Abstract
The high antibacterial activity and selectivity of aminoglycosides and their low activity against intracellular bacteria associated with eukaryotic cells make them the antibiotics of choice for the elimination of extracellular bacteria during intracellular studies. Given the evidence that aminoglycosides can penetrate the eukaryotic cell membrane, the goal of this study was to examine the influence of aminoglycosides on macrophage-associated Salmonella. Herein, we show that gentamicin, kanamycin, and tobramycin at concentrations between 15 to 150 μg ml−1 do not kill intracellular Salmonella but have other effects on the bacterial physiology. By using Salmonella enterica serovars Typhimurium and Virchow harboring luciferase reporter plasmid, we observed that the light produced by intracellular Salmonella declined immediately upon exposure to aminoglycosides, indicating that the bacteria were under stress. The extent of this effect was dependent on the macrophage host, on the identity of the aminoglycoside and its concentration, on the exposure time, and on the Salmonella serovar. Salmonella associated with Nramp1-negative macrophages, in which the phagosomal pH is higher, were more susceptible to aminoglycosides than Salmonella associated with Nramp1-expressing macrophages. These results verify that aminoglycosides affect intracellular bacteria and that the extent of this effect is dependent on the acidity level within the phagosome, suggesting that for the study of intracellular bacteria, the aminoglycoside concentration should be limited to two to five times the MIC for the bacterial strain studied. This precaution should guarantee the complete execution of extracellular bacteria with minimal effects on the intracellular bacteria and the host cells.
Aminoglycosides are broad-spectrum antibiotics that exert their bactericidal activity by selectively binding to the bacterial ribosome and interfering with protein synthesis (22, 39). These antibiotics are considered gold standard drugs against serious bacterial pathogens. On the other hand, they fail to treat intracellular diseases, such as chlamydial disease (3, 18, 25), or to kill intracellular bacteria, such as Staphylococcus aureus and Salmonella enterica, in vitro (18, 33). This result was attributed mostly to the reduced permeability of aminoglycosides through the eukaryotic cell membrane (3, 21) which, in turn, led researchers to develop several techniques to investigate in vitro the invasion and intracellular persistence of bacterial pathogens such as Shigella, Salmonella, Listeria, and Yersinia (7, 23, 27, 28, 35). All these techniques share a common step, an incorporation of aminoglycosides (usually gentamicin) into the medium for removal of the free bacteria and for consistent prevention of growth of extracellular bacteria in the infected eukaryotic cell cultures, while the intracellular pathogens are protected (21).
Other evidence suggested that aminoglycosides are able to penetrate the eukaryotic host cells and can even bind to the A site of the eukaryotic ribosome, promoting mistranslation (4, 12, 41). Aminoglycosides are thought to penetrate the eukaryotic cell through pinocytosis and to reach the phagosomes by fusion of the endosomes with the phagosomes (10). Therefore, the antibiotics that accumulate within the phagosomes may affect the growth and survival of intraphagosomal pathogens (2, 25). Moreover, small amounts of antibiotics can be released into the cytosol of specific eukaryotic cells (i.e., kidney cells) via the endoplasmic reticulum (31) or penetrate the cells via endosome-independent mechanisms, such as ion channels, transporters, or pores in the plasma membrane (26).
These observations triggered us to hypothesize that at certain intracellular concentrations aminoglycosides might affect the pathogens. To these ends, we developed an assay to follow and quantify in real time the activity of aminoglycosides against intracellular S. enterica. Our purpose was to analyze their antibacterial activity, as well as to explore their other possible effects on the intracellular pathogen. S. enterica is a gram-negative pathogen that causes a wide variety of illnesses, including systemic diseases, in multiple hosts. It is able to invade, survive, and replicate within the phagosome of macrophages and in other types of cells. Although aminoglycosides are widely used by researchers during intracellular studies of S. enterica, only the antibacterial activity of aminoglycosides, determined by viable counts, was examined, providing a limited scope for aminoglycoside effects (18). Other potential actions of these antibiotics had rarely been examined.
MATERIALS AND METHODS
Bacterial strains, plasmids, and eukaryotic cell lines.
S. enterica serovar Typhimurium ATCC 14028 and S. enterica serovar Virchow 101591 (a clinical isolate) (40) were used. The bacteria were transformed with the pChν1 plasmid, which carries the entire Vibrio fischeri luciferase system, and luxABCDE and luxIR operons (36, 37). The plasmid pGFP (Clontech, Palo Alto, CA) was used to obtain green fluorescent protein (GFP)-labeled Salmonella in order to determine the yield of infection.
Macrophage-like cell lines used in this study were J774A.1, RAW 264.7 R37, which expresses functional natural resistance-associated macrophage protein 1 (Nramp1+), and RAW 264.7 R21, which is deficient in a functional Nramp1 (Nramp1−) (5). All cell lines were a gift from B. Z. Levi (Technion).
Determination of aminoglycoside MICs in the broth.
