Abstract
Clostridium perfringens-induced gas gangrene is mediated by potent extracellular toxins, especially alpha toxin (a phospholipase C [PLC]) and theta toxin (perfringolysin O [PFO], a thiol-activated cytolysin); and antibiotic-induced suppression of toxin synthesis is an important clinical goal. The production of PLC and PFO by a gatifloxacin-induced, fluoroquinolone-resistant mutant strain of C. perfringens, strain 10G, carrying a stable mutation in DNA gyrase was compared with that of the wild-type (WT) parent strain. Zymography (with sheep red blood cell and egg yolk overlays) and time course analysis [with hydrolysis of egg yolk lecithin and O-(4 nitrophenyl-phosphoryl)choline] demonstrated that strain 10G produced more PLC and PFO than the WT strain. Increased toxin production in strain 10G was not related either to differences in growth characteristics between the wild-type and the mutant strain or to nonsynonymous polymorphisms in PLC, PFO, or their known regulatory proteins. Increased PLC and PFO production by strain 10G was associated with increased cytotoxic activity for HT-29 human adenocarcinoma cells and with increased platelet-neutrophil aggregate formation. Four other gatifloxacin-induced gyrase mutants did not show increased toxin production, suggesting that gatifloxacin resistance was not always associated with increased toxin production in all strains of C. perfringens. This is the first report of increased toxin production in a fluoroquinolone-resistant strain of C. perfringens.
Clostridium perfringens is the most common pathogen isolated from patients with trauma-induced gas gangrene (9, 33). The local and systemic manifestations of gas gangrene are mediated by potent extracellular protein toxins, especially alpha toxin (phospholipase C [PLC]) and theta toxin (perfringolysin O [PFO]), a thiol-activated cytolysin (9, 33). PLC is a zinc metalloproteinase that possesses both PLC and sphingomyelinase activities (35). PFO is a cholesterol-dependent cytolysin that forms lytic pores in membranes after oligomerization (reviewed in reference 13). At high concentrations, both PLC and PFO are cytotoxic for multiple cell types. At sublytic concentrations, both toxins contribute to the pathogenesis of gas gangrene through their actions on host cell function (9). PLC contributes to the rapid progression of tissue destruction by inducing the formation of intravascular aggregates of platelets and leukocytes that block blood flow and cause tissue ischemia (7) and by inhibiting the tissue inflammatory response (8, 32, 34). In addition, PLC contributes to hemodynamic collapse by directly suppressing cardiac contractility (31). PFO has profound adverse effects on leukocyte and endothelial cell function (6) and indirectly mediates cardiac dysfunction, likely via the induction of a myocardial depressant factor (31).
Gatifloxacin is a newer broad-spectrum fluoroquinolone that has extended activity against clinically important anaerobes (30). However, resistance to gatifloxacin has recently been found among clostridial isolates (30).
Among 110 toxicogenic strains of C. difficile isolated from 1983 to 2004 in various hospitals, 17 strains were resistant to 8 μg or more of gatifloxacin (14).
In addition, hospital formulary changes from levofloxacin to gatifloxacin have resulted in increases in Clostridium difficile-associated diseases, which have been directly correlated with the duration of gatifloxacin therapy (12). Furthermore, a fluoroquinolone-resistant strain of highly virulent C. difficile with enhanced toxin production has been found in health care facilities (18, 21). Adams et al. (2) showed that gatifloxacin and moxifloxacin promoted more growth and toxin production in C. difficile than ciprofloxacin and levofloxacin.
C. perfringens antibiotic-associated diarrhea (AAD) has also been reported (1, 4, 5, 11, 15, 16, 19, 29). In one study, 92% of C. perfringens-positive stool specimens came from hospitalized patients who did have symptoms of food poisoning but who had received antibiotics, including fluoroquinolones, before the onset of diarrhea (37). Those authors concluded that since the detection of C. perfringens and its alpha toxin is not part of routine laboratory testing, the incidence of C. perfringens AAD may often be underreported.
Irrespective of the etiology, the pathogenesis of AAD is attributed to the disturbance of the intestinal ecological system (5, 16) and the increased production of potent cytotoxins. However, fluoroquinolone-induced toxin upregulation has not been investigated in strains of Clostridium. The exposure of C. perfringens to fluoroquinolones induces the formation of stable gyrase mutants that are highly resistant to gatifloxacin and other fluoroquinolones (24). In the present study, we report on the changes in PLC and PFO production observed in one gatifloxacin-resistant gyrase mutant strain of C. perfringens.
MATERIALS AND METHODS
Bacterial growth.
