sexually transmitted infections such as HSV can become persistent rapidly, a microbicide must target HSV prior to the initial infection of susceptible cells in the reproductive tract. A topical microbicide could also be used to reduce mother-to-child (vertical) transmission of HSV since the virus is usually transmitted perinatally as the neonate is exposed to HSV during passage through an infected birth canal (21) and potentially will also reduce vertical HIV transmission in the birth canal (47).
Green, black, and oolong teas are produced from the tea plant Camellia sinensis by different manufacturing processes (8, 27). As a consequence of these differences, only green tea maintains its original composition of polyphenols (27). Polyphenolic compounds in green tea include flavanols, flavandiols, flavanoids, and phenolic acids and account for 30% of the dry weight of green tea leaves (27). Most of the polyphenols in green tea are flavanols commonly known as catechins. These mainly consist of four compounds, (−)-epicatechin (EC), (−)-epigallocatechin (EGC), EC gallate (ECG), and EGC gallate (EGCG), that can be present at concentrations of up to 1 mg/ml in a cup of tea (19). The concentration of EGCG and other catechins in green tea can vary slightly, depending upon the variety of tea plant and the harvesting season (8). EGCG is not found in other plants and is the major catechin in tea (14). EGCG (molecular weight, 458.4) is the largest of the simple isoflavanoids found in tea but is still a small molecule and, along with the other tea polyphenols, appears to lack toxicity following human consumption (14). In fact, tea, and therefore EGCG, is on the FDA's list of compounds generally recognized as safe and approved for human consumption (34).
EGCG binds to a wide variety of proteins, especially to nonglobular extended proteins and particularly to proteins with a high proline content (10, 11, 18, 24, 31, 33, 37, 44). EGCG inhibits the binding of HIV envelope glycoprotein gp120 to the CD4 receptor (15, 20, 52) and inhibits influenza virus replication by specific interaction with the hemagglutinin envelope glycoprotein and potentially altering the physical properties of the viral envelope (29, 40). HSV encodes 11 or 12 glycoproteins, and there is a large body of evidence that 4 of these glycoproteins, gD, gB, and gH/gL, are required for virion entry into cells (4). gD is the glycoprotein that interacts with cell surface receptors, but all four glycoproteins are required to mediate virus-cell fusion (43). If EGCG interacts with any of these glycoproteins, it could inhibit their function in the fusion process, thereby inactivating the HSV virion. A number of proteins shown to reduce HSV infectivity, including cytokines and retrocyclins, have been shown to strongly bind gB (30, 53).
Due to the limited bioavailability of EGCG following oral consumption (3), studies were undertaken to directly test EGCG and other purified green tea catechins as topical agents against HSV. The present studies establish that EGCG inactivates multiple clinical isolates of HSV-1 and HSV-2. Inactivation is rapid and occurs in a concentration range that can easily be delivered to the female reproductive tract, where EGCG would be able to directly inactivate extracellular HSV virions in the vaginal lumen, thereby helping to prevent the initial infection of HSV-susceptible cells.
MATERIALS AND METHODS
Cells, viruses, and HSV proteins.
HSV-1 (strain F1) was obtained from R. Rubenstein (New York State Institute For Basic Research), and HSV-2 (strain 333) was obtained from Mary K. Howett (Penn State University). HSV-1 and HSV-2 were grown in Vero and CV-1 cells, respectively. Cells were obtained from ATCC (Manassas, VA). Cells were grown in RPMI 1640 medium (26) with 10% fetal calf serum (FCS). The maintenance medium for Vero and CV-1 cells was as described above but with 1% FCS. Clinical isolates of HSV-1 and HSV-2 were obtained from Jeanne Jordan at the Magee-Womens Hospital (Pittsburgh, PA) by using an institutional review board-approved protocol. In response to a physician order, independent of this protocol, swabs were collected from patients and put in M-4 medium and samples not needed for HSV diagnosis were frozen at −20°C and shipped to our laboratory coded but without identifiers, as outlined in our institutional review board protocol. Purified HSV-1 glycoproteins gB (730) (Nwe C#8) and gD-1 (306) (C#226) were provided by Gary H. Cohen and Roselyn J. Eisenberg (University of Pennsylvania).
Treatment of cells with EGCG.
Vero and CV-1 cell monolayers were grown in 96-well plates (Falcon) and incubated with 100 μM EGCG in cell culture medium for the indicated periods of time. Cells were then washed with medium without EGCG, and Vero and CV1 cells were infected with HSV-1 and HSV-2, respectively. After 48 h, the titer of virus produced was determined as described below.
HSV-1 and HSV-2 titration.
HSV-1 and HSV-2 were incubated with the appropriate catechin at the indicated concentration for 1 h in RPMI 1640 medium with 1% FCS at pH 7.4. Addition of catechins to the medium did not change the pH. HSV-1 was titrated by inoculation of 10-fold dilutions into Vero cell cultures in 96-well microtiter tissue culture plates (Falcon). HSV-2 was titrated by using CV1 cells. A virus dilution (0.1 ml) in maintenance medium was inoculated into each well with three wells per dilution (45). Each experiment was performed in triplicate, and each datum point presented is the mean ± the standard deviation of three separate experiments. The plates were incubated for 5 days at 37°C and examined daily for a cytopathic effect. Virus titers were calculated by the method of Reed and Muench (36).
Electron microscopy (EM) of HSV-infected cells.
HSV-1 (strain F1) was allowed to adsorb to Vero cells for 1 h at a multiplicity of infection (MOI) of 10 in RPMI 1640 medium with 1% FCS. The medium was then removed, and the cells were washed two times with RPMI 1640 medium. Medium with 1% FCS was then added to control cells, medium with 1% FCS and 100 μM EGCG was added to treated cells, and control and EGCG-treated cells were incubated at 37° for 24 h. They were then placed in microcentrifuge vials and spun at 1,000 rpm in an Eppendorf microcentrifuge for 5 min to obtain cell pellets. The pellets were first rinsed in 0.1 M phosphate-buffered saline (PBS) (pH 7.4) for 30 min and then fixed in a 4% solution of glutaraldehyde in 0.1 M PBS (pH 7.4) for 60 min as previously described (49). Briefly, cells were rinsed again in 0.1 M PBS (pH 7.4 for 10 min), postfixed with 1% osmium tetroxide for 90 min, rinsed with PBS for 10 min, dehydrated twice with graded (50, 70, 85, 95, and 100%) ethanol for 10 min in each step, infiltrated with propylene oxide and plastic Spurr medium resin (41), and finally embedded in BEEM capsules and polymerized in a 70°C oven overnight. Ultrathin sections were obtained by using a Sorvall MT-2 or MT-5000 ultramicrotome with a diamond knife. The ultrathin sections obtained were placed on uncoated 200-mesh copper grids, stained with saturated uranyl acetate in 50% ethanol for 5 min, rinsed with 0.22-μm Millipore-filtered distilled water for at least 50 s to obtain a clean section without any residue of uranyl acetate and then stained with Reynold's lead citrate for 5 min and rinsed again with Millipore-filtered distilled water for 50 s. They were then air dried, examined with a Hitachi 7500 transmission electron microscope operated at 80 kV, and photographed with an Advanced Microscopy Techniques digital camera system.