A broth dilution method was carried out to determine the MICs of gentamicin, kanamycin, and tobramycin. Overnight cultures of Salmonella were diluted (1:100) in fresh Luria-Bertani (LB) broth, adjusted with phosphate buffer to pH 7 or 5.7, and aliquoted into 96-well microtiter plates that contained increasing concentrations of the antibiotics (0 to 500 μg ml−1). The final inoculum contained approximately 106 CFU ml−1. The absorbance of the cultures (at 600 nm) was measured after 24 h of incubation at 37°C with shaking.
Determination of aminoglycoside EC50s in the broth.
Overnight cultures of Salmonella harboring the pChν1 plasmid were diluted (1:100) in fresh LB broth containing 30 μg ml−1 chloramphenicol (pH 7 or pH 5.7) and aliquoted into 96-well, clear-bottomed, white microtiter plates to obtain a final titer of approximately 106 CFU ml−1 in medium with increasing concentrations of gentamicin, kanamycin, or tobramycin (0 to 500 μg ml−1). After 1 h of incubation at 30°C, homoserine-lactone (100 ng ml−1) was added in order to induce light emission, and plates were incubated in a luminometer (Victor2; PerkinElmer) at 30°C with shaking. Light emission was measured every 5 min to identify the time points at which emission was maximal and steady. The effective antibiotic dose that reduced half of the light emission (EC50) was calculated from a graph of light emission after 60 min versus antibiotic concentration.
Influence of aminoglycosides on light production by planktonic Salmonella.
The time needed to observe a significant reduction in light emission was studied. In these experiments, homoserine-lactone (100 ng ml−1) was added 20 min prior to the addition of antibiotics in order to achieve equilibrium before the antibiotic treatment. Bacteria were then treated with 8, 15, 30, 50, 100, and 150 μg ml−1 of each antibiotic. Light emission was measured immediately after adding the antibiotic and at subsequent 5-min intervals.
Culture preparations and infection.
The macrophages were grown in RPMI medium (Sigma) supplemented with 10% heat-inactivated fetal calf serum, 40 μM β-mercaptoethanol, 50 U ml−1 penicillin, and 50 μg ml−1 streptomycin (Biological Industries, Ltd., Israel). Prior to infection, the medium was replaced with antibiotic-free RPMI solution. Macrophages (2 ml) were seeded on six-well plates at approximately 105 cells ml−1 and incubated overnight in 5% CO2 at 37°C. The approximate final density was 5 × 105 cell ml−1. Viable cells were counted using trypan blue.
Bacterial cultures harboring either the pChν1 or the pGFP plasmid were prepared by dilution (1:100) of cultures grown overnight in RPMI medium containing chloramphenicol or ampicillin and incubated with shaking at 37°C to an optical density at 600 nm of approximately 1. Bacteria were harvested, washed, and resuspended in antibiotic-free RPMI medium. The final concentration of Salmonella was adjusted so that the ratio of bacteria to macrophages would be 100:1. This 100:1 multiplicity of infection was selected over other ratios tested (1:10, 1:50, 1:200, 1:500) because it was the minimal multiplicity of infection that gave the highest percentages of infected macrophages. High infection rates were needed to obtain detectable levels of bioluminescence when antibiotics were added.
Infection of the macrophages was obtained by a method described previously with a few modifications (9, 29). Following infection, the macrophages were washed with phosphate-buffered saline (PBS), incubated with fresh RPMI medium supplemented with 5 μg ml−1 polymyxin B (five times the MIC) for 10 min at 37°C to remove the extracellular bacteria, and washed twice with PBS. Polymyxin B has been used for invasion studies of different pathogens, usually when the bacteria are resistant to gentamicin (1, 8, 9, 16, 19, 32). Plates of macrophages infected with GFP-expressing Salmonella were visualized with an epifluorescence microscope (TE 2000-S, T-SAM; Nikon, Japan) to calculate the efficacy of infection. Under these conditions, about 55% of the macrophages were infected with about 106 bacteria, and we did not observe extracellular bacteria in the microscope images. Additional tests to determine the number of extracellular bacteria that remained in the medium were conducted by plate counting of the washing solution. The bacterial counts of the washing solution were below 0.04% of the intracellular counts; thus, the contribution of the extracellular bacteria to the results was negligible.
Viable counts of intracellular bacteria.
Bacteria were released from the macrophages by using lysis buffer (1% Triton X-100, 20 mM Tris, 0.2 M NaCl, 2 mM EDTA). The lysates of the macrophages containing the released bacteria were serially diluted (1:10 in PBS) and plated on LB agar for bacterial counting.
Influence of aminoglycosides on light production by intracellular Salmonella.
Infected macrophages were resuspended in antibiotic-free RPMI medium and incubated at 37°C for 10 min in the case of J774 and for 10 to 150 min in the case of RAW cells. Next, 500 ng ml−1 homoserine lactone was added to the medium. Following an incubation of 20 min, the antibiotic was added in concentrations of 15, 50, 100, and 150 μg ml−1, and the cells were reincubated for an additional 5 min. Finally, the plates were sealed with tape to prevent CO2 leakage and were incubated in the laminator. Every 5 min, up to 40 or 60 min, orbital shaking was performed for 5 s followed by a luminescence measurement. In each plate, three wells contained an aminoglycoside-treated culture (of a single concentration), and three wells contained the untreated control culture. Each experiment was conducted at least three times.
