Abstract
We used an in vitro model of continuous venovenous hemofiltration (CVVH) to characterize amikacin adsorption by polyacrylonitrile (PAN) and polyamide filters. A blood-crystalloid mixture dosed with amikacin was pumped from a reservoir through a hemofiltration circuit and back to the reservoir. All ultrafiltrate was also returned to the reservoir. The level of adsorption was calculated from the fall in the amikacin concentration. The dose and the initial concentration of amikacin were varied, as were the pH, the type of hemofilter, and the hemofilter surface area. The reversibility of adsorption and the effect of repeated dosing were also studied. The level of adsorption by 0.6-m2 PAN filters was significantly greater than that by 0.6-m2 polyamide filters. Adsorption was increased by increasing the dose of amikacin even when the initial concentration was unchanged. It was unaffected by the pH (pH 6.8 or 7.4) or the hemofilter surface area (0.6 m2 or 0.9 m2). Repeated doses of amikacin resulted in further adsorption. In a saturation experiment, the maximum adsorptive capacity of 0.6-m2 PAN hemofilters was at least 546.9 mg (range, 427.6 to 577.5 mg). The adsorption of amikacin by hemofilters is irreversible and was associated with the dose and the hemofilter material but not the hemofilter surface area. Close monitoring of peak amikacin levels should be considered for patients receiving CVVH with PAN hemofilters.
Critical illness is frequently associated with severe sepsis and septic shock, necessitating the use of broad-spectrum antibiotics such as aminoglycosides. Patients with these conditions often also develop acute renal failure, resulting in a requirement for renal replacement therapy (17). Outside North and South America, the most common mode of renal replacement therapy for critically ill patients is continuous venovenous hemofiltration (CVVH). Thus, it is common for critically ill patients to be receiving both aminoglycosides and CVVH, and it is important to understand the elimination of aminoglycosides by CVVH. Although the elimination of drugs is largely due to ultrafiltration, there is a largely unexplored possibility that there may be significant adsorption of the drugs onto the hemofilter membrane.
Limited data indicate that there is significant adsorption of tobramycin by polyacrylonitrile (PAN) membranes and that the level of tobramycin adsorption onto PAN/AN69 membranes is concentration dependent (4, 12). Kraft and Lode found that gentamicin adsorption onto the PAN membrane might contribute to the shorter serum half-life time of gentamicin during hemofiltration in vivo and the paradoxical increase in its concentration in the filtrate (11). There are, however, no data on the adsorption of other aminoglycosides. We carried out an in vitro experiment to characterize the adsorption of amikacin by hemofilters. The aim of the study was to determine the extent and the time course of amikacin adsorption and to determine the effects of the membrane material, pH, the amikacin concentration, the amount of circulating amikacin, the membrane surface area, and repeated dosing on adsorption.
MATERIALS AND METHODS
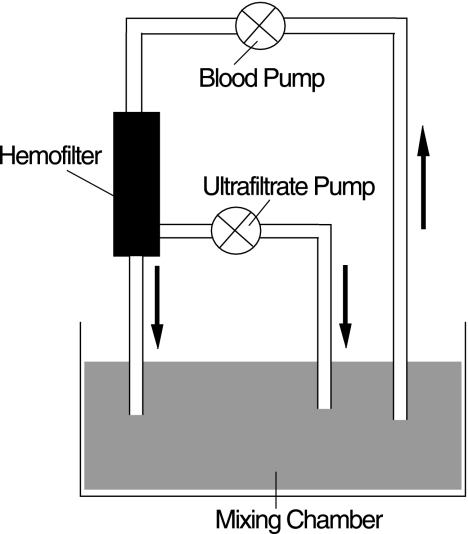
The study used a previously described one-compartment model of CVVH to determine the adsorption of amikacin (3, 19). The model involves filtration of a blood-crystalloid mixture of low hemoglobin concentration and low albumin concentration (to simulate the condition in critically ill patients) spiked with an appropriate amount of amikacin. Blood was pumped from a glass mixing chamber (containing a known volume of blood-crystalloid mixture) through a hemofiltration circuit (of known volume) and back to the mixing chamber. The ultrafiltrate was also returned to the mixing chamber. No replacement fluid was