A highly fluoroquinolone-resistant mutant of C. perfringens, strain 10G (reported previously as Gat-NCTR-10 [24]), was developed by exposure of wild-type (WT) strain NCTR to increasing concentrations of gatifloxacin up to 10 μg/ml (24). Strain 10G has a double mutation in gyrA, resulting in the substitutions Gly81Cys and Asp87Tyr, which protect the cells from fluoroquinolones (24, 25). Both strain 10G and the WT strain were grown in brain heart infusion (BHI) medium in an anaerobic chamber under an atmosphere of 85% N2, 5% H2, and 10% CO2 (24). Growth was monitored spectrophotometrically under anaerobic conditions. After 24 h, the cultures were cleared of organisms by centrifugation and were sterilized by addition of penicillin and streptomycin (final concentrations, 10 U/ml and 100 μg/ml, respectively). The presence of PLC and PFO and the sterility of the exotoxin preparations were verified by duplicate plating on egg yolk agar and sheep blood agar plates, followed by 48 h of anaerobic and aerobic incubation. For some applications, the proteins in the cleared supernatants were precipitated with 70% ammonium sulfate, followed by extensive dialysis against phosphate-buffered saline (PBS). Protein concentrations were determined by a Bio-Rad (Hercules, CA) assay with bovine serum albumin as the standard. The exotoxin preparations were divided and frozen at −70°C until use.
Detection of PLC and PFO activities.
PLC activity in stationary-phase culture supernatants of the WT and mutant C. perfringens strains was qualitatively assessed by standard zymographic techniques with egg yolk lecithin agar overlays of nondenaturing 6% polyacrylamide gels containing 10 μg total protein/lane. The overlays were incubated at 37°C, and the zones of diacylglycerol precipitation for the egg yolk plate were photographed.
PFO activity was also detected by zymography, as described above for PLC, except that 0.5% sheep red blood cell agar overlays containing PFO buffer (2 mM EDTA, 20 mM dithiothreitol, 64 mM KH2PO4, 150 mM NaCl, pH 6.8) were used (23). In addition, PFO was quantitated by measurement of the release of hemoglobin from sheep red blood cells in the presence of PFO buffer. Briefly, the prepared exotoxins were mixed with washed, packed sheep red blood cells (25:75, vol/vol), followed by anaerobic incubation at 37°C for 1 h. Sterile BHI medium was used as a control. Cell debris was removed by centrifugation at 1,000 × g for 5 min, and the amount of hemoglobin in the supernatant was measured spectrophotometrically at 550 nm. One unit of PFO activity (1 hemolytic unit [HU]) was defined as the difference in the absorbance at 550 nm between the bacterial samples and the control per microgram of protein present in each sample (23).
Kinetic analysis of PLC activity.
The kinetics of PLC-induced hydrolysis of O-(4-nitrophenyl phosphoryl)choline (NPPC) were measured by the method of Kurioka and Matsuda (17). Briefly, a 100-μl sample of exotoxins prepared from the WT or mutant C. perfringens strain was added, with or without NPPC (final concentration, 1.25 mg/ml in 100 μl of PBS), to duplicate wells of a 96-well microtiter plate. Appropriate uninoculated medium samples, also with and without NPPC, were used as negative controls. Recombinant PLC (rPLC; 31,100 U/ml; specific activity, 19,438 U/mg; kindly provided by Richard Titball, Defense Science and Technology Laboratory, Porton Down, Salisbury, United Kingdom) served as a positive control. A standard curve was prepared by dilution of 4-nitrophenol (Sigma, St. Louis, MO) and was used to determine the amount of PLC activity in each sample tested. The kinetics of 4-nitrophenol release at 37°C was monitored at 30-min intervals over 24 h by measuring the absorbance at 410 nm in a temperature-controlled PowerWave X spectrophotometer (Bio-Tek Instruments, Winooski, VT).
The kinetics of egg yolk lecithin hydrolysis were similarly assessed. Ammonium sulfate-concentrated exotoxins (0.8 μg in a total volume of 100 μl PBS) from the WT or 10G strain were added to duplicate wells of a microtiter plate. Concentrated uninoculated BHI medium, with or without rPLC, was tested in parallel. Egg yolk emulsion (10% in PBS; 100 μl/well) was added, and the plates were incubated at 37°C in the spectrophotometer. The hydrolysis of lecithin was monitored every 2 min for 1 h at 620 nm. The dynamics of NPPC and lecithin hydrolysis were plotted with Microsoft Excel software.
Effects of C. perfringens exotoxins on HT-29 cells.
The effects of C. perfringens exotoxins on human colon carcinoma cells were measured according to the methods of Arimochi et al. (3), except that the supernatants were not heat inactivated. Briefly, cells of the HT-29 cell line (a human colon carcinoma cell line; American Type Culture Collection, Manassas, VA) were maintained in continuous culture in McCoy's 5A medium (Invitrogen, Carlsbad, CA) with penicillin and streptomycin, as recommended by the supplier. One day before experimentation, the cells were harvested with trypsin-EDTA. The concentration was adjusted to 5 × 106 cells/ml, and 1 ml was plated into duplicate wells of a 24-well tissue culture plate. The next day, the culture medium was replaced with a solution containing bacterial supernatants (80 μl) in 420 μl of McCoy's 5A medium (Invitrogen) containing 1% fetal bovine serum (HyClone, Logan, UT) and 0.004% neutral red dye (Sigma). The plates were incubated in a CO2 cell culture incubator overnight at 37°C. The medium containing excess dye was removed, and the cells were washed three times with PBS. The cell-associated dye was extracted with 200 μl/well of phosphate-buffered ethanol (0.1 M Na2HPO4-ethanol, 1:1, vol/vol). The well contents were collected and briefly centrifuged, and the amount of dye that was extracted was measured by determination of the absorbance at 460 nm.
