Abstract
Rhesus macaque θ-defensins (RTDs) are unique macrocyclic antimicrobial peptides. The three RTDs (RTD 1-3), isolated from macaque leukocytes, have broad-spectrum antimicrobial activities in vitro and share certain structural features with acyclic porcine protegrins, which are microbicidal peptides of the cathelicidin family. To understand the structural features that confer the respective cytocidal properties to θ-defensins and protegrins, we determined and compared the biological properties of RTD 1-3 and protegrin 1 (PG-1) in assays for antimicrobial activity, bacterial membrane permeabilization, and toxicity to human cells. RTD 1-3 and PG-1 had similar microbicidal potencies against Escherichia coli, Staphylococcus aureus, and Candida albicans in low-ionic-strength (10 mM) buffers at pH 7.4. The inclusion of physiologic sodium chloride partially inhibited the microbicidal activities of the RTDs, and the degree of inhibition depended on the buffer used in the assay. Similarly, the inclusion of 10% normal human serum partially antagonized the bactericidal activities of all four peptides. In contrast, the microbicidal activities of PG-1 and RTD 1-3 against E. coli were unaffected by physiologic concentrations of calcium chloride and magnesium chloride. Treatment of E. coli ML35 cells with RTD 1-3 or PG-1 rapidly rendered the bacteria permeable to ο-nitrophenyl-β-d-galactopyranoside, and this was accompanied by the rapid entry of the RTDs. Finally, although PG-1 was toxic to human fibroblasts and caused a marked lysis of erythrocytes, the RTDs were not cytotoxic or hemolytic. Thus, compared to PG-1, RTD 1-3 possess substantially greater cytocidal selectivity against microbes. Surprisingly, the low cytotoxicity of the RTDs did not depend on the peptides’ cyclic conformation.
Antimicrobial peptides (AMPs) are essential components of innate immunity. Numerous AMPs have been identified in mammals; and they can generally be divided into three groups: histatins, cathelicidins, and defensins. Histatins are histidine-rich peptides found in saliva, and they exhibit antifungal properties in vitro (19). Cathelicidins are structurally diverse peptides that are derived from the carboxyl-terminal sequences of cathelin-related precursors (40, 59). Defensins are cationic, tridisulfide-containing AMPs that are produced by leukocytes and various epithelia (43). They are subdivided into the α-, β-, and θ-defensin subfamilies, which are distinguished by peptide size and different disulfide motifs (43, 47, 48). In humans, four α-defensins (HNP-1 to HNP-4) have been isolated from neutrophils (12, 42) and two enteric α-defensins (HD-5 and HD-6) are expressed by Paneth cells in crypts of the small intestine (35, 36). The expression of HD-5 has also been detected in the female urogenital tract (37). Three human β-defensins (hBD-1 to hBD-3) have been isolated from epithelial and nonepithelial cell types of various organs (2, 4, 13, 16), and the expression of several others has been deduced by cDNA analysis or from analysis of the human genome (39). Numerous lines of evidence suggest that defensins provide an antimicrobial effector function in skin (16); the respiratory epithelium (2); the urogenital tract (13); and various leukocytes, i.e., neutrophils (42), monocytes (41), and NK cells (7, 32). Furthermore, defensins activate cells involved in both the innate and the adaptive immune responses, suggesting that they may serve to link the two branches of immunity (5, 56).
The antimicrobial activities of human α- and β-defensins are generally antagonized by physiologic sodium chloride in vitro (2, 13, 24). Thus, it was hypothesized that salt-induced inhibition of the antimicrobial activities of hBD-1 and hBD-2 underlie the increased incidence of bacterial infections in patients with cystic fibrosis (2). Attempts to develop AMPs as therapeutics have focused on peptides with salt-resistant properties (1, 3, 8, 11, 14, 15). In this regard, the microbicidal activities of hBD-3 are salt insensitive in vitro (16), as are the activities of several linear, cysteine-free peptides, e.g., magainin, LL37, and cecropin-melittin hybrids (9, 11, 14, 17). Porcine protegrin 1 (PG-1), a two-disulfide cathelicidin, also possesses salt-insensitive microbicidal activities in vitro. Structure-activity analyses of PG-1 revealed that its disulfide bonds are required for optimal peptide microbicidal activity under assay conditions that included physiologic salt, divalent cations, and serum (8).
θ-Defensins are cyclic octadecapeptides formed by the posttranslational splicing of two nonapeptides derived from 76-amino-acid α-defensin-related precursors (48). Three θ-defensins that have been isolated from acid extracts of leukocytes and bone marrow of rhesus monkeys (RTD 1-3) (25, 48, 50) exhibit broad-spectrum, salt-insensitive microbicidal properties against bacteria, fungi, herpes simplex virus, and human immunodeficiency virus type 1 (25, 30, 48, 58). Humans do not express θ-defensin peptides since the expression of θ-defensins ceased near the time that orangutans emerged in evolution due to a mutation that introduced a premature stop codon in the peptide precursor (31).
Although θ-defensins and protegrins are products of unrelated genes, they are structurally similar. Both are arginine-rich octadecapeptides in which the peptide backbone structure is dominated by a disulfide-stabilized β sheet (48, 49); Fig. 1). Despite these similarities, recent studies of the interactions of the peptides with model lipid bilayers suggest that θ-defensins and protegrins employ different mechanisms to achieve their antimicrobial activities (52). The studies reported here demonstrate the distinct functional properties of three closely related RTDs (RTD 1-3), provide insights into the mechanisms of antibacterial activity of the RTDs, and reveal that RTD 1-3 and PG-1 have markedly different selectivity profiles.
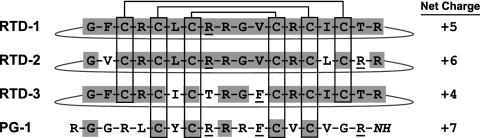
FIG. 1.
Structural features of RTDs and PG-1. The amino acid sequences of RTD 1-3 are aligned with the amino acid sequence of PG-1, and the net charge at pH 7 for each peptide is listed. The cyclic conformations of the RTDs are indicated by lines signifying the Arg-Gly peptide bond. The C-terminal amide of PG-1 is indicated by —NH. Disulfide bonds between cysteine pairs are shown, and the amino acids conserved in RTD 1-3 and shared with PG-1 are shaded. Additional residues shared by PG-1 and at least one of the RTDs are underlined.
MATERIALS AND METHODS
Peptide synthesis, disulfide formation, and cyclization.
The synthesis of PG-1 and RTD 1-3 was performed as described previously (48, 50). Briefly, the peptides (Fig. 1) were assembled on 9-fluorenylmethoxycarbonyl (Fmoc)-arginine-2,2,4,6,7-pentamethyldi-hydrobenzofuran-5-sulfonyl (Pbf)-polyethylene glycol graft polystyrene (PEG-PS) resins (RTD 1-3) or Fmoc-peptide amide linker PEG-PS (PG-1) resins and were cleaved from the solid support by treatment with reagent K (for RTD 1-3, and PG-1) or reagent R (for RTD-2) (48, 50, 51). Linear peptides were purified by preparative C18 reversed-phase high-performance liquid chromatography (RP-HPLC) from crude synthetic products, and the disulfide bonds were formed by air oxidation in 17.4 mM ammonium acetate, pH 8.0, with stirring (48). Peptide cyclization (RTD 1-3) was carried out in 0.1% diisopropylethylamine-dimethyl sulfoxide with excess 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide and 1-hydroxybenzotriazole (48). The extent of peptide cyclization was determined by C18 RP-HPLC and matrix-assisted laser desorption ionization-time-of-flight mass spectroscopy. RTD 1-3 and PG-1 were purified to homogeneity by C18 RP-HPLC and were characterized by acid-urea polyacrylamide gel electrophoresis, mass spectroscopy, and amino acid analysis (48, 50).
