Abstract
Serum (1→3)-β-d-glucan concentrations were serially measured in the presence and absence of antifungal therapy in a murine model of invasive pulmonary aspergillosis. Serum (1→3)-β-d-glucan was detected early during the course of infection, and reductions in this biomarker were associated with improved survival in animals treated with antifungal agents.
Early diagnosis and initiation of antifungal therapy have been demonstrated to improve outcomes in invasive aspergillosis (2, 3). Serial screening of serum for galactomannan and computed tomography have been shown to result in the earlier initiation of antifungal therapy in patients with invasive aspergillosis while avoiding unnecessary empirical antifungal use (4). However, the sensitivity of galactomannan is reduced in patients receiving antifungal agents (5). An assay for serum (1→3)-β-d-glucan is available and has been shown to be a useful diagnostic marker for invasive fungal infections (6, 7). However, there are few data for this assay regarding the diagnosis of invasive aspergillosis and its performance in the setting of drug therapy.
(This work was presented in part at the 47th Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, 17 to 20 September 2007.)
The objectives of our study were to measure the serum concentrations of (1→3)-β-d-glucan over time following pulmonary inoculation of an animal model of invasive pulmonary aspergillosis and to examine the utility of this assay as a biomarker of disease burden in the presence of antifungal therapy. To achieve these objectives, outbred ICR mice (Harlan), weighing between 18 and 22 g, were immunosuppressed by cortisone acetate and cyclophosphamide and inoculated via an inhalation chamber on day 0 with either Aspergillus fumigatus clinical isolate AF 293 or CEA 10 as previously described (8). To assess changes in serum (1→3)-β-d-glucan concentrations over time, mice were randomly chosen on days 1, 3, 5, and 7 postinoculation and euthanized, and blood samples were collected by cardiac puncture. The effects of antifungal therapy on serum (1→3)-β-d-glucan concentrations were measured in mice randomly assigned to one of four treatment regimens beginning 1 day after inoculation: (i) control, (ii) 3 mg/kg amphotericin B deoxycholate intraperitoneally once a day (QD), (iii) 40 mg/kg posaconazole orally QD, or (iv) 10 mg/kg liposomal amphotericin B intravenously QD. In the survival arm, therapy was continued until day 7 postinoculation, and animals were monitored off therapy until day 12. For fungal burden, therapy was continued until day 4. Mice were euthanized on day 5, and blood and lung samples were collected. Serum (1→3)-β-d-glucan concentrations were measured using a commercially available kit (Fungitell, Associates of Cape Cod). Five microliters of each sample was transferred in duplicate to a 96-well cell culture tray and processed according to the manufacturer's instructions [package insert, Fungitell assay for (1,3)-β-d-glucan in serum; Associates of Cape Cod, East Falmouth, MA]. The mean rate of change at an optical density of 405 nm for each sample was read over 40 min with a microplate spectrophotometer (Synergy HT; Biotek Instruments, Inc., Winooski, VT), and unknowns were interpolated from a standard curve. To measure the pulmonary fungal burden, lungs were homogenized in sterile saline and DNA was extracted using a commercially available kit (QIAamp DNA mini kit; Qiagen, Valencia, CA). Fungal DNA was measured by a real-time PCR assay using a probe and primers specific for the A. fumigatus FKS gene (GenBank accession no. U79728) and reported as conidial equivalents per gram of lung tissue (9). Each study was conducted in duplicate on separate occasions to ensure reproducibility. Survival was plotted by the Kaplan-Meier analysis, and differences in percent survival were analyzed by the chi-square test. Differences in serum (1→3)-β-d-glucan and the conidial equivalents were assessed for significance using the Kruskal-Wallis test with Dunn's test for multiple comparisons. The Spearman rank correlation test was used for correlations between serum (1→3)-β-d-glucan and the conidial equivalents.
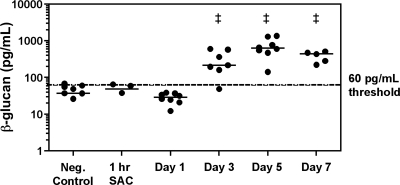
Serum (1→3)-β-d-glucan was detected early during the course of infection, with significant increases in concentrations by day 3 postinoculation (218 pg/ml, range of 49.2 to 609 pg/ml) compared to the thresholds of 60 pg/ml and 80 pg/ml set by the manufacturer as the cutoffs for negative and positive results in humans, respectively (Fungitell package insert; Associates of Cape Cod), and to the negative controls (37.6 pg/ml, range of 0 to 68.3 pg/ml, P < 0.01) (Fig. 1). Increases in this biomarker occurred 2 days prior to the first observed death in infected mice. (1→3)-β-d-Glucan concentrations remained elevated on days 5 (635 pg/ml, range of 142 to 1,375 pg/ml) and 7 (442 pg/ml, range of 225 to 510 pg/ml) postinoculation.