Platelet-neutrophil complex formation.
The platelet-neutrophil complex formation induced by exotoxins in the bacterial supernatants from the WT or 10G strain was measured in whole blood by flow cytometry, as described previously (7). Briefly, heparinized whole blood was obtained from healthy human volunteers who had given signed informed consent and who had denied taking any medication for the previous 10 days. Blood (100 μl) was mixed with 10 μl each of fluorescein isothiocyanate (FITC)-conjugated anti-human CD42b (a panplatelet marker; green; Coulter, Hialeah, FL) and phycoerythrin (PE)-conjugated anti-human CD11b (a neutrophil marker; red; Coulter) or the relevant conjugated control antibodies. One unit of rPLC (diluted in PBS containing 2.0 mM CaCl2 and 100 μM ZnCl2) or each bacterial supernatant either alone or in combination with neutralizing anti-PLC antibody (clone 1C6; final concentration, 20 μg/ml; kindly provided by Hiroko Sato, Japanese National Institute of Health, Tokyo, Japan [26]) was added and the tubes were incubated for 10 min at 37°C. Red blood cells were removed by formic acid lysis, and the remaining cells were fixed in 2% (vol/vol) paraformaldehyde in PBS for flow cytometric analysis on an Epics XL flow cytometer (Beckman Coulter, Fullerton, CA). Polymorphonuclear leukocytes (PMNLs) were gated by use of the characteristic forward and side scatter, and their purity was assessed by red fluorescent staining (PE-conjugated anti-CD11b). The percentage of PMNLs bearing platelets (i.e., dual-color-positive events) and the mean fluorescence intensity of the green platelet marker (FITC-conjugated CD42b; a measure of the number of platelets/PMNL) were evaluated for an average of 10,000 events.
Amplification of plc, pfo, and their regulatory genes for DNA sequencing.
DNA was extracted by the method described previously (24). The primers used for amplification of plc and pfo and their known regulatory genes, virR and virS, are listed in Table 1. One microgram of DNA was used in a reaction mixture consisting of the primers and the other reagents (24). The PCR conditions were initial heating at 95°C for 2 min, followed by 30 cycles of denaturation at 95°C for 30 s, annealing at 48°C for 1 min, and extension at 72°C for 2 min and 1 cycle of 72°C for 7 min for final extension. After electrophoresis, the DNA amplicons were purified with a QIAquick gel extraction kit (Qiagen Sciences, Georgetown, MD), and the DNA was sequenced with an Applied Biosystems (Foster City, CA) sequencer and an Applied Biosystems dideoxy terminator.
TABLE 1.
Primers used for the amplification and sequencing of pfo, plc, and vir genes from C. perfringens
| Gene location | Sequence |
|---|---|
| pfo −372 Fa | TATTCACGATTAAAGCCCAGTTCT |
| pfo 586R | AAGGGCACTTGATATTTGTGATTT |
| pfo 413 F | AAGGGTGAAAATAGCATAAAGGTT |
| pfo 1341 R | TTGGCTTCAAGAGGTATTACTGTT |
| pfo 1265 F | TTAACTCATAAAACATGGGATGG |
| pfo 1666R | CTCGGCTTCATAAACACTTTT |
| cpa −493F | CTTCATTAAGCCCAAGTTC |
| cpa 1317R | TAAAGTAATAATCCCTAAATCTC |
| vir −682F | TTTGGTAGAAGTTTGTTTTTGTGA |
| vir −222F | TGGGCTGGGTGAAAAATG |
| vir 300F | AACTGCTTTATGGGATTA |
| vir 766F | AATGTTTTGGGACTTCA |
| vir 2076R | TAAATAACAAAAAGAAGCGTAGAT |
The minus sign indicates the number of bases upstream from the plc and pfo regulatory genes, virR and virS.
RESULTS
Bacterial growth and toxin production.