used (Fig. 1). The system thus constitutes a closed circuit in which drug elimination can occur only by spontaneous degradation, metabolism by blood, or adsorption to the circuit or hemofilter. The circuit was primed with heparinized normal saline, and the mixing chamber was filled with a known volume of the blood-crystalloid mixture. The mixture was prepared by adding heparinized lactated Ringer's solution (5,000 U heparin/liter) to 1 to 2 units of human blood (deemed unfit for transfusion) to produce a predetermined volume (Table 1). The pH of the mixture was titrated to 6.8 or 7.4 with sodium bicarbonate (8.4%) (Table 1). The circuit blood flow was set at 200 ml/min, and the ultrafiltration rate was set at 1,000 ml/h. Heparin (1,000 U/ml) was infused at 5 ml/h. The temperature of the blood-crystalloid mixture was maintained at 36°C with an automatic water bath. After an equilibration period of 10 min and after the temperature of the blood-crystalloid mixture had reached 36 to 38°C, 5 ml of blood was taken for measurement of the hemoglobin and albumin concentrations. Thereafter, amikacin was infused into the mixing chamber over 10 min. Immediately after 10 min, the CVVH procedure was started. Blood samples (exactly 5 ml each) were taken at predetermined times (Table 1) for measurement of the amikacin concentration. After blood sampling at 90 min, the circulating volume was increased by the addition of 500 ml of lactated Ringer's solution or a second dose of amikacin was infused (over 10 min) (Table 1). Due to the variability in the amount of amikacin in commercial preparations, the doses given were calculated from the measured concentrations (Table 1). Three types of hemofilters were used: a 0.6-m2 PAN hemofilter (Multiflow 60; Hospal, Meyzieu, France), a 0.9-m2 PAN hemofilter (Multiflow 100; Hospal), and a 0.6-m2 polyamide hemofilter (Hemofilter 6S; Gambro, Hechingen, Germany). There were eight sets of experimental conditions, with the experiment being repeated four times under each set of conditions (Table 1).
FIG. 1.
In vitro model.
TABLE 1.
Experimental summary of the eight groups
| Groupa | Membrane | pH | No. of units of blood | Vol (ml) of blood-crystalloid mixture | Circuit vol (ml) | Total vol (ml) | Dose (mg [range])b |
|---|---|---|---|---|---|---|---|
| 1 | 0.6 m2 PAN | 7.40 | 1 | 500 | 220 | 720 | 68.9 (65.1-75.0) |
| 2 | 0.6 m2 PAN | 6.80 | 1 | 500 | 220 | 720 | 69.5 (64.4-84.7) |
| 3 | 0.6 m2 PAN | 7.40 | 1 | 500 | 220 | 720 | 34.9 (33.9-38.4) |
| 4 | 0.6 m2 polyamide | 7.40 | 1 | 500 | 230 | 730 | 58.3 (51.4-67.7) |
| 5 | 0.6 m2 PAN | 7.40 | 2 | 1,500 | 220 | 1,720 | 156.7 (154.8-158.0) |
| 6 | 0.9 m2 PAN | 7.40 | 1 | 500 | 240 | 740 | 73.1 (69.7-78.0) |
| 7 | 0.9 m2 PAN | 7.40 | 2 | 1,500 | 240 | 1,740 | 149.7 (138.5-151.8) |
| 8 | 0.6 m2 PAN | 7.40 | 1 | 500 | 220 | 720 | 74.7 (64.9-75.6) |
For groups 1 to 4, the intervention was volume expansion with 500 ml lactated Ringer's solution after 90 min and the blood sampling times (the time from the start of the 10-min amikacin infusion) were 10, 60, 90, and 150 min. For groups 5 to 7, the intervention was a repeat dose of amikacin given after 90 min and the blood sampling times were 10, 20, 30, 40, 60, 90, and 150 min. For group 8, the intervention was repeat dosing every 45 min (10 doses) and the blood sampling times were 10, 45, 90, 135, 180, 225, 270, 315, 360, 405, and 450 min.
Dose = concentration at 10 min × initial volume of blood-crystalloid mixture.
Effect of pH.
Adsorption was studied at pH 7.4 (group 1) and pH 6.8 (group 2) (Table 1).
Effects of dose and concentration.
Adsorption was studied at three different doses and two different circulating volumes (groups 1, 3, and 5) (Table 1) to differentiate between the effect of changes in the dose and the effect of changes in the concentration.