Microbicidal assays.
The antimicrobial activities of RTD 1-3 and PG-1 were determined in cell-suspension assays (48, 50). Serial dilutions of each peptide (final concentrations, 0.05 to 10.0 μg/ml) were prepared in 0.01% HOAc, incubated with 1 × 106 to 2 × 106 CFU/ml of the indicated log-phase bacterial or fungal organism in 10 mM 1,4-piperazinediethanesulfonic acid (PIPES), pH 7.4, containing 5 mM glucose in 96-well polystyrene plates (Corning, Corning, NY) in a final volume of 100 μl. After 2 h of incubation at 37°C, 35 μl of the incubation mixture was removed and diluted 1:50 in 10 mM PIPES, pH 7.4, and plated on Trypticase soy agar (bacteria) or Sabouraud dextrose agar by using an Autoplate 4000 instrument (Spiral Biotech). Samples of the incubation mixtures were examined at ×1,000 magnification with a phase-contrast microscope to confirm the absence of cell clumping under all conditions report here. After 18 to 24 h incubation at 37°C, the microbicidal activities were determined by colony counting and are expressed as CFU/ml or the percent killing relative to that of the no-peptide controls. The effects of ionic strength, divalent cations, and serum on the peptide activities were also determined by use of this assay format, as described in Results. Heat-inactivated human serum was prepared by incubation in a 55°C water bath for 30 min, followed by centrifugation to clarify the sample.
The minimum microbicidal concentrations (MMCs) of RTD 1-3 and PG-1 were determined by using a modification of the assay described above. Following the 2-h incubation step, 15 μl of the incubation mixture was removed and serially diluted (1:10 to 1:106) in Trypticase soy broth (bacteria) or Sabouraud dextrose broth (fungi) in a 96-well plate and grown for 24 to 48 h at 37°C until cell pellets were visible. Microbicidal activities were determined as the absence of growth and were correlated to the CFU/ml in control experiments, in which the activities were determined in parallel by colony counting (48, 50). The absence of growth at the 1:103 dilution was equivalent to a 3-log reduction in microbial viability. The MMC was determined for each peptide as the lowest concentration that reduced cell viability by 99.9% in at least two experiments.
Membrane permeabilization assays.
The effect of RTD 1-3 and PG-1 on bacterial envelope integrity was determined by measuring the hydrolysis of ο-nitrophenyl-β-d-galactopyranoside (ONPG) in Escherichia coli ML35 (23, 44). Peptides (final concentrations, 0 to 8 μg/ml) in 10 mM PIPES, pH 7.4, containing 3 mM ONPG were incubated with 1 × 106 to 2 × 106 CFU/ml of log-phase bacteria in a final volume of 100 μl. ONPG hydrolysis was measured at 405 nm for 60 min with a SpectraMAX 190 plate reader and SOFTmaxPRO 3.1 software (Molecular Devices Corp.). The effect of each peptide on β-galactosidase-dependent ONPG hydrolysis was separately determined by incubating 0 to 4 μg/ml of each peptide with recombinant β-galactosidase in 10 mM PIPES, pH 7.4, containing 3 mM ONPG. The dose-dependent inhibition of 10 nM β-galactosidase was determined for each peptide and was used to calculate an adjusted rate of ONPG hydrolysis by whole bacteria as R + (−log10C) × m + Δb, where R is the measured rate of hydrolysis, C is the peptide concentration, m is the slope obtained from a log-linear plot of the dose-dependent β-galactosidase inhibition by each peptide, and Δb is the difference in the y-axis intercepts obtained from plots of the ONPG hydrolysis rates by purified β-galactosidase in 10 mM PIPES buffer and identical mixtures containing 1 μg/ml of each peptide.
Hemolysis and cytotoxicity assays.
The hemolytic activity of each peptide was determined by the method of Tam et al. (46). EDTA-anticoagulated human blood was obtained from a healthy donor, in accordance with a protocol approved by the University of California, Irvine, Institutional Review Board. Erythrocytes were harvested by centrifugation at 234 × g for 10 min at 22°C, washed four times with phosphate-buffered saline (PBS; 10 mM sodium phosphate, 150 mM NaCl, pH 7.4) or PIPES-buffered saline (PiBS; 10 mM PIPES, 150 mM NaCl, pH 7.4) containing 4 mM EDTA, and resuspended in buffer without EDTA. Peptides in 10 μl 0.01% HOAc were diluted with PBS or PiBS to final concentrations of 0.3 to 100 μg/ml, and erythrocytes were added to 2% (vol/vol) in 100 μl. Incubations were conducted with and without addition of 10% autologous serum. After 1 h of incubation at 37°C, the cell suspensions were centrifuged at 234 × g for 10 min at 22°C. Fifty-microliter supernatant samples were removed, and the absorbance was measured at 405 nm. The hemolytic activity of each peptide was calculated relative to the 100% hemolysis obtained by incubation in 1% Nonidet P-40. A selectivity index (SI) was calculated and was the ratio of the peptide concentrations that produced 3% hemolysis (obtained from a dose-dependent hemolysis plot over 0 to 10 μg/ml for PG-1 and 0 to 30 μg/ml for RTD 1-3 of each peptide in PBS) to the lowest MMC value (μg/ml) that generated 99.9% killing of any of the three test organisms.
Peptide cytotoxicities against HS68 human fibroblasts were evaluated by using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method of Li and Zhang (26). HS68 cells were grown in Dulbecco's minimum essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 units/ml penicillin G sodium, and 100 μg/ml streptomycin sulfate and were maintained in a humidified incubator at 37°C with 5% CO2. A total of 5 × 103 cells was added to each well in 96-well plates (Becton Dickinson Labware), and the plates were incubated at 37°C for 18 h. Medium was replaced with DMEM containing 0.4% or 10% FBS without antibiotics, and peptides were added to final concentrations of 0 to 100 μg/ml. After 1 h of incubation at 37°C, the peptide solutions were replaced with fresh growth medium, MTT was added, and cell viability was quantified spectrophotometrically (26) in triplicate with a SpectraMAX 190 plate reader and by using cells in test wells lacking peptide as controls.
RESULTS
Synthetic RTD 1-3 and PG-1.
Three RTDs (RTD 1-3) and protegrin PG-1 (Fig. 1) were synthesized, purified, and characterized as described in Materials and Methods. Each preparation was homogeneous (≥99% pure by RP-HPLC) and indistinguishable from the corresponding natural peptide, as assessed by RP-HPLC, acid-urea polyacrylamide gel electrophoresis, amino acid analysis, and matrix-assisted laser desorption ionization-time-of-flight mass spectroscopy (48, 50).
In vitro microbicidal activities of RTD 1-3 and PG-1.
