Abstract
Human African trypanosomiasis (HAT) is a fatal tropical disease caused by infection with protozoans of the species Trypanosoma brucei gambiense and T. b. rhodesiense. An oral prodrug, DB289, is a promising new therapy undergoing phase III clinical trials for early-stage HAT. DB289 is metabolically converted to the active trypanocidal diamidine DB75 [2,5-bis(4-amidinophenyl)furan]. We previously determined that DB75 inhibits yeast mitochondrial function (C. A. Lanteri, B. L. Trumpower, R. R. Tidwell, and S. R. Meshnick, Antimicrob. Agent Chemother. 48:3968-3974, 2004). The purpose of this study was to investigate if DB75 targets the mitochondrion of T. b. brucei bloodstream forms. DB75 rapidly accumulates within the mitochondria of living trypanosomes, as indicated by the fluorescent colocalization of DB75 with a mitochondrion-specific dye. Fluorescence-activated cell sorting analysis of rhodamine 123-stained living trypanosomes shows that DB75 and other trypanocidal diamidines (pentamidine and diminazene) collapse the mitochondrial membrane potential. DB75 inhibits ATP hydrolysis within T. brucei mitochondria and appears to inhibit the oligomycin-sensitive F1F0-ATPase and perhaps other ATPases. DB75 is most likely not an inhibitor of electron transport within trypanosome mitochondria, since DB75 fails to inhibit mitochondrial respiration when glycerol-3-phosphate is used as the respiratory substrate. However, DB75 inhibits whole-cell respiration (50% inhibitory concentration, 20 μM) at drug concentrations and incubation durations that also result in the dissipation of the mitochondrial membrane potential. Taken together, these findings suggest that the mitochondrion is a target of the trypanocidal action of DB75.
New therapies are desperately needed for the treatment of human African trypanosomiasis (HAT). This reemerging tropical disease afflicts as many as 500,000 people (2, 5). Current drugs, such as pentamidine, suramin, melarsoprol, eflornithine, and nifurtimox, are often associated with deleterious side effects and cannot be administered orally (5, 16, 19, 34).
A promising new therapy, DB289 [2,5-bis-(4-amidinophenyl)-furan bis-O-methylamidoxime], is undergoing phase III clinical trials for registration as the first orally administered drug for early-stage HAT (19). The oral prodrug DB289 is metabolically converted to the trypanocidal agent DB75 [2,5-bis(4-amidinophenyl)furan] (6, 50, 51). DB75 is active against Trypanosoma spp. in vitro (11, 12) and is a structural analogue of the aromatic diamidine drug pentamidine.
DB75 and pentamidine are antimicrobial diamidine-type compounds that are active against various fungal and protozoal infections. However, the mechanism through which diamidine drugs exert their activity is still not entirely known and continues to be a focus of extensive research (46). We previously investigated the mechanisms of action of DB75 and pentamidine in yeast cells and found that both drugs appear to disrupt the mitochondrial function in Saccharomyces cerevisiae by collapsing the mitochondrial membrane potential (Ψm) and inhibiting mitochondrial respiration (24). We also showed that DB75 and pentamidine inhibit oxidative phosphorylation in isolated rat liver mitochondria (24), which is consistent with the results attained with pentamidine in a previous study (31).
The purpose of this investigation was to gain insight into the mechanism of trypanocidal action of DB75. In particular, we investigated if DB75 acts against Trypanosoma brucei bloodstream forms (BFs) by targeting the mitochondrion.
MATERIALS AND METHODS
Chemicals.
DB75 [2,5-bis(4-amidinophenyl)furan dihydrochloride] was obtained from David Boykin at Georgia State University, Atlanta, and pentamidine isethionate [1,5-di(4-amidinophenoxy)pentane isethionate] was prepared by LyphoMed, Inc. (Melrose Park, IL). All other chemicals were of the highest quality available and were from Sigma-Aldrich (St. Louis, MO), unless otherwise stated.
Collection and isolation of trypanosomes from infected blood.
Male Sprague-Dawley rats and Swiss-Webster mice (Charles River Laboratories, Cambridge, MA) were infected by intraperitoneal injection of approximately 2 × 106 and 5 × 105 BFs of Trypanosoma brucei brucei strain 427 cells, respectively. Infected blood was collected through cardiac puncture at the time of peak parasitemia (usually day 5 postinfection for rats and day 4 postinfection for mice). The blood was centrifuged in heparinized tubes at 2,200 × g for 10 min at 4°C, and the buffy coat was removed and diluted 1:3 in phosphate saline glucose buffer, pH 8.0. The trypanosomes were purified from the blood by the use of DE52 anion-exchange columns (22).
Fluorescence microscopy.
DB75 has an inherent blue fluorescence, and it excites at 365 nm and emits at 465 nm under UV light. UV fluorescence microscopy was used to track the intracellular distribution of DB75 in T. b. brucei S427 cells. Approximately 106 cells were treated with 1.0 μM DB75 (10 μl of a 1 mM DB75 solution in sterile distilled water) and were incubated in a T25 flask in a humidified atmosphere of 5.0% CO2 at 37°C in complete Baltz's minimal essential medium (cBMEM), consisting of Eagle's minimal essential medium with Earle's salts supplemented with 0.4 mM l-threonine, 0.1 mM hypoxanthine, 0.016 mM thymidine, 0.08 mM adenosine, 0.2 mM sodium pyruvate, 6 mM d-glucose, 25 mM HEPES, 25 mM NaHCO3, nonessential amino acids, 0.2 mM 2-mercaptoethanol, 2 mM l-glutamine, and 10% heat-inactivated HyClone fetal bovine serum. An aliquot of 1.5 ml was removed after predetermined drug incubation times and was centrifuged at 1,400 × g for 5 min. The resultant pellet was resuspended, and dried films were prepared for UV fluorescence microscopy with a Nikon Microphot FXA microscope fitted with a Nikon UV2A filter cube. Subsequent UV fluorescence microscopy experiments were conducted to determine if DB75 localizes to T. b. brucei mitochondria. Briefly, 106 cells per ml were incubated at 37°C in an atmosphere of 5.0% CO2 for 30 min in cBMEM containing 25 nM MitoTracker Red CMXRos (Molecular Probes Inc., Eugene, OR). The cells were then collected by centrifugation (1,000 × g, 10 min) and resuspended in fresh prewarmed medium lacking dye. These cells were then treated with 100 μM DB75 to determine if the DB75 fluorescence colocalizes with MitoTracker. To slow down the motility of the trypanosomes so that they could be viewed under the microscope, 20 μl of sample was mixed in 20 μl of 3.0% agarose (at 37°C), and the mixture was then spun briefly in a microcentrifuge. Next, 20 μl of the resultant cell-agar mixture was placed on an ice-cold glass slide and a coverslip was mounted on the slide. The samples were viewed with a Nikon Microphot FXA microscope fitted with a Nikon multiband triple-filter block for 4′,6′-diamidino-2-phenylindole-fluorescein isothiocyanate-Texas Red. The intracellular localization of pink fluorescence indicated the distribution of DB75 and MitoTracker Red together within the T. b. brucei mitochondrion. Images were captured with Scion Image software.