Effect of hemofilter material.
Adsorption was studied by using a PAN hemofilter (group 1) and a polyamide hemofilter (group 4).
Effect of membrane surface area.
Adsorption was studied by using 0.6-m2 and 0.9-m2 PAN hemofilters (group 1 and group 6, respectively, and group 5 and group 7, respectively).
Reversibility of adsorption.
Adsorption was compared before and after the reduction of the circulating amikacin concentration, achieved by the addition of 500 ml of lactated Ringer's solution (groups 1 to 4).
Time course of adsorption.
Blood samples were taken at 10, 20, 30, 40, 60, 90, and 150 min for groups 5 to 7 to characterize the time course of adsorption.
Effect of repeated dosing and measurement of maximum adsorptive capacity.
The levels of adsorption before and after a second dose of amikacin were compared (groups 5 to 7). To determine the maximum binding capacity of a 0.6-m2 PAN membrane, 10 doses of amikacin were infused over 10 min at 45-min intervals (group 8). The amikacin concentration was measured at 10 min and at the end of each 45-min interval to determine the adsorption of each dose.
To exclude the possibility of spontaneous degradation or metabolism by blood, 20 mg of amikacin was added to 500 ml of the blood-crystalloid mixture. The temperature was maintained at 36°C and the pH was adjusted to 7.4. The mixture was not circulated through the hemofilter circuit. Samples were taken immediately after the mixture was spiked and then 120 and 240 min later to determine the amikacin concentrations. This degradation control experiment was repeated four times.
Blood samples were collected in heparinized tubes, and the tubes were centrifuged at 3,000 rpm for 15 min. The serum was separated and stored at −80°C until measurement of the amikacin concentrations by a fluorescence polarization immunoassay (TDX; Abbott Laboratories, Diagnostic Division, Abbott Park, IL).
Drug adsorption was calculated as follows: dose − (concentration × circulating volume of blood-crystalloid mixture), where the dose is equal to the concentration at 10 min × the initial volume of the blood-crystalloid mixture.
All results are described as medians (ranges). Comparison of the results for the different experimental groups was performed by the Kruskal-Wallis test, and when the difference was significant, the results were tested by the Nemenyi test. Regression analysis was performed by the curve estimation method. The level of significance was defined as a P value of <0.05.
RESULTS
Both hemoglobin and albumin concentrations were lower than the normal range of concentrations for healthy humans (Tables 2 and 3). There was no statistically significant difference between the groups.
TABLE 2.
Hemoglobin, albumin, and amikacin concentrations in groups 1 to 4a
| Group | Hemoglobin concn (g/dl) | Albumin concn (g/liter) | Amikacin concn (mg/liter [range]) at:
|
|||
|---|---|---|---|---|---|---|
| 10 min | 60 min | 90 min | 150 min | |||
| 1 | 6.8 (6.0-7.7) | 22.1 (20.0-29.2) | 93.8 (88.6-102.0) | 8.7 (7.9-10.3) | 8.3 (7.4-9.9) | 5.7 (5.1-6.6) |
| 2 | 7.2 (6.7-8.6) | 21.6 (10.9-33.7) | 94.6 (87.6-115.2) | 3.8 (3.7-4.8) | 3.4 (3.4-4.5) | 2.9 (2.8-3.4) |
| 3 | 6.9 (5.7-8.3) | 19.9 (12.8-32.5) | 47.4b (46.1-52.3) | 4.5 (3.9-6.6) | 4.1 (3.6-6.5) | 2.9 (2.8-3.9) |
| 4 | 7.1 (6.6-8.3) | 21.4 (14.1-38.7) | 78.3 (68.9-90.0) | 59.1 (56.9-71.4) | 60.5 (43.6-71.6) | 34.7 (20.7-38.6) |
The concentration at 10 min is an adjusted concentration. The concentration in the mixing chamber was measured immediately prior to the start of circulation through the CVVH circuit. This value was then adjusted for the additional volume of the circuit, assuming an instantaneous distribution throughout the circulating volume.
P < 0.05 compared with the results for group 1 and P < 0.05 compared with the results for group 2.
TABLE 3.