Data were analyzed with Microsoft Excel version 7 and statistically processed using the one-way analysis of variance (ANOVA) method followed by Tukey-Kramer multiple comparison tests. The two-tailed P values were reported, and P values of ≤0.01 were regarded as significant.
RESULTS
Comparison between MICs and EC50s within the broth.
Our initial goal was to tune the luminescence-based assay so that it could be applied to the investigation of aminoglycoside-induced effects on intracellular pathogens. Bacterial light emission was used as a reporter for protein expression rates and for the general bacterial physiology, since the expression of the luciferase proteins is expected to decrease when damaged proteins are produced (direct effect), and their activity might decline when the bacteria are subjected to energetic stress or other indirect effects (38). Therefore, to evaluate the feasibility of this luminescence-based assay as a reporter for the effects of aminoglycosides, we compared the MICs to the EC50s of three aminoglycosides, gentamicin, tobramycin, and kanamycin, against two S. enterica serovars, Typhimurium and Virchow. Serovar Typhimurium ATCC 14028 has been widely used for studying host-pathogen interactions. Serovar Virchow is a clinical isolate that is slightly less susceptible to antibiotics and produces more intense light than serovar Typhimurium. A strong correlation was observed between the MICs and EC50s for both serovars (EC50 = 0.367 × MIC; R2 = 0.9) (Table 1). For each antibiotic, the EC50 was always below the MIC, indicating that the bioluminescence assay is a good reporter system for the physiology of the cells; not only are the results obtained immediately but also the bioluminescence assay is more sensitive than the measurements of the inhibition of bacterial growth.
TABLE 1.
Aminoglycoside MICs and EC50s for serovars Virchow and Typhimurium in natural and acidic LB brotha
| Antibiotic | Serovar Virchow
|
Serovar Typhimurium
|
||||||
|---|---|---|---|---|---|---|---|---|
| MIC
|
EC50
|
MIC
|
EC50
|
|||||
| pH 7.0 | pH 5.7 | pH 7.0 | pH 5.7 | pH 7.0 | pH 5.7 | pH 7.0 | pH 5.7 | |
| Gentamicin | 8 | 125 | 2.0 (0.5) | 85 (7) | 4 | 125 | 1.0 (0.5) | 34 (5) |
| Kanamycin | 16 | 250 | 6.5 (1.5) | 130 (14) | 8 | 250 | 3.5 (2.5) | 29 (6) |
| Tobramycin | 8 | 250 | 2.5 (1.5) | 75 (7) | 4 | 125 | 1.0 (0.5) | 19 (6) |
EC50s were measured at 1 h; standard deviations are shown in parentheses. All data are mean concentrations (μg ml−1) from three experiments.
Since the environment within the phagosomes is usually acidic (30), and the antibacterial activity of aminoglycosides is inhibited by low pH (42), we sought to test the feasibility of the bioluminescence assay in an acidic environment (pH 5.7) (Table 1). The antibiotics affected both the growth of Salmonella and the light production at pH 5.7, an effect which was significantly poorer than at pH 7.0 (P < 0.001). The correlation between the EC50 and the MIC was very good, although as expected, these values were approximately 15- to 40-fold higher at pH 7.0 than at pH 5.7.
Aminoglycosides affect Salmonella in a broth within minutes.
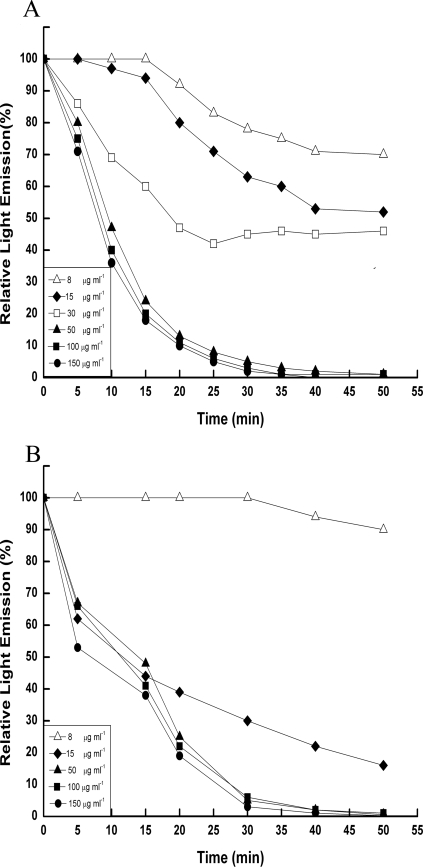
The time course for the effects of aminoglycosides on light production of planktonic bacteria was determined at different concentrations of the antibiotics. A rapid decrease in light production was observed for both Salmonella serovars within less than 5 min, while in the untreated control cells, the light emission was stable. Figure 1 illustrates the results obtained with gentamicin as a representative antibiotic. The light emission at the higher concentrations of gentamicin (≥50 μg ml−1 for serovar Virchow and >30 μg ml−1 for serovar Typhimurium) declined to undetectable levels within 40 min or less. The lower concentrations of gentamicin (<50 μg ml−1 for serovar Virchow and ≤30 μg ml−1 for serovar Typhimurium) were less effective, and the light intensity was stabilized at low but detectable levels.
