Abstract
Clostridium difficile-associated colitis is an increasing cause of morbidity and mortality in hospitalized patients, with high relapse rates following conventional therapy. We sought to determine the efficacy of rifaximin, a novel nonabsorbed antibiotic, in the hamster model of C. difficile-associated diarrhea (CDAD). Hamsters received clindamycin subcutaneously and 24 h later were infected by gavage with one of two C. difficile strains: a reference strain (VPI 10463) and a current epidemic strain (BI17). Vancomycin (50 mg/kg of body weight) or rifaximin (100, 50, and 25 mg/kg) were then administered orally for 5 days beginning either on the same day as infection (prevention) or 24 h later (treatment). Therapeutic effects were assessed by weight gain, histology, and survival. We found that rifaximin was as effective as vancomycin in the prevention and treatment of colitis associated with the two C. difficile strains that we examined. There was no relapse after treatment with vancomycin or rifaximin in hamsters infected with the BI17 strain. Hamsters infected with the VPI 10463 strain and treated with rifaximin did not develop relapsing infection within a month of follow-up, whereas the majority of vancomycin-treated animals relapsed (0% versus 75%, respectively; P < 0.01). In conclusion, rifaximin was found to be an effective prophylactic and therapeutic agent for CDAD in hamsters and was not associated with disease recurrence. These findings, in conjunction with the pharmacokinetic and safety profiles of rifaximin, suggest that it is an attractive candidate for clinical use for CDAD.
Clostridium difficile is the most commonly identified cause of hospital-acquired infectious diarrhea in developed nations, accounting for up to 20% of cases of nosocomial diarrhea (4, 15). There are approximately 500,000 annual cases in the United States alone, with an estimated annual C. difficile-associated hospital cost of $3.2 billion (17, 26). The incidence of C. difficile-associated diarrhea (CDAD) has increased dramatically in the last 5 years, and serious outbreaks with high mortality have been reported (18, 21, 30, 31). Two of the current epidemic C. difficile strains, BI6 and BI17, belong to the BI/NAPI group according to restriction endonuclease analysis and pulsed-field gel electrophoresis, respectively, and to toxinotype III according to restriction fragment length polymorphism analysis. They are characterized by the presence of the binary toxin CDT, by a deletion in the tcdC locus whose gene product negatively regulates the production of toxins A and B, and often by resistance to fluoroquinolones (21, 33). The initiating factor in the vast majority of cases is prior antibiotic therapy, which disrupts normal colonic flora allowing colonization by C. difficile (6) and production of two toxins, A and B, that cause intestinal inflammation (34). Almost any antibiotic can predispose to CDAD, but clindamycin, penicillin, cephalosporins, and fluoroquinolones are most commonly implicated (15, 18). Patients who acquire C. difficile infection may be asymptomatic carriers or may develop diarrhea, pseudomembranous colitis, or toxic megacolon. Mortality rates of 2 to 15% have been reported due to toxic megacolon, colonic perforation, sepsis, systemic inflammatory response syndrome, and requirement for emergency colectomy (1, 18, 21, 24, 27, 30).
The primary treatment for CDAD is administration of metronidazole or oral vancomycin (15). Prior to 2000, both metronidazole and vancomycin had reported efficacies of approximately 95% in CDAD (16). More recent data indicate that a failure to respond to metronidazole, the usual first-line agent, is now more common, raising concerns that the current treatment approach may be inadequate (3). Of further concern is the fact that relapse after treatment of initial infection is common, occurring in approximately 20% of cases overall and in some series in as many as 50% (29, 35). Recurrent CDAD may result from persistence of bacterial spores, reinfection from the environment, and failure to develop a protective immune response (17, 38). Although intermediate resistance of C. difficile strains to metronidazole and vancomycin has been reported, almost all episodes of recurrent CDAD result from strains susceptible to these antimicrobial agents and develop shortly after therapy has been completed (28).
Rifaximin, a nonabsorbed antibiotic when administered orally (8), is well tolerated and is almost completely excreted in the feces in its original form, making it ideally suited for use against C. difficile. It inhibits bacterial RNA synthesis, with activity against gram-positive and gram-negative aerobic and anaerobic bacteria (19). Rifaximin has been proven efficacious in preventing or treating traveler's diarrhea, caused by diarrheagenic and enterotoxigenic strains of Escherichia coli (10) and by Shigella (36). It also has excellent in vitro activity against C. difficile (19) and is associated with low rates of mutagenesis and resistance (19). In view of these characteristics, we sought to determine the effects of rifaximin at three different doses (25 mg/kg, 50 mg/kg, and 100 mg/kg of body weight) in a hamster model of CDAD, in which clindamycin administration, followed by exposure to C. difficile, leads to hemorrhagic cecitis similar to fulminant antibiotic-associated pseudomembranous colitis in humans (2, 9).
MATERIALS AND METHODS
Clindamycin-induced C. difficile colitis.