Hemoglobin, albumin, and amikacin concentrations in groups 5 to 7a
| Group | Hemoglobin concn (g/dl) | Albumin concn (g/liter) | Amikacin concn (mg/liter [range]) at:
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| 10 min | 20 min | 30 min | 40 min | 60 min | 90 min | 150 min | |||
| 5 | 6.4 (5.6-7.8) | 23.9 (17.7-29.3) | 90.3 (89.2-91.1) | 38.1 (37.0-46.2) | 27.1 (23.4-28.8) | 25.2 (22.5-27.3) | 24.0 (20.5-26.5) | 23.8 (21.4-27.2) | 62.0 (55-64.9) |
| 6 | 7.1 (5.9-8.3) | 18.7 (16.2-21.9) | 96.8 (92.3-103) | 7.8 (7.1-14.7) | 4.3 (3.8-4.6) | 5.8 (5.5-6.4) | 6.1 (5.8-7.0) | 8.5 (6.2-11.7) | 14.7 (10.7-18.6) |
| 7 | 6.7 (5.4-7.1) | 17.2 (12.4-20.9) | 84.2 (77.9-85.4) | 39.8 (34.1-47.3) | 22.0 (19.4-30.5) | 18.0 (15.2-17.1) | 18.2 (15.7-22.0) | 18.6 (15.4-18.8) | 43.1 (37.4-49.9) |
The concentration at 10 min is an adjusted concentration. The concentration in the mixing chamber was measured immediately prior to the start of circulation through the CVVH circuit. This value was then adjusted for the additional volume of the circuit, assuming an instantaneous distribution throughout the circulating volume.
The degradation experiment showed that the concentration of amikacin did not change over time: 42.1 mg/liter (range, 40.1 to 46.4 mg/liter), 43.0 mg/liter (range, 38.2 to 47.5 mg/liter), and 44.7 mg/liter (range, 39.9 to 45.4 mg/liter) at 0, 2, and 4 h, respectively. These results indicate the stability of amikacin in the blood-crystalloid mixture.
The time courses of the amikacin concentration and adsorption by hemofilters are shown in Tables 2 and 3 and Fig. 2 and 3.
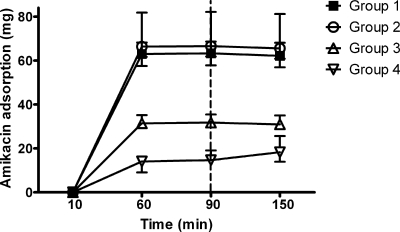
FIG. 2.
Amikacin adsorption by PAN filters against time. Immediately after 90 min, 500 ml of Ringer's solution was infused into the mixing chamber. P was <0.05 for group 1 versus group 3, and P was <0.01 for group 1 versus group 4. All data are shown as medians (ranges).
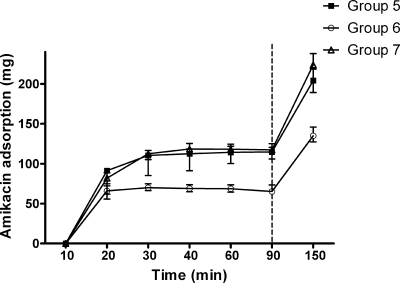
FIG. 3.
Amikacin adsorption by hemofilters against time. Immediately after 90 min, the second dose was infused repeatedly into the mixing chamber. P was <0.01 for group 5 versus group 6, for group 7 versus group 6, and for adsorption at 150 min versus adsorption at 90 min in the same group. All data are shown as medians (ranges).
Effect of pH.
There was no significant difference in adsorption at pH 7.4 (group 1) and pH 6.8 (group 2) (Fig. 2).
Effects of dose and concentration.
A reduction in the dose from 68.9 mg (range, 65.1 to 75.0 mg) (group 1) to 34.9 mg (range, 33.9 to 38.4 mg) (group 3) was associated with a significant reduction in adsorption (63.4 mg [range, 57.9 to 68.7 mg] and 31.7 mg [range, 29.8 to 35.4 mg], respectively; P < 0.05). An increase in the initial dose from 68.9 mg (range, 65.1 to 75.0 mg) (group 1) to 156.7 mg (range, 154.8 to 158.0 mg) (group 5) was associated with an increase in adsorption by PAN filters (63.4 mg [range, 57.9 to 68.7 mg] and 114.7 mg [range, 111.4 to 120.5 mg], respectively; P < 0.01), even though the initial circulating amikacin concentrations were similar. Regression analysis revealed the following relationships between adsorption and initial dose: adsorption (mg) = 1.931 × initial dose0.811 for 0.6-m2 PAN hemofilters (r2 = 0.991) and adsorption (mg) = 2.450 × initial dose0.772 for 0.9-m2 PAN hemofilters (r2 = 0.981).