Preparation of T. b. brucei mitochondrial fraction.
The trypanosome mitochondrial fraction was prepared by a differential centrifugation protocol (30, 39), with all steps performed at 0 to 4°C. Isolated trypanosomes were washed once (by centrifugation at 2,000 × g for 10 min at 4°C) in resuspension buffer, which consisted of 0.12 M sodium phosphate buffer (pH 8.0) containing 86.3 mM NaCl and 56 mM glucose. The cells were then resuspended in 2.0 ml of buffer and placed on ice. These packed cells were suspended in an equal volume of glass beads (diameter, 0.10 to 0.11 mm) and 4.0 ml of homogenization buffer containing 50 mM HEPES-NaOH buffer (pH 7.2) with 0.27 M sucrose, 1.0 mM EDTA, and 1.0 mM MgCl2 and 80 μl of a freshly prepared concentrated stock solution of a protease inhibitor cocktail (Roche complete EDTA-free cocktail). The cells were then mixed vigorously with a Vortex mixer for 2 min; approximately 100% of the cells were lysed after this treatment. The resultant homogenate was collected (by centrifugation at 2,000 × g for 10 min at 4°C), added to a fresh 15-ml conical tube, and centrifuged again at 900 × g for 5 min to remove any residual cells. The cell extract was collected from this spin, and a crude mitochondrial fraction was obtained by centrifugation at 12,000 × g for 10 min at 4°C. The precipitate was resuspended in 5.0 ml of washing buffer, consisting of 10 mM potassium phosphate buffer (pH 7.5) containing 0.25 M sucrose and 1.0 mM EDTA, and was washed again by centrifugation at 12,000 × g for 10 min. The resultant pellet was resuspended in a minimal volume (250 to 500 μl) of washing buffer, and the total protein concentration was determined by using a Bio-Rad protein assay kit with bovine serum albumin (BSA) as the standard. If the samples were not used on the same day of preparation, they were frozen at −80°C until further use; no observable differences in the results of the oxygen consumption experiments were obtained when fresh or frozen mitochondrial preparations were used.
Measurement of oxygen consumption.
The oxygen consumption of T. b. brucei whole cells and mitochondrial preparations was measured at 37°C by using a Hansatech Instruments Oxytherm system equipped with an oxygen electrode disc, a borosilicate reaction chamber, and a Teflon-coated magnetic stirring bar. Parasites isolated from infected rat blood were washed (by centrifugation at 2,000 × g, 10 min, 4°C) once in mannitol-based assay buffer (pH 7.4) containing 20 mM glucose as the carbon source and consisting of 220 mM mannitol, 25 mM HEPES, 2 mM EGTA, and 5 mM MgSO4. The cells were then resuspended, and the oxygen consumed by 2.0 × 106 cells per ml was recorded in the presence and the absence of the test compounds. The respiration of T. b. brucei mitochondrial preparations was measured by following a protocol adapted from that described in previous publications (4, 30). Briefly, the mitochondria were suspended to 0.8 mg/ml in mannitol-based assay buffer (pH 7.4) lacking glucose, and a final concentration of 20 mM l-α-glycerol phosphate was then added as the respiratory substrate. The samples were next loaded into the reaction chamber, and after a stable reading was achieved (usually after 2 min), various test compounds were added to the reaction chamber and respiration was recorded for an additional several minutes. The data were analyzed with Oxyg32 (Hansatech) software.
Flow cytometric analysis of ΔΨm.
Fluorescence-activated cell sorting (FACS) analysis was used to assess changes in the Ψm of T. b. brucei cells, as determined from the mean fluorescence of cells treated with the fluorescent ΔΨm-sensitive dye rhodamine 123 (Rh123) (47). The assay is based upon the principle that the mitochondrial accumulation and retention of Rh123 are dependent upon the ΔΨm across the inner mitochondrial membrane and that the mitochondrial uptake of Rh123 is directly proportional to the magnitude of ΔΨm (15, 21, 47). Trypanosomes isolated from rat blood were resuspended and washed once (by centrifugation at 2,000 × g, 10 min, 4°C) in Tes buffer, which consisted of 118 mM NaCl, 5 mM KCl, 1.2 mM MgSO4, 16 mM Na2HPO4, 1.2 mM KH2PO4, 5 mM NaHCO3, 10 mM glucose, and 30 mM N-[Tris(hydroxymethyl)methyl]-2-aminoethanesulfonic acid, which was adjusted to pH 7.5 with 1.0 M NaOH, and which contained fresh 1.0% fatty acid-free BSA (35, 37). The cells were resuspended to 108 per ml in Tes buffer and warmed to room temperature. Aliquots of the cells (107 per ml) were then treated for 10 min with various test compounds, after which time a final concentration of 250 nM Rh123 was added to each sample for a further 2 min. The cells were diluted 1:10 in buffer immediately before FACS analysis with a Becton Dickinson FACScan flow cytometer with fluorescence settings appropriate for the detection of Rh123 emission (λ = 529 nm) and excitation (λ = 507 nm). Summit (version 3.1) software was used to plot the fluorescence distribution for 20,000 cells as a frequency histogram, and the mean fluorescence channel was recorded for each sample treatment.
Measurement of ATP consumption within T. b. brucei mitochondrial preparations.