Golden Syrian hamsters purchased from Charles River were housed in cages in groups of two with free access to chow (Purina 5000) and tap water. Hamsters were conditioned with a single subcutaneous injection of clindamycin phosphate (10 mg/kg) (Sigma) to eliminate the normal flora and one day later (day 1) were infected by gavage with 105 CFU of a reference toxinogenic (binary toxin-negative, toxin A-positive, toxin B-positive) C. difficile strain (VPI 10463, ATCC 43255) or of a hypervirulent epidemic strain of toxinotype III (BI17-6443) (2, 21). Control animals received no C. difficile. The animal studies were approved by the institutional animal care and use committee of Beth Israel Deaconess Medical Center.
Antibiotic treatment.
Previous in vitro studies have reported that rifaximin was one of the most-effective antibiotics (MIC90 of 0.015 mg/liter) when tested against 110 toxinogenic C. difficile clinical isolates, including the one (BI17-6443) from the recent epidemic that we tested in our model (12, 19). Moreover, the incidence of C. difficile mutants spontaneously resistant to rifaximin was found to be particularly low (<1 × 10−9) (19), while 3 out of the 110 toxinogenic clinical isolates were found to be resistant to rifaximin (12). Rifaximin (Salix Pharmaceutical Inc.) was fully suspended in an aqueous solution of 0.1 M phosphate buffer (pH of 7.4) plus 4.5‰ sodium dodecyl sulfate. Vancomycin is also effective against toxinogenic strains of C. difficile (MIC90 of 1 mg/liter), and a dose of 50 mg/kg has previously been shown to be effective in the hamster model of CDAD (2, 23, 37). Hamsters (n = 10/group) were treated by gavage with daily doses of vancomycin (50 mg/kg) (Sigma), rifaximin (100, 50, and 25 mg/kg), or vehicle (4.5‰ sodium dodecyl sulfate in buffer) for a total of 5 doses. The administration of antibiotics was initiated at day 1 (prevention study) or at day 2 (treatment and relapse studies) as depicted in Fig. 1. The animals were weighed daily for 1 week and two to three times per week thereafter and observed twice per day for signs of morbidity or diarrhea. At the end of the observation period (day 7 or day 27), or at the time of death, the cecum was collected from each animal for histological evaluation of inflammation.
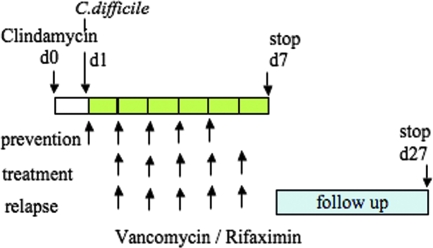
FIG. 1.
Schematic representation of induction and treatment of C. difficile colitis in hamsters. At day 0, hamsters were treated subcutaneously with clindamycin phosphate (10 mg/kg). At day 1, they received by gavage 105 CFU of toxigenic C. difficile strain VPI 10463 or BI17-6443. Hamsters received daily antibiotic treatments for days 1 to 5 in the prevention study and for days 2 to 6 in the treatment and relapse studies. Surviving hamsters were sacrificed on day 7 (prevention and treatment studies) or were monitored for disease relapse up to day 27.
Histological examination.
Hematoxylin and eosin-stained paraffin sections of the cecum were blindly evaluated by a gastrointestinal pathologist (M. O'Brien) and scored (0 to 3) for each of the following parameters associated with C. difficile colitis, as previously described by us (14): (i) epithelial damage, (ii) congestion and hemorrhage of the mucosa, and (iii) neutrophil infiltration. Histological analysis was performed in all animals included in the study, either at the time of their death due to C. difficile infection or at the end of the experiment.
Statistical analysis.
Data were analyzed by Kaplan-Meier survival analysis and the log rank test, analysis of variance with Bonferroni correction, Kruskal-Wallis nonparametric analysis, and a χ2 test using the StatView statistical software program (Abacus Concepts, Berkeley, CA). Results are expressed as mean ± standard error unless otherwise indicated.
RESULTS
Rifaximin and vancomycin prevent C. difficile-associated colitis.