Virus purification.
Vero cells were grown in RPMI 1640 medium (Cellgro) supplemented with 1% FCS (46). The monolayer of Vero cells was infected with HSV-1 at an MOI of 10. After 1 h of adsorption, the cells were washed and then incubated for 24 h at 37°C in 5% CO2. The cells were frozen at −80°C and thawed at 4°C. The suspensions were clarified by centrifugation at 1,000 rpm for 25 min and then by centrifugation at 3,000 rpm for 10 min. The virus was pelleted from the supernatant by centrifugation at 12,500 rpm for 2 h in a Beckman SS34 rotor. The pellet was suspended in TNE buffer (50 mM Tris-HCl [pH 7.4], 100 mM NaCl, 1 mM EDTA) and laid onto a three-step discontinuous sucrose gradient consisting of 2 ml each of 50, 40, and 30% sucrose in TNE buffer. After a 2-h centrifugation at 22,000 rpm in a Beckman SW 41 rotor, virus at the interphase between the 40 and 50% sucrose layers was aspirated, diluted 1:4 with PBS, and pelleted in a Beckman SW 50.2 rotor for 1 h at 35,000 rpm. The virus pellet was suspended in PBS, left untreated or treated with EGCG (100 μM) for 2 h at 37°C, and then spun in a Beckman TLA 100.4 rotor for 2 h at 20,000 rpm. The virus pellet was fixed in a 4% glutaraldehyde buffer.
EM and immunogold labeling of purified virus.
EGCG-treated and control HSV-1 virions were fixed with 1% glutaraldehyde and 1% paraformaldehyde in 0.1 M PBS (pH 7.4) for 60 min and washed with 0.1 M PBS (pH 7.4). The samples were dehydrated through 70% ethanol and then embedded into LR White embedding medium directly from 70% ethanol. Osmium tetroxide was not used in order to preserve antigenicity. Sections were cut with either a Sorvall MT-2 or MT-5000 ultramicrotome and collected on nickel grids. Grids were immersed in 1% bovine serum albumin (BSA)-0.1 M PBS-NaN3 for 30 min and then placed into a blocking solution of 20% normal goat serum. Photographs were taken of purified control and EGCG-treated virions. Primary monoclonal antibodies (1 mg/ml) against gB (clone 1105; EastcoastBio, Inc., North Berwick, ME), gD (clone 1103; EastcoastBio, Inc., North Berwick, ME), and the 155-kDa capsid protein (Biodesign International, Saco, ME) were incubated overnight at a dilution of 1:15 and then rinsed in PBS. Treatment with a 1:20 dilution of colloidal gold with a particle diameter of 15 nm (Amersham Life Science, NJ) in PBS was done for 60 min. Following the colloidal gold incubation, the grids were washed with 30 drops per grid of 1% BSA-PBS-NaN3, followed by an immersion rinse in the same solution. The final wash was done with distilled H2O and followed the same routine as the previous wash. The grids were stained with 1% aqueous uranyl acetate.
SDS-PAGE of EGCG-treated proteins.
A mixture of EGCG (250 μg/ml) and BSA, gB, or gD (500 to 700 μg/ml) in a 0.1 M K2HPO4-KH2PO4 buffer (pH 7.0) was incubated at 35°C in a water bath for 24 h, and the products were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (22). Ten microliters of each product was mixed with an equal volume of sample buffer and denatured at 50°C for 15 min. The samples were separated on 6.5% polyacrylamide gel and visualized with Coomassie brilliant blue R250.
RESULTS
Inactivation of HSV by catechins.
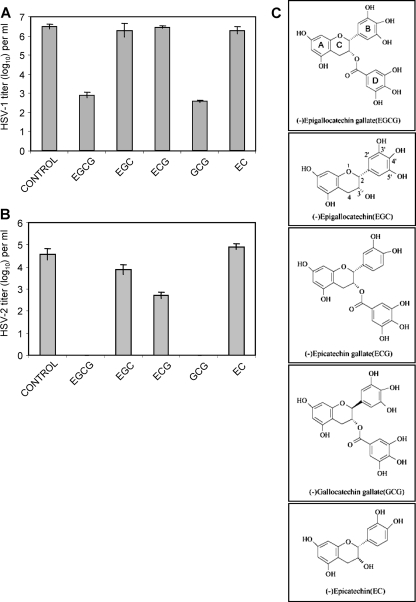
The results in Fig. 1 show that EGCG and its epimer GCG inactivate HSV-1 titers by 3.5 log10 (3,000-fold) and HSV-2 titers by 4.0 log10 (10,000-fold). ECG inactivated HSV-2 by ≥1 log10, while EGC and EC showed little or no effect against HSV-2. ECG, EGC, and EC were all ineffective against HSV-1. Therefore, only EGCG and GCG were effective against both HSV-1 and HSV-2. These studies indicated that the gallolyl moiety (ring D) (Fig. 1C) is essential for significant anti-HSV activity since EC and EGC were ineffective against HSV-1 and EGC showed minimal activity against HSV-2. While ECG and EGCG both have the gallate moiety and only differ by the absence of one hydroxyl group in the B ring of ECG, EGCG has much greater antiviral activity. The importance of both the gallate moiety and the presence of three hydroxyl groups on the B ring has also been found for the antibacterial effects of these catechins (54). While EGCG and GCG have comparable anti-HSV activities, EGCG is more readily available than GCG, which is produced by epimerization of EGCG, and therefore EGCG was utilized in subsequent experiments.
FIG. 1.
Inactivation of HSV virions by green tea catechins. Each catechin was used at a concentration of 100 μM and incubated with virus from clarified cell supernatant for 1 h at 37°C prior to titration with Vero cells for HSV-1 (strain F1) and CV1 cells for HSV-2 (strain 333). (A) HSV-1. (B) HSV-2. (C) Structures of EGCG, EGC, ECG, GCG, and EC. Titers are the mean ± standard deviation of three separate experiments.