ATP concentrations were measured by using an ATP bioluminescence assay kit (CLS II; Roche), which uses a method based upon the ATP-dependent bioluminescence of luciferase. T. b. brucei mitochondrial fractions were diluted to 100 μg of protein in 150 μl reaction buffer consisting of 20 mM Tris-HCl (pH 7.4) containing 15 mM KH2PO4, 0.6 M sorbitol, 10 mM MgSO4, and freshly added 2.5 mg/ml fatty acid-free BSA. The effects of mitochondrial inhibitors and DB75 on mitochondrial ATP consumption were tested by incubating the samples for 10 min on ice in the presence of 50 μM oligomycin, added as 2.4 μl of 2.5 mg/ml solution in ethanol; 50 μM carbonylcyanide m-chlorophenylhydrazone (CCCP), added as 1.5 μl of 5 mM solution in ethanol; and 10, 50, or 100 μM DB75, added as 1.5 μl of 1 mM, 5 mM, or 10 mM DB75, respectively, in sterile distilled water. Non-drug-treated samples were also incubated on ice for 10 min, in order to determine if the drugs were stimulating or inhibiting ATP consumption. To initiate ATPase activity, the samples were then incubated for 30 min at 30°C in the presence of 0.20 mM ATP. Following the incubation, the samples were processed for the luciferase assay, according to previously reported methods (1). Briefly, samples were treated with 3.5 μl of 60% perchloric acid and were immediately vortexed. The samples were then incubated on ice for at least 10 min or until a white precipitate appeared and were spun in an Eppendorf centrifuge (model 5417C) for 5 min at full speed (20,000 × g). From each sample, 120 μl of supernatant was removed and placed in a new tube, to which 23 μl of 1.0 N KOH was added. This was immediately followed by mixing on a Vortex mixer and incubation on ice for 3 min. The samples were then spun again, and the resultant supernatant was transferred to a fresh tube and kept on ice. ATP consumption was assessed in a 100-μl total volume consisting of 10 μl of sample, 40 μl 0.5 M Tris-acetate (pH 7.75), and 50 μl of luciferase reagent. ATP standards in the range of 10−3 to 10−11 M were also tested. Samples were read with a luminometer in a 96-well white microtiter plate by using a 1-s delay and an integration time of 1 to 10 s. The data for the blank (no ATP) were subtracted from the raw data, and the ATP concentrations for each sample were calculated from a log-log plot of the standard curve with GraphPad Prism software.
RESULTS
Fluorescent localization of DB75 within BFs of T. b. brucei.
The inherent UV fluorescence of DB75 was used to study its intracellular distribution within BFs of T. b. brucei cells. The parasites were treated in vitro with 1.0 μM DB75, and dried smears were prepared for microscopy at various time points. UV fluorescence microscopy showed that DB75 distributes within the DNA-containing organelles, the nucleus and the kinetoplast (Fig. 1A). Specifically, the blue fluorescence of DB75 was observed within a prominent intracellular organelle located roughly at the midpoint of the cell (Fig. 1A), which is characteristic of the position of the nucleus (17). In addition, DB75 fluorescence was observed within a smaller organelle near the base of the flagellum (Fig. 1A) that corresponded to the position of the trypanosome organelle containing mitochondrial DNA, the kinetoplast (17). The accumulation of DB75 occurred relatively rapidly, with blue fluorescence being observed in the nucleus and the kinetoplast within 5 min.
FIG. 1.
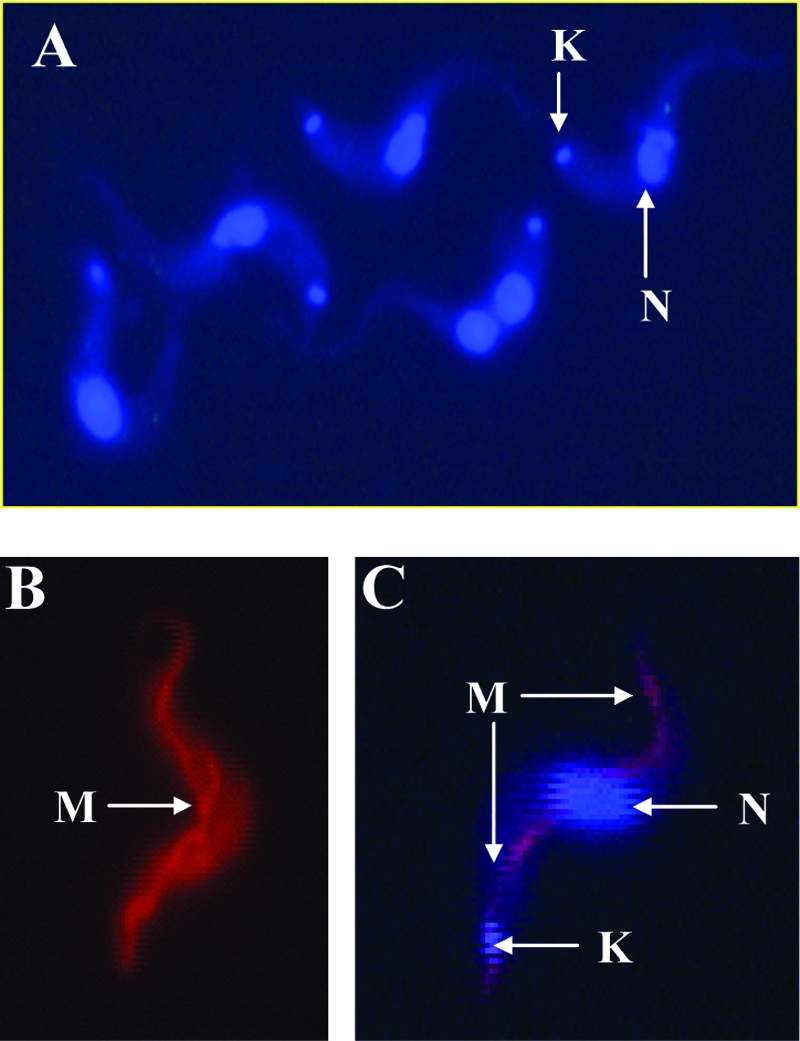
Cellular distribution of DB75 fluorescence within T. b. brucei BFs. (A) Fluorescent localization of DB75 within the nucleus (N) and kinetoplast (K) of trypanosomes. Cells were incubated at 37°C and 5.0% CO2 in cBMEM containing 1.0 μM DB75, and after 30 min, dried films were prepared for UV fluorescence microscopy with a Nikon Microphot FXA microscope fitted with a Nikon UV2A cube. (B) The elongated, tubular structure of the mitochondrion (M), typical of BF parasites, was observed in living cells treated with the mitochondrion-specific dye MitoTracker Red. Cells were incubated with 25 nM MitoTracker Red for 30 min, washed, and suspended in agarose before UV fluorescence microscopy was performed with living cells with a Nikon Texas Red filter. (C) Fluorescent colocalization of MitoTracker Red and DB75 within the trypanosome mitochondrion. Cells were first treated with 25 nM MitoTracker Red and were next incubated with 100 μM DB75 for 5 min, and wet mounts of agarose mixtures were viewed with a Nikon multiband triple filter block for 4′,6′-diamidino-2-phenylindole-fluorescein isothiocyanate-Texas Red. Fluorescent colocalization, indicated by pink fluorescence, of MitoTracker with DB75 suggests that DB75 enters the mitochondrion. Magnifications, ×63.