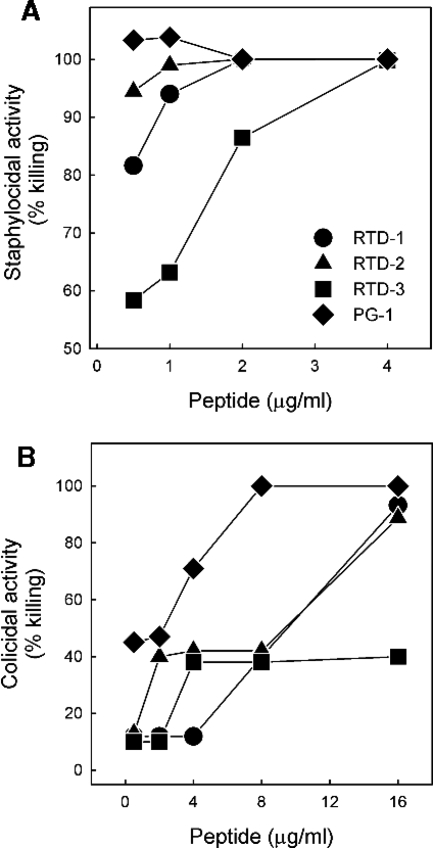
The microbicidal activities of RTD 1-3 and PG-1 were analyzed by determining the MMCs for each peptide against Staphylococcus aureus 502a, E. coli ML35, and Candida albicans 16820. The peptides were initially tested for their activities against the three test organisms in 10 mM PIPES-5 mM glucose, pH 7.4. As summarized in Table 1 (also see Fig. 3), the MMCs for all four peptides ranged from 0.4 to 3 μg/ml (0.2 to 1.4 μM) against the three organisms, consistent with the findings described in previous reports (8, 29, 48, 50).
TABLE 1.
MMCs of RTDs and PG-1
| Peptide | MMC (μg/ml)a
|
||
|---|---|---|---|
| S. aureus | E. coli | C. albicans | |
| RTD-1 | 1.0 ± 0 | 2.0 ± 0 | 1.0 ± 0 |
| RTD-2 | 1.0 ± 0.71 | 2.0 ± 0 | 1.0 ± 0 |
| RTD-3 | 1.5 ± 0 | 2.3 ± 2.5 | 3.0 ± 1.4 |
| PG-1 | 0.4 ± 0.18 | 1.0 ± 0 | 0.8 ± 0.35 |
Average values obtained from two or more independent experiments, expressed as the means ± standard errors of the means.
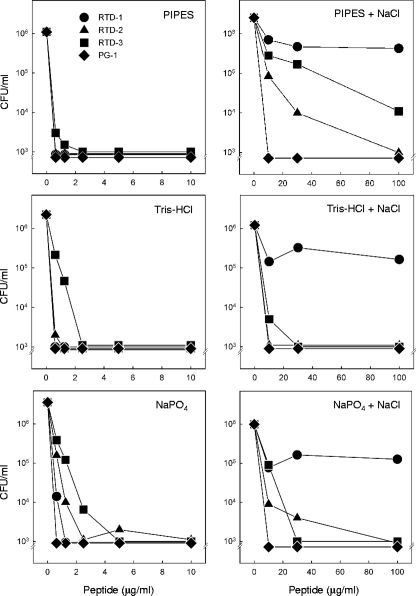
FIG. 3.
Effect of NaCl on staphylocidal activities of RTDs and PG-1 in different buffers. Log-phase bacteria were incubated for 2 h at 37°C with increasing concentrations of RTD 1-3 and PG-1 in 10 mM solutions of the indicated buffers with or without addition of 154 mM NaCl. Bactericidal activity was quantified by colony counting.
Preliminary studies suggest that RTDs function as antimicrobial effector molecules in the phagolysosome and may also play an extracellular role in innate immunity (unpublished data). Therefore, we evaluated the antimicrobial and cytotoxic activities of RTD 1-3 and the structurally similar peptide PG-1 under conditions more closely resembling those of the extracellular environment. We therefore tested the four peptides for their in vitro biologic activities in the presence of NaCl, divalent cations, and 10% normal human serum.
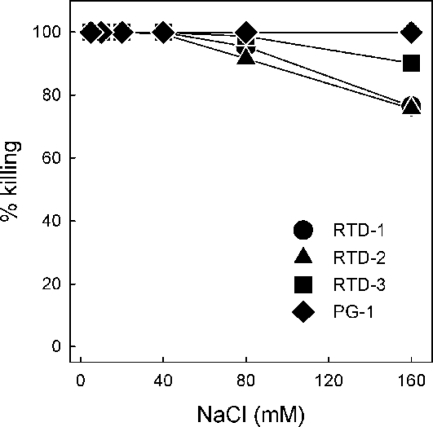
We first determined the staphylocidal activities of 5 μg/ml of RTD 1-3 and PG-1 in the presence of increasing concentrations of NaCl. We previously reported that RTD-1 (10 μg/ml) retained nearly all of its staphylocidal activity in normal PiBS, whereas an acyclic analog of RTD-1 was rendered inactive by physiologic concentrations of NaCl (48). Even when the lower peptide concentration was used, no salt inhibition of killing was observed for any of the peptides until the NaCl concentration reached 80 mM (Fig. 2). At 160 mM NaCl, the staphylocidal activities of RTD-1 and RTD-2 were reduced by 24%, and the rate of killing by RTD-3 was reduced by 10%. Over the range of concentrations tested, addition of salt had no effect on the microbicidal activity of PG-1 (Fig. 2). Analogous experiments were performed with C. albicans as the target organism, and similar results were obtained. While 10 μg/ml of each of the four peptides killed more than 3 log units of the input C. albicans yeast in PIPES buffer alone, the addition of physiologic saline reduced the rates of killing by RTD 1-3 by 37%, 16%, and 7%, respectively; NaCl addition had no effect on the killing activity of PG-1. We were unable to analyze the effect of NaCl addition on the activities of the peptides against E. coli ML35, as this organism was unstable in physiologic saline. The modest salt-mediated inhibition of RTD 1-3 observed is consistent with the findings described in earlier reports (48, 50), demonstrating that the bactericidal activity of θ-defensin is substantially more resistant to salt inhibition than the activities of most α- and β-defensins studied to date.
FIG. 2.
Effect of NaCl on the staphylocidal activities of the RTDs and PG-1. Log-phase bacteria were incubated with 5 μg/ml of RTD 1-3 and PG-1 in 10 mM PIPES, pH 7.4, containing increasing concentrations of sodium chloride for 2 h at 37°C. Killing activities were determined by colony counting and were calculated as a percentage of the killing obtained in buffer alone.
Previous studies in our laboratory revealed that different buffering agents substantially affected the microbicidal potencies of various AMPs, especially when these activities were evaluated in the presence of physiologic saline. Thus, we determined the staphylocidal activities of RTD 1-3 and PG-1 in the presence or absence of 154 mM NaCl using 10 mM PIPES, 10 mM Tris-HCl, or 10 mM sodium phosphate, pH 7.4, as buffer systems. All four peptides were potently bactericidal in each of the three buffers in the absence of NaCl, as greater than 3 log units of killing was observed at peptide concentrations ≤2.5 μg/ml (Fig. 3). In the presence of 154 mM NaCl, higher concentrations of peptide were required for equivalent killing, and the relative activities of the RTDs were markedly affected by the buffer system. For example, the staphylocidal activity of RTD-3 was substantially inhibited by salt in PIPES buffer but was relatively unaffected by NaCl in Tris-HCl or sodium phosphate buffer (Fig. 3). In 154 mM NaCl, RTD-1 killed approximately 90% of the input bacteria in all buffers, similar to the results shown in Fig. 2. Of note was the fact that RTD-2 and RTD-3 were substantially more active than RTD-1 in the presence of physiologic saline in all three buffer systems. Unlike the RTDs, the staphylocidal activity of PG-1 was unaffected by buffer-salt combinations (Fig. 3). These data reveal that the ionic milieu of the incubation medium has a marked influence on the microbicidal potencies of the RTDs and demonstrate significant differences in the relative activities of RTD 1-3.