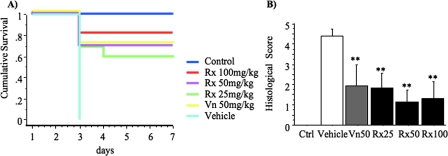
Hamsters (n = 10/group) were conditioned with clindamycin (day 0) and 24 h later (day 1) were infected with C. difficile (VPI 10463) and received the first dose of antibiotic or vehicle treatment which was continued daily for a total of five antibiotic doses (Fig. 1). Surviving animals were sacrificed at day 7. The survival rate in noninfected animals was 100%. All vehicle-treated animals developed severe colitis after infection with C. difficile and either died or were euthanized in a moribund state by day 3. In contrast, 80%, 70%, and 60% of animals receiving rifaximin treatment (100, 50, and 25 mg/kg, respectively) survived (Fig. 2A), indicating a dose-dependent effect of rifaximin (Table 1). Similar survival rates (70%) were also observed in the vancomycin-treated animals. In the hamster C. difficile challenge model, antibiotics do not completely prevent intestinal disease, as evidenced by weight loss in all infected animals compared to controls. Control animals gained weight (6.2% ± 1.2% of initial body weight) during the course of the experiment. The mean weight loss among the vancomycin-treated animals was −6.6% ± 1.8% of their initial weight. Similar weight loss was observed in the rifaximin-treated animals (−8.1% ± 1.3%, −7.9% ± 1.1%, and −8.4% ± 1.3% in the 100-, 50-, and 25-mg/kg groups, respectively). Hamsters that did not survive the infection up to day 7 were not included in the analysis of weights. There was no statistically significant difference in weights between any of the antibiotic-treated groups. All C. difficile-challenged, vehicle-treated animals quickly developed severe colitis as assessed by histology score (4.4 ± 0.3) (Fig. 2B). The mean histology score, including sick animals that had to be euthanized prior to the completion of the experiment, in the vancomycin-treated group was 1.9 ± 1.0. Similar scores were observed in the rifaximin-treated animals (1.3 ± 0.9, 1.1 ± 0.6, and 1.8 ± 0.8 in the 100-, 50-, and 25-mg/kg groups, respectively) (Fig. 2B). The difference between antibiotic-treated and vehicle-treated animals was statistically significant (P < 0.01), but there was no difference between the vancomycin- and rifaximin-treated hamsters. All control animals had uniformly normal cecal histology (0.0 ± 0.0 total histology score) at sacrifice. These results demonstrate that rifaximin at doses of 100, 50, and 25 mg/kg once daily was similar to vancomycin 50 mg/kg once daily in protecting against C. difficile-associated fatal cecitis, weight loss, and intestinal injury.
FIG. 2.
Rifaximin prevents C. difficile-induced enterocolitis in hamsters. At day 0, hamsters were treated subcutaneously with clindamycin phosphate (10 mg/kg). At day 1, they received by gavage 105 CFU of toxigenic C. difficile strain VPI 10463 and at the same time antibiotic at various doses (Rx100, 100 mg/kg of rifaximin; Rx50, 50 mg/kg of rifaximin; Rx25, 25 mg/kg of rifaximin; and Vn50, 50 mg/kg of vancomycin). Control hamsters were treated with clindamycin but not exposed to C. difficile and received vehicle treatments for 5 days. (A) Kaplan-Meier survival analysis up to day 7 for hamsters receiving various antibiotic treatments (P of <0.001 by log rank). (B) Hematoxylin and eosin-stained histological sections of cecal biopsies from all hamsters included in the study were blindly evaluated by an experienced pathologist and scored (0 to 3) for each of the following parameters: (i) epithelial damage, (ii) congestion and hemorrhage of the mucosa, and (iii) neutrophil infiltration. Histological score represents the sum of the above scores. **, P of <0.001 compared to vehicle treatment.
TABLE 1.
Rifaximin and vancomycin efficacy against C. difficile strain VPI 10463a
| Cohort | Survival (n) with:
|
||||
|---|---|---|---|---|---|
| No infection | Vancomycin (50 mg/kg) | Rifaximin (mg/kg)
|
|||
| 100 | 50 | 25 | |||
| A | 10 | 7 | 8 | 7 | 6 |
| B | 10 | 10 | 10 | 10 | 8 |
| C | 10 | 8 | 8 | 3 | 1 |
| Total (%) | 30 (100) | 25 (83) | 26 (87) | 20 (67) | 15 (50) |
Hamsters were treated with clindamycin, followed 24 h later by infection with C. difficile strain VPI 10463 (day 1). In cohort A, antibiotic treatment (a total of five daily doses) was given simultaneously with C. difficile; in cohorts B and C, treatment was given 24 h later. Survival was evaluated at day 7. Presented are the percent and the actual number of hamsters that survived out of 10 animals per group enrolled in each study. The hamster survival rate between the doses of rifaximin used was significantly different (P of <0.01 by χ2), indicating a dose effect. All vehicle values were 0.
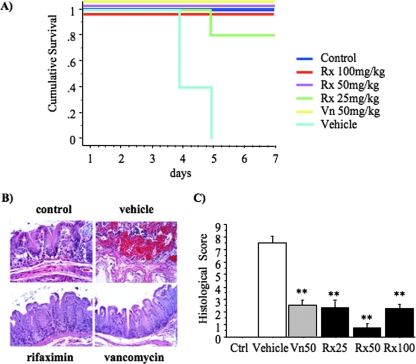
Rifaximin and vancomycin are equally effective in treatment of C. difficile-associated colitis.