FIG. 1.
Kinetic study of light emission during exposure to gentamicin in broth. Ratio of light emission (%) between gentamicin-treated bacteria and untreated control bacteria. (A) Serovar Virchow. (B) Serovar Typhimurium. Bacteria were incubated in LB broth for 30 min, and then the inducer, homoserine lactone (100 ng ml−1), was added to produce a basal level of light emission. Gentamicin (8 to 150 μg ml−1) was added 30 min after induction. Light emission was measured immediately and every 5 min.
Effects of aminoglycosides on intracellular Salmonella.
To determine the effects of aminoglycosides on intracellular Salmonella, J774 macrophages were infected by Salmonella and treated with gentamicin, tobramycin, or kanamycin for 1 h. Several preliminary experiments to establish optimal experimental conditions for phagocytosis, elimination of extracellular bacteria, and bioluminescence detection were carried out. The final experimental assay is described in Materials and Methods. The treated macrophages were lysed, and the supernatants were plated to estimate viable bacteria. When we used a 50 μg ml−1 or lower concentration of aminoglycoside, the bacterial viable counts did not change significantly. Higher antibiotic concentrations (up to 150 μg ml−1) resulted in only a 1-log reduction in viable counts (P < 0.01). Note that such high concentrations of antibiotics (150 μg ml−1) resulted in a >6-log reduction in viable counts of Salmonella in the broth, to undetectable levels. We did not increase the antibiotic concentration beyond 150 μg ml−1, because higher concentrations also changed the morphology of the host cells.
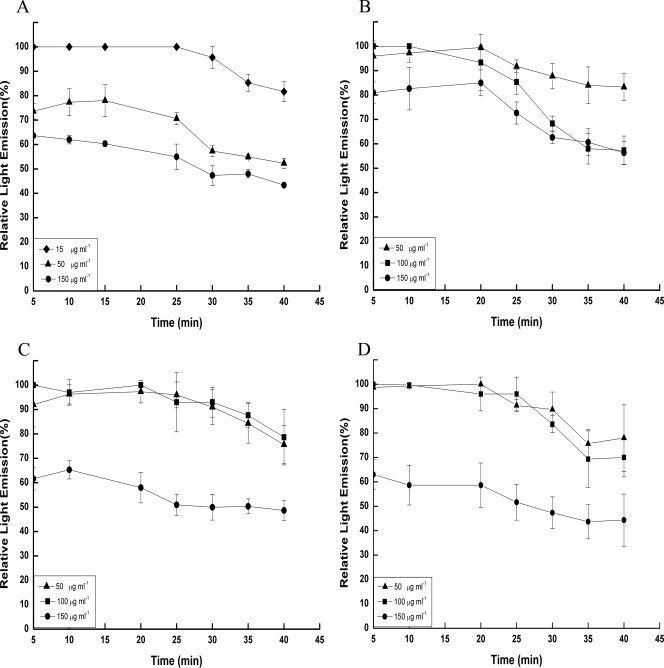
It is highly noteworthy that, in contrast to the small effect of aminoglycosides on the viability of intracellular Salmonella, we observed a substantial decrease in the intensity of light production even with the lower concentrations (≤15 or 50 μg ml−1, depending on the serovar or antibiotic) (Fig. 2). The extent of emitted light was dependent on the initial antibiotic concentrations. The experiments were terminated after 40 min of exposure to the antibiotics, because after a longer incubation time, the light intensity started to decline also in the untreated control experiments, probably due to a reduction in the available concentrations of the inducer. The light emitted by intracellular serovar Typhimurium treated with 15 μg ml−1 gentamicin started to decrease after about 30 min of exposure to the antibiotic, achieving less than 20% reduction after 40 min. Treatment with 50 μg ml−1 gentamicin or higher concentrations resulted in a steeper reduction of light emission. The first reduction step was observed within less than 5 min, and the second was observed after approximately 30 min (Fig. 2A). However, light intensity was still detectable even after 40 min. A similar effect was also observed for serovar Virchow, although the time necessary to observe a substantial change in the emitted light was generally longer (Fig. 2B). Similar results were also observed with the other two antibiotics, although they were more effective in reducing their light during the first 5 min (Fig. 2C and D). These results demonstrate that aminoglycosides affect intracellular bacteria and that this effect is concentration dependent. Since the rates of light reduction were lower than those in the broth with the same antibiotic concentration, we assume that the effective intracellular antibiotic concentrations were lower than those in the medium.
FIG. 2.
Light emission of aminoglycoside-treated intracellular (within J774 macrophages) serovar Typhimurium and serovar Virchow harboring the pChν1 plasmid in comparison to untreated bacteria. Results are expressed as the ratio of light emission (%) between the antibiotic-treated cultures and the untreated control cultures. (A) Serovar Typhimurium treated with gentamicin. (B) Serovar Virchow treated with gentamicin. (C) Serovar Virchow treated with tobramycin. (D) Serovar Virchow treated with kanamycin. Each trial was conducted at least three times in triplicate. Bars represent the standard deviations for the experiments.