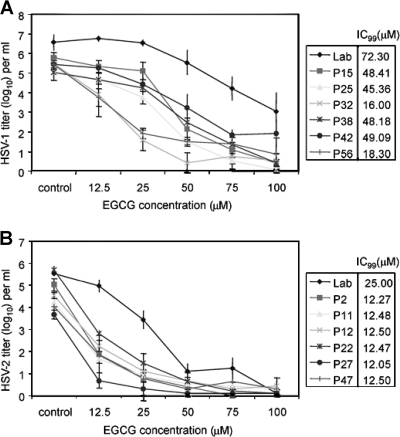
Determination of the antiviral concentration range of EGCG against clinical isolates of HSV-1 and HSV-2.
Various concentrations of EGCG were tested against laboratory strains and clinical isolates of HSV-1 and HSV-2. Clinical isolates of HSV-2 showed greater sensitivity to EGCG than the laboratory isolate (Fig. 2B). At a concentration of 12.5 μM, all of the HSV-2 clinical isolates showed a titer reduction of ≥2 log10 (100-fold) corresponding to an approximate 99% inhibitory concentration (IC99) of 12.5 μM, while the IC99 for the laboratory isolate was 25 μM. When 50 μM EGCG was used, all of the HSV-2 clinical isolates were almost completely inactivated and the laboratory isolate titer was reduced by ≥4 log10. All HSV-2 isolates were inactivated by EGCG, and the clinical isolates appeared to be more sensitive on a concentration basis than the laboratory strain. This was not solely due to the lower titers of HSV-2 clinical isolates, since the zero time point titers of clinical isolate p2 (105.03) and the laboratory strain (105.52) were comparable and yet at an EGCG concentration of 12.5 μg/ml the p2 titer decreased by 3.15 log10 while the laboratory strain titer dropped by 0.56 log10. These intrinsic isolate differences in susceptibility to EGCG inactivation at a low concentration disappeared at higher EGCG concentrations.
FIG. 2.
EGCG inactivates clinical isolates of HSV-1 (A) and HSV-2 (B). Clinical isolates were prepared as clarified cell supernatants. HSV-1 isolates were grown and their titers were determined in Vero cells, and HSV-2 isolates were grown and their titers were determined in CV-1 cells. Titers are the mean ± standard deviation of three separate experiments. The IC99s of EGCG, i.e., the concentrations that reduced viral titers by 99% from the zero concentration titer, were calculated graphically.
HSV-1 isolates (Fig. 2A) were more resistant to EGCG inactivation than HSV-2 isolates were. The HSV-1 titers were usually higher than those of HSV-2, so that this may be a viral titer effect, as well as due to any inherent difference in sensitivity to EGCG between HSV-1 and HSV-2. However, all of the HSV-1 clinical isolates were inactivated by 2 log10 to 5 log10 at 50 μM EGCG with IC99s ranging from 16 to 49 μM and were almost completely inactivated at 100 μM EGCG. As with HSV-2, the laboratory strain of HSV-1 (IC99, 72.3 μM) was the most resistant to EGCG inactivation but an EGCG concentration of 100 μM reduced the laboratory strain titer by ≥3 log10.
HSV-1 and HSV-2 were routinely grown and their titers were determined in Vero and CV-1 cells, respectively. To determine whether the cell type affected viral sensitivity, the laboratory strain of HSV-2 was grown and titers were determined in both Vero and CV-1 cells and compared to those of the laboratory strain of HSV-1, which was grown and whose titer was determined in Vero cells (Table 1). The initial titers of HSV-2 grown in Vero and CV-1 cells were 104.75 and 105.52, respectively. Following viral treatment with 100 μM EGCG, the titer of HSV-2 grown in both Vero and CV-1 cells was decreased by ≅4 to 5 log10. HSV-1, which had an initial titer of 106.54, showed a titer reduction of ≅2 log10. The results obtained with HSV-2, which was grown and whose titers were determined in Vero cells are therefore comparable to those obtained with CV-1 cells (Fig. 2B and 3B).
TABLE 1.
Effect of cell type on the susceptibility of HSV-2 to EGCG inactivationa
| Virus | Cell type used
|
TCID50b
|
Titer decrease | ||
|---|---|---|---|---|---|
| Growth | Titration | Control | EGCG | ||
| HSV-2 | Vero | Vero | 104.75 ± 0.22 | 100.38 ± 0.00 | 104.37 |
| HSV-2 | CV-1 | CV-1 | 105.52 ± 0.09 | 100.10 ± 0.17 | 105.42 |
| HSV-1 | Vero | Vero | 106.54 ± 0.07 | 104.50 ± 0.13 | 102.04 |
Treated virus was incubated with 100 μM EGCG for 60 min. Laboratory isolates of HSV-1 and HSV-2 were used. Values are means ± standard deviations of three separate experiments.
TCID50, 50% tissue culture infective dose.
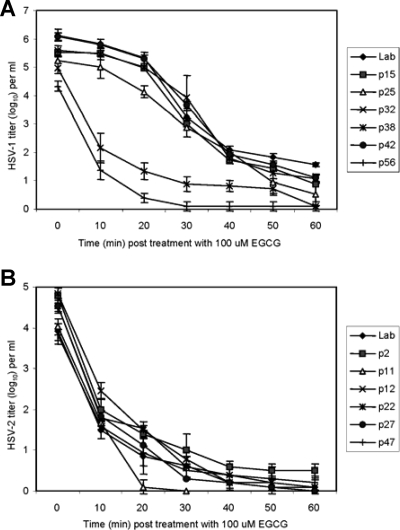
FIG. 3.
Times required for EGCG to inactivate HSV-1 (A) and HSV-2 (B) clinical isolates. Isolates were prepared and titers were determined as described in the legend to Fig. 2. Titers are the mean ± standard deviation of three separate experiments.
Time course of EGCG-dependent HSV inactivation.
Since it is essential to inactivate HSV as rapidly as possible to prevent infection of susceptible cells, studies were done with 100 μM EGCG to determine how rapidly HSV-1 and HSV-2 isolates could be inactivated (Fig. 3). The titers of all of the clinical HSV-2 isolates and the laboratory isolate were decreased by at least 2 log10 within 10 min and by 3 log10 within 20 min. Inactivation of HSV-1 isolates, which had higher titers than HSV-2 isolates, was more varied. Two of the isolates (p32 and p56) showed the same inactivation profile as the HSV-2 isolates, whereas the other HSV-1 isolates (p15, p25, p38, p42) needed 40 to 60 min of EGCG exposure to be decreased to the same low titers as HSV-2 after 20 min of EGCG exposure. It should be noted, however, that the infectivity of high-titer HSV-1 isolates was reduced by 2 to 3 log10 within 30 min by EGCG. The p32 and p56 HSV-1 isolates, whose titer decreases paralleled those of HSV-2 isolates, were the two lowest-titer HSV-1 isolates.