We were then interested in determining if DB75 also distributed within the trypanosome mitochondrion, which in the BF of T. brucei is a thin, elongated structure that runs along the length of the cell (17). The blue fluorescence of DB75 within the nucleus and the kinetoplast is very intense (Fig. 1A), making it difficult to resolve if DB75 distributes within the much thinner structure of the mitochondrion. The mitochondrion-specific dye MitoTracker Red CMXRos (17, 43) was therefore used to reveal DB75 fluorescence within the mitochondrion. The thin, tubular structure of the mitochondrion was observed in living BFs treated with MitoTracker Red alone (Fig. 1B). To determine if DB75 also enters the mitochondrion, cells pretreated with MitoTracker Red were then incubated with 100 μM DB75 (Fig. 1C). This higher concentration of DB75 was optimal for distinguishing the fluorescent distribution of DB75 within the mitochondrion from the more intense staining of DB75 in other organelles. In these cells, the colocalization of the blue fluorescence of DB75 with the red-orange fluorescence of MitoTracker Red within the mitochondrion was evidenced by pink fluorescence (Fig. 1C). DB75 appears to distribute within the mitochondrion relatively rapidly, with the fluorescent colocalization of DB75 and MitoTracker Red observed within 5 min (Fig. 1C).
Effect of DB75 on T. b. brucei whole-cell and mitochondrial respiration.
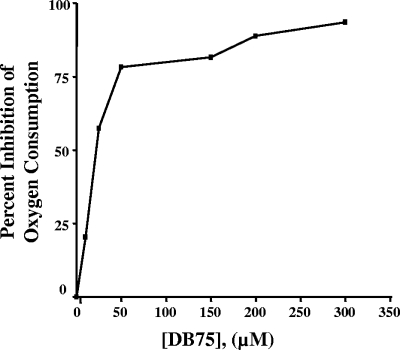
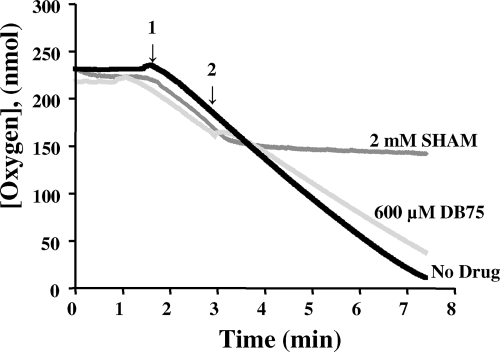
We next investigated the effect of DB75 on the whole-cell and mitochondrial respiration of T. b. brucei BFs. DB75 inhibited the oxygen consumption of whole cells in a mannitol-based buffer containing glucose in a dose-dependent manner, with a 50% inhibitory concentration of approximately 20 μM DB75 (Fig. 2). DB75 at 300 μM appeared to completely inhibit cellular respiration (Fig. 2). The effect of DB75 on the respiration of isolated mitochondria was then investigated by using glycerol-3-phosphate (G-3-P) as the substrate. As expected, 2 mM salicylhydroxamic acid (SHAM), a specific inhibitor of the trypanosome mitochondrial α-glycerophosphate oxidase system (9, 33), completely inhibited mitochondrial respiration (Fig. 3). In contrast, DB75 at concentrations up to 600 μM did not alter G-3-P-dependent oxygen consumption in isolated trypanosome mitochondria (Fig. 3), whereas concentrations of SHAM as low as 10 μM were shown to inhibit at least some oxygen consumption in isolated mitochondria (data not shown). These results indicate that DB75 is not an inhibitor of the SHAM-sensitive mitochondrial alternative oxidase.
FIG. 2.
DB75 inhibits glucose-dependent cellular respiration in T. b. brucei BFs. The oxygen consumed by 2 × 106 cells per ml in mannitol-based buffer containing 20 mM glucose was recorded with an oxygen electrode, and the rates of oxygen consumption were determined with Oxyg32 software. The percent inhibition of respiration was determined by comparing the oxygen consumption rates immediately before and after the addition of DB75. The graph depicts the results from two independent experiments; error bars associated with the standard deviations are too small to be represented on the graph.
FIG. 3.
DB75 does not inhibit respiration in T. b. brucei mitochondrial preparations. An oxygen electrode was used to measure the oxygen consumed by mitochondria in mannitol-based buffer containing 20 mM G-3-P as the respiratory substrate in the presence or the absence of DB75 or SHAM. The arrows indicate the time points at which 0.8 mg/ml of T. b. brucei mitochondria (arrow 1) and 600 μM DB75 or 2 mM SHAM (arrow 2) were added to the reaction chamber.
DB75 collapses T. b. brucei ΔΨm.
Rh123 is a mitochondrion-specific dye that accumulates only within energized mitochondria and that is commonly used as a fluorescent probe to monitor the drug-induced collapse of ΔΨm in trypanosomes (15, 47). FACS analysis of the Rh123 fluorescence within T. congolense cells was previously used to characterize the ΔΨm of various trypanosome populations (47). We therefore adapted this protocol to determine the effect of DB75 on the ΔΨm of T. b. brucei BFs in situ. Histograms of total Rh123 fluorescence in 20,000 trypanosomes were generated (Fig. 4A), and the mean fluorescence for each sample served as a relative measure of ΔΨm in the presence and the absence of various test compounds.
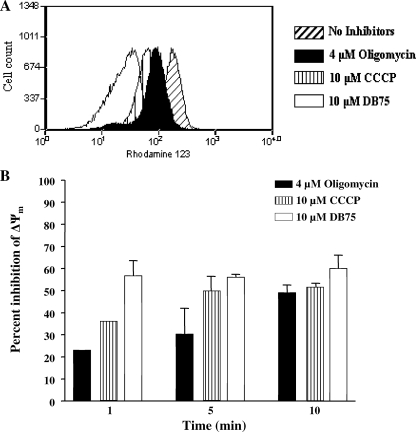
FIG. 4.