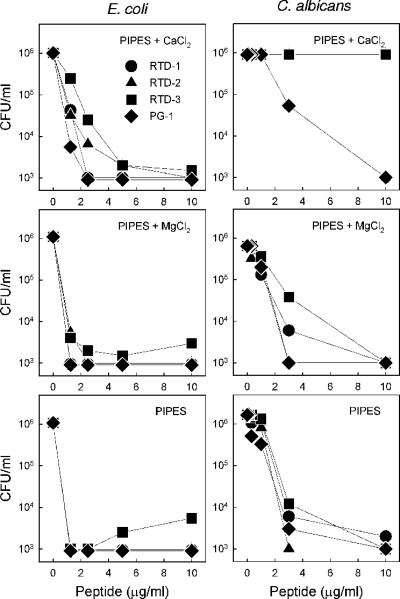
Previous studies demonstrated that RTD 1-3 and PG-1 are also potently microbicidal against gram-negative bacteria such as E. coli and Salmonella enterica serovar Typhimurium (20, 48). Since previous studies have shown that the killing of gram-negative bacteria and fungi by α- and β-defensins is generally inhibited by divalent cations (2, 24), we evaluated the effect of magnesium and calcium ions on the killing of E. coli and C. albicans by the four test peptides (Fig. 4). Potent killing of E. coli by PG-1 and RTD 1-3 occurred in the presence of physiologic Ca2+ and Mg2+ concentrations, and the extent of killing was similar to that obtained in the absence of divalent cations (Fig. 4, left panels). Similar results (i.e., no inhibitory effect) were obtained when S. aureus was the target organism (data not shown). Although the peptide concentrations required for the equivalent killing of C. albicans tended to be slightly higher for all four peptides, the addition of MgCl2 had little effect, similar to the result obtained with E. coli (Fig. 4). However, the effect of added calcium was markedly different, as the three RTDs were completely inactive against C. albicans in the presence of 1.2 mM CaCl2 (Fig. 4, right panels).
FIG. 4.
Effect of divalent cations on the killing of E. coli and C. albicans by the RTDs and PG-1. The killing of E. coli ML35 and C. albicans was measured by incubating increasing concentrations of RTD 1-3 with log-phase organisms in 10 mM PIPES, pH 7.4, with 1.2 mM CaCl2 and 0.8 mM MgCl2 or in 10 mM PIPES lacking added divalent salts.
The effect of serum on the bactericidal activities of peptides was tested by determining the staphylocidal activities of RTD 1-3 and PG-1 in 10 mM PIPES, pH 7.4, in the presence or absence of 10% normal human serum (higher concentrations of serum had confounding effects due to clumping or antimicrobial effects on the test organisms). While 10% serum alone had no effect on S. aureus, it slightly augmented the activity of PG-1 (Fig. 5A). In contrast, serum partially inhibited the activities of RTD-1 and RTD-3 at low peptide concentrations (0.5 to 2 μg/ml), but the inhibitory effect was not seen once the RTD concentration was increased to ≥ 2 μg/ml.
FIG. 5.
Bactericidal activities of the RTDs and PG-1 in the presence of normal human serum. The dose-dependent bactericidal activities of RTD 1-3 and PG-1 were determined in 10 mM PIPES, pH 7.4, containing 10% normal human serum (S. aureus) (A) or 10% heat-inactivated human serum (E. coli) (B). Bactericidal activities were determined by colony counting and are expressed as the percentage of killing relative to that for control incubations lacking serum.
Similar experiments were performed with E. coli as the test organism. For these experiments, 10% heat-inactivated human serum was used to circumvent the complement-mediated effects against E. coli (33). At peptide concentrations ≤8 μg/ml, the bactericidal activities of RTD 1-3 and PG-1 were antagonized by 10% serum to different degrees (Fig. 5B). However, this inhibition was overcome as the concentration of the RTD-1, RTD-2, and PG-1 peptides was increased to 16 μg/ml. Interestingly, the activity of RTD-3 was inhibited by at least 60% regardless of the peptide concentration employed in this assay.
Permeabilization of E. coli cytoplasmic membranes.
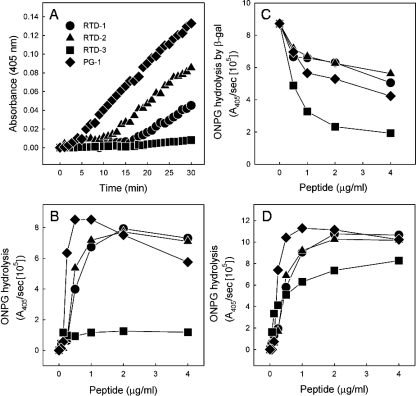
Previous studies showed that the microbicidal action of PG-1 correlated with the permeabilization of the bacterial cytoplasmic membrane (27, 57). We tested RTD 1-3 for the ability to permeabilize E. coli ML35 cells by measuring the rates of ONPG hydrolysis by cytoplasmic β-galactosidase (23, 44). Incubation of bacteria with 1 μg/ml of RTD 1-3 or PG-1 induced the apparent influx of ONPG into treated cells (Fig. 6A). In the case of PG-1, there was a very short lag before ONPG hydrolysis was detected, whereas RTD-1 and RTD-2 induced similar rates of hydrolysis, but after a 10- to 15-min lag. However, RTD-3 appeared to induce no bacterial permeabilization (Fig. 6A), a result that was unexpected given that the MMC of RTD-3 against E. coli is nearly identical to those of RTD-1 and RTD-2 (Table 1; Fig. 4). In separate experiments, we analyzed the concentration-dependent rate of ONPG hydrolysis for each peptide (Fig. 6B). These experiments revealed a bimodal relationship between the peptide concentration and maximal ONPG hydrolysis, as increasing the PG-1, RTD-1, and RTD-2 concentrations inhibited the production of O-nitrophenol by bacteria exposed to peptide. Consistent with the data shown in Fig. 6A, there was little RTD-3-induced permeabilization even at the highest peptide concentration tested. The basis for these observations was revealed when we tested the effect of each peptide on the hydrolysis of ONPG by purified β-galactosidase. As shown in Fig. 6C, RTD 1-3 and PG-1 inhibited the β-galactosidase hydrolysis of ONPG to various degrees in a concentration-dependent manner. RTD-1, RTD-2, and PG-1 inhibited β-galactosidase activity by 36 to 52% at the highest peptide concentration tested (4 μg/ml). However, RTD-3, at 2 μg/ml, inhibited ONPG hydrolysis by 78%, raising the possibility that the low level of ONPG hydrolysis observed when E. coli ML35 cells were incubated with bactericidal concentrations of RTD-3 was the result of this inhibition. To determine whether the apparent β-galactosidase inhibition was intracellular and/or extracellular, ONPG hydrolysis was measured in the supernatants of incubation mixtures of E. coli ML35 cells with 1 μg/ml of RTD 1-3. In each case, no ONPG hydrolysis was detected in the bacterial supernatants, indicating that β-galactosidase (465 kDa) remains within bacterial cells that are permeabilized by peptide treatment.
FIG. 6.
Permeabilization of E. coli cells by RTD 1-3 and PG-1. (A) The kinetics of ONPG hydrolysis in E. coli ML35 cells were measured by incubating 1 × 106 to 2 × 106 log-phase bacteria with 1 μg/ml of RTD 1-3 and PG-1. (B) The dose-dependent rates of ONPG hydrolysis (A405/s) were determined as the highest slope from the kinetic data for each peptide, as shown in panel A. (C) Peptide-mediated inhibition of β-galactosidase activity was determined by incubating 10 nM of the cell-free enzyme with the indicated concentrations of each peptide and determining the rate of ONPG hydrolysis. (D) The dose-dependent permeabilization of E. coli ML35 was determined by adjusting the measured rates of ONPG hydrolysis obtained (see panel B) by using the inhibition coefficient determined for each peptide.