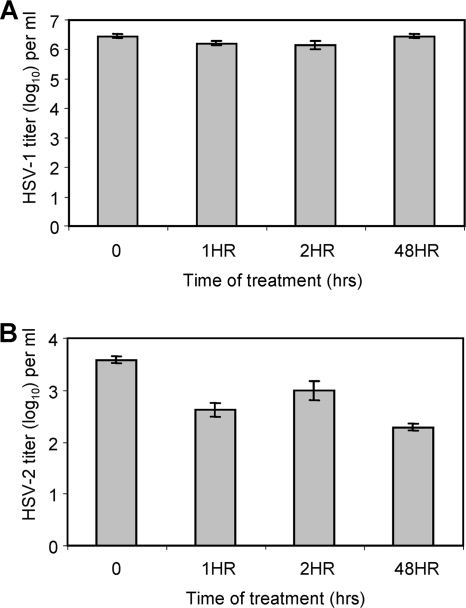
Treatment of HSV target cells with EGCG.
The results in Fig. 1 to 3 show that EGCG directly inactivates HSV particles. However, it has been previously established that EGCG reduces HIV replication by binding to the CD4 receptor and preventing HIV binding but that EGCG does not directly inactivate HIV virions (20). The results in Fig. 4A indicate that treatment of Vero cells for periods ranging from 1 to 48 h, followed by removal of EGCG from the cell medium, prior to HSV-1 exposure did not reduce the amount of infectious HSV-1 produced by the Vero cells. Treatment of CV1 cells with EGCG prior to infection with HSV-2 (Fig. 4B) had only a minimal effect on the virus titer produced, even though a 100 μM EGCG concentration reduced the titer of the laboratory isolate of HSV-2 by ≥3 log10 in 20 min when the cells were incubated with virions (Fig. 3). These results show that treating Vero and CV1 cells with EGCG for up to 48 h, washing out the EGCG, and then adding HSV-1 or HSV-2 does not prevent viral replication. Also, EGCG does not reduce the viability of Vero and CV1 cells (results not presented), since following a 24- or 48-h exposure to 100 μM EGCG, Vero and CV1 cell viability remained between 80 and 97%.
FIG. 4.
Treatment of Vero cells (A) and CV-1 cells (B) with EGCG (100 μM). Cells were treated with EGCG for the indicated periods, EGCG was removed, and then Vero cells were infected with HSV-1 (strain F1) and CV-1 cells were infected with HSV-2 (strain 333). HSV production was determined after 2 days as described in Materials and Methods.
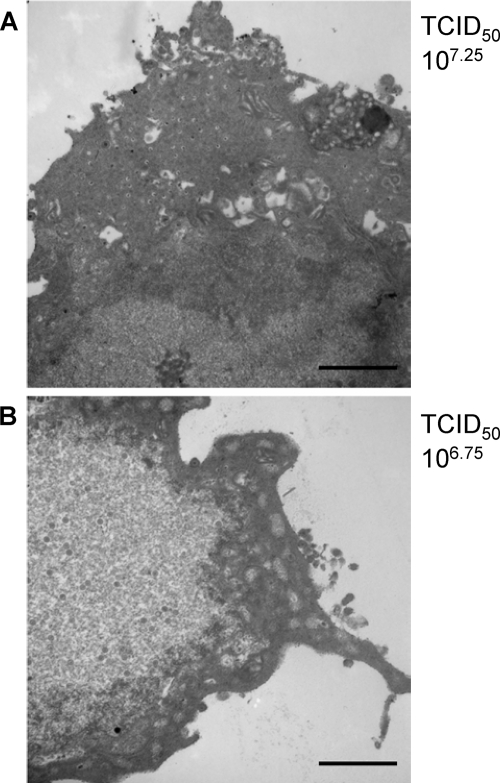
EM studies were also done to examine the effect of the presence of EGCG over a 24-h period on the production of HSV-1 in Vero cells previously infected with the virus (Fig. 5). Both control (Fig. 5A) and EGCG (100 μM)-treated (Fig. 5B) cells produced infectious virus. Control cells produced a 50% tissue culture infective dose of 107.25, and the EGCG-treated cells yielded a titer of 106.75. The presence of EGCG following HSV-1 adsorption and entry into Vero cells did not prevent virus production.
FIG. 5.
The presence of EGCG during HSV-1 replication does not inhibit virus replication. Vero cells were infected with HSV-1 (MOI, 10) (strain F1), and 1 h later EGCG (100 μM) was added to the medium of treated cells. Cells were processed as described in Materials and Methods, and the cells were examined by transmission EM after a 24-h incubation. Scale bars on the electron micrographs are 1,160 nm. (A) Control. (B) EGCG treated. TCID50, 50% tissue culture infective dose.
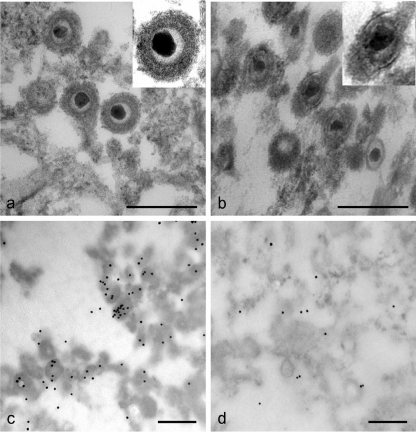
EGCG exposure damages HSV-1 virions.
EM studies were done with HSV-1 purified in sucrose gradients (Fig. 6). Figure 6A shows intact untreated HSV-1 particles, while Fig. 6B shows examples of virus particle damage found following EGCG treatment (100 μM) of purified HSV-1. The envelopes of EGCG-treated particles are damaged in the presence of EGCG. Further evidence for a direct effect of EGCG on HSV-1 particles can be seen in Fig. 6C (control) and D (EGCG treated), which show immunogold labeling of virus particles with a monoclonal antibody against the gD envelope glycoprotein. The electron micrographs show far more labeling of gD in control virus particles than with the EGCG-treated particles, providing further evidence for a direct effect of EGCG on the virion. Similar results were obtained when antibody was used against the gB envelope glycoprotein (micrographs not shown).
FIG. 6.
EGCG damages HSV-1 virions. (a) Purified virus (strain F1) was incubated in tissue culture medium for 2 h at 37°C and then prepared for EM (see Materials and Methods). (b) Purified virus was incubated with EGCG (100 μM) for 2 h and then prepared for EM. (c) Purified virions were incubated with monoclonal antibody specific for the gD envelope glycoprotein (clone 1103, 67 μg/ml) and labeled with immunogold (see Materials and Methods). (d) Purified virions were incubated with EGCG (100 μM) for 2 h and treated as for panel C. Scale bars are 240 nm. The inserts in the upper right corners of panels a and b show representative virions magnified ×1.6.