DB75 collapses the mitochondrial membrane potential in T. b. brucei BFs. FACS analysis of the mean Rh123 fluorescence was conducted as a measure of the drug-induced changes in ΔΨm. (A) Rh123 fluorescence distribution plotted as frequency histograms. Trypanosomes (107/ml) were pretreated with 10 μM DB75, 4 μM oligomycin, or 10 μM CCCP for 10 min in Tes buffer supplemented with 1.0% BSA. Next, the cells were treated with 250 nM Rh123 for a further 2 min, diluted 1:10, and subjected to FACS analysis. The results indicate that the mean Rh123 fluorescence (and, therefore, ΔΨm) was decreased in drug-treated cells compared to the fluorescence of the untreated cells. The results shown are representative of at least four independent experiments. (B) FACS analysis results expressed as the percent inhibition of ΔΨm. Trypanosomes were pretreated with drug for the times indicated on the graph. The percent inhibition of ΔΨm was determined by calculating the percent decrease in the mean Rh123 fluorescence of drug-treated cells relative to the mean fluorescence of the untreated cells. The results shown represent the averages of at least three independent experiments and indicate that DB75 inhibits ΔΨm to a similar extent as the mitochondrial inhibitors oligomycin and CCCP.
This method proved to be an effective measure of ΔΨm within trypanosomes, as indicated by the ability of mitochondrial inhibitors to decrease the mean Rh123 fluorescence (Fig. 4). For example, oligomycin is a specific and potent inhibitor of the T. brucei mitochondrial F1F0-ATPase that generates the ΔΨm within the inner mitochondrial membrane (7, 37). As expected, treatment of the trypanosomes for 10 min with 4 μM oligomycin resulted in an approximately 50% reduction in Rh123 fluorescence relative to that for the untreated cells (Fig. 4B). Likewise, 10 μM the protonophore CCCP reduced the Rh123 fluorescence to approximately 51% of that detected in untreated parasites after a 5-min incubation (Fig. 4B). In addition, a 10-min incubation with 10 μM the ionophore valinomycin reduced Rh123 to approximately 70% of that observed in the no-drug controls (results not shown). In our assay, these mitochondrial inhibitors behaved as expected, since the drug concentrations and incubation times that we observed for ΔΨm collapse were identical to those documented in a previous report that used UV fluorescence microscopy of Rh123 to monitor drug-mediated ΔΨm collapse in T. brucei BFs (15). Furthermore, compounds not expected to affect the mitochondrial function in BFs failed to alter the Rh123 fluorescence in our FACS analysis. Specifically, no change in ΔΨm was observed in cells treated with the cytochrome oxidase inhibitor potassium cyanide at 1 mM or the electron transport chain inhibitor antimycin A at 10 μM (results not shown). Taken together, these results validated our FACS assay for use in the measurement of drug-induced ΔΨm collapse in trypanosomes.
Through FACS analysis of Rh123 fluorescence, we discovered that DB75 is a potent inhibitor of ΔΨm in BFs of T. b. brucei (Fig. 4). A minimal concentration of 1.0 μM DB75 was required for a detectable decrease in Rh123 fluorescence after at least 1 min of incubation with drug (results not shown). DB75 appears to collapse the ΔΨm relatively quickly, since only 1 min of incubation with 10 μM DB75 was required for the maximal reduction (approximately 57%) in Rh123 fluorescence relative to the incubation time required for the untreated cells (Fig. 4B). In addition, DB75 seems to be a more powerful inhibitor of ΔΨm than oligomycin and CCCP, since a 1-min incubation with 4 μM oligomycin or 10 μM CCCP resulted in decreases in Rh123 fluorescence of only 23% and 36%, respectively, compared to the fluorescence of the untreated cells (Fig. 4B).
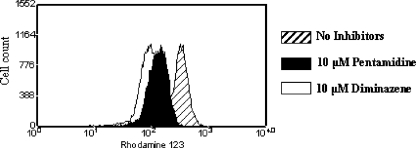
In addition to DB75, other trypanocidal diamidine drugs were shown to collapse ΔΨm through our FACS analysis. For example, 10 min of incubation with 10 μM pentamidine or diminazene aceturate resulted in the dissipation of Ψm (Fig. 5).
FIG. 5.
Other trypanocidal diamidine drugs, pentamidine isethionate and diminazene aceturate, collapse the mitochondrial membrane potential in T. b. brucei BFs. Trypanosomes (107/ml) were pretreated with 10 μM pentamidine isethionate or diminazene aceturate for 10 min in Tes buffer supplemented with 1.0% BSA. Next, the cells were treated with 250 nM Rh123 for an additional 2 min, diluted 1:10, and analyzed on the flow cytometer. The Rh123 fluorescence distribution was plotted as frequency histograms. The results indicate that the mean Rh123 fluorescence (and, therefore, ΔΨm) was decreased in drug-treated cells compared to the fluorescence of the untreated controls. The experiment was performed once.
DB75 inhibits ATP consumption in mitochondria isolated from T. b. brucei BFs.
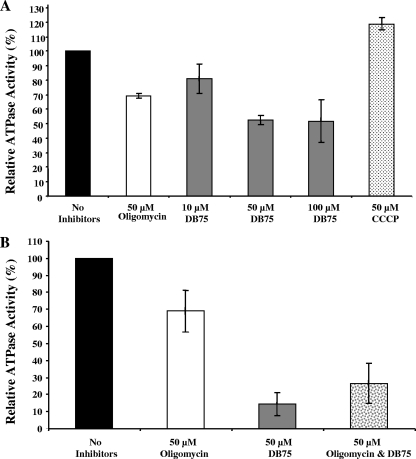
We next investigated the mechanism through which DB75 dissipates the ΔΨm in T. b. brucei BFs. One possibility is that DB75 is an inhibitor of the T. brucei mitochondrial F1F0-ATPase. We found that 50 μM oligomycin inhibited approximately 30% of the mitochondrial ATPase activity (Fig. 6A). This level of inhibition for oligomycin-treated T. brucei mitochondrial preparations is similar to that reported previously (37, 48). Also as expected, treatment with the protonophore CCCP stimulated ATPase activity in the trypanosome mitochondrial preparations (Fig. 6A). DB75 at 10 μM inhibited approximately 20% of the mitochondrial ATPase activity, whereas treatment with either 50 or 100 μM DB75 resulted in inhibition of roughly 47% of the ATPase activity (Fig. 6A). DB75 is therefore a more potent inhibitor of mitochondrial ATPase than oligomycin when their activities are compared on a micromolar basis.