Effects of aminoglycosides on Salmonella viability and on light production within Nramp1+ and Nramp1− macrophages.
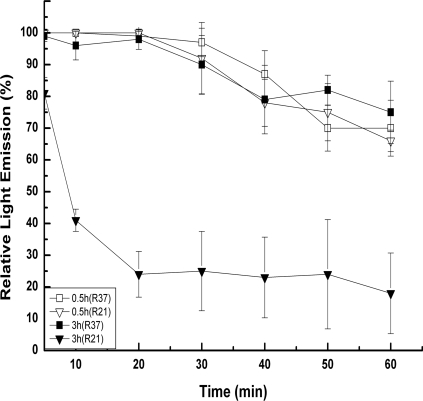
Nramp1 is a divalent metal transporter that is located in the phagosome membrane of infected macrophages. Nramp1 plays a direct role in the control of phagosomal acidification after internalization of the pathogens. Mutations in Nramp1 result in increased susceptibility to intracellular pathogens and an increase of the phagosome pH from 5.5 to 6.6 or from 5.1 to 5.8 (14, 15). In order to investigate the activity of gentamicin against intracellular Salmonella in a less-acidic environment, we compared the bacterial viability and light production levels after infection of Nramp1− and Nramp1+ RAW 264.7 macrophages. Although Nramp1− cells are expected to be more sensitive to Salmonella (14), we found that Salmonella associated with Nramp1− was more sensitive to gentamicin. About 0.4- and 1.6-log reductions were obtained for the intracellular counts of Salmonella after treatment of infected Nramp1+ and Nramp1−, respectively, with 100 μg ml−1 gentamicin (P < 0.01). When gentamicin was added to RAW 264.7 a short time after infection with Salmonella (30 to 90 min), the light declined, but the time until we observed a significant reduction (about 20 min) was longer than with the infected J774 macrophages and the magnitude of light reduction was lower (Fig. 3). In these short experiments, Salmonella behaved similarly in Nramp1+ and Nramp1− cell lines. However, when we added the antibiotic 2 h after infection, a difference was observed between Nramp1+ and Nramp1− cell lines, which was even more significant after 3 h (Fig. 3). In this case, the light emitted by Salmonella within Nramp1− declined immediately and reached a maximal reduction of 80%, while in Nramp1+, light emission started to decline after 20 min and reached a maximal reduction of 25% (Fig. 3).
FIG. 3.
Light emission of gentamicin-treated serovar Typhimurium in Nramp1+ (R37) and Nramp1− (R21) RAW 264.7 macrophages. RAW 264 macrophages were infected with serovar Typhimurium and were treated with gentamicin (100 μg ml−1) 0.5 h and 3 h after infection. Results are expressed as the ratio of light emission (%) between the gentamicin-treated cultures and the untreated control cultures of Salmonella in R37 or R21. Each trial was conducted at least three times in triplicate. Bars represent the standard deviations for the experiments.
DISCUSSION
The effects of aminoglycosides on S. enterica serovars located inside macrophages were investigated by employing the highly sensitive luciferase reporter system. In agreement with previous reports (2, 18, 33), the observed data demonstrate that, even at the higher concentrations tested (up to 35 times the MIC), aminoglycosides do not have a significant bactericide effect on the intracellular bacteria. However, the results clearly indicate that under the experimental conditions used, the aminoglycosides affect the bacteria in other ways.
We observed significant changes in the total amount of light emitted by S. enterica serovars Typhimurium and Virchow within macrophages upon exposure of aminoglycosides. Note that although parts of these observations were reported in the past in control studies of intracellular serovar Typhimurium, the observed effects did not receive sufficient interpretation and attention (13). By tuning the experimental conditions appropriate for utilizing the luciferase assay for quantitative characterization of aminoglycoside-induced effects on intracellular bacteria, we further showed that these effects occurred rapidly (within 5 to 30 min) for both J774 and RAW 264 macrophages. The time course and the degree of the reduction of emitted light were different for each type of host cell, probably because each cell line regulates differently the penetration and the intracellular location of the aminoglycosides (10, 20, 26, 31). Other important variables were the identity of aminoglycoside and its concentration, as well as the Salmonella serovar and its susceptibility to the particular antibiotic.