Counts were done of the immunogold labeling of control and EGCG-treated HSV-1 virions (Table 2) with antibodies against gB, gD, and the 155-kDa capsid protein. Glycoproteins gB and gD showed the same distribution of labeled and unlabeled virus in the control samples with approximately 70% of the virus particles labeled. Virus samples treated with EGCG (100 μM) showed approximately 30% and 40% drops in gB and gD labeling, respectively, indicating that following EGCG treatment these HSV-1 virion envelope glycoproteins had a reduced ability to bind the monoclonal antibodies. Antibody binding to the capsid protein (Table 2), however, was not diminished by EGCG treatment. This is in agreement with the results in Fig. 6B, which show that the viral capsid is clearly present following EGCG exposure. EGCG reduces HSV infectivity (Fig. 2 and 3) by ≥3 log10, which is an even greater effect than is indicated by the decreased immunogold labeling. This suggests that many virus particles which appear to be undamaged visually and on the basis of immunogold labeling are inactivated by EGCG.
TABLE 2.
Quantitation of immunogold labeling of envelope and capsid proteins in HSV-1 particles in the presence or absence of EGCGa
| Proteinb | % Labeled
|
P value | |
|---|---|---|---|
| Control | EGCG treated | ||
| gB | 72.0 ± 4.11 | 43.4 ± 6.45 | 1 × 10−9 |
| gD | 71.5 ± 3.63 | 33.5 ± 2.46 | 1 × 10−9 |
| 155-kDa nucleocapsid | 63.7 ± 4.11 | 61.1 ± 8.68 | 0.40c |
Ten separate fields and 100 virus particles in each field were counted. Values are means ± standard deviations. A 100 μM EGCG concentration was used.
gB and gD were labeled with monoclonal antibodies.
No statistically significant difference.
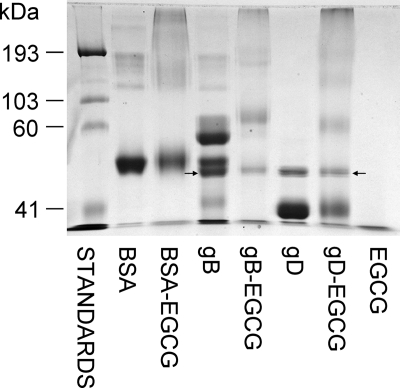
Production of macromolecular complexes with gB and gD in the presence of EGCG.
EGCG has been shown to bind BSA and produce macromolecular complexes (31) (Fig. 7). The results in Fig. 7 show that EGCG also produces molecular weight shifts with purified HSV-1 gB and gD. Two of the three bands in the gB control lane are almost gone following incubation with EGCG, and higher-molecular-weight bands have appeared in the gB-EGCG lane. When gD was incubated with EGCG (Fig. 7), the ≅41-kDa band seen in the gD-only lane was greatly decreased and new higher-molecular-weight bands appeared. When BSA and gB were incubated with EGC, which does not inactivate HSV (Fig. 1), macromolecular complexes were not found (results not shown).
FIG. 7.
Envelope glycoproteins gB and gD form macromolecular complexes in the presence of EGCG. EGCG (545 μM) was incubated with recombinant purified gB or gD or with purified BSA for 24 h and then analyzed by SDS-PAGE, followed by Coomassie blue staining. All of the bands in lanes with gB and gD (with or without EGCG) were analyzed by tandem mass spectrometry (Mass Spectrometry Facility, New York State Institute for Basic Research). Arrows indicate the presence of endochitinase either alone in the gD lanes or mixed with gB.
There is no protein staining in the EGCG-only lane, and the higher-molecular-weight bands that appeared following EGCG incubation with gB and gD are likely complexes of each glycoprotein. The new band seen at approximately 60 kDa following EGCG incubation with gD may be a dimer of the ≅41-kDa band in the gD control lane. The other new gD bands are at 150 and 200 kDa, which are also approximate multiples of the gD control band at 41 kDa. Similar molecular weight changes are seen when gB is incubated with EGCG where the new EGCG-dependent protein bands are approximately molecular weight multiples of the bands in the gB control lane. The molecular weights of the control gB bands were unchanged following the 24-h incubation whether or not protease inhibitors were used (results not shown). This indicates that the two higher-molecular-weight primary bands in the gB control lane are not breakdown products produced during incubation. As indicated in Fig. 7 (arrows), the lower-molecular-weight band is endochitinase, some of which remains in the EGCG-treated gB lane. However, both of the primary gB bands in the control lane are gone following EGCG treatment and higher-molecular-weight bands are found. Following exposure to EGCG, both gB and gD show protein staining at the top of the gel at the boundary of the stacking gel which is not observed in the control lanes.
DISCUSSION
The present studies establish that EGCG inactivates multiple clinical isolates of HSV-1 and HSV-2. HSV-2 isolates can be inactivated by ≥1,000-fold in 10 to 20 min and HSV-1 isolates can be inactivated in 30 to 40 min. The greater sensitivity of HSV-2 to inactivation by EGCG appears to be due, in large part, to the lower titers of this virus. It has been shown for both HSV (28) and HIV (35) that the viral load in genital secretions is the chief predictor of viral transmission. In the case of HSV transmission, primary infection is associated with the excretion of 106 to 108 PFU, whereas recurrent infection with previously established HSV is associated with the excretion of 102 PFU of virus (51). As a result of this difference in virus production, it has been found that the incidence of vertical (mother-to-child) herpesvirus transmission is at least 10-fold greater during a primary infection than during a secondary infection. This suggests that if a microbicidal agent such as EGCG reduces the concentration of HSV in cervicovaginal fluid, the incidence of viral transmission will be reduced.
Topical application, such as in a vaginal microbicide, may provide the ideal environment in which to utilize the antiviral activity of EGCG. EGCG has been shown to inhibit carcinogenesis induced by a wide variety of carcinogens in rodent cancer models (39), but the oral bioavailability of EGCG is low in rodents and humans, resulting in concentrations in plasma that are 5 to 50 times less than the concentration shown to exert biological activity in in vitro systems (5, 7). Due to the limited bioavailability of EGCG following oral consumption, it is often unclear which EGCG-dependent in vitro effects are relevant in vivo. The intestine appears to be a major factor in limiting the bioavailability of EGCG due to poor absorption and extensive metabolism. As part of a topical microbicide, EGCG will remain in the female reproductive tract and function in the vaginal lumen and at the mucosal surface.