Effect of hemofilter material.
Changing the filter material from PAN (group 1) to polyamide (group 4) was associated with a marked reduction in adsorption (63.4 mg [range, 57.9 to 68.7 mg] and 14.7 mg [range, 12.5 to 19.1 mg], respectively; P < 0.01) (Fig. 2).
Effect of membrane surface area.
An elevation in the membrane surface area from 0.6 m2 (group 1) to 0.9 m2 (group 6) was not associated with an elevation in adsorption (63.4 mg [range, 57.9 to 68.7 mg] and 65.2 mg [range, 64.3 to 73.5 mg], respectively). Similarly, there was no difference in adsorption between group 5 (0.6-m2 membrane) and group 7 (0.9-m2 membrane) (114.7 mg [range, 111.4 to 120.5 mg] and 117.4 mg [range, 106.0 to 125.1 mg], respectively).
Reversibility of adsorption.
Despite a significant decrease in the amikacin concentration following the addition of 500 ml fluid after 90 min (Table 2), the adsorption remained unchanged in all four groups (Fig. 2).
Time course of adsorption.
Adsorption was very rapid, occurring within 30 min of the initial dose (Fig. 3).
Effect of repeated dosing.
The infusion of a second dose of amikacin was associated with a significant increase in adsorption from 114.7 mg (range, 111.4 to 120.5 mg) to 204.2 mg (range, 201.6 to 218.6 mg) in group 5, 65.2 mg (range, 64.3 to 73.5 mg) to 135.0 mg (range, 127.3 to 145.8 mg) in group 6, and 117.4 mg (range, 106.0 to 125.1 mg) to 223.8 mg (range, 189.2 to 237.9 mg) in group 7 (P < 0.01 for all groups) (Fig. 3).
Maximum adsorptive capacity.
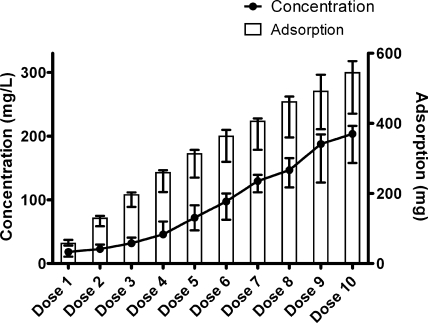
In group 8, repeated amikacin dosing resulted in cumulative adsorption which did not reach a plateau even after 10 doses (total dose, 747.1 mg [range, 649.2 to 755.5 mg]) had been given and a total of 546.9 mg (range, 427.6 to 577.5) had been adsorbed (Fig. 4). The maximum adsorption at a clinically relevant circulating concentration (146.7 mg/liter [range, 119.6 to 165.5 mg/liter]) was 463.2 mg (range, 360.1 to 476.3 mg).
FIG. 4.
Cumulative amikacin adsorption (n = 4) by hemofilters with repeated dosing and the circulating concentration in vitro when blood was taken to calculate adsorption (group 8). Data are presented as medians and ranges.
DISCUSSION
The major finding of our study is that there is significant adsorption of amikacin by PAN hemofilter membranes in vitro (at least 115 mg may be adsorbed after a single dose), and the results of our experiments with repeated doses suggest that the median adsorptive capacity of a 0.6-m2 PAN hemofilter is at least 564 mg. As the recommended doses of amikacin in patients with renal failure range from 7.5 mg/kg of body weight to 10 mg/kg (2, 10, 20), the adsorption of these amounts would represent a significant proportion of the dose.
The likely clinical significance of our finding depends not only on the absolute amount adsorbed but also on the characteristics of the adsorption. Our data indicate that adsorption is rapid, with adsorption being complete by 30 min and not reversed by a fall in the circulating amikacin concentration. Aminoglycosides exhibit concentration-dependent bacterial killing, with the postdistribution peak concentration being closely related to bacteriological cure (1, 9, 13). The rapidity of amikacin adsorption combined with the significant amount adsorbed suggests that amikacin adsorption might significantly reduce the peak amikacin concentration in vitro, with a resultant negative impact on bacterial killing. Aminoglycoside toxicity is related to the trough concentration. Reversible adsorption would likely increase the trough concentration and thus might increase toxicity. However, our data indicate that amikacin adsorption is not reversed by a subsequent reduction in the circulating drug concentration.