Gentamicin, for example, is thought to enter eukaryotic cells through pinocytosis. It is accumulated within the pinocytotic endosomes, reaching the phagosomes by fusion of the endosomes with the pathogen-containing phagosomes (10). Why, then, do we observe only a very low killing effect and partial reduction in light emission in the presence of gentamicin? The main reason for such a low bactericidal activity cannot be attributed to very low concentrations of antibiotics within the host, since it was shown that the ratio between intracellular and extracellular gentamicin within macrophages is more than 0.5 (17). Since the majority of the Salmonella-containing phagosomes acidify within less than 60 min after their formation (30), the acidity within the phagosomes could be the main reason for the observed poor effects of gentamicin and of other aminoglycosides on the intracellular bacteria. This hypothesis is based on the well-known observation that the antimicrobial activity of aminoglycosides is pH dependent and is strongly inhibited at a low pH. Low pH changes the electrostatic potential across the bacterial cytoplasmic membrane, and this electrostatic potential is crucial for the uptake of aminoglycosides into the bacterial cell (2, 11, 34, 42). Thus, the low pH in phagosomes could affect both the uptake of aminoglycosides into the bacterial cell and their antibacterial activity. Indeed, a previous observation indicated that a direct increase in the pH of the phagosomes was sufficient to significantly increase the intracellular killing effect of Staphylococcus aureus by the aminoglycoside amikacin (24). In fact, our data support this hypothesis by showing that Salmonella within Nramp1− macrophages, which have a higher pH (14, 15), were more susceptible to gentamicin than Salmonella associated with Nramp1+ macrophages. Note that this difference between the two macrophage cell lines was seen not immediately but at about 2 h after infection, probably because of the time needed for the recruitment of functional Nramp1 to the membrane of the phagosome (14). While the diminished effects of aminoglycosides on intracellular bacteria are probably due mainly to the low pH of the phagosomes, it is possible that under the conditions within the phagosomes, aminoglycosides exhibit interactions with phospholipids and phosphoproteins in the host cells (6) which, in turn, further contribute to their reduced antibacterial activity performance.
It was demonstrated that about 20% of the Salmonella-containing phagosomes in murine macrophages do not undergo a pH change (30). Such a distribution of the pH within the phagosomes could explain the initial rapid decline—about 20%—in the light emission associated with the J774 macrophages and the persistence of about 50% of the light, which probably reflects the survival of Salmonella within phagosomes with a pH below 5.5.
Two alternative processes might explain the observed profiles of the light emitted by the macrophages-associated bacteria: (i) a direct penetration of the antibiotics through the phagosome into the bacteria and inhibition of the bacterial protein translation process; in this case, the light reduction is caused by direct inhibition of the translation of the luciferase proteins; (ii) an indirect effect that is caused by changes in the physiology of the macrophages which then affect the bacterial physiology; this effect might reduce light production by indirect inhibition of the translation of the genes or by causing energetic stress that reduces the bacterial ATP reservoir and influences the activity of the luciferase proteins. Based on the influence of the phagosomal pH on the activity of the antibiotics, we suggest that at least part of the light reduction is caused by a direct influence of aminoglycosides on the intracellular bacteria. However, final conclusions may be achieved by proteomic analysis of whole cells that will identify the bacterial proteins whose expression is influenced by aminoglycosides.
In summary, we have demonstrated that aminoglycosides affect macrophage-associated Salmonella. The extent of this effect depends on the aminoglycoside concentration and may occur in a short time period after treatment. Gentamicin is widely used to eliminate the extracellular bacteria in studies of intracellular pathogens. In these studies, it is common to incubate the infected culture with 50 to 150 μg ml−1 (in some cases, with 400 μg ml−1) of gentamicin for at least 1 h (7, 23, 27, 28, 35). According to our results, the treatment with gentamicin can affect the intracellular pathogen, and these effects might be different within different host cells at different stages of infection. For example, Salmonella located within less-acidic phagosomes or in the cytosol will be more susceptible than Salmonella within highly acidic phagosomes. In general, for the study of intracellular bacteria, we reinforce previous suggestions for other intracellular bacteria to limit the gentamicin concentration to less than five times the MIC for the specific bacterial strain. Particular attention should be paid when experimental conditions that might influence the pH of the phagosomes are used or when the pH of phagosomes in the host cells is less acidic, such as that for Nramp1 null mutants.
Acknowledgments
We thank Shimon Ulitzur and Jonathan Kuhn for kindly providing us with the pChν1 plasmid.
This work was supported by the Israel Science Foundation, founded by the Israel Academy of Sciences and Humanities (grant nos. 766/04 and 1275/04).
Footnotes
Published ahead of print on 2 January 2008.