FIG. 1.
Changes in serum (1→3)-β-d-glucan concentrations over time in mice following pulmonary inoculation with A. fumigatus clinical isolate AF 293. ‡, P < 0.01 versus negative controls (Neg. Control) and infected mice 1 h after inoculation (1 h SAC) and on day 1 postinoculation. n ≥ 5 for each time point.
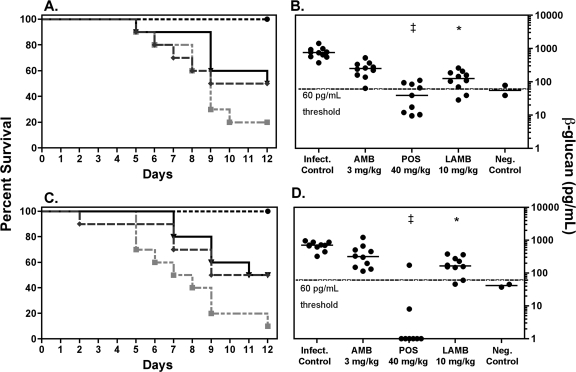
Decreases in serum (1→3)-β-d-glucan were associated with improved survival in animals treated with antifungal agents. Posaconazole resulted in 100% survival and significant reductions in serum (1→3)-β-d-glucan concentrations in mice infected with AF 293 (28.5 pg/ml) or CEA 10 (0 pg/ml) compared to controls infected with AF 293 (758 pg/ml) or CEA 10 (707 pg/ml) (P < 0.01) (Fig. 2). Although both amphotericin B formulations resulted in improved survival against both isolates (Fig. 2A and C), only liposomal amphotericin B significantly reduced serum (1→3)-β-d-glucan concentrations (Fig. 2B and D), and neither formulation resulted in a median concentration below 127 pg/ml. Posaconazole therapy also resulted in significant reductions in conidial equivalents compared to controls against both isolates (Table 1). However, only liposomal amphotericin B treatment led to a reduction in the pulmonary fungal burden and only against AF 293. Although good correlation was found between (1→3)-β-d-glucan and conidial equivalents for both isolates (AF 293, Spearman's rho, 0.81, P < 0.0001; CEA 10 Spearman's rho, 0.48, P = 0.0019), no treatment regimen completely sterilized lungs when measured by conidial equivalents. This discrepancy between (1→3)-β-d-glucan serum concentrations and residual lung tissue fungal burden may possibly reflect colonization within the upper airways following pulmonary inoculation, as previously described (1).
FIG. 2.
Survival (A and C) of and serum (1→3)-β-d-glucan concentrations (B and C) in mice treated with antifungal agents following pulmonary inoculation with A. fumigatus clinical isolate AF 293 (A and B) and CEA 10 (C and D). Treatment groups consisted of infected controls (Infect. Control) (gray squares), 3 mg/kg amphotericin B deoxycholate (AMB) intraperitoneally QD (▾), 40 mg/kg posaconazole (POS) orally QD (•), 10 mg/kg liposomal amphotericin B (LAMB) intravenously QD (♦), and uninfected controls (Neg. Control). ‡, P < 0.01 versus infected controls, AMB, and LAMB. *, P ≤ 0.01 versus infected controls. n = 10 per treatment group and infected controls.
TABLE 1.
Pulmonary fungal burdens as measured by quantitative real-time PCR and reported as conidial equivalentsa
| Treatment group | AF 293
|
CEA 10
|
||||
|---|---|---|---|---|---|---|
| Median log10 CE | Range | P value vs control | Median log10 CE | Range | P value vs control | |
| Control | 10 | 9.1-11 | 9.6 | 0-11 | ||
| POS 40 mg/kg | 3.6 | 0-8.7 | <0.01 | 7.3 | 0-8.7 | <0.01 |
| AMB 3 mg/kg | 9.6 | 8.8-11 | NS | 9.0 | 7.6-10 | NS |
| LAMB 10 mg/kg | 8.9 | 8.3-9.8 | <0.01 | 9.5 | 7.7-10 | NS |
CE, conidial equivalent; POS, posaconazole; AMB, amphotericin B deoxycholate; LAMB, liposomal amphotericin B, NS, not significant (P > 0.05).
While the results of this study are promising, there are limitations that must be considered. Although clinical studies have shown the serum (1→3)-β-d-glucan assay to be a useful diagnostic marker for invasive fungal infections with a high negative predictive value (6, 7), it is not specific for Aspergillus species, as (1→3)-β-d-glucan is a component of the cell wall of a number of pathogenic fungi. In addition, one study reported false positive reactions in patients with gram-positive or gram-negative bacteremia (7). Several substances can also result in false positives, including gauze exposed to serum, as well as cellulose filters used in patients undergoing hemodialysis (Fungitell package insert; Associates of Cape Cod). In addition, we did not evaluate the effects of echinocandins on serum (1→3)-β-d-glucan concentrations.