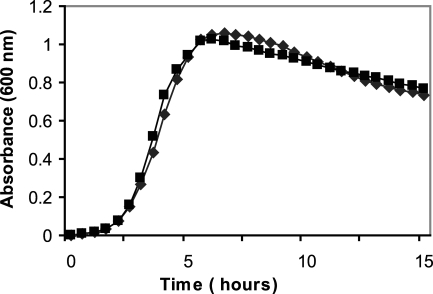
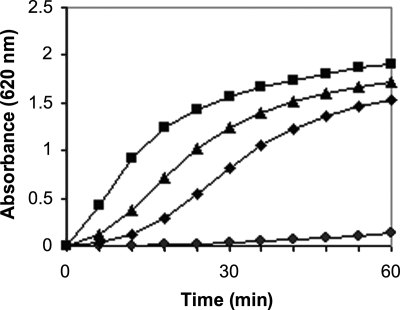
Strain 10G, the gatifloxacin-resistant mutant strain of C. perfringens, grew equally as well in BHI as WT strain NCTR (Fig. 1). The same volume of supernatant from the mutant produced larger zones of diacylglycerol precipitation and red blood cell hemolysis than that from the WT strain on egg yolk and sheep blood agars, respectively. When equal amounts of total extracellular protein from the WT and 10G strains were run on nondenaturing gels, zymograms with egg yolk agar overlays demonstrated a larger zone of precipitation for the mutant, indicating the increased production of PLC by this strain (Fig. 2). Quantitation of the PLC activity by NPPC and egg yolk lecithin hydrolysis (Fig. 3 and 4, respectively) confirmed this finding. Specific PLC activities in the mutant and WT supernatants were 11 and 3 units per milligram of total protein, respectively.
FIG. 1.
Growth of the C. perfringens WT strain (⧫) and gatifloxacin-resistant mutant 10G (▪) in BHI medium under anaerobic conditions at 37°C.
FIG. 2.
Zymographic comparison of PLC and PFO activities in extracellular proteins of the WT and gatifloxacin-resistant mutant of C. perfringens. (Left panel) Lysis of red blood cells by PFO; (right panel) precipitation of diacylglycerol following egg yolk lecithin hydrolysis by PLC.
FIG. 3.
PLC-induced hydrolysis of NPPC by either 50 μl (⧫) or 100 μl (•) of an unconcentrated stationary-phase culture supernatant from the gatifloxacin-resistant mutant of C. perfringens (10G), 100 μl (▪) of the identically prepared culture supernatant from the WT C. perfringens strain, or 1 U (▴) of rPLC.
FIG. 4.
PLC-induced hydrolysis of egg yolk lecithin by 0.8 μg (▪) of ammonium sulfate-concentrated protein from the gatifloxacin-resistant mutant of C. perfringens (strain 10G) or its WT parent strain (•) or 1.0 (▴) or 0.5 U (⧫) of rPLC.
PFO activity was also increased in mutant strain 10G, as evidenced by the larger regions of beta-hemolysis in the red blood cell zymogram (Fig. 2). Quantitation of PFO by a red blood cell-hemoglobin release assay confirmed this finding (6.70 and 2.47 HU per μg of protein in the mutant and WT strains, respectively).
Biological effects of WT and mutant C. perfringens exotoxins.
When the biological effects of the WT and mutant exotoxins were assessed by the HT-29 cell cytotoxicity assay and by measurement of the toxin-induced formation of platelet-neutrophil complexes, more cytotoxicity was observed in HT-29 cells treated with exotoxins from mutant strain 10G than in those treated with toxins from the WT strain (Fig. 5). This was confirmed by the microscopic examination of the cultures, in addition to measurement of a decrease in the amount of neutral red associated with the cells treated with the supernatants of the mutants in comparison with that associated with the cells treated with the supernatants of the WT.
FIG. 5.
Amount of neutral red associated with viable cells after 18 h incubation of HT-29 cells with the supernatants from the WT strain or the gatifloxacin-resistant mutant of C. perfringens (strain 10G). Uninoculated BHI medium served as a negative control. OD460, optical density at 460 nm.
In assays of toxin-induced platelet-neutrophil aggregation, exotoxins from the WT strain induced significant platelet-neutrophil complex formation (Table 2). However, the intensity of the platelet signal on these complexes (indicative of the relative number of platelets bound per neutrophil) was only slightly greater than the baseline intensity. Inclusion of a neutralizing anti-PLC monoclonal antibody had little effect on WT exotoxin-induced complex formation, suggesting that other WT exotoxins may mediate this activity. In contrast, the WT exotoxin-induced surface expression of neutrophil CD11b was significantly greater than that in unstimulated cells or in cells stimulated with rPLC, suggesting that exotoxins from the WT, most likely PFO, caused the marked degranulation of these cells. This finding is in agreement with the findings of previous studies by Bryant et al. (6) and others (38), who demonstrated that members of the thiol-activated cytolysin family are potent neutrophil degranulating agents. The inability of anti-PLC antibody to suppress the CD11b response supports this notion.
TABLE 2.