FIG. 6.
Effects of DB75 and mitochondrial inhibitors on ATP consumption by mitochondria isolated from T. b. brucei BFs. (A) Effects of a 10-min incubation with oligomycin, DB75, and CCCP on ATPase activity. DB75 is a more potent inhibitor of mitochondrial ATPase activity than oligomycin. (B) Effect of a 10-min pretreatment with oligomycin on the ability of DB75 to inhibit ATPase activity. The combination of oligomycin plus DB75 inhibited the activity to the same degree as DB75 alone did. Mitochondria (100 μg protein) were incubated on ice in the absence or the presence of drugs in 150 μl of buffer and were then processed for luciferase-based measurement of ATP consumption (see Materials and Methods). The ATP concentration within the untreated controls was set as 100% ATPase activity, and the relative activity (%) was determined for each drug treatment. The results shown are representative of those from three independent experiments, with error bars representing the standard deviations of duplicate samples.
We then investigated if DB75 specifically targets the oligomycin-sensitive ATPase. The effect of pretreatment with oligomycin on the ability of DB75 to inhibit ATPase activity was investigated with isolated T. brucei mitochondria (Fig. 6B). The results indicate that oligomycin pretreatment did not completely prevent DB75 from inhibiting mitochondrial ATPase activity. Thus, perhaps DB75 inhibits other mitochondrial ATPases in addition to the oligomycin-sensitive F1F0-ATPase.
DISCUSSION
Here we report that the mitochondrion of T. b. brucei BFs appears to be a cellular target of the trypanocidal diamidine DB75. The results from this investigation correlate well with the findings from our previous study (24) in which we used S. cerevisiae as a model for investigating the antimicrobial action of DB75. As observed in yeast, DB75 rapidly accumulates within the mitochondrion of living trypanosomes, as evidenced by the immediate fluorescent colocalization of DB75 with MitoTracker Red in cells treated simultaneously with both drugs (Fig. 1C). DB75 was previously shown to collapse the ΔΨm in yeast cells (24), and here we show that DB75 is also a potent inhibitor of ΔΨm in trypanosomes (Fig. 4). As occurs in yeast (24), DB75 inhibits whole-cell respiration (Fig. 2) at micromolar drug concentrations that also result in the collapse of ΔΨm (Fig. 4). We also discovered that DB75 inhibits ATP hydrolysis in mitochondrial fractions from BF trypanosomes and appears to be a more potent inhibitor than oligomycin (Fig. 6). Taken together, the results of this investigation suggest that the killing effect of DB75 in T. brucei BFs involves ΔΨm collapse and the inhibition of mitochondrial ATPase activity.
The functional peculiarities of the mitochondrion in T. brucei BFs make this organelle an attractive candidate as a parasite-specific drug target. The trypanosome mitochondrion alters the biochemistry of the parasite to facilitate life within the distinct environments of the tsetse fly vector and the mammalian host (38, 40, 41). For example, a major functional difference between the mitochondrion in fly and mammalian infective forms is the mechanism through which these forms generate and maintain an energized ΔΨm (7, 36, 37, 45, 49). The oligomycin-sensitive F1F0-ATPase of fly-infective forms (procyclics) functions similarly to the mammalian ATPase, whereas the ATPase of T. brucei BFs functions differently. In particular, the establishment of an energized ΔΨm in procyclics results in the generation of ATP, since the mitochondrial F1F0-ATPase (complex 5) couples ΔΨm generation with the phosphorylation of ADP (1, 45). In contrast, maintenance of the ΔΨm in BFs requires energy and occurs through ATP hydrolysis, mediated by the mitochondrial F1F0-ATPase (7, 37, 45). Given the functional uniqueness of the mitochondrial F1F0-ATPase in T. brucei BFs relative to the ATPase in mammalian host cells, the inhibition of ATP hydrolysis by DB75 is likely to represent a mechanism of killing that is parasite selective.
Another major distinction of BF trypanosomes relative to mammalian cells or procyclics is that the mitochondria of BFs lack cytochromes and respiration is entirely dependent on the trypanosome alternative oxidase (TAO) system, consisting of d-glycerol-3-phosphate dehydrogenase and a terminal oxidase (3, 7, 9, 42). Therefore, inhibitors of TAO activity in vitro are expected to reduce respiration in BFs. SHAM is a potent TAO inhibitor that diminishes whole-cell and mitochondrial respiration in BFs; this trend was demonstrated in our oxygen consumption assays. DB75 inhibited glucose-dependent cellular respiration in BF trypanosomes (Fig. 2) but did not inhibit respiration in isolated mitochondria (Fig. 3). These results could potentially be explained by the fact that mitochondrial preparations obtained from trypanosomes do not retain an energized ΔΨm (15). Therefore, any effects of DB75 upon mitochondrial respiration resulting from ΔΨm collapse cannot be observed in trypanosomal mitochondrial preparations. However, the abbreviated electron transport chain and the TAO system of BFs remain intact in such mitochondrial preparations (4, 30). We therefore conclude that DB75 is probably not an inhibitor of mitochondrial electron transport or TAO activity in BF trypanosomes.
How, then, does DB75 inhibit whole-cell respiration in BF trypanosomes? One possibility is that the DB75-mediated dissipation of ΔΨm accounts for the observed inhibition of glucose-dependent whole-cell respiration occurring in T. brucei BFs. This is a reasonable mechanism, because of the similarities in the DB75 μM concentrations and the incubation durations required to observe the drug-induced inhibition of cellular oxygen uptake (Fig. 2) and ΔΨm collapse (Fig. 4). The connection between the DB75-induced dissipation of ΔΨm and inhibition of cellular respiration is further supported by the fact that oligomycin also inhibits glucose-dependent oxygen uptake (37). It has been suggested that the oligomycin-mediated inhibition of cellular respiration is a result of this drug indirectly inhibiting glycolysis by limiting the cellular supplies of ADP and inorganic phosphate required to maintain carbon flux through pyruvate kinase (29). Like oligomycin, DB75 inhibited ATP hydrolysis within T. brucei mitochondrial preparations (Fig. 6), suggesting that perhaps the DB75-mediated dissipation of ΔΨm indirectly inhibits glycolysis through the depletion of cellular ADP stores. Another explanation for the DB75-mediated inhibition of cellular respiration is that DB75 has cellular targets in addition to the oligomycin-sensitive F1F0-ATPase. This explanation is plausible, considering that DB75 appears to cause a more rapid onset of ΔΨm collapse and is a more potent inhibitor of the mitochondrial F1F0-ATPase than oligomycin.