We hypothesized that peptide entering permeabilized bacteria could inhibit cytosolic β-galactosidase, thereby reducing the rate of ONPG hydrolysis observed in incubations containing higher peptide concentrations (Fig. 6B). We tested this hypothesis by determining the β-galactosidase inhibition coefficient for each peptide using data similar to those shown in Fig. 6C (also see Materials and Methods) and used this factor to correct the rates of ONPG hydrolysis observed for RTD 1-3 and PG-1. As shown in Fig. 6D, this correction produces dose-response data that are consistent with substrate conversion as a function of substrate (ONPG) influx. Indeed, the corrected rate of hydrolysis rose in proportion to the concentration of each peptide and then plateaued at the approximate E. coli MMC for each peptide (Fig. 6D; Table 1). Since β-galactosidase inhibition must occur intracellularly, these data suggested that the RTDs and PG-1 permeabilize E. coli cells and mediate their own uptake.
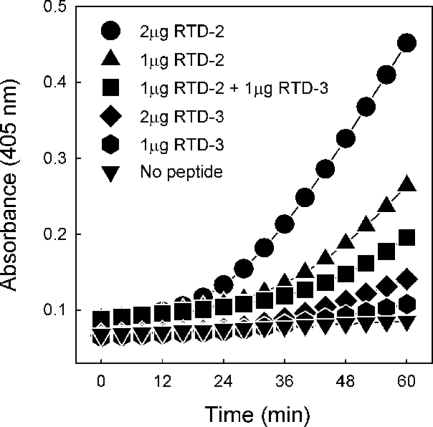
To further characterize the interaction of the RTDs with E. coli cells, we exploited the different β-galactosidase inhibitory activities of RTD-2 and RTD-3. E. coli ML35 cells were incubated with different concentrations (1.0 or 2.0 μg/ml) of RTD-2 and RTD-3 or a 1:1 mixture of the two peptides. Under each set of conditions, ONPG hydrolysis was monitored spectrophotometrically for 60 min. As shown in Fig. 7, the incubation containing 1 μg/ml each of RTD-2 and RTD-3 produced a rate of hydrolysis that was less than that achieved with 1 μg/ml of RTD-2 alone, clearly demonstrating the RTD-3-mediated inhibition of ONPG hydrolysis. Since no β-galactosidase was released by RTD-treated cells, the observed inhibition must have occurred in the cytosol of the permeabilized bacteria. The results provide further support for the conclusion that the RTDs mediate their own uptake and enter bacteria on the same time scale as the influx of ONPG.
FIG. 7.
Permeabilization of E. coli cells and intracellular inhibition of β-galactosidase by internalized RTD. The kinetics of bacterial ONPG hydrolysis resulting from incubation with RTD-2, RTD-3, or a 1:1 mixture of the two peptides was analyzed as described in Materials and Methods.
Hemolytic and cytotoxicity profiles of RTDs and PG-1.
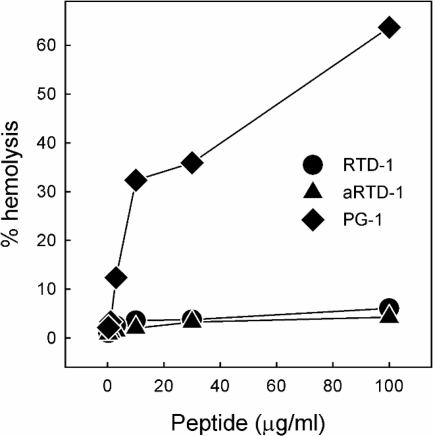
Preliminary experiments indicated that the RTDs and protegrins possess different cytotoxicity profiles. We evaluated these properties further by determining the hemolytic activities of RTD 1-3 and PG-1 in the presence and the absence of autologous serum. In the absence of serum, PG-1 induced dose-dependent hemolysis that reached 65% at a peptide concentration of 100 μg/ml, whereas RTD-1 was nearly devoid of hemolytic activity at all concentrations tested (Fig. 8). RTD-2 and RTD-3 had activities identical to the activity of RTD-1 (data not shown). In the presence of 10% autologous serum, 100 μg/ml of PG-1 induced 42% hemolysis, whereas RTD 1-3 (100 μg/ml) were completely nonhemolytic under these conditions (data not shown). The cytotoxic activities of the RTDs and PG-1 were assessed further by analyzing their effects on human fibroblasts. No cytotoxicity was observed for RTD-1 at concentrations up to 100 μg/ml in the presence of 0.4% FBS (Fig. 9) or in the presence of 10% FBS (data not shown). On the other hand, PG-1 was highly cytotoxic for fibroblasts in 0.4% FBS (Fig. 9), and this activity was only modestly attenuated by higher serum concentrations, as 50 μg/ml and 100 μg/ml of PG-1 killed 90% and 100% of the fibroblasts, respectively, in the presence of 10% FBS (data not shown).
FIG. 8.
Hemolytic activities of the RTDs and PG-1. Human red blood cells were incubated with 0 to 100 μg/ml of RTD 1, aRTD-1, and PG-1 in PBS for 1 h at 37°C. The absorbance of supernatants from the incubation mixtures was measured at 405 nm, and the percent hemolysis was calculated relative to the 100% hemolysis produced by 1% Nonidet P-40.
FIG. 9.
Cytotoxicity of RTDs and PG-1 for human fibroblasts. Human fibroblast (HS68) cells were exposed to 0 to 100 μg/ml of RTD-1, aRTD-1, and PG-1 in DMEM containing 0.4% FBS for 1 h at 37°C. After a 24-h recovery period, cell viability was determined by the MTT method, and viability was enumerated as the percent viability compared to the viability of incubations lacking peptide.
The relative in vitro selectivities of RTD 1-3 and PG-1 were determined by calculating a SI for each peptide (see Materials and Methods). The SI for each peptide (Table 2) was determined by dividing the peptide concentration that produced 3% hemolysis by the lowest MMC value obtained (Table 1). As summarized in Table 2, the SIs ranged from 2.1 (PG-1) to 14.0 (RTD-3). A similar analysis was attempted by using the cytotoxicity results obtained with fibroblasts (Fig. 9); however, it was not possible to generate SI values for the RTDs because no cytotoxicity was observed at the highest concentration of RTD 1-3 tested.
TABLE 2.
SIs for cytoxicity of RTD 1-3 and PG-1
| Peptide | Ma (μg/ml) | Hb (μg/ml) | SI (H/M) |
|---|---|---|---|
| RTD-1 | 1.0 | 5.5 | 5.5 |
| RTD-2 | 1.0 | 6.2 | 6.2 |
| RTD-3 | 1.5 | 21.0 | 14.0 |
| PG-1 | 0.4 | 0.8 | 2.0 |
M, lowest peptide concentration that killed 99.9% of either S. aureus, E. coli, or C. albicans, taken from the mean MMC values in Table 1.
H, lowest peptide concentration that caused 3% hemolysis. The concentrations used to determine the lowest peptide concentration were 0 to 10 μg/ml for PG-1 and 0 to 30 μg/ml for RTD 1-3 (see Fig. 8).
We postulated that the marked differences in the hemolytic/cytocidal activities of RTD 1-3 and PG-1 were a function of the cyclic conformations of the RTDs. To test this hypothesis, we determined the hemolytic and cytotoxic activities of acyclic RTD-1 (aRTD-1), a synthetic peptide identical in sequence to RTD-1 but lacking the Arg-Gly peptide bond in the macrocyclic backbone (Fig. 1). Surprisingly, aRTD-1 was indistinguishable from RTD-1 in its low hemolytic (Fig. 8) and noncytotoxic (Fig. 9) properties.