In this experiment, antibiotic treatment was started 24 h after infection with C. difficile (VPI 10463) (Fig. 1), at which time all animals were developing severe cecitis. All noninfected control animals survived to day 7, but none of the C. difficile-challenged, vehicle-treated animals survived. All hamsters treated with 50 mg/kg or 100 mg/kg of rifaximin and 50 mg/kg of vancomycin survived, and 80% of those treated with 25 mg/kg of rifaximin survived (Fig. 3A). On histological examination (Fig. 3B), vehicle-treated animals showed severe mucosal necrosis with hemorrhage, while no C. difficile-exposed hamsters and the surviving rifaximin (100 mg/kg)- or vancomycin-treated hamsters had normal histology. All antibiotic-treated animals had significantly lower histological scores than vehicle-treated animals (Fig. 3C) (P < 0.001). The histological scores, including animals that did not survive the infection, were 7.5 ± 0.6 for the vehicle-treated hamsters and 2.2 ± 0.3 and 2.3 ± 0.8 for the rifaximin-treated hamsters (100 mg/kg and 25 mg/kg, respectively) (Fig. 3C). The group treated with 50 mg/kg of rifaximin had a significantly (P < 0.05) reduced total histological score (0.6 ± 0.3) compared to those with other doses of rifaximin or vancomycin (2.5 ± 0.6). Overall, rifaximin at doses of 100 and 50 mg/kg administered after the establishment of C. difficile infection was similar to the 50-mg/kg dose of vancomycin in its efficacy in treating cecitis, weight loss, and intestinal injury in the hamster CDAD model.
FIG. 3.
Rifaximin effectively treats C. difficile-induced enterocolitis. At day 0, hamsters were treated subcutaneously with clindamycin phosphate (10 mg/kg). At day 1, they received by gavage 105 CFU of toxigenic C. difficile strain VPI 10463. Antibiotic treatments were initiated the day after C. difficile exposure, at day 2, and continued daily for a total of five doses. Rx100, 100 mg/kg of rifaximin; Rx50, 50 mg/kg of rifaximin; Rx25, 25 mg/kg of rifaximin; and Vn50, 50 mg/kg of vancomycin. (A) Kaplan-Meier survival analysis up to day 7 of C. difficile-infected hamsters receiving various antibiotic treatments (P of <0.001 by log rank). (B) Hematoxylin and eosin-stained sections of cecal mucosa (magnification, 20× objective). Control (top left): histological normal cecal mucosa. Vehicle (top right): hemorrhagic necrosis of the full thickness of the mucosa. Rifaximin (100 mg/kg) (bottom left): histological normal cecal mucosa. Vancomycin (50 mg/kg) (bottom right): histological normal cecal mucosa. (C) Hematoxylin and eosin-stained histological biopsies of cecal tissue from all hamsters enrolled in the study were blindly evaluated by an experienced pathologist and scored (0 to 3) for (i) epithelial damage, (ii) congestion and hemorrhage of the mucosa, and (iii) neutrophil infiltration. Histological score represents the sum of the above scores. **, P of <0.01 compared to vehicle treatment.
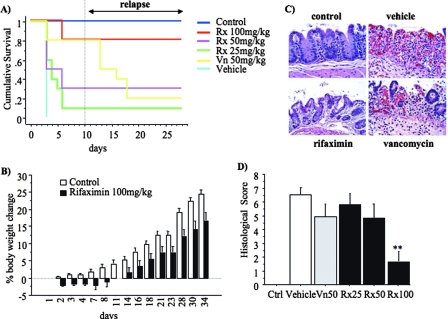
Rifaximin prevents recurrence of C. difficile-associated colitis.
We then studied a third cohort of hamsters with the intention of examining whether rifaximin and vancomycin were associated with differing rates of CDAD recurrence. Antibiotic treatment was administered on days 2 to 6 (Fig. 1). The 20 animals that survived their initial episode of C. difficile (VPI 10463) infection were maintained under observation for an additional 20 days after the termination of antibiotic treatment with rifaximin (either dose, n = 12) or vancomycin (n = 8) on day 6 (Fig. 1 and 4A). In a previous study with hamsters, we observed recurrence of C. difficile approximately 10 to 15 days after initial infection (2). As illustrated in Fig. 4A, 100% of rifaximin-treated hamsters (regardless of dosage level) survived to day 28 without recurrence of CDAD, while only 25% (two out of eight) of the vancomycin-treated animals survived the relapse (P < 0.01). After a period of weight loss immediately following C. difficile challenge, hamsters treated with 100 mg/kg of rifaximin recovered and started gaining weight, although at a lower rate than control animals (Fig. 4B). Overall, control hamsters gained 24.4% ± 1.2% and rifaximin-treated hamsters gained 16.7% ± 2.4% of their initial body weight (P < 0.01).
FIG. 4.