Effect of exotoxins from the C. perfringens WT strain and its gatifloxacin-resistant mutant, strain 10G, on platelet-leukocyte aggregation in whole blood
| Agonist and/or toxin inhibitor | % Complex formationa | Platelet CD42b intensityb | All neutrophil CD11b intensityc |
|---|---|---|---|
| Vehicle buffer | 11.38 ± 1.21d | 3.92 ± 0.74 | 34.42 ± 25.6 |
| rPLC (1 U) | 91.38 ± 3.99 | 28.58 ± 4.18 | 88.67 ± 12.7 |
| Anti-PLC 1C6 (20 μg/ml) | 12.8 ± 4.4 | 3.68 ± 0.52 | 37.2 ± 17.6 |
| WT exotoxinse | 64.57 ± 10.26 | 5.03 ± 0.85 | 118.52 ± 17.31 |
| WT exotoxins + anti-PLC 1C6 | 50.58 ± 10.89 | 4.13 ± 0.26 | 110.0 ± 16.7 |
| 10G exotoxins | 90.77 ± 2.80f | 8.92 ± 0.84f | 113.68 ± 11.88 |
| 10G exotoxins + anti-PLC 1C6 | 53.53 ± 9.88 | 5.95 ± 0.31 | 108.2 ± 24.61 |
The percentage of neutrophils binding to one or more platelets (i.e., the percentage of dual-color events).
The relative number of platelets bound per neutrophil, as measured by the mean fluorescence intensity of the FITC-labeled CD42b signal.
The relative amount of surface-expressed PE-labeled CD11b on all neutrophils in the population, irrespective of whether or not they are complexed with platelets.
Results are given as the means ± standard deviations of three assays performed in duplicate with blood from different donors.
Ammonium sulfate-concentrated exotoxins (10 μl/test) were prepared as described in Materials and Methods and diluted 1:10 in vehicle buffer before addition to whole blood.
The result is significantly different from that for the WT parent strain, as determined by Student's t test, with the level of significance chosen as a P value of <0.05.
Significantly more platelet-neutrophil complexes were formed in response to exotoxins from the 10G mutant strain than in response to exotoxins from the WT strain (Table 2). In addition, the number of platelets per neutrophil was significantly increased compared to both the baseline and the WT exotoxin-induced values. The ability of the anti-PLC antibody to reduce the strain 10G exotoxin-induced formation of large aggregates of platelets and neutrophils supports our finding that this strain produces significantly more PLC than its WT parent strain.
Genetic analysis of plc and pfo.
The genes for PLC and PFO, along with the sequences 370 bp upstream of pfo and 493 bp upstream of plc, in addition to the known regulatory genes for these toxins, were amplified from the WT and 10G strains by PCR, sequenced, and compared. No differences in the amino acid sequences of PLC, PFO, or their upstream regions and their known regulatory proteins, VirR and VirS, were found.
DISCUSSION
Gatifloxacin-induced stable mutant strain 10G of C. perfringens (formerly reported as strain Gat-NCTR-10 [24]) with double mutations in gyrA grows well in the presence of high concentrations of different fluoroquinolones (24), with the MIC of gatifloxacin for this mutant being 256 times greater than that for WT strains. In this study, we compared the amounts of PLC and PFO produced by this mutant strain to that produced by the WT parent strain. The results demonstrated that this mutant produced higher levels of both toxins. Alterations in the amounts of PFO and PLC produced were accompanied by the increased cytotoxic activity for HT-29 human adenocarcinoma cells and with increased platelet-neutrophil aggregate formation. The increased levels of toxins were not related to different growth characteristics between the two strains or to differences in the amino acid sequences of these toxins or their known regulatory proteins.
Differences in PLC activity have been observed among different strains of C. perfringens (36) and even among individual strains at different stages of bacterial growth (22). Furthermore, the conditions of the assay influence the enzymatic activity (17). Nord et al. (22) showed that cultures of C. perfringens reached stationary phase before the PLC activity started to decline, whereas the beta-hemolytic activity of PFO was stable. In our experiments, we showed higher PLC and PFO activities in the mutant strain than in the WT strain under identical conditions of bacterial growth in a toxin assay.
The cytotoxicity for HT-29 cells was greater with exotoxins from the mutant strain than with those from the WT strain. This human colon adenocarcinoma cell line has previously been used to demonstrate the effects of a heat-stable C. perfringens extracellular factor on cell proliferation (3). Those authors heat treated the bacterial toxin preparation and reported cytostatic activities for a heat-stable substance(s). In the present work, exotoxin-induced cell death (cytotoxicity) likely accounted for the reduced vital dye staining in cells exposed to the mutant exotoxins.
Sublytic concentrations of both PLC and PFO differentially induce the formation of the large aggregates of platelets and neutrophils that have been shown to impede blood flow in vivo and to contribute to ischemic necrosis and a reduced tissue inflammatory response (7, 8). Significantly more platelet-neutrophil complexes were formed in response to exotoxins from the 10G mutant strain than in response to the WT strain. This is consistent with elevated amounts of PLC and PFO in the mutant toxin preparation.