DISCUSSION
PG-1 and RTD 1-3 are broad-spectrum microbicides with activities in the micromolar ranges against bacteria and fungi (Table 1) (45, 48). While these peptides are predominantly expressed by phagocytic leukocytes and are believed to contribute to the microbicidal milieu of the phagolysosome, they may also exert an antimicrobial effect extracellularly in a manner analogous to that of enteric α-defensins, which are secreted by Paneth cells into the lumen of the small intestine (34). At least two in vivo studies demonstrate that enteric α-defensins play a critical role in antibacterial mucosal immunity (38, 53). To study the potential extracellular activities of θ-defensins, we assessed the relative microbicidal and cytotoxic activities of RTD 1-3 and the structurally related peptide PG-1 under various conditions, analyzing the effects of sodium chloride, divalent cations, and serum on peptide functions. RTD 1-3 and PG-1 were highly effective microbicides against gram-positive (S. aureus), gram-negative (E. coli), and fungal (C. albicans) target organisms under low-ionic-strength conditions. To various degrees, increasing the ionic strength (with NaCl) of the incubation mixture antagonized the staphylocidal activities of RTD 1-3, but the differential effects of physiologic NaCl on the activities of RTD 1-3 were rather unexpected. Even at the low peptide concentrations used (5 μg/ml), there was no inhibitory effect of NaCl supplementation up to 40 mM salt, and a very modest effect was noted with 80 mM salt. Increasing the NaCl concentration to 160 mM inhibited RTD-1- and RTD-2-mediated killing by approximately 24% and inhibited RTD-3-mediated killing by only 10%. Interestingly, there seemed to be no correlation between staphylocidal potency and the net charge of the three RTDs in 160 mM NaCl. Furthermore, the buffering salts (10 mM PIPES, Tris-HCl, and sodium phosphate) also influenced the activities of the peptides in the presence or the absence of physiologic NaCl (Fig. 3). RTD-1 (net charge, +5) was the most potent RTD in low-ionic-strength medium. In 154 to 160 mM NaCl, RTD-2 (net charge, +6) or RTD-3 (net charge, +4) was the most active, but the relative potency depended on the buffer. RTD-2 was the most active in PIPES, whereas RTD-3 was more active in Tris-HCl. In sodium phosphate, the relative potencies of RTD-2 and RTD-3 actually varied, depending on the peptide concentration being tested. These data highlight the complexity of the peptide-target interaction, one that appears to be markedly affected by the ionic milieu. In this regard, we found that the colicidal activities of RTD 1-3 and PG-1 were only slightly affected by physiologic concentrations of Ca2+ and Mg2+ but that calcium was highly inhibitory for RTD killing of C. albicans. Similarly, the antibacterial activities of RTD-1 and RTD-2 were inhibited to various degrees by normal human serum. However, with the exception of the anti-E. coli activity of RTD-3, the full activity of each peptide was achieved by modestly increasing the peptide concentration to levels ranging from 1 to 16 μg/ml (Fig. 5). The relative insensitivity of RTD 1-3 to salt, divalent cations, and serum seems to differentiate the activities of the θ-defensins from those of the α- and β-defensins, which are generally antagonized by these additives in vitro (2, 24).
The killing of bacteria by protegrins and by AMPs in general has been attributed to the peptide-induced permeabilization of cytoplasmic membranes (6, 10, 18, 22, 27, 28, 44, 54, 55). We previously reported that equal amounts of RTD 1-3 bind to a fixed number of E. coli cells, despite the fact that the MMCs of the three peptides vary slightly (50). This indicated that postbinding events determine the bactericidal efficacy. In this study, ONPG hydrolysis experiments disclosed that RTD 1-3, like the protegrins, permeabilize E. coli cells. Maximum ONPG hydrolysis occurred at peptide concentrations approximating the MMC for each peptide, suggesting that bacterial killing is dependent on or closely linked to permeabilization of the bacterial membranes (Table 1 and Fig. 6).
The fact that higher concentrations of RTD 1-3 and PG-1 reduced β-galactosidase activity in peptide-permeabilized E. coli ML35 (Fig. 6) led to the hypothesis that θ-defensins are internalized in the cytoplasm of permeabilized bacteria. ONPG hydrolysis experiments with an RTD-2-RTD-3 mixture provided further evidence to support this hypothesis (Fig. 7). We also performed similar ONPG hydrolysis experiments with RTD 1-3 and observed similarly reduced ONPG hydrolysis (data not shown). In separate ONPG hydrolysis experiments, the addition of isopropyl-β-d-thiogalactopyranoside (IPTG), a nonhydrolyzable analog of ONPG that inhibits β-galactosidase activity, significantly reduced the rate of ONPG hydrolysis (data not shown). The observed influx of ONPG, IPTG, and RTD 1-3 demonstrates that θ-defensins permeabilize the E. coli cells to an extent that allows the passage of 500-Da molecules (ONPG and IPTG) and 2-kDa cationic peptides (θ-defensins) across the cytoplasmic membranes. These results are similar to data showing that PG-1 (27), indolicidin (21), and α-defensins (18, 54) permeabilize target cell membranes. Moreover, the cytoplasmic uptake of θ-defensins is consistent with the “self-promoted uptake” mechanism, wherein internalized AMPs disrupt essential cellular functions (28, 55).
The structural similarities between RTD 1-3 and PG-1 support the concept that host defense peptides possess conserved molecular features necessary for their microbicidal effects. However, θ-defensins and protegrins have different cytotoxicity profiles and membranolytic selectivities. PG-1 reportedly permeabilizes Xenopus oocytes (27) and model membranes (57), induces significant hemolysis (46), and is cytotoxic to human fibroblasts (Fig. 9). In contrast, θ-defensins caused very little hemolysis and were virtually noncytotoxic for human fibroblasts. Trabi et al. reported that the solution structures of PG-1 and RTD-1 showed significant differences in the flexibility of the peptide backbones and the distribution of the positively charged arginine residues within the peptide sequences (49). The more rigid peptide PG-1 backbone may produce a peptide-membrane interaction that is poorly tolerated by host cells. Weiss et al. suggested that differences in the transition of RTD-1 and PG-1 within two distinct stages of the peptide-membrane interaction were due to peptide amphipathicity (52). In PG-1, arginine residues are clustered in the β turn and at the chain termini. In contrast, the positively charged residues in θ-defensins are located throughout the peptide chain (Fig. 1) (48). The fact that aRTD-1 was just as nontoxic to mammalian cells as RTD-1 demonstrates that the macrocyclic structure per se is not a major determinant of low toxicity. This finding may facilitate structure-activity studies aimed at delineating the sequence and other structural requirements needed to produce potentially useful therapeutic molecules.
Acknowledgments
This work was supported by the Hewitt Foundation for Biomedical Research and NIH grants AI22931, AI58120, and DE15517.
Footnotes
Published ahead of print on 26 December 2007.