Rifaximin prevents relapse of C. difficile-induced enterocolitis after discontinuation of treatment. At day 0, hamsters were treated subcutaneously with clindamycin phosphate (10 mg/kg). At day 1, they received by gavage 105 CFU of toxigenic C. difficile strain VPI 10463. Antibiotic treatments (Rx100, 100 mg/kg of rifaximin; Rx50, 50 mg/kg of rifaximin; Rx25, 25 mg/kg of rifaximin; and Vn50, 50 mg/kg of vancomycin) were initiated the day after C. difficile exposure, at day 2, and continued daily for a total of five doses. (A) Kaplan-Meier survival analysis up to day 27 of C. difficile-infected hamsters receiving various antibiotic treatments (P of <0.001 by log rank). (B) Body weight change (as a percent of the initial body weight) over time. (C) Representative hematoxylin and eosin-stained histological sections (magnification, 20× objective) of cecum collected at the end of the study or at death from control hamsters (noninfected) and hamsters treated with vehicle, rifaximin (100 mg/kg), or vancomycin (50 mg/kg). (D) Hematoxylin and eosin-stained histological sections of cecal biopsy specimens from all hamsters included in the study were blindly evaluated by an experienced pathologist and scored (0 to 3) for (i) epithelial damage, (ii) congestion and hemorrhage of the mucosa, and (iii) neutrophil infiltration. Histological score represents the sum of the above scores. **, P of <0.01 compared to vehicle-treated hamsters.
On histological examination (Fig. 4C), control animals had no lesions in the mucosal or submucosal areas while vehicle-treated animals exhibited extensive necrosis, congestion, and hemorrhage with reparative changes in residual crypts. Vancomycin-treated animals developed complete mucosal necrosis with hemorrhage, in contrast to rifaximin (100 mg/kg)-treated hamsters which had normal mucosa. As illustrated in Fig. 4D, control animals had uniformly normal cecal histology (0.0 ± 0 total histology score). All C. difficile-infected, vehicle-treated animals quickly developed severe colitis and had high histology scores (6.6 ± 0.5). The mean histological score among all the vancomycin-treated animals was 4.9 ± 1.0. Similar scores were observed in the hamsters treated with 25 mg/kg and 50 mg/kg of rifaximin (5.8 ± 0.8 and 4.8 ± 1.1, respectively). The histology scores of hamsters treated with 100 mg/kg of rifaximin, including the 2 out of 10 that did not survive, were significantly lower (1.6 ± 0.7) than those for each of the other four C. difficile-challenged groups (all groups, P < 0.001; and P of 0.02 compared to vancomycin). Histological appearance was completely normal in hamsters surviving to the end of the study.
Rifaximin and vancomycin are effective in preventing and treating infection with an epidemic strain of C. difficile (BI17) and preventing disease relapse.
In addition to a reference toxinogenic C. difficile strain (VPI 10463), we also examined the effectiveness of rifaximin in treatment of CDAD caused by an epidemic strain (BI17-6443) (21). We conducted two studies, one for prevention of disease development and the other for treatment (for rifaximin and vancomycin treatments, n = 10/group; and for vehicle, n = 8/group); their design was as described in the legend for Fig. 1. Both cohorts were monitored for one month, to assess rates of recurrent infection. All mice treated with either rifaximin (100 mg/kg) or vancomycin (50 mg/kg) survived the acute infection with this hypervirulent strain. Moreover, we did not observe any disease relapse with this particular strain of C. difficile in antibiotic-treated hamsters during the follow-up period.
DISCUSSION
We report here that rifaximin was equivalent to vancomycin in prevention and treatment of weight loss, histological inflammation, and fatal CDAD in hamsters caused by two different C. difficile strains. However, hamsters treated effectively with rifaximin for acute infection with the C. difficile strain VPI 10463 did not develop recurrent fatal cecitis after discontinuation of therapy, whereas the majority of vancomycin-treated animals relapsed (0% versus 75%, respectively; P < 0.01). In humans, the standard management of CDAD is discontinuation of the precipitating antibiotic(s) and treatment with metronidazole or vancomycin (20). However, one recent study reported overall efficacy rates of only 50% with metronidazole, with 22% of patients remaining symptomatic despite treatment (25). In the present study, the overall efficacy in treating initial infection in hamsters, including data from all three cohorts, was 87% for rifaximin (100 mg/kg), similar to the 83% for vancomycin (50 mg/kg) (Table 1). In humans, about 15 to 30% of those treated for their first episode of C. difficile infection will experience a second episode of the disease, usually within 2 to 10 days after the completion of their antibiotic therapy (29). Relapse rates of up to 56% with vancomycin and 45% with metronidazole treatment have been reported (29, 39). Disease relapse may originate from persistence of spores in the gut after the initial infection or from reinfection from the environment (38), and in some patients more than ten recurrence episodes have occurred. About two-thirds of patients with a first incident of relapse are at risk for subsequent relapses (22). In our experience, the recurrence rate in hamsters infected with the VPI 10463 strain of C. difficile after vancomycin treatment was 75% in the present study and 50% in a previous one (2), while no relapse with either antibiotic treatment was observed when hamsters were infected with the new epidemic strain BI17. This is unexpected since it has been reported that in humans, the BI strains responsible for recent epidemics of CDAD have been associated with increased relapse rates (5). To our knowledge, studies of recurrent CDAD in hamsters have not been reported previously for a BI strain, and our findings may reflect interspecies differences in disease manifestations and severity between humans and hamsters. The most-effective dose of rifaximin used in this study (100 mg/kg) is almost 10-fold higher than the dosage used in patients with CDAD (400 to 800 mg daily, in two to three divided doses) (11, 13). The dose of vancomycin used in hamsters (50 mg/kg) is also higher than the one used in humans (500 mg) (2, 23, 37); therefore, it is plausible that it results in a greater disturbance of the normal flora and an increased risk for relapse in this model. However, studies in humans do not support this concern, since they report high-dose vancomycin to be at least equivalent to low-dose vancomycin in preventing relapse (22). Moreover, the same study concluded that the duration of vancomycin therapy, more than the dose per se, is the most important determinant of risk for relapse (22).