One probable mechanism through which DB75 collapses ΔΨm in BF trypanosomes is via inhibition of the oligomycin-sensitive mitochondrial F1F0-ATPase. DB75 is a more powerful inhibitor of F1F0-ATPase activity than oligomycin when their activities are compared on a micromolar basis (Fig. 6). Interestingly, a combination of oligomycin plus DB75 fails to completely prevent DB75 from inhibiting ATP hydrolysis, perhaps indicating that DB75 has additional enzymatic targets in the mitochondrion besides the oligomycin-sensitive F1F0-ATPase. The possibility that DB75 has other cellular targets in BFs in addition to the mitochondrial F1F0-ATPase is further supported by our finding that DB75 collapses the ΔΨm in yeast cells (24). Because the ΔΨm generated in yeast cells is independent of F1F0-ATPase activity, it is possible that DB75 deenergizes mitochondria via multiple mechanisms.
Besides the mitochondrion, other cellular components could potentially be the targets of action of DB75. Indeed, UV fluorescence microscopy indicates that DB75 distributes within other organelles in addition to the mitochondrion (Fig. 1A and C). DB75 is a known DNA minor groove binder (10, 20, 27) and therefore is anticipated to interact with the nucleus and kinetoplast. However, the DNA binding affinity of diphenyl and aza analogs related to DB75 was recently shown not to be predictive of trypanocidal potency (25), thereby suggesting that DNA binding alone does not explain the mechanism through which diamidines kill trypanosomes.
The collapse of the ΔΨm by diamidines is a highly plausible mechanism of trypanocidal activity. We have shown that DB75, pentamidine, and diminazene potently collapse the ΔΨm in trypanosomes (Fig. 5). Pentamidine was expected to disrupt ΔΨm, since pentamidine collapses ΔΨm in Leishmania donovani promastigotes (28, 44) and in mitochondria isolated from S. cerevisiae (24). However, this is the first known report of the ΔΨm-inhibitory action of diminazene aceturate, the active agent of a drug used to treat trypanosomiasis in livestock and occasionally to treat early-stage HAT. The diamidine-induced collapse of ΔΨm is expected to cause many deleterious downstream effects in BFs. The maintenance of an energized mitochondrion in BF trypanosomes is absolutely necessary for the mitochondrial importation of nuclear DNA-encoded proteins required for the roles that mitochondria play in cellular functions, such as glycolysis, transcription, polyadenylation, and RNA editing (18, 40). The maintenance of an energized ΔΨm is also critical to metabolic functions, such as fatty acid metabolism (40) and intracellular calcium homeostasis (32, 45). Future studies should investigate the consequences of diamidine-induced ΔΨm collapse to definitively determine if these actions are responsible for BF trypanosome death.
The selective accumulation and concentration of DB75 and other diamidines within BF trypanosomes is likely to play an important role in trypanocidal action. DB75 in vivo accumulates and concentrates within trypanosomes to intracellular concentrations that are 15,000-fold greater than mouse plasma concentrations (26). A similar concentrative effect was reported in an earlier study with infected mice dosed with pentamidine (8), which accumulates within trypanosomes via the P2 amino purine transporter and two other plasma membrane transporters (8, 13, 14). We recently determined that DB75 is rapidly accumulated by trypanosomes primarily through uptake mediated by the trypanosome-specific P2 amino purine transporter (23). We can therefore speculate that in vivo treatment with nanomolar concentrations of DB75 should result in P2 transporter-mediated accumulation of DB75 within BF trypanosomes to the micromolar levels that we report are required for the collapse of ΔΨm.
In summary, we suggest a mechanism of DB75 action in T. brucei BFs as follows: (i) DB75 is selectively taken up and accumulated within parasites by the P2 transporter; (ii) DB75 enters the mitochondrion, probably as a result of the fact that the dicationic nature of DB75 is attracted by the negatively charged environment of the inner mitochondrial matrix; (iii) DB75 collapses ΔΨm via inhibition of the mitochondrial F1F0-ATPase and possibly other mechanisms; and (iv) the deenergization of the mitochondrion results in deleterious downstream effects that lead to trypanosome death. This report paves the way for many interesting avenues of research aimed at investigating the consequences of the antimitochondrial effects of DB75 and other clinically relevant diamidines.
Acknowledgments
This work was supported by a Bill and Melinda Gates Foundation grant to the University of North Carolina, Chapel Hill (principal investigator, R. Tidwell), for “treatment of African trypanosomiasis and leishmaniasis.”
We thank Amanda Mathis for infecting mice with trypanosomes.
Footnotes
Published ahead of print on 17 December 2007.