EGCG is stable under acidic conditions but degrades more rapidly at pH levels above 6.5 (55). The vaginal pH is normally in the range of 3.8 to 4.5 but can vary to a more neutral range as a result of vaginal infection with Trichomonas vaginalis and other pathogens or during menses (17). However, the vaginal pH, even with an infection, is still usually below 6.0 and EGCG is stable at this pH so that 90 to 95% of the EGCG applied remains after 18 h (23, 38, 55). It is therefore possible to deliver EGCG to the vaginal lumen at concentrations that we have shown in this study to inactivate HSV, and other in vitro studies have shown it to reduce HIV replication (20) and reduce carcinogenesis (39). Introducing EGCG in a buffered gel or film will also maintain an acidic pH in the presence of semen.
HSV enters cells by fusion with plasma or endocytic membranes through a process carried out by the sequential activity of envelope glycoproteins, including gB, gD, and gH/gL (12, 16, 25, 43). Both gB and gD are essential for HSV entry into cells (16, 25) since gD needs to interact with a cognate receptor prior to virus-to-cell fusion, and recent studies suggest that gB is required for content mixing and full virus-cell fusion (43). A number of studies provide evidence that compounds with anti-HSV activity function by binding to gB from either HSV-1 or HSV-2. Among 22 human cytokines examined, MIP-1α/CCL3, MIP-1β/CCL4, and RANTES/CCL5 had anti-HSV-1 activity and were shown to strongly bind to HSV-1 virions via envelope gB (30) and generate pores in the HSV-1 envelope which often led to the disintegration of virus particles. The θ defensin retrocyclin 2, which protects cells from infection by inhibiting viral adhesion and entry, was shown by surface plasmon resonance studies to strongly bind to gB from HSV-2 (53). Also, a number of sulfated or sulfonated polysaccharides which inhibit the cell-to-cell spread of HSV bind to gB (6). It is therefore possible that EGCG binding to gB has a similar antiviral effect on HSV infectivity. EGCG is potentially a lower-molecular-weight and inexpensive alternative to polyanions, chemokines, and defensins for reducing HSV transmission.
The formation of higher-molecular-weight complexes following EGCG incubation with gB and gD does not prove that EGCG interaction with gB or gD is solely responsible for HSV inactivation. It is also possible that EGCG binding to other HSV glycoproteins, for example, gH/gL, may be necessary to inactivate HSV (43). However, the ability of EGCG to produce aggregation of any HSV glycoproteins on the virion envelope or possibly intracellularly, as was found with purified gB and gD on SDS-polyacrylamide gels (Fig. 7), could prevent virion fusion with target cell membranes. The abilities of EGCG to form water-soluble complexes with BSA (21), gB, or gD and bind to a large number of proteins which are involved in the control of biological function (10, 11, 18, 20, 24, 31, 33, 37, 44, 52) suggest that there may be a structural component that is well conserved among proteins of markedly different genetic sequences (32) and is recognized by EGCG.
The results presented in Fig. 2 and 6 and Table 2 show that far more virions are inactivated by EGCG than indicated visually. This suggests that many HSV particles which appear intact are no longer infectious following EGCG treatment. Determining whether these morphologically undamaged particles are inactivated as a result of EGCG interaction with an envelope glycoprotein, e.g., gB, causing loss of glycoproteins, denaturation of glycoproteins, or possibly some other change that is not readily observable by EM requires further studies.
EGCG can undergo oxidation and dimerization in solution, and it is possible that its oxidative reaction products could disrupt the HSV envelope (38). This is unlikely, however, since a direct membrane-disruptive effect would also directly inactivate HIV virions and it has been established that EGCG does not inactivate HIV virions but prevents receptor binding (20, 52). If inhibition of both HSV and HIV by EGCG were due to degradative products of EGCG, e.g., the dimer theasinensin A, then allowing EGCG to sit in pH 7.4 phosphate buffer for 2 h prior to use should increase its anti-HSV activity (38, 55). We have found, though, that the longer EGCG sits in a buffer at pH 7.4, the greater the loss of anti-HSV activity (results not shown), whereas at pH ≤6.0, where EGCG is stable, anti-HSV activity is diminished minimally or not at all.
EGCG is stable at vaginal pH and appears to be a promising candidate for use in a topical microbicide to reduce the transmission of HSV and potentially disrupt the synergistic link between HSV and HIV. Studies are under way to determine whether EGCG formulations can be developed which will deliver concentrations of this catechin sufficient to inactivate HSV in the vaginal lumen.
Acknowledgments
We thank Gary H. Cohen and Roselyn J. Eisenberg for providing purified HSV-1 gB and gD. We also thank David C. Bolton for performing mass spectrometry analysis of gB and gD bands following SDS-PAGE, Richard A. Kascsak for helpful suggestions during the preparation of the manuscript, Jeanne Jordan for providing HSV clinical isolates, and Anna Parese for helping to prepare the manuscript.
This work was supported by Public Health Service grant AI 51611-05 from the National Institute of Allergy and Infectious Diseases and by the New York State Office of Mental Retardation and Developmental Disabilities.
Footnotes
Published ahead of print on 14 January 2008.
REFERENCES
- 1.Aurelian, L. 2004. Minireview. Herpes simplex virus type 2 vaccines: new ground for optimism? Clin. Diagn. Lab. Immunol. 11:437-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barton, S. 2005. The role of anti-HSV therapeutics in the HIV-infected host and in controlling the HIV epidemic. Herpes 12:15-22. [PubMed] [Google Scholar]
- 3.Cai, Y., N. D. Anavy, and H.-H. S. Chow. 2002. Contribution of presystemic hepatic extraction to the low oral bioavailability of green tea catechins in rats. Drug Metab. Dispos. 30:1246-1249. [DOI] [PubMed] [Google Scholar]
- 4.Campadelli-Fiume, G., M. Amasio, E. Avitabile, A. Cerretani, C. Forghieri, T. Gianni, and L. Menotti. 2007. The multipartite system that mediates entry of herpes simplex virus into the cell. Rev. Med. Virol. 17:313-326. [DOI] [PubMed] [Google Scholar]
- 5.Chen, L., M.-J. Lee, H. Li, and C. S. Yang. 1997. Absorption distribution and elimination of tea polyphenols in rats. Drug Metab. Dispos. 25:1045-1050. [PubMed] [Google Scholar]
- 6.Cheshenko, N., M. J. Keller, V. MasCasullo, G. A. Jarvis, H. Cheng, M. John, J.-H. Li, K. Hogarty, R. A. Anderson, D. P. Waller, L. J. D. Zaneveld, A. T. Profy, M. E. Klotman, and B. C. Herold. 2004. Candidate topical microbicides bind herpes simplex virus glycoprotein B and prevent viral entry and cell-to-cell spread. Antimicrob. Agents Chemother. 48:2025-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow, H.-H. S., I. A. Hakim, D. R. Vining, J. A. Crowell, J. Ranger-Moore, W. M. Chew, C. A. Celaya, S. R. Rodney, Y. Hara, and D. S. Alberts. 2005. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of polyphenon E in healthy individuals. Clin. Cancer Res. 11:4627-4633. [DOI] [PubMed] [Google Scholar]
- 8.Chu, D.-C., and L. R. Juneja. 1997. General chemical composition of green tea and its infusion, p. 13-22. In T. Yamamoto, L. R. Juneja, D.-C. Chu, and M. Kim (ed.), Chemistry and applications of green tea. CRC Press, New York, NY.