The management of recurrent CDAD remains problematic, and in addition to repeat courses of vancomycin, therapies such as probiotics, which restore the normal flora, agents that block toxin A binding, such as cholestyramine, and immunotherapy with anti-toxin A antibodies have been applied (20). Rifaximin treatment of the initial infection might prove to be beneficial for preventing relapse in clinical practice. Alternatively, it could be used to treat the first relapse and thus reduce the risk for subsequent episodes.
Due to minimal systemic absorption (<1%) (8), rifaximin was found in randomized clinical trials to be a safe drug, with adverse effects not different from those of placebo (10, 32). It has also been reported that rifaximin had minimal effects in altering the intestinal microflora with respect to coliforms and enterococci (7, 10). The ability of rifaximin to preserve elements of the colonic flora while eradicating C. difficile may be important in restoring colonization resistance and provides another possible mechanism for the absence of recurrent CDAD after rifaximin therapy. Studies of bacterial resistance to rifaximin have demonstrated that C. difficile has a very low incidence of mutants spontaneously resistant to rifaximin (19). However, among 110 toxigenic clinical isolates evaluated, three of them (two from Argentina in 1998 and one from Chicago in 1995) were found to be resistant to rifaximin in vitro (12).
In conclusion, rifaximin is effective for prevention and treatment of fulminant C. difficile-associated colitis in clindamycin-treated hamsters. Our major finding was that, compared to vancomycin, rifaximin was associated with significantly lower rates of recurrent CDAD after completion of therapy for the initial infection with the VPI 10463 strain of C. difficile. Lack of systemic absorption and a good safety profile make rifaximin an attractive candidate for use in the treatment of CDAD in humans. Indeed, while this paper was under revision, the first report of rifaximin preventing recurrence of C. difficile infection in seven out of eight women with a history of multiple episodes of CDAD was published (13). These data indicate the need for prospective controlled trials of rifaximin both for primary therapy and for secondary prevention of CDAD.
Acknowledgments
This work was supported by a grant from Salix Pharmaceuticals to C.P.K. The company had no direct input in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
We would like to thank Dale N. Gerding for kindly providing us with the BI17-6443 strain of C. difficile and J. Thomas Lamont for his critical review of the manuscript.
Footnotes
Published ahead of print on 14 January 2008.
REFERENCES
- 1.Al-Eidan, F. A., J. C. McElnay, M. G. Scott, and M. P. Kearney. 2000. Clostridium difficile-associated diarrhoea in hospitalised patients. J. Clin. Pharm. Ther. 25:101-109. [DOI] [PubMed] [Google Scholar]
- 2.Anton, P. M., M. O'Brien, E. Kokkotou, B. Eisenstein, A. Michaelis, D. Rothstein, S. Paraschos, C. P. Kelly, and C. Pothoulakis. 2004. Rifalazil treats and prevents relapse of Clostridium difficile-associated diarrhea in hamsters. Antimicrob. Agents Chemother. 48:3975-3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aslam, S., R. J. Hamill, and D. M. Musher. 2005. Treatment of Clostridium difficile-associated disease: old therapies and new strategies. Lancet Infect. Dis. 5:549-557. [DOI] [PubMed] [Google Scholar]
- 4.Barbut, F., and J. C. Petit. 2001. Epidemiology of Clostridium difficile-associated infections. Clin. Microbiol. Infect. 7:405-410. [DOI] [PubMed] [Google Scholar]
- 5.Bartlett, J. G. 2006. Narrative review: the new epidemic of Clostridium difficile-associated enteric disease. Ann. Intern. Med. 145:758-764. [DOI] [PubMed] [Google Scholar]
- 6.Bartlett, J. G., N. Moon, T. W. Chang, N. Taylor, and A. B. Onderdonk. 1978. Role of Clostridium difficile in antibiotic-associated pseudomembranous colitis. Gastroenterology 75:778-782. [PubMed] [Google Scholar]
- 7.Brigidi, P., E. Swennen, F. Rizzello, M. Bozzolasco, and D. Matteuzzi. 2002. Effects of rifaximin administration on the intestinal microbiota in patients with ulcerative colitis. J. Chemother. 14:290-295. [DOI] [PubMed] [Google Scholar]