REFERENCES
- 1.Abromaitis, S., S. Faucher, M. Beland, R. Curtiss III, and F. Daigle. 2005. The presence of the tet gene from cloning vectors impairs Salmonella survival in macrophages. FEMS Microbiol. Lett. 242:305-312. [DOI] [PubMed] [Google Scholar]
- 2.Barcia-Macay, M., C. Seral, M. P. Mingeot-Leclercq, P. M. Tulkens, and F. Van Bambeke. 2006. Pharmacodynamic evaluation of the intracellular activities of antibiotics against Staphylococcus aureus in a model of THP-1 macrophages. Antimicrob. Agents Chemother. 50:841-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonventre, P. F., R. Hayes, and J. Imhoff. 1967. Autoradiographic evidence for the impermeability of mouse peritoneal macrophages to tritiated streptomycin. J. Bacteriol. 93:445-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bottger, E. C., B. Springer, T. Prammananan, Y. Kidan, and P. Sander. 2001. Structural basis for selectivity and toxicity of ribosomal antibiotics. EMBO Rep. 2:318-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowen, H., T. E. Biggs, E. Phillips, S. T. Baker, V. H. Perry, D. A. Mann, and C. H. Barton. 2002. c-Myc represses and Miz-1 activates the murine natural resistance-associated protein 1 promoter. J. Biol. Chem. 277:34997-35006. [DOI] [PubMed] [Google Scholar]
- 6.Carlier, M. B., G. Laurent, P. J. Claes, H. J. Vanderhaeghe, and P. M. Tulkens. 1983. Inhibition of lysosomal phospholipases by aminoglycoside antibiotics: in vitro comparative studies. Antimicrob. Agents Chemother. 23:440-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan, K., C. C. Kim, and S. Falkow. 2005. Microarray-based detection of Salmonella enterica serovar Typhimurium transposon mutants that cannot survive in macrophages and mice. Infect. Immun. 73:5438-5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Craig, J. E., A. Cliffe, K. Garnett, and N. J. High. 2001. Survival of nontypeable Haemophilus influenzae in macrophages. FEMS Microbiol. Lett. 203:55-61. [DOI] [PubMed] [Google Scholar]
- 9.Craig, J. E., A. Nobbs, and N. J. High. 2002. The extracytoplasmic sigma factor, ςE, is required for intracellular survival of nontypeable Haemophilus influenzae in J774 macrophages. Infect. Immun. 70:708-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drevets, D. A., B. P. Canono, P. J. Leenen, and P. A. Campbell. 1994. Gentamicin kills intracellular Listeria monocytogenes. Infect. Immun. 62:2222-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenberg, E. S., L. J. Mandel, H. R. Kaback, and M. H. Miller. 1984. Quantitative association between electrical potential across the cytoplasmatic membrane and early gentamicin uptake and killing in Staphylococcus aureus. J. Bacteriol. 157:863-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eustice, D. C., and J. M. Wilhelm. 1984. Fidelity of the eukaryotic codon-anticodon interaction: interference by aminoglycoside antibiotics. Biochemistry 23:1462-1467. [DOI] [PubMed] [Google Scholar]
- 13.Francis, K. P., and M. P. Gallagher. 1993. Light emission from a Mudlux transcriptional fusion in Salmonella typhimurium is stimulated by hydrogen peroxide and by interaction with the mouse macrophage cell line J774.2. Infect. Immun. 61:640-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Govoni, G., F. Canonne-Hergaux, C. G. Pfeifer, S. L. Marcus, S. D. Mills, D. J. Hackam, S. Grinstein, D. Malo, B. B. Finlay, and P. Gros. 1999. Functional expression of Nramp1 in vitro in the murine macrophage line RAW264.7. Infect. Immun. 67:2225-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hackam, D. J., O. D. Rotstein, W. Zhang, S. Gruenheid, P. Gros, and S. Grinstein. 1998. Host resistance to intracellular infection: mutation of natural resistance-associated macrophage protein 1 (Nramp1) impairs phagosomal acidification. J. Exp. Med. 188:351-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henderson, S. C., D. I. Bounous, and M. D. Lee. 1999. Early events in the pathogenesis of avian salmonellosis. Infect. Immun. 67:3580-3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson, J. D., W. L. Hand, J. B. Francis, N. King-Thompson, and R. W. Corwin. 1980. Antibiotic uptake by alveolar macrophages. J. Lab. Clin. Med. 95:429-439. [PubMed] [Google Scholar]
- 18.Kihlstrom, E., and L. Andaker. 1985. Inability of gentamicin and fosfomycin to eliminate intracellular Enterobacteriaceae. J. Antimicrob. Chemother. 15:723-728. [DOI] [PubMed] [Google Scholar]
- 19.Lee, C. K., A. L. Roberts, T. M. Finn, S. Knapp, and J. J. Mekalanos. 1990. A new assay for invasion of HeLa 229 cells by Bordetella pertussis: effects of inhibitors, phenotypic modulation, and genetic alterations. Infect. Immun. 58:2516-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Pocher, H., P. Brouqui, and D. Raoult. 1998. Killing kinetics of intracellular Afipia felis treated with amikacin. J. Antimicrob. Chemother. 42:825-829. [DOI] [PubMed] [Google Scholar]
- 21.Lissner, C. R., R. N. Swanson, and A. D. O'Brien. 1983. Genetic control of the innate resistance of mice to Salmonella typhimurium: expression of the Ity gene in peritoneal and splenic macrophages isolated in vitro. J. Immunol. 131:3006-3013. [PubMed] [Google Scholar]