The difference in the absolute adsorption between groups 1 and 3 indicates that absolute absorption is either concentration or dose dependent. However, the increase in absolute adsorption between group 1 and group 5 when the dose was increased but the initial concentration was not suggests that the dose rather than the initial concentration is most closely associated with adsorption. Regression analysis suggests that at the doses tested in our experiment, there is a power relationship between the dose and adsorption. The regression equations suggest that adsorption will be even greater following larger doses but that the increase in adsorption will be disproportionately less than the increase in the dose. However it is important to understand that the relationship demonstrated in our study may not hold for a large single dose, although the results of our experiment with repeated small doses indicate that the adsorptive capacity of a 0.6-m2 PAN hemofilter is at least 546 mg in vitro.
We are unable to explain the mechanism underlying the dose-dependent adsorption. The amikacin concentration data shown in Tables 2 and 3 do not provide any support for a threshold concentration below which adsorption no longer occurs, with a markedly higher trough concentration in group 5 than in groups 1 and 3. This lack of an apparent threshold is not due to saturation of the membrane, as repeated doses of amikacin resulted in further adsorption in groups 5 and 8.
The adsorption by polyamide hemofilters is much less than that by PAN filters. This difference in adsorption between polyamide and PAN filters may be due to an ionic interaction between amikacin and the membrane. Polyamide filters carry no net charge. In contrast, the sodium methallyl sulfonate radicals in the PAN membrane carry a fixed negative charge (14). At physiological pH, the amino groups of amikacin largely carry a positive charge (8), which may promote the interaction of the amino groups of amikacin with the sulfonate radicals of the PAN membranes and, hence, the adsorption of amikacin onto the PAN membrane. However, we found no difference in adsorption at pH 6.8 and pH 7.4, suggesting that ionic interactions are not the sole mechanism. Polyamide and PAN hemofilters also differ in structure (6). Hemofilters consist of a series of parallel hollow fibers through which the blood is pumped. The ultrafiltrate is formed by the passage of fluid across the wall of the fibers. PAN fibers have walls that are homogeneous in structure, with the result that the passage of fluid through the walls is likely to be uniformly distributed. This results in a large number of potential binding sites for amikacin adsorption. In contrast, the walls of polyamide fibers have a thin internal “skin” layer and, adjacent to the skin, a sponge-like layer with pores that become larger as they radiate outwards (6). After the ultrafiltrate is formed by the passage of fluid across the skin layer, it may preferentially pass through the pores, with the result that the amikacin contained in the ultrafiltrate will be exposed to only a limited number of binding sites (i.e., those lining the pores).
The PAN filters with a larger surface area (0.9 m2) did not show increased adsorption compared to that for the PAN filters with a 0.6-m2 surface area. This initially unexpected finding may be explicable if the major sites of adsorption are within the wall of the hollow fibers rather than the inner surface of the fiber, as suggested by a study of adsorption of β2-microglobulin (5). The hollow fibers of the 0.6-m2 and 0.9-m2 PAN hemofilters differ only in fiber length (19 cm and 28 cm, respectively). They have the same internal diameter, wall thickness, and width. At a constant blood flow and ultrafiltrate rate, the pressure across the filter wall is higher for shorter hollow fibers than for longer fibers (7). This may result in the greater penetration of amikacin into the walls of shorter fibers, such that the effective area for adsorption is the same, despite the difference in the nominal surface area. However, we did not measure transmembrane pressure and therefore cannot confirm that the pressure across the walls of the fibers was higher for the 0.6-m2 fibers in our study.