The PLC and PFO genes are associated with a variable region of the chromosome, located at the same locus near the putative origin of replication (oriC) in all serotypes of C. perfringens (10). The promoter of plc is one of the strongest known promoters (36). The transcription of plc and pfo is regulated by the products of the regulatory genes, virR and virS (20, 27, 28). No differences in the sequences of plc, pfo, or their promoter regions were observed between the WT and mutant strains used. Furthermore, there was no difference in the amino acid sequences of the known regulatory proteins, VirR and VirS, in the mutant and WT strains.
In addition to comparing the activities of PLC and PFO of the 10G gyrA mutant strain with the activities of PLC and PFO of WT parent strain NCTR, we have compared the activities of PLC of four other gatifloxacin-resistant mutant strains developed in our laboratory with those of their WT parent strains (data not shown). Two of these strains had the same genotype as mutant strain 10G (C. perfringens type B), and two were type A. Two of the mutants had the same double mutations in gyrA as strain 10G, resulting in the substitution of G81C and D87 plus other mutations in parC (24). One strain had a single mutation in gyrA, resulting in G81C, and another strain had both the G81C mutation in gyrA and other mutations in parC. None of the culture supernatants of these mutants showed increased toxin activity compared to the activities of the WT parent strains. Thus, increases in the production of PLC and PFO in strain 10G were not attributed to the mutation in gyrA.
Although all of the resistant mutants from five wild-type strains were generated in the laboratory under the same conditions, only one gatifloxacin-resistant mutant showed increased levels of toxin production. This suggests that there is not always a correlation between gatifloxacin resistance and increased toxin production in C. perfringens. It appears that, in addition to changes that occur in the fluoroquinolone target as the result of exposure to fluoroquinolones, other changes occur in different strains, and these changes may be unique to each strain.
An increase in the prevalence of more virulent strains of C. difficile has been found in hospitals after the use of gatifloxacin (18, 21). In a mouse model, increases in toxin production in some strains of C. difficile have been demonstrated after treatment with gatifloxacin (2). In summary, this is the first report showing that fluoroquinolone exposure and the development of resistance can be associated with increased toxin production in C. perfringens; however, elucidation of the mechanism responsible requires further work. At this point the implication of our findings in patients with C. perfringens infections is not known.
Acknowledgments
Special thanks go to John B. Sutherland, Robert Heflich, and Carl E. Cerniglia for comments on the manuscript and research support. We also thank Cliff Bayer for technical assistance with flow cytometric analyses.
This work was supported in part by an appointment (to M.P. and S.J.J.) to the Science Internship Program at the National Center for Toxicological Research, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the U.S. Food and Drug Administration. Additional support for this work was provided, in part, by grants (to A.E.B.) from the Office of Research and Development, Medical Research Service, U.S. Department of Veterans Affairs, and from the National Institutes of Health (grant NCRR P20RR15587).
The views presented in this article do not necessarily reflect those of the U.S. Food and Drug Administration.
Footnotes
Published ahead of print on 26 December 2007.
REFERENCES
- 1.Ackermann, G., S. Thomalla, F. Ackermann, R. Schaumann, A. C. Rodloff, and B. R. Ruf. 2005. Prevalence and characteristics of bacteria and host factors in an outbreak situation of antibiotic-associated diarrhoea. J. Med. Microbiol. 54:149-153. [DOI] [PubMed] [Google Scholar]
- 2.Adams, D. A., M. M. Riggs, and C. J. Donskey. 2007. Effect of fluoroquinolone treatment on growth of and toxin production by epidemic and nonepidemic Clostridium difficile strains in the cecal contents of mice. Antimicrob. Agents Chemother. 51:2674-2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arimochi, H., K. Morita, K. Kataoka, S. Nakanishi, T. Kuwahara, and Y. Ohnishi. 2006. Suppressive effect of Clostridium perfringens-produced heat-stable substance(s) on proliferation of human colon adenocarcinoma HT29 cells in culture. Cancer Lett. 241:228-234. [DOI] [PubMed] [Google Scholar]
- 4.Asha, N. J., and M. H. Wilcox. 2002. Laboratory diagnosis of Clostridium perfringens antibiotic-associated diarrhoea. J. Med. Microbiol. 51:891-894. [DOI] [PubMed] [Google Scholar]
- 5.Beaugerie, L., and J. C. Petit. 2004. Microbial-gut interactions in health and disease. Antibiotic-associated diarrhoea. Best Pract. Res. Clin. Gastroenterol. 18:337-352. [DOI] [PubMed] [Google Scholar]
- 6.Bryant, A. E., R. Bergstrom, G. A. Zimmerman, J. L. Salyer, H. R. Hill, R. K. Tweten, H. Sato, and D. L. Stevens. 1993. Clostridium perfringens invasiveness is enhanced by effects of theta toxin upon PMNL structure and function: the roles of leukocytotoxicity and expression of CD11/CD18 adherence glycoprotein. FEMS Immunol. Med. Microbiol. 7:321-336. [DOI] [PubMed] [Google Scholar]
- 7.Bryant, A. E., R. Y. Chen, Y. Nagata, Y. Wang, C. H. Lee, S. Finegold, P. H. Guth, and D. L. Stevens. 2000. Clostridial gas gangrene. II. Phospholipase C-induced activation of platelet gpIIbIIIa mediates vascular occlusion and myonecrosis in Clostridium perfringens gas gangrene. J. Infect. Dis. 182:808-815. [DOI] [PubMed] [Google Scholar]
- 8.Bryant, A. E., C. R. Bayer, M. J. Aldape, R. J. Wallace, R. W. Titball, and D. L. Stevens. 2006. Clostridium perfringens phospholipase C-induced platelet/leukocyte interactions impede neutrophil diapedesis. J. Med. Microbiol. 55:495-504. [DOI] [PubMed] [Google Scholar]
- 9.Bryant, A. E., and D. L. Stevens. 2006. Clostridial toxins in the pathogenesis of gas gangrene, p. 919-929. In J. E. Alouf and M. R. Popoff (ed.), The comprehensive sourcebook of bacterial protein toxins, 3rd ed. Elsevier, Amsterdam, The Netherlands.