- 8.Cellai, L., M. Colosimo, E. Marchi, A. P. Venturini, and G. Zanolo. 1984. Rifaximin (L/105), a new topical intestinal antibiotic: pharmacokinetic study after single oral administration of 3H-rifaximin to rats. Chemioterapia 3:373-377. [PubMed] [Google Scholar]
- 9.Chang, T. W., J. G. Bartlett, S. L. Gorbach, and A. B. Onderdonk. 1978. Clindamycin-induced enterocolitis in hamsters as a model of pseudomembranous colitis in patients. Infect. Immun. 20:526-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DuPont, H. L., Z. D. Jiang, P. C. Okhuysen, C. D. Ericsson, F. J. de la Cabada, S. Ke, M. W. DuPont, and F. Martinez-Sandoval. 2005. A randomized, double-blind, placebo-controlled trial of rifaximin to prevent travelers' diarrhea. Ann. Intern. Med. 142:805-812. [DOI] [PubMed] [Google Scholar]
- 11.Gionchetti, P., F. Rizzello, A. Venturi, F. Ugolini, M. Rossi, P. Brigidi, R. Johansson, A. Ferrieri, G. Poggioli, and M. Campieri. 1999. Antibiotic combination therapy in patients with chronic, treatment-resistant pouchitis. Aliment. Pharmacol. Ther. 13:713-718. [DOI] [PubMed] [Google Scholar]
- 12.Hecht, D. W., M. A. Galang, S. P. Sambol, J. R. Osmolski, S. Johnson, and D. N. Gerding. 2007. In vitro activities of 15 antimicrobial agents against 110 toxigenic Clostridium difficile clinical isolates collected from 1983 to 2004. Antimicrob. Agents Chemother. 51:2716-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson, S., C. Schriever, M. Galang, C. P. Kelly, and D. N. Gerding. 2007. Interruption of recurrent Clostridium difficile-associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin. Infect. Dis. 44:846-848. [DOI] [PubMed] [Google Scholar]
- 14.Kelly, C. P., S. Becker, J. K. Linevsky, M. A. Joshi, J. C. O'Keane, B. F. Dickey, J. T. LaMont, and C. Pothoulakis. 1994. Neutrophil recruitment in Clostridium difficile toxin A enteritis in the rabbit. J. Clin. Investig. 93:1257-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly, C. P., C. Pothoulakis, and J. T. LaMont. 1994. Clostridium difficile colitis. N. Engl. J. Med. 330:257-262. [DOI] [PubMed] [Google Scholar]
- 16.Kyne, L., R. J. Farrell, and C. P. Kelly. 2001. Clostridium difficile. Gastroenterol. Clin. N. Am. 30:753-777. [DOI] [PubMed] [Google Scholar]
- 17.Kyne, L., M. Warny, A. Qamar, and C. P. Kelly. 2001. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet 357:189-193. [DOI] [PubMed] [Google Scholar]
- 18.Loo, V. G., L. Poirier, M. A. Miller, M. Oughton, M. D. Libman, S. Michaud, A. M. Bourgault, T. Nguyen, C. Frenette, M. Kelly, A. Vibien, P. Brassard, S. Fenn, K. Dewar, T. J. Hudson, R. Horn, P. Rene, Y. Monczak, and A. Dascal. 2005. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N. Engl. J. Med. 353:2442-2449. [DOI] [PubMed] [Google Scholar]
- 19.Marchese, A., A. Salerno, A. Pesce, E. A. Debbia, and G. C. Schito. 2000. In vitro activity of rifaximin, metronidazole and vancomycin against Clostridium difficile and the rate of selection of spontaneously resistant mutants against representative anaerobic and aerobic bacteria, including ammonia-producing species. Chemotherapy 46:253-266. [DOI] [PubMed] [Google Scholar]
- 20.Maroo, S., and J. T. Lamont. 2006. Recurrent Clostridium difficile. Gastroenterology 130:1311-1316. [DOI] [PubMed] [Google Scholar]
- 21.McDonald, L. C., G. E. Killgore, A. Thompson, R. C. Owens, Jr., S. V. Kazakova, S. P. Sambol, S. Johnson, and D. N. Gerding. 2005. An epidemic, toxin gene-variant strain of Clostridium difficile. N. Engl. J. Med. 353:2433-2441. [DOI] [PubMed] [Google Scholar]
- 22.McFarland, L. V., G. W. Elmer, and C. M. Surawicz. 2002. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am. J. Gastroenterol. 97:1769-1775. [DOI] [PubMed] [Google Scholar]
- 23.McVay, C. S., and R. D. Rolfe. 2000. In vitro and in vivo activities of nitazoxanide against Clostridium difficile. Antimicrob. Agents Chemother. 44:2254-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris, A. M., B. A. Jobe, M. Stoney, B. C. Sheppard, C. W. Deveney, and K. E. Deveney. 2002. Clostridium difficile colitis: an increasingly aggressive iatrogenic disease? Arch. Surg. 137:1096-1100. [DOI] [PubMed] [Google Scholar]