The major limitation of our study is that it was an in vitro study. However, in vivo measurement is difficult. In theory, it should be possible to measure adsorption by extracting the drug from the hemofilter, but it is difficult to be sure that extraction is complete. Although we attempted to simulate the conditions seen in critically ill patients, who are often anemic and hypoalbuminemic, we were unable to produce a uremic model or a model that completely mimicked the acute-phase response seen in septic patients. Furthermore, amikacin was infused over 10 min and prior to the start of CVVH. It is conceivable that these factors might affect adsorption. For example, the duration of filter use prior to drug dosing may affect the extent of adsorption, with greater adsorption in the first few hours of filter use (18). The small circulating volume of our model limited our ability to fully explore the effects of the dose and the concentration on adsorption and to determine the maximum adsorptive capacity of the PAN hemofilter at clinically relevant concentrations. Even after 10 doses, the cumulative adsorption had not reached a plateau but the circulating concentration had reached 203.6 mg/liter (range, 157.9 to 216.2 mg/liter), which exceeds the usual peak amikacin concentration in patients of 33 to 157 mg/liter (15, 21). Although our data suggest that adsorption is more closely related to the dose than to the concentration, the limitations of our model prevent us from concluding that this is true for a wide range of doses and concentrations. It might, therefore, be considered more appropriate to use the maximum adsorptive capacity at a clinically relevant concentration as an estimate of the likely maximum adsorptive capacity in vivo. After eight doses the circulating concentration (146.7 mg/liter [range, 119.6 to 165.5 mg/liter]) had reached the upper end of this range and the cumulative adsorption had reached 463.2 mg (range, 360.1 to 476.3 mg). Finally, only two types of hemofilter were included in this study, and the results should not be extended to filters made of other materials, such as polysulfone and polymethyl methacrylate filters.
The application of in vitro data to the clinical situation should be undertaken only with great caution. Nevertheless, we feel that, in the absence of contrary in vivo data, peak amikacin concentrations should be routinely measured in patients receiving CVVH with PAN hemofilters. Furthermore, it may be preferable to delay the start of CVVH with a PAN hemofilter for at least 1 h after amikacin infusion in vivo, in order to attenuate the effect of adsorption on the peak amikacin concentration.
Our findings have significance for investigators studying amikacin clearance by CVVH. We would recommend that the amikacin concentration in samples taken in the first 30 min after amikacin dosing or the initiation of CVVH not be used to calculate clearance by ultrafiltration, due to the confounding effect of adsorption. If these samples are taken in the first 30 min, both the filter inlet and the filter outlet blood concentrations should be used to calculate clearance (16).
In summary, there is significant, rapid adsorption of amikacin onto PAN hemofilters in vitro. The adsorption is dose related, is not reversed by a subsequent decrease in concentration, and is dependent on the filter material, with greater adsorption onto PAN filters than onto polyamide filters.
Footnotes
Published ahead of print on 17 December 2007.
REFERENCES
- 1.Bartal, C., A. Danon, F. Schlaeffer, K. Reisenberg, M. Alkan, R. Smoliakov, A. Sidi, and Y. Almog. 2003. Pharmacokinetic dosing of aminoglycosides: a controlled trial. Am. J. Med. 114:194-198. [DOI] [PubMed] [Google Scholar]
- 2.Bressolle, F., J. M. Kinowski, J. E. de la Coussaye, N. Wynn, J. J. Eledjam, and M. Galtier. 1994. Clinical pharmacokinetics during continuous haemofiltration. Clin. Pharmacokinet. 26:457-471. [DOI] [PubMed] [Google Scholar]