- 10.Canard, B., B. Saint-Joanis, and S. T. Cole. 1992. Genomic diversity and organization of virulence genes in the pathogenic anaerobe Clostridium perfringens. Mol. Microbiol. 6:1421-1429. [DOI] [PubMed] [Google Scholar]
- 11.Collie, R. E., J. F. Kokai-Kun, and B. A. McClane. 1998. Phenotypic characterization of enterotoxigenic Clostridium perfringens isolates from non-foodborne human gastrointestinal diseases. Anaerobe 4:69-79. [DOI] [PubMed] [Google Scholar]
- 12.Gaynes, R., D. Rimland, E. Killum, H. K. Lowery, T. M. Johnson, G. Killgore, and F. C. Tenover. 2004. Outbreak of Clostridium difficile infection in a long-term care facility: association with gatifloxacin use. Clin. Infect. Dis. 38:640-645. [DOI] [PubMed] [Google Scholar]
- 13.Giddings, K. S., A. E. Johnson, and R. K. Tweten. 2006. Perfringolysin O and intermedilysin: mechanisms of pore formation by the cholesterol-dependent cytolysins, p. 671-679. In J. E. Alouf and M. R. Popoff (ed.), The comprehensive sourcebook of bacterial protein toxins, 3rd ed. Elsevier, Amsterdam, The Netherlands.
- 14.Hecht, D. W., M. A. Galang, S. P. Sambol, J. R. Osmolski, S. Johnson, and D. N. Gerding. 2007. In vitro activities of 15 antimicrobial agents against 110 toxigenic Clostridium difficile clinical isolates collected from 1983 to 2004. Antimicrob. Agents Chemother. 51:2716-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heimesaat, M. M., K. Granzow, H. Leidinger, and O. Liesenfeld. 2005. Prevalence of Clostridium difficile toxins A and B and Clostridium perfringens enterotoxin A in stool samples of patients with antibiotic-associated diarrhea. Infection 33:340-344. [DOI] [PubMed] [Google Scholar]
- 16.Hogenauer, C., H. F. Hammer, G. J. Krejs, and E. C. Reisinger. 1998. Mechanisms and management of antibiotic-associated diarrhea. Clin. Infect. Dis. 27:702-710. [DOI] [PubMed] [Google Scholar]
- 17.Kurioka, S., and M. Matsuda. 1976. Phospholipase C assay using p-nitrophenylphosphoryl-choline together with sorbitol and its application to studying the metal and detergent requirement of the enzyme. Anal. Biochem. 75:281-289. [DOI] [PubMed] [Google Scholar]
- 18.Loo, V. G., L. Poirier, M. A. Miller, M. Oughton, M. D. Libman, S. Michaud, A. M. Bourgault, T. Nguyen, C. Frenette, M. Kelly, A. Vibien, P. Brassard, S. Fenn, K. Dewar, T. J. Hudson, R. Horn, P. Rene, Y. Monczak, and A. Dascal. 2005. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N. Engl. J. Med. 353:2442-2449. [DOI] [PubMed] [Google Scholar]
- 19.Loy, C. E. 2005. Antibiotic-associated diarrhoea: an overlooked aetiology? Br. J. Biomed. Sci. 62:166-169. [DOI] [PubMed] [Google Scholar]
- 20.Lyristis, M., A. E. Bryant, J. Sloan, M. M. Awad, I. T. Nisbet, D. L. Stevens, and J. I. Rood. 1994. Identification and molecular analysis of a locus that regulates extracellular toxin production in Clostridium perfringens. Mol. Microbiol. 12:761-777. [DOI] [PubMed] [Google Scholar]
- 21.McDonald, L. C., G. E. Killgore, A. Thompson, R. C. Owens, Jr., S. V. Kazakova, S. P. Sambol, S. Johnson, and D. N. Gerding. 2005. An epidemic, toxin gene-variant strain of Clostridium difficile. N. Engl. J. Med. 353:2433-2441. [DOI] [PubMed] [Google Scholar]
- 22.Nord, C. E., R. Mollby, C. Smyth, and T. Wadstrom. 1974. Formation of phospholipase C and theta-haemolysin in pre-reduced media in batch and continuous culture of Clostridium perfringens type A. J. Gen. Microbiol. 84:117-127. [DOI] [PubMed] [Google Scholar]