- 25.Musher, D. M., S. Aslam, N. Logan, S. Nallacheru, I. Bhaila, F. Borchert, and R. J. Hamill. 2005. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin. Infect. Dis. 40:1586-1590. [DOI] [PubMed] [Google Scholar]
- 26.O'Brien, J. A., B. J. Lahue, J. J. Caro, and D. M. Davidson. 2007. The emerging infectious challenge of Clostridium difficile-associated disease in Massachusetts hospitals: clinical and economic consequences. Infect. Control Hosp. Epidemiol. 28:1219-1227. [DOI] [PubMed] [Google Scholar]
- 27.Olson, M. M., C. J. Shanholtzer, J. T. Lee, Jr., and D. N. Gerding. 1994. Ten years of prospective Clostridium difficile-associated disease surveillance and treatment at the Minneapolis VA Medical Center, 1982-1991. Infect. Control Hosp. Epidemiol. 15:371-381. [DOI] [PubMed] [Google Scholar]
- 28.Pelaez, T., L. Alcala, R. Alonso, M. Rodriguez-Creixems, J. M. Garcia-Lechuz, and E. Bouza. 2002. Reassessment of Clostridium difficile susceptibility to metronidazole and vancomycin. Antimicrob. Agents Chemother. 46:1647-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pepin, J., M. E. Alary, L. Valiquette, E. Raiche, J. Ruel, K. Fulop, D. Godin, and C. Bourassa. 2005. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin. Infect. Dis. 40:1591-1597. [DOI] [PubMed] [Google Scholar]
- 30.Pepin, J., L. Valiquette, M. E. Alary, P. Villemure, A. Pelletier, K. Forget, K. Pepin, and D. Chouinard. 2004. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. Can. Med. Assoc. J. 171:466-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pindera, L. 2004. Quebec to report on Clostridium difficile in 2005. Can. Med. Assoc. J. 171:715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prantera, C., H. Lochs, M. Campieri, M. L. Scribano, G. C. Sturniolo, F. Castiglione, and M. Cottone. 2006. Antibiotic treatment of Crohn's disease: results of a multicentre, double blind, randomized, placebo-controlled trial with rifaximin. Aliment. Pharmacol. Ther. 23:1117-1125. [DOI] [PubMed] [Google Scholar]
- 33.Rupnik, M., B. Dupuy, N. F. Fairweather, D. N. Gerding, S. Johnson, I. Just, D. M. Lyerly, M. R. Popoff, J. I. Rood, A. L. Sonenshein, M. Thelestam, B. W. Wren, T. D. Wilkins, and C. von Eichel-Streiber. 2005. Revised nomenclature of Clostridium difficile toxins and associated genes. J. Med. Microbiol. 54:113-117. [DOI] [PubMed] [Google Scholar]
- 34.Savidge, T. C., W. H. Pan, P. Newman, M. O'Brien, P. M. Anton, and C. Pothoulakis. 2003. Clostridium difficile toxin B is an inflammatory enterotoxin in human intestine. Gastroenterology 125:413-420. [DOI] [PubMed] [Google Scholar]
- 35.Tal, S., A. Gurevich, V. Guller, I. Gurevich, D. Berger, and S. Levi. 2002. Risk factors for recurrence of Clostridium difficile-associated diarrhea in the elderly. Scand. J. Infect. Dis. 34:594-597. [DOI] [PubMed] [Google Scholar]
- 36.Taylor, D. N., R. McKenzie, A. Durbin, C. Carpenter, C. B. Atzinger, R. Haake, and A. L. Bourgeois. 2006. Rifaximin, a nonabsorbed oral antibiotic, prevents shigellosis after experimental challenge. Clin. Infect. Dis. 42:1283-1288. [DOI] [PubMed] [Google Scholar]
- 37.Tyrrell, K. L., D. M. Citron, Y. A. Warren, H. T. Fernandez, C. V. Merriam, and E. J. Goldstein. 2006. In vitro activities of daptomycin, vancomycin, and penicillin against Clostridium difficile, C. perfringens, Finegoldia magna, and Propionibacterium acnes. Antimicrob. Agents Chemother. 50:2728-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilcox, M. H., W. N. Fawley, C. D. Settle, and A. Davidson. 1998. Recurrence of symptoms in Clostridium difficile infection—relapse or reinfection? J. Hosp. Infect. 38:93-100. [DOI] [PubMed] [Google Scholar]
- 39.Young, G. P., P. B. Ward, N. Bayley, D. Gordon, G. Higgins, J. A. Trapani, M. I. McDonald, J. Labrooy, and R. Hecker. 1985. Antibiotic-associated colitis due to Clostridium difficile: double-blind comparison of vancomycin with bacitracin. Gastroenterology 89:1038-1045. [DOI] [PubMed] [Google Scholar]