REFERENCES
- 1.Ahmad, I., W. R. Perkins, D. M. Lupan, M. E. Selsted, and A. S. Janoff. 1995. Liposomal entrapment of the neutrophil-derived peptide indolicidin endows it with in vivo antifungal activity. Biochim. Biophys. Acta 1237:109-114. [DOI] [PubMed] [Google Scholar]
- 2.Bals, R., X. Wang, Z. Wu, T. Freeman, V. Bafna, M. Zasloff, and J. M. Wilson. 1998. Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J. Clin. Investig. 102:874-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bals, R., D. J. Weiner, R. L. Meegalla, and J. M. Wilson. 1999. Transfer of a cathelicidin peptide antibiotic gene restores bacterial killing in a cystic fibrosis xenograft model. J. Clin. Investig. 103:1113-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bensch, K. W., M. Raida, H. J. Magert, P. Schulz-Knappe, and W. G. Forssmann. 1995. hBD-1: a novel beta-defensin from human plasma. FEBS Lett. 368:331-335. [DOI] [PubMed] [Google Scholar]
- 5.Biragyn, A., M. Surenhu, D. Yang, P. A. Ruffini, B. A. Haines, E. Klyushnenkova, J. J. Oppenheim, and L. W. Kwak. 2001. Mediators of innate immunity that target immature, but not mature, dendritic cells induce antitumor immunity when genetically fused with nonimmunogenic tumor antigens. J. Immunol. 167:6644-6653. [DOI] [PubMed] [Google Scholar]
- 6.Bulet, P., C. Hetru, J. L. Dimarcq, and D. Hoffmann. 1999. Antimicrobial peptides in insects; structure and function. Dev. Comp. Immunol. 23:329-344. [DOI] [PubMed] [Google Scholar]
- 7.Chalifour, A., P. Jeannin, J. F. Gauchat, A. Blaecke, M. Malissard, T. N′Guyen, N. Thieblemont, and Y. Delneste. 2004. Direct bacterial protein PAMP recognition by human NK cells involves TLRs and triggers alpha-defensin production. Blood 104:1778-1783. [DOI] [PubMed] [Google Scholar]
- 8.Chen, J., T. J. Falla, H. Liu, M. A. Hurst, C. A. Fujii, D. A. Mosca, J. R. Embree, D. J. Loury, P. A. Radel, C. Cheng Chang, L. Gu, and J. C. Fiddes. 2000. Development of protegrins for the treatment and prevention of oral mucositis: structure-activity relationships of synthetic protegrin analogues. Biopolymers 55:88-98. [DOI] [PubMed] [Google Scholar]
- 9.Cowland, J. B., A. H. Johnsen, and N. Borregaard. 1995. hCAP-18, a cathelin/pro-bactenecin-like protein of human neutrophil specific granules. FEBS Lett. 368:173-176. [DOI] [PubMed] [Google Scholar]
- 10.Falla, T. J., D. N. Karunaratne, and R. E. Hancock. 1996. Mode of action of the antimicrobial peptide indolicidin. J. Biol. Chem. 271:19298-19303. [DOI] [PubMed] [Google Scholar]
- 11.Friedrich, C., M. G. Scott, N. Karunaratne, H. Yan, and R. E. Hancock. 1999. Salt-resistant alpha-helical cationic antimicrobial peptides. Antimicrob. Agents Chemother. 43:1542-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabay, J. E., R. W. Scott, D. Campanelli, J. Griffith, C. Wilde, M. N. Marra, M. Seeger, and C. F. Nathan. 1989. Antibiotic proteins of human polymorphonuclear leukocytes. Proc. Natl. Acad. Sci. USA 86:5610-5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia, J. R., A. Krause, S. Schulz, F. J. Rodriguez-Jimenez, E. Kluver, K. Adermann, U. Forssmann, A. Frimpong-Boateng, R. Bals, and W. G. Forssmann. 2001. Human beta-defensin 4: a novel inducible peptide with a specific salt-sensitive spectrum of antimicrobial activity. FASEB J. 15:1819-1821. [PubMed] [Google Scholar]
- 14.Ge, Y., D. L. MacDonald, K. J. Holroyd, C. Thornsberry, H. Wexler, and M. Zasloff. 1999. In vitro antibacterial properties of pexiganan, an analog of magainin. Antimicrob. Agents Chemother. 43:782-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hancock, R. E., and M. G. Scott. 2000. The role of antimicrobial peptides in animal defenses. Proc. Natl. Acad. Sci. USA 97:8856-8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harder, J., J. Bartels, E. Christophers, and J. M. Schroder. 2001. Isolation and characterization of human beta-defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 276:5707-5713. [DOI] [PubMed] [Google Scholar]
- 17.Hirata, M., Y. Shimomura, M. Yoshida, J. G. Morgan, I. Palings, D. Wilson, M. H. Yen, S. C. Wright, and J. W. Larrick. 1994. Characterization of a rabbit cationic protein (CAP18) with lipopolysaccharide-inhibitory activity. Infect. Immun. 62:1421-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kagan, B. L., M. E. Selsted, T. Ganz, and R. I. Lehrer. 1990. Antimicrobial defensin peptides form voltage-dependent ion-permeable channels in planar lipid bilayer membranes. Proc. Natl. Acad. Sci. USA 87:210-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kavanagh, K., and S. Dowd. 2004. Histatins: antimicrobial peptides with therapeutic potential. J. Pharm. Pharmacol. 56:285-289. [DOI] [PubMed] [Google Scholar]
- 20.Kokryakov, V. N., S. S. Harwig, E. A. Panyutich, A. A. Shevchenko, G. M. Aleshina, O. V. Shamova, H. A. Korneva, and R. I. Lehrer. 1993. Protegrins: leukocyte antimicrobial peptides that combine features of corticostatic defensins and tachyplesins. FEBS Lett. 327:231-236. [DOI] [PubMed] [Google Scholar]
- 21.Ladokhin, A. S., M. E. Selsted, and S. H. White. 1997. Bilayer interactions of indolicidin, a small antimicrobial peptide rich in tryptophan, proline, and basic amino acids. Biophys. J. 72:794-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehrer, R. I., A. Barton, K. A. Daher, S. S. Harwig, T. Ganz, and M. E. Selsted. 1989. Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J. Clin. Investig. 84:553-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehrer, R. I., A. Barton, and T. Ganz. 1988. Concurrent assessment of inner and outer membrane permeabilization and bacteriolysis in E. coli by multiple-wavelength spectrophotometry. J. Immunol. Methods 108:153-158. [DOI] [PubMed] [Google Scholar]
- 24.Lehrer, R. I., T. Ganz, D. Szklarek, and M. E. Selsted. 1988. Modulation of the in vitro candidacidal activity of human neutrophil defensins by target cell metabolism and divalent cations. J. Clin. Investig. 81:1829-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leonova, L., V. N. Kokryakov, G. Aleshina, T. Hong, T. Nguyen, C. Zhao, A. J. Waring, and R. I. Lehrer. 2001. Circular minidefensins and posttranslational generation of molecular diversity. J. Leukoc. Biol. 70:461-464. [PubMed] [Google Scholar]
- 26.Li, H., and S. Zhang. 2001. In vitro cytotoxicity of the organophosphorus pesticide parathion to FG-9307 cells. Toxicol. In Vitro 15:643-647. [DOI] [PubMed] [Google Scholar]
- 27.Mangoni, M. E., A. Aumelas, P. Charnet, C. Roumestand, L. Chiche, E. Despaux, G. Grassy, B. Calas, and A. Chavanieu. 1996. Change in membrane permeability induced by protegrin 1: implication of disulphide bridges for pore formation. FEBS Lett. 383:93-98. [DOI] [PubMed] [Google Scholar]
- 28.Matsuzaki, K., Y. Mitani, K. Y. Akada, O. Murase, S. Yoneyama, M. Zasloff, and K. Miyajima. 1998. Mechanism of synergism between antimicrobial peptides magainin 2 and PGLa. Biochemistry 37:15144-15153. [DOI] [PubMed] [Google Scholar]