These data suggest the potential use of the serum (1→3)-β-d-glucan assay for the screening and early diagnosis of invasive aspergillosis. Furthermore, the results from our model also suggest that this assay may be useful for monitoring treatment efficacy. Further clinical studies are warranted to confirm our results.
Acknowledgments
This project was funded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under contract no. N01-AI-30041.
N.P.W. has received research support from Pfizer, Inc. L.K.N. has served as a consultant for Merck & Co. J.R.G. has received research support from Pfizer, Inc., Schering-Plough Corporation, Merck & Co., and Fujisawa and has served as a speaker for Merck & Co. and Schering-Plough Corporation and as a consultant for Merck & Co., Schering-Plough Corporation, Indevus, Vicuron, and Nektar Therapeutics. T.F.P. has received research support from Merck & Co., Pfizer, Inc., Schering-Plough Corporation, and Nektar Therapeutics and has served as a speaker for Merck & Co. and Pfizer, Inc., and as a consultant for Astellas Pharma US, Inc., Basilea, Merck & Co., Nektar Therapeutics, Pfizer, Inc., Schering-Plough Corporation, and Stiefel Laboratories.
Footnotes
Published ahead of print on 26 December 2007.
REFERENCES
- 1.Alvarez, C. A., N. P. Wiederhold, J. T. McConville, J. I. Peters, L. K. Najvar, J. R. Graybill, J. J. Coalson, R. L. Talbert, D. S. Burgess, R. Bocanegra, K. P. Johnston, and R. O. Williams III. 2007. Aerosolized nanostructured itraconazole as prophylaxis against invasive pulmonary aspergillosis. J. Infect. 55: 68-74. [DOI] [PubMed] [Google Scholar]
- 2.Caillot, D., O. Casasnovas, A. Bernard, J. F. Couaillier, C. Durand, B. Cuisenier, E. Solary, F. Piard, T. Petrella, A. Bonnin, G. Couillault, M. Dumas, and H. Guy. 1997. Improved management of invasive pulmonary aspergillosis in neutropenic patients using early thoracic computed tomographic scan and surgery. J. Clin. Oncol. 15:139-147. [DOI] [PubMed] [Google Scholar]
- 3.Caillot, D., L. Mannone, B. Cuisenier, and J. F. Couaillier. 2001. Role of early diagnosis and aggressive surgery in the management of invasive pulmonary aspergillosis in neutropenic patients. Clin. Microbiol. Infect. 7(Suppl. 2): 54-61. [DOI] [PubMed] [Google Scholar]
- 4.Maertens, J., K. Theunissen, G. Verhoef, J. Verschakelen, K. Lagrou, E. Verbeken, A. Wilmer, J. Verhaegen, M. Boogaerts, and J. Van Eldere. 2005. Galactomannan and computed tomography-based preemptive antifungal therapy in neutropenic patients at high risk for invasive fungal infection: a prospective feasibility study. Clin. Infect. Dis. 41:1242-1250. [DOI] [PubMed] [Google Scholar]
- 5.Marr, K. A., M. Laverdiere, A. Gugel, and W. Leisenring. 2005. Antifungal therapy decreases sensitivity of the Aspergillus galactomannan enzyme immunoassay. Clin. Infect. Dis. 40:1762-1769. [DOI] [PubMed] [Google Scholar]
- 6.Odabasi, Z., G. Mattiuzzi, E. Estey, H. Kantarjian, F. Saeki, R. J. Ridge, P. A. Ketchum, M. A. Finkelman, J. H. Rex, and L. Ostrosky-Zeichner. 2004. β-d-Glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin. Infect. Dis. 39:199-205. [DOI] [PubMed] [Google Scholar]
- 7.Pickering, J. W., H. W. Sant, C. A. P. Bowles, W. L. Roberts, and G. L. Woods. 2005. Evaluation of a (1→3)-β-d-glucan assay for diagnosis of invasive fungal infections. J. Clin. Microbiol. 43:5957-5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheppard, D. C., G. Rieg, L. Y. Chiang, S. G. Filler, J. E. Edwards, Jr., and A. S. Ibrahim. 2004. Novel inhalational murine model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 48:1908-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vallor, A. C., M. L. Herrera, D. I. McCarthy, W. R. Kirkpatrick, L. K. Najvar, R. Bocanegra, M. C. Olivo, D. D. Molina, A. W. Fothergill, B. L. Wickes, J. R. Graybill, and T. F. Patterson. 2005. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-1382.