- 9.Cohen, J. 2005. Prevention cocktails: combining tools to stop HIV's spread. Science 309:1002-1005. [DOI] [PubMed] [Google Scholar]
- 10.Ermakova, S., B. Y. Choi, H. S. Choi, B. S. Kang, A. M. Bode, and Z. Dong. 2005. The intermediate filament protein vimentin is a new target for epigallocatechin gallate. J. Biol. Chem. 29:16882-16890. [DOI] [PubMed] [Google Scholar]
- 11.Fang, M. Z., Y. Wang, N. Ai, Z. Hou, Y. Sun, H. Lu, W. Welsh, and C. S. Yang. 2003. Tea polyphenol (−)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 67:7563-7570. [PubMed] [Google Scholar]
- 12.Farnsworth, A., T. W. Wisner, M. Webb, R. Roller, G. Cohen, R. Eisenberg, and D. C. Johnson. 2007. Herpes simplex virus glycoproteins gB and gH function in fusion between the virion envelope and the outer nuclear membrane. Proc. Natl. Acad. Sci. USA 204:10187-10192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleming, D. G., G. M. McQuillan, R. E. Johnson, A. J. Nahmias, S. O. Aral, F. K. Lee, and M. E. St. Louis. 1997. Herpes simplex virus type 2 in the United States, 1976 to 1994. N. Engl. J. Med. 337:1105-1111. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton-Miller, J. M. T. 1995. Antimicrobial properties of tea (Camellia sinensis L.). Antimicrob. Agents Chemother. 39:2375-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamza, A., and C.-G. Zhan. 2006. How can (−)-epigallocatechin gallate from green tea prevent HIV-1 infection? Mechanistic insights from computational modeling and the implication for rational design of anti-HIV-1 entry inhibitors. J. Phys. Chem. B 110:2910-2917. [DOI] [PubMed] [Google Scholar]
- 16.Heldwein, E. E., H. Lou, F. C. Bender, G. H. Cohen, R. J. Eisenberg, and S. C. Harrison. 2006. Crystal structure of glycoprotein B from herpes simplex virus 1. Science 313:217-220. [DOI] [PubMed] [Google Scholar]
- 17.Hillier, S., and K. K. Holmes. 1999. Bacterial vaginosis, p. 563-586. In K. K. Holmes, et al. (ed.), Sexually transmitted diseases, third edition. McGraw-Hill, New York, NY.
- 18.Jöbstl, E., J. R. Howse, J. P. A. Fairclough, and M. P. Williamson. 2006. Noncovalent cross-linking of casein by epigallocatechin gallate characterized by single molecule force microscopy. J. Agric. Food Chem. 54:4077-4081. [DOI] [PubMed] [Google Scholar]
- 19.Kanaka, S., M. Kim, M. Taniguchi, and T. Yamamoto. 1989. Antibacterial substances in Japanese green tea extract against streptococcus mutans, a cariogenic bacterium. Agric. Biol. Chem. 53:2307-2311. [Google Scholar]
- 20.Kawai, K., N. H. Tsuno, J. Kitayama, Y. Okaji, K. Yazawa, M. Asakage, N. Hori, T. Watanabe, K. Takahashi, and H. Nagawa. 2003. Epigallocatechin gallate, the main component of tea polyphenol, binds to CD4 and interferes with gp120 binding. J. Allergy Clin. Immunol. 112:951-957. [DOI] [PubMed] [Google Scholar]
- 21.Koelle, D. M. 2006. Vaccines for herpes simplex virus infections. Curr. Opin. Investig. Drugs 7:136-141. [PubMed] [Google Scholar]
- 22.Kusuda, M., T. Hatano, and T. Yoshida. 2006. Water-soluble complexes formed by natural polyphenols and bovine serum albumin: evidence from gel electrophoresis. Biosci. Biotechnol. Biochem. 70:152-160. [DOI] [PubMed] [Google Scholar]
- 23.Lambert, J. D., D. H. Kim, R. Zheng, and C. S. Yang. 2006. Transdermal delivery of (−)-epigallocatechin-3-gallate, a green tea polyphenol, in mice. J. Pharm. Pharmacol. 58:599-604. [DOI] [PubMed] [Google Scholar]
- 24.Li, C., A. Allen, J. Kwagh, N. M. Doliba, W. Qin, H. Najafi, H. W. Collins, F. M. Matschinsky, C. A. Stanley, and T. J. Smith. 2006. Green tea polyphenols modulate insulin secretion by inhibiting glutamate dehydrogenase. J. Biol. Chem. 281:10214-10221. [DOI] [PubMed] [Google Scholar]
- 25.Manoj, S., C. R. Jogger, D. Myscofski, M. Yoon, and P. G. Spear. 2004. Mutations in herpes simplex virus glycoprotein D that prevent cell entry via nectins and alter cell tropism. Proc. Natl. Acad. Sci. USA 101:12414-12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mindel, A. 1998. Genital herpes—how much of a public-health problem? Lancet 351:16-18. [DOI] [PubMed] [Google Scholar]
- 27.Mukhtar, H., and N. Ahmad. 2000. Tea polyphenols: prevention of cancer and optimizing health. Am. J. Clin. Nutr. 71:1698S-1702S. [DOI] [PubMed] [Google Scholar]
- 28.Nagot, N., A. Ouédraogo, V. Foulongne, I. Konaté, H. A. Weiss, L. Vergne, M.-C. Defer, Sanon A. Djagbaré, J.-B. Andonaba, P. Becquart, M. Segondy, R. Vallo, A. Sawadogo, P. Van de Perre, and P. Mayaud. 2007. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N. Engl. J. Med. 356:790-799. [DOI] [PubMed] [Google Scholar]
- 29.Nakayama, M., K. Suzuki, M. Toda, S. Okubo, Y. Hara, and T. Shimamura. 1993. Inhibition of the infectivity of influenza virus by tea polyphenols. Antivir. Res. 21:289-299. [DOI] [PubMed] [Google Scholar]
- 30.Nakayama, T., J. Shirane, K. Hieshima, M. Shibano, M. Watanabe, Z. Jin, D. Nagakubo, T. Saito, Y. Shimomura, and O. Yoshie. 2006. Novel antiviral activity of chemokines. Virology 350:484-492. [DOI] [PubMed] [Google Scholar]
- 31.Nam, S., D. M. Smith, and Q. P. Dou. 2001. Ester bond-containing tea polyphenols potently inhibit proteasome activity in vitro and in vivo. J. Biol. Chem. 276:13322-13330. [DOI] [PubMed] [Google Scholar]