- 29.Mosca, D. A., M. A. Hurst, W. So, B. S. Viajar, C. A. Fujii, and T. J. Falla. 2000. IB-367, a protegrin peptide with in vitro and in vivo activities against the microflora associated with oral mucositis. Antimicrob. Agents Chemother. 44:1803-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munk, C., G. Wei, O. O. Yang, A. J. Waring, W. Wang, T. Hong, R. I. Lehrer, N. R. Landau, and A. M. Cole. 2003. The theta-defensin, retrocyclin, inhibits HIV-1 entry. AIDS Res. Hum. Retrovir. 19:875-881. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen, T. X., A. M. Cole, and R. I. Lehrer. 2003. Evolution of primate theta-defensins: a serpentine path to a sweet tooth. Peptides 24:1647-1654. [DOI] [PubMed] [Google Scholar]
- 32.Obata-Onai, A., S. Hashimoto, N. Onai, M. Kurachi, S. Nagai, K. Shizuno, T. Nagahata, and K. Matsushima. 2002. Comprehensive gene expression analysis of human NK cells and CD8+ T lymphocytes. Int. Immunol. 14:1085-1098. [DOI] [PubMed] [Google Scholar]
- 33.O'Hara, A. M., A. P. Moran, R. Wurzner, and A. Orren. 2001. Complement-mediated lipopolysaccharide release and outer membrane damage in Escherichia coli J5: requirement for C9. Immunology 102:365-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ouellette, A. J. 2005. Paneth cell alpha-defensins: peptide mediators of innate immunity in the small intestine. Springer Semin. Immunopathol. 27:133-146. [DOI] [PubMed] [Google Scholar]
- 35.Porter, E. M., L. Liu, A. Oren, P. A. Anton, and T. Ganz. 1997. Localization of human intestinal defensin 5 in Paneth cell granules. Infect. Immun. 65:2389-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porter, E. M., M. A. Poles, J. S. Lee, J. Naitoh, C. L. Bevins, and T. Ganz. 1998. Isolation of human intestinal defensins from ileal neobladder urine. FEBS Lett. 434:272-276. [DOI] [PubMed] [Google Scholar]
- 37.Quayle, A. J., E. M. Porter, A. A. Nussbaum, Y. M. Wang, C. Brabec, K. P. Yip, and S. C. Mok. 1998. Gene expression, immunolocalization, and secretion of human defensin-5 in human female reproductive tract. Am. J. Pathol. 152:1247-1258. [PMC free article] [PubMed] [Google Scholar]
- 38.Salzman, N. H., D. Ghosh, K. M. Huttner, Y. Paterson, and C. L. Bevins. 2003. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature 422:522-526. [DOI] [PubMed] [Google Scholar]
- 39.Schutte, B. C., J. P. Mitros, J. A. Bartlett, J. D. Walters, H. P. Jia, M. J. Welsh, T. L. Casavant, and P. B. McCray, Jr. 2002. Discovery of five conserved beta-defensin gene clusters using a computational search strategy. Proc. Natl. Acad. Sci. USA 99:2129-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scocchi, M., S. Wang, and M. Zanetti. 1997. Structural organization of the bovine cathelicidin gene family and identification of a novel member. FEBS Lett. 417:311-315. [DOI] [PubMed] [Google Scholar]
- 41.Selsted, M. E., D. M. Brown, R. J. DeLange, and R. I. Lehrer. 1983. Primary structures of MCP-1 and MCP-2, natural peptide antibiotics of rabbit lung macrophages. J. Biol. Chem. 258:14485-14489. [PubMed] [Google Scholar]
- 42.Selsted, M. E., S. S. Harwig, T. Ganz, J. W. Schilling, and R. I. Lehrer. 1985. Primary structures of three human neutrophil defensins. J. Clin. Investig. 76:1436-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Selsted, M. E., and A. J. Ouellette. 2005. Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 6:551-557. [DOI] [PubMed] [Google Scholar]
- 44.Sitaram, N., V. Krishnakumari, and R. Nagaraj. 1992. The antibacterial peptide seminal plasmin alters permeability of the inner membrane of E. coli. FEBS Lett. 303:265-268. [DOI] [PubMed] [Google Scholar]
- 45.Steinberg, D. A., M. A. Hurst, C. A. Fujii, A. H. Kung, J. F. Ho, F. C. Cheng, D. J. Loury, and J. C. Fiddes. 1997. Protegrin-1: a broad-spectrum, rapidly microbicidal peptide with in vivo activity. Antimicrob. Agents Chemother. 41:1738-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tam, J. P., C. Wu, and J. L. Yang. 2000. Membranolytic selectivity of cystine-stabilized cyclic protegrins. Eur. J. Biochem. 267:3289-3300. [DOI] [PubMed] [Google Scholar]
- 47.Tang, Y. Q., and M. E. Selsted. 1993. Characterization of the disulfide motif in BNBD-12, an antimicrobial beta-defensin peptide from bovine neutrophils. J. Biol. Chem. 268:6649-6653. [PubMed] [Google Scholar]
- 48.Tang, Y. Q., J. Yuan, G. Osapay, K. Osapay, D. Tran, C. J. Miller, A. J. Ouellette, and M. E. Selsted. 1999. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated alpha-defensins. Science 286:498-502. [DOI] [PubMed] [Google Scholar]
- 49.Trabi, M., H. J. Schirra, and D. J. Craik. 2001. Three-dimensional structure of RTD-1, a cyclic antimicrobial defensin from rhesus macaque leukocytes. Biochemistry 40:4211-4221. [DOI] [PubMed] [Google Scholar]
- 50.Tran, D., P. A. Tran, Y. Q. Tang, J. Yuan, T. Cole, and M. E. Selsted. 2002. Homodimeric theta-defensins from rhesus macaque leukocytes: isolation, synthesis, antimicrobial activities, and bacterial binding properties of the cyclic peptides. J. Biol. Chem. 277:3079-3084. [DOI] [PubMed] [Google Scholar]
- 51.van Abel, R. J., Y. Q. Tang, V. S. Rao, C. H. Dobbs, D. Tran, G. Barany, and M. E. Selsted. 1995. Synthesis and characterization of indolicidin, a tryptophan-rich antimicrobial peptide from bovine neutrophils. Int. J. Pept. Protein Res. 45:401-409. [DOI] [PubMed] [Google Scholar]
- 52.Weiss, T. M., L. Yang, L. Ding, A. J. Waring, R. I. Lehrer, and H. W. Huang. 2002. Two states of cyclic antimicrobial peptide RTD-1 in lipid bilayers. Biochemistry 41:10070-10076. [DOI] [PubMed] [Google Scholar]
- 53.Wilson, C. L., A. J. Ouellette, D. P. Satchell, T. Ayabe, Y. S. Lopez-Boado, J. L. Stratman, S. J. Hultgren, L. M. Matrisian, and W. C. Parks. 1999. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science 286:113-117. [DOI] [PubMed] [Google Scholar]
- 54.Wimley, W. C., M. E. Selsted, and S. H. White. 1994. Interactions between human defensins and lipid bilayers: evidence for formation of multimeric pores. Protein Sci. 3:1362-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu, M., E. Maier, R. Benz, and R. E. Hancock. 1999. Mechanism of interaction of different classes of cationic antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli. Biochemistry 38:7235-7242. [DOI] [PubMed] [Google Scholar]
- 56.Yang, D., O. Chertov, and J. J. Oppenheim. 2001. Participation of mammalian defensins and cathelicidins in anti-microbial immunity: receptors and activities of human defensins and cathelicidin (LL-37). J. Leukoc. Biol. 69:691-697. [PubMed] [Google Scholar]
- 57.Yang, L., T. M. Weiss, R. I. Lehrer, and H. W. Huang. 2000. Crystallization of antimicrobial pores in membranes: magainin and protegrin. Biophys. J. 79:2002-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yasin, B., W. Wang, M. Pang, N. Cheshenko, T. Hong, A. J. Waring, B. C. Herold, E. A. Wagar, and R. I. Lehrer. 2004. Theta defensins protect cells from infection by herpes simplex virus by inhibiting viral adhesion and entry. J. Virol. 78:5147-5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zanetti, M., R. Gennaro, and D. Romeo. 1995. Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 374:1-5. [DOI] [PubMed] [Google Scholar]