- 22.Magnet, S., and J. S. Blanchard. 2005. Molecular insights into aminoglycoside action and resistance. Chem. Rev. 105:477-498. [DOI] [PubMed] [Google Scholar]
- 23.Martin-Orozco, N., N. Touret, M. L. Zaharik, E. Park, R. Kopelman, S. Miller, B. B. Finlay, P. Gros, and S. Grinstein. 2006. Visualization of vacuolar acidification-induced transcription of genes of pathogens inside macrophages. Mol. Biol. Cell 17:498-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maurin, M., and D. Raoult. 1994. Phagolysosomal alkalinization and intracellular killing of Staphylococcus aureus by amikacin. J. Infect. Dis. 169:330-336. [DOI] [PubMed] [Google Scholar]
- 25.Maurin, M., and D. Raoult. 2001. Use of aminoglycosides in treatment of infections due to intracellular bacteria. Antimicrob. Agents Chemother. 45:2977-2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myrdal, S. E., K. C. Johnson, and P. S. Steyger. 2005. Cytoplasmic and intra-nuclear binding of gentamicin does not require endocytosis. Hear Res. 204:156-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan, Q., X.-L. Zhang, H.-Y. Wu, P.-W. He, F. Wang, M.-S. Zhang, J.-M. Hu, B. Xia, and J. Wu. 2005. Aptamers that preferentially bind type IVB pili and inhibit human monocytic-cell invasion by Salmonella enterica serovar Typhi. Antimicrob. Agents Chemother. 49:4052-4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parsons, D. A., and F. Heffron. 2005. sciS, an icmF homolog in Salmonella enterica serovar Typhimurium, limits intracellular replication and decreases virulence. Infect. Immun. 73:4338-4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rathman, M., L. P. Barker, and S. Falkow. 1997. The unique trafficking pattern of Salmonella typhimurium-containing phagosomes in murine macrophages is independent of the mechanism of bacterial entry. Infect. Immun. 65:1475-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rathman, M., M. D. Sjaastad, and S. Falkow. 1996. Acidification of phagosomes containing Salmonella typhimurium in murine macrophages. Infect. Immun. 64:2765-2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandoval, R. M., and B. A. Molitoris. 2004. Gentamicin traffics retrograde through the secretory pathway and is released in the cytosol via the endoplasmic reticulum. Am. J. Physiol. Renal Physiol. 286:F617-F624. [DOI] [PubMed] [Google Scholar]
- 32.Schaeffer, L. M., and A. A. Weiss. 2001. Pertussis toxin and lipopolysaccharide influence phagocytosis of Bordetella pertussis by human monocytes. Infect. Immun. 69:7635-7641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seral, C., F. Van Bambeke, and P. M. Tulkens. 2003. Quantitative analysis of gentamicin, azithromycin, telithromycin, ciprofloxacin, moxifloxacin, and oritavancin (LY333328) activities against intracellular Staphylococcus aureus in mouse J774 macrophages. Antimicrob. Agents Chemother. 47:2283-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taber, H. W., J. P. Mueller, P. F. Miller, and A. S. Arrow. 1987. Bacterial uptake of aminoglycoside antibiotics. Microbiol. Rev. 51:439-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takaya, A., T. Tomoyasu, H. Matsui, and T. Yamamoto. 2004. The DnaK/DnaJ chaperone machinery of Salmonella enterica serovar Typhimurium is essential for invasion of epithelial cells and survival within macrophages, leading to systemic infection. Infect. Immun. 72:1364-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ulitzur, S., and J. Kuhn. 1988. The transcription of bacterial luminescence is regulated by sigma 32. J. Biolumin. Chemilumin. 2:81-93. [DOI] [PubMed] [Google Scholar]
- 37.Ulitzur, S., A. Matin, C. Fraley, and E. Meighen. 1997. H-NS protein represses transcription of the lux systems of Vibrio fischeri and other luminous bacteria cloned into Escherichia coli. Curr. Microbiol. 35:336-342. [DOI] [PubMed] [Google Scholar]
- 38.Unge, A., R. Tombolini, L. Molbak, and J. K. Jansson. 1999. Simultaneous monitoring of cell number and metabolic activity of specific bacterial populations with a dual gfp-luxAB marker system. Appl. Environ Microbiol. 65:813-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vakulenko, S. B., and S. Mobashery. 2003. Versatility of aminoglycosides and prospects for their future. Clin. Microbiol. Rev. 16:430-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinberger, M., H. Solnik-Isaac, D. Shachar, A. Reisfeld, L. Valinsky, N. Andorn, V. Agmon, R. Yishai, R. Bassal, A. Fraser, S. Yaron, and D. Cohen. 2006. Salmonella enterica serotype Virchow: epidemiology, resistance patterns and molecular characterisation of an invasive Salmonella serotype in Israel. Clin. Microbiol. Infect. 12:999-1005. [DOI] [PubMed] [Google Scholar]
- 41.Wilhelm, J. M., J. J. Jessop, and S. E. Pettitt. 1978. Aminoglycoside antibiotics and eukaryotic protein synthesis: stimulation of errors in the translation of natural messengers in extracts of cultured human cells. Biochemistry 17:1149-1153. [DOI] [PubMed] [Google Scholar]
- 42.Xiong, Y. Q., J. Caillon, H. Drugeon, G. Potel, and D. Baron. 1996. Influence of pH on adaptive resistance of Pseudomonas aeruginosa to aminoglycosides and their postantibiotic effects. Antimicrob. Agents Chemother. 40:35-39. [DOI] [PMC free article] [PubMed] [Google Scholar]