- 32.Overington, J. P., B. Al-Lazikani, and A. L. Hopkins. 2006. How many drug targets are there? Nature 5:993-996. [DOI] [PubMed] [Google Scholar]
- 33.Palermo, C. M., C. A. Westlake, and T. A. Gasiewicz. 2005. Epigallocatechin gallate inhibits aryl hydrocarbon receptor gene transcription through an indirect mechanism involving binding to a 90 kDa heat shock protein. Biochemistry 44:5041-5052. [DOI] [PubMed] [Google Scholar]
- 34.Paterson, I., and E. A. Anderson. 2005. The renaissance of natural products as drug candidates. Science 310:451-453. [DOI] [PubMed] [Google Scholar]
- 35.Quinn, T. C., M. J. Wawer, N. Sewankambo, D. Serwadda, C. Li, F. Wabwire-Mangen, M. O. Meehan, T. Lutalo, and R. H. Gray. 2000. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N. Engl. J. Med. 342:921-929. [DOI] [PubMed] [Google Scholar]
- 36.Reed, L. J., and M. Muench. 1938. A simple method of estimating 50 per cent end points. Am. J. Hyg. 27:493-497. [Google Scholar]
- 37.Sah, J. F., S. Balasubramanian, R. L. Eckert, and E. A. Rorke. 2004. Epigallocatechin-3-gallate inhibits epidermal growth factor receptor signaling pathway. J. Biol. Chem. 279:12755-12762. [DOI] [PubMed] [Google Scholar]
- 38.Sang, S., M.-J. Lee, C.-T. Ho, and C. S. Yang. 2005. Stability of tea polyphenol (−)-epigallocatechin-3-gallate and formation of dimers and epimers under common experimental conditions. J. Agric. Food Chem. 53:9478-9484. [DOI] [PubMed] [Google Scholar]
- 39.Shimizu, M., A. Deguchi, J. T. E. Lim, H. Moriwaki, L. Kopelovich, and I. B. Weinstein. 2005. (−)-Epigallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clin. Cancer Res. 11:2735-2746. [DOI] [PubMed] [Google Scholar]
- 40.Song, J.-M., K.-H. Lee, and B.-L. Seong. 2005. Antiviral effect of catechins in green tea on influenza virus. Antivir. Res. 68:66-74. [DOI] [PubMed] [Google Scholar]
- 41.Spurr, A. R. 1969. A low viscosity epoxy resin and embedding medium for electron microscopy. J. Ultrastruct. Res. 26:31-43. [DOI] [PubMed] [Google Scholar]
- 42.Stanberry, L. R. 2004. Clinical trials of prophylactic and therapeutic herpes simplex virus vaccines. Herpes 11:161A-169A. [PubMed] [Google Scholar]
- 43.Subramanian, R. P., and R. J. Geraghty. 2007. Herpes simplex virus type 1 mediates fusion through a hemifusion intermediate by sequential activity of glycoproteins D, H, L, and B. Proc. Natl. Acad. Sci. USA 104:2903-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tachibana, H., K. Koga, Y. Fujimura, and K. Yamada. 2004. A receptor for green tea polyphenol EGCG. Nat. Struct. Mol. Biol. 4:380-381. [DOI] [PubMed] [Google Scholar]
- 45.Thormar, H., C. E. Isaacs, H. R. Brown, M. R. Barshatzky, and T. Pessolano. 1987. Inactivation of enveloped viruses and killing of cells by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 31:27-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trybala, E., J.-Å. Liljeqvist, B. Svennerholm, and T. Bergström. 2000. Herpes simplex virus types 1 and 2 differ in their interaction with heparan sulfate. J. Virol. 74:9106-9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vermund, S. H. 2004. Prevention of mother-to-child transmission of HIV in Africa. Top. HIV Med. 12:130-134. [PubMed] [Google Scholar]
- 48.Wald, A. 2004. Synergistic interactions between herpes simplex virus type-2 and human immunodeficiency virus epidemics. Herpes 11:70-76. [PubMed] [Google Scholar]
- 49.Wen, G. Y., S. Y. Yang, W. Kaczmarski, X. Y. He, and K. Pappas. 2002. Presence of hydroxysteroid dehydrogenase type 10 in Hsia's APP-Sw transgenic mouse brains, but absent in amyloid plaques of Alzheimer's disease brains. Brain Res. 954:115-122. [DOI] [PubMed] [Google Scholar]
- 50.White, M. K., T. S. Gorrill, and K. Khalili. 2006. Reciprocal transactivation between HIV-1 and other human viruses. Virology 352:1-13. [DOI] [PubMed] [Google Scholar]
- 51.Whitley, R. J. 1994. Herpes simplex virus infections of women and their offspring: implications for a developed society. Proc. Natl. Acad. Sci. USA 91:2441-2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williamson, M. P., T. G. McCormick, C. L. Nance, and W. T. Shearer. 2006. Epigallocatechin gallate, the main polyphenol in green tea, binds to the T-cell receptor, CD4: potential for HIV-1 therapy. J. Allergy Clin. Immunol. 118:1369-1374. [DOI] [PubMed] [Google Scholar]
- 53.Yasin, B., W. Wang, M. Pang, N. Cheshenko, T. Hong, A. J. Waring, B. C. Herold, E. A. Wagar, and R. I. Lehrer. 2004. θ defensins protect cells from infection by herpes simplex virus by inhibiting viral adhesion and entry. J. Virol. 78:5147-5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang, Y.-M., and C. O. Rock. 2004. Evaluation of epigallocatechin gallate and related plant polyphenols as inhibitors of the FabG and FabI reductases of bacterial type II fatty-acid synthase. J. Biol. Chem. 279:30994-31001. [DOI] [PubMed] [Google Scholar]
- 55.Zhu, Q. Y., A. Zhang, D. Tsang, Y. Huang, and Z. Y. Chen. 1997. Stability of green tea catechins. J. Agric. Food Chem. 45:4624-4628. [Google Scholar]