- 3.Choi, G., C. D. Gomersall, J. Lipman, A. Wong, G. M. Joynt, P. Leung, S. J. Ramsay, and O. M. Ho. 2004. The effect of adsorption, filter material and point of dilution on antibiotic elimination by haemofiltration: an in vitro study of levofloxacin. Int. J. Antimicrob. Agents 24:468-472. [DOI] [PubMed] [Google Scholar]
- 4.Cigarran-Guldris, S., M. E. Brier, and T. A. Golper. 1991. Tobramycin clearance during simulated continuous arteriovenous hemodialysis. Contrib. Nephrol. 93:120-123. [DOI] [PubMed] [Google Scholar]
- 5.Clark, W. R., W. L. Macias, B. A. Molitoris, and N. H. Wang. 1994. Membrane adsorption of beta 2-microglobulin: equilibrium and kinetic characterization. Kidney Int. 46:1140-1146. [DOI] [PubMed] [Google Scholar]
- 6.Clark, W. R., R. J. Hamburger, and M. J. Lysaght. 1999. Effect of membrane composition and structure on solute removal and biocompatibility in hemodialysis. Kidney Int. 56:2005-2015. [DOI] [PubMed] [Google Scholar]
- 7.Dungen, H. D., C. von Heymann, C. Ronco, W. J. Kox, and C. D. Spies. 2001. Renal replacement therapy: physical properties of hollow fibers influence efficiency. Int. J. Artif. Organs 24:357-366. [PubMed] [Google Scholar]
- 8.Forge, A., and J. Schacht. 2000. Aminoglycoside antibiotics. Audiol. Neurootol. 5:3-22. [DOI] [PubMed] [Google Scholar]
- 9.Hatala, R., T. Dinh, and D. J. Cook. 1996. Once-daily aminoglycoside dosing in immunocompetent adults: a meta-analysis. Ann. Intern. Med. 124:717-725. [DOI] [PubMed] [Google Scholar]
- 10.Joos, B., M. Schmidli, and G. Keusch. 1996. Pharmacokinetics of antimicrobial agents in anuric patients during continuous venovenous haemofiltration. Nephrol. Dial. Transplant. 11:1582-1585. [PubMed] [Google Scholar]
- 11.Kraft, D., and H. Lode. 1979. Elimination of ampicillin and gentamicin by hemofiltration. Klin. Wochenschr. 57:195-196. [DOI] [PubMed] [Google Scholar]
- 12.Kronfol, N. O., A. H. Lau, and M. M. Barakat. 1987. Aminoglycoside binding to polyacrylonitrile hemofilter membranes during continuous hemofiltration. ASAIO Trans. 33:300-303. [PubMed] [Google Scholar]
- 13.Lacy, M. K., D. P. Nicolau, C. H. Nightingale, and R. Quintiliani. 1998. The pharmacodynamics of aminoglycosides. Clin. Infect. Dis. 27:23-27. [DOI] [PubMed] [Google Scholar]
- 14.Lau, A. H., and N. O. Kronfol. 1994. Determinants of drug removal by continuous hemofiltration. Int. J. Artif. Organs 17:373-378. [PubMed] [Google Scholar]
- 15.Maller, R., B. Isaksson, L. Nilsson, and L. Soren. 1988. A study of amikacin given once versus twice daily in serious infections. J. Antimicrob. Chemother. 22:75-79. [DOI] [PubMed] [Google Scholar]
- 16.Meyer, B., S. Ahmed el Gendy, G. Delle Karth, G. J. Locker, G. Heinz, W. Jaeger, and F. Thalhammer. 2003. How to calculate clearance of highly protein-bound drugs during continuous venovenous hemofiltration demonstrated with flucloxacillin. Kidney Blood Press Res. 26:135-140. [DOI] [PubMed] [Google Scholar]
- 17.Ronco, C., P. Inguaggiato, V. D'Intini, L. Cole, R. Bellomo, S. Poulin, V. Bordoni, C. Crepaldi, F. Gastaldon, A. Brendolan, P. Trairak, and T. Khajohn. 2003. The role of extracorporeal therapies in sepsis. J. Nephrol. 16(Suppl. 7):S34-S41. [PubMed] [Google Scholar]
- 18.Rumpf, K. W., J. Rieger, R. Ansorg, B. Doht, and F. Scheler. 1977. Binding of antibiotics by dialysis membranes and its clinical relevance. Proc. Eur. Dial. Transplant Assoc. 14:607-609. [PubMed] [Google Scholar]
- 19.Tian, Q., C. D. Gomersall, A. Wong, P. Leung, G. Choi, G. M. Joynt, P. Tan, and J. Lipman. 2006. Effect of drug concentration on adsorption of levofloxacin by polyacrylonitrile haemofilters. Int. J. Antimicrob. Agents 28:147-150. [DOI] [PubMed] [Google Scholar]
- 20.Trotman, R. L., J. C. Williamson, D. M. Shoemaker, and W. L. Salzer. 2005. Antibiotic dosing in critically ill adult patients receiving continuous renal replacement therapy. Clin. Infect. Dis. 41:1159-1166. [DOI] [PubMed] [Google Scholar]
- 21.Van der Auwera, P., and J. Klastersky. 1987. Serum bactericidal activity and postantibiotic effect in serum of patients with urinary tract infection receiving high-dose amikacin. Antimicrob. Agents Chemother. 31:1061-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]