- 23.O'Brien, D. K., and S. B. Melville. 2004. Effects of Clostridium perfringens alpha-toxin (PLC) and perfringolysin O (PFO) on cytotoxicity to macrophages, on escape from the phagosomes of macrophages, and on persistence of C. perfringens in host tissues. Infect. Immun. 72:5204-5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rafii, F., M. Park, and J. S. Novak. 2005. Alterations in DNA gyrase and topoisomerase IV in resistant mutants of Clostridium perfringens found after in vitro treatment with fluoroquinolones. Antimicrob. Agents Chemother. 49:488-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rafii, F., and M. Park. 2007. Substitutions of amino acids in alpha-helix-4 of gyrase A confer fluoroquinolone resistance on Clostridium perfringens. Arch. Microbiol. 187:137-144. [DOI] [PubMed] [Google Scholar]
- 26.Sato, H., J. Chiba, and Y. Sato. 1989. Monoclonal antibodies against alpha toxin of Clostridium perfringens. FEMS Microbiol. Lett. 50:173-176. [DOI] [PubMed] [Google Scholar]
- 27.Shimizu, T., W. Ba-Thein, M. Tamaki, and H. Hayashi. 1994. The virR gene, a member of a class of two-component response regulators, regulates the production of perfringolysin O, collagenase, and hemagglutinin in Clostridium perfringens. J. Bacteriol. 176:1616-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimizu, T., H. Yaguchi, K. Ohtani, S. Banu, and H. Hayashi. 2002. Clostridial VirR/VirS regulon involves a regulatory RNA molecule for expression of toxins. Mol. Microbiol. 43:257-265. [DOI] [PubMed] [Google Scholar]
- 29.Sparks, S. G., R. J. Carman, M. R. Sarker, and B. A. McClane. 2001. Genotyping of enterotoxigenic Clostridium perfringens fecal isolates associated with antibiotic-associated diarrhea and food poisoning in North America. J. Clin. Microbiol. 39:883-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stein, G. E., and E. J. C. Goldstein. 2006. Fluoroquinolones and anaerobes. Clin. Infect. Dis. 42:1598-1602. [DOI] [PubMed] [Google Scholar]
- 31.Stevens, D. L., B. E. Troyer, D. T. Merrick, J. E. Mitten, and R. D. Olson. 1988. Lethal effects and cardiovascular effects of purified alpha- and theta-toxins from Clostridium perfringens. J. Infect. Dis. 157:272-279. [DOI] [PubMed] [Google Scholar]
- 32.Stevens, D. L., R. K. Tweten, M. M. Awad, J. I. Rood, and A. E. Bryant. 1997. Clostridial gas gangrene: evidence that alpha and theta toxins differentially modulate the immune response and induce acute tissue necrosis. J. Infect. Dis. 176:189-195. [DOI] [PubMed] [Google Scholar]
- 33.Stevens, D. L., and A. E. Bryant. 1999. The pathogenesis of shock and tissue injury in clostridial gas gangrene, p. 623-636. In J. E. Alouf and J. H. Freer (ed.), The comprehensive sourcebook of bacterial toxins, 2nd ed. Elsevier, Amsterdam, The Netherlands.
- 34.Stevens, D. L., R. W. Titball, M. Jepson, C. R. Bayer, S. M. Hayes-Schroer, and A. E. Bryant. 2004. Immunization with the C-domain of alpha-toxin prevents lethal infection, localizes tissue injury, and promotes host response to challenge with Clostridium perfringens. J. Infect. Dis. 190:767-773. [DOI] [PubMed] [Google Scholar]
- 35.Titball, R. W., C. E. Naylor, and A. K. Basak. 1999. The Clostridium perfringens alpha-toxin. Anaerobe 5:51-64. [DOI] [PubMed] [Google Scholar]
- 36.Tsutsui, K., J. Minami, O. Matsushita, S. Katayama, Y. Taniguchi, S. Nakamura, M. Nishioka, and A. Okabe. 1995. Phylogenetic analysis of phospholipase C genes from Clostridium perfringens types A to E and Clostridium novyi. J. Bacteriol. 177:7164-7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaishnavi, C., S. Kaur, and K. Singh. 2005. Clostridium perfringens type A & antibiotic associated diarrhoea. Indian J. Med. Res. 122:52-56. [PubMed] [Google Scholar]
- 38.Zucker-Franklin, D. 1965. Electron microscope study of the degranulation of polymorphonuclear leukocytes following treatment with streptolysin. Am. J. Pathol. 47:419-433. [PMC free article] [PubMed] [Google Scholar]