REFERENCES
- 1.Allemann, N., and A. Schneider. 2000. ATP production in isolated mitochondria of procyclic Trypanosoma brucei. Mol. Biochem. Parasitol. 111:87-94. [DOI] [PubMed] [Google Scholar]
- 2.Barrett, M. P., R. J. Burchmore, A. Stich, J. O. Lazzari, A. C. Frasch, J. J. Cazzulo, and S. Krishna. 2003. The trypanosomiases. Lancet 362:1469-1480. [DOI] [PubMed] [Google Scholar]
- 3.Bienen, E. J., G. C. Hill, and K. O. Shin. 1983. Elaboration of mitochondrial function during Trypanosoma brucei differentiation. Mol. Biochem. Parasitol. 7:75-86. [DOI] [PubMed] [Google Scholar]
- 4.Bienen, E. J., R. K. Maturi, G. Pollakis, and A. B. Clarkson, Jr. 1993. Non-cytochrome mediated mitochondrial ATP production in bloodstream form Trypanosoma brucei brucei. Eur. J. Biochem. 216:75-80. [DOI] [PubMed] [Google Scholar]
- 5.Bouteille, B., O. Oukem, S. Bisser, and M. Dumas. 2003. Treatment perspectives for human African trypanosomiasis. Fundam. Clin. Pharmacol. 17:171-181. [DOI] [PubMed] [Google Scholar]
- 6.Boykin, D. W., A. Kumar, J. E. Hall, B. C. Bender, and R. R. Tidwell. 1996. Anti-Pneumocystis activity of the bis-amidoximes and bis-O-alkylamidoximes prodrugs. Bioorg. Med. Chem. Lett. 6:3017-3020. [Google Scholar]
- 7.Brown, S. V., P. Hosking, J. Li, and N. Williams. 2006. ATP synthase is responsible for maintaining mitochondrial membrane potential in bloodstream form Trypanosoma brucei. Eukaryot. Cell 5:45-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter, N. S., B. J. Berger, and A. H. Fairlamb. 1995. Uptake of diamidine drugs by the P2 nucleoside transporter in melarsen-sensitive and -resistant Trypanosoma brucei brucei. J. Biol. Chem. 270:28153-28157. [DOI] [PubMed] [Google Scholar]
- 9.Clarkson, A. B., Jr., E. J. Bienen, G. Pollakis, and R. W. Grady. 1989. Respiration of bloodstream forms of the parasite Trypanosoma brucei brucei is dependent on a plant-like alternative oxidase. J. Biol. Chem. 264:17770-17776. [PubMed] [Google Scholar]
- 10.Coury, J. E., L. McFail-Isom, L. D. Williams, and L. A. Bottomley. 1996. A novel assay for drug-DNA binding mode, affinity, and exclusion number: scanning force microscopy. Proc. Natl. Acad. Sci. USA 93:12283-12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dann, O., H. Fick, B. Pietzner, E. Walkenhorst, R. Fernbach, and D. Zeh. 1975. Trypanocidal diamidine with three isolated ring systems. Justus Liebigs Ann. Chem. 1975:160-194. [DOI] [PubMed] [Google Scholar]
- 12.Das, B. P., and D. W. Boykin. 1977. Synthesis and antiprotozoal activity of 2,5-bis(4-guanylphenyl)furans. J. Med. Chem. 20:531-536. [DOI] [PubMed] [Google Scholar]
- 13.De Koning, H. P. 2001. Uptake of pentamidine in Trypanosoma brucei brucei is mediated by three distinct transporters: implications for cross-resistance with arsenicals. Mol. Pharmacol. 59:586-592. [DOI] [PubMed] [Google Scholar]
- 14.de Koning, H. P., and S. M. Jarvis. 2001. Uptake of pentamidine in Trypanosoma brucei brucei is mediated by the P2 adenosine transporter and at least one novel, unrelated transporter. Acta Trop. 80:245-250. [DOI] [PubMed] [Google Scholar]
- 15.Divo, A. A., C. L. Patton, and A. C. Sartorelli. 1993. Evaluation of rhodamine 123 as a probe for monitoring mitochondrial function in Trypanosoma brucei spp. J. Eukaryot. Microbiol. 40:329-335. [DOI] [PubMed] [Google Scholar]
- 16.Fairlamb, A. H. 2003. Chemotherapy of human African trypanosomiasis: current and future prospects. Trends Parasitol. 19:488-494. [DOI] [PubMed] [Google Scholar]
- 17.Field, M. C., C. L. Allen, V. Dhir, D. Goulding, B. S. Hall, G. W. Morgan, P. Veazey, and M. Engstler. 2004. New approaches to the microscopic imaging of Trypanosoma brucei. Microsc. Microanal. 10:621-636. [DOI] [PubMed] [Google Scholar]
- 18.Hauser, R., M. Pypaert, T. Hausler, E. K. Horn, and A. Schneider. 1996. In vitro import of proteins into mitochondria of Trypanosoma brucei and Leishmania tarentolae. J. Cell Sci. 109(Pt 2):517-523. [DOI] [PubMed] [Google Scholar]
- 19.Jannin, J., and P. Cattand. 2004. Treatment and control of human African trypanosomiasis. Curr. Opin. Infect. Dis. 17:565-571. [DOI] [PubMed] [Google Scholar]
- 20.Jansen, K., P. Lincoln, and B. Norden. 1993. Binding of DAPI analogue 2,5-bis(4-amidinophenyl)furan to DNA. Biochemistry 32:6605-6612. [DOI] [PubMed] [Google Scholar]
- 21.Johnson, L. V., M. L. Walsh, B. J. Bockus, and L. B. Chen. 1981. Monitoring of relative mitochondrial membrane potential in living cells by fluorescence microscopy. J. Cell Biol. 88:526-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lanham, S. M. 1968. Separation of trypanosomes from the blood of infected rats and mice by anion-exchangers. Nature 218:1273-1274. [DOI] [PubMed] [Google Scholar]
- 23.Lanteri, C. A., M. L. Stewart, J. M. Brock, V. P. Alibu, S. R. Meshnick, R. R. Tidwell, and M. P. Barrett. 2006. Roles for the Trypanosoma brucei P2 transporter in DB75 uptake and resistance. Mol. Pharmacol. 70:1585-1592. [DOI] [PubMed] [Google Scholar]
- 24.Lanteri, C. A., B. L. Trumpower, R. R. Tidwell, and S. R. Meshnick. 2004. DB75, a novel trypanocidal agent, disrupts mitochondrial function in Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 48:3968-3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathis, A. M., A. S. Bridges, M. A. Ismail, A. Kumar, I. Francesconi, M. Anbazhagan, Q. Hu, F. A. Tanious, T. Wenzler, J. Saulter, W. D. Wilson, R. Brun, D. W. Boykin, R. R. Tidwell, and J. E. Hall. 2007. Diphenyl furans and aza analogs: effects of structural modification on in vitro activity, DNA binding, and accumulation and distribution in trypanosomes. Antimicrob. Agents Chemother. 51:2801-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathis, A. M., J. L. Holman, L. M. Sturk, M. A. Ismail, D. W. Boykin, R. R. Tidwell, and J. E. Hall. 2006. Accumulation and intracellular distribution of antitrypanosomal diamidine compounds DB75 and DB820 in African trypanosomes. Antimicrob. Agents Chemother. 50:2185-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazur, S., F. A. Tanious, D. Ding, A. Kumar, D. W. Boykin, I. J. Simpson, S. Neidle, and W. D. Wilson. 2000. A thermodynamic and structural analysis of DNA minor-groove complex formation. J. Mol. Biol. 300:321-337. [DOI] [PubMed] [Google Scholar]
- 28.Mehta, A., and C. Shaha. 2004. Apoptotic death in Leishmania donovani promastigotes in response to respiratory chain inhibition: complex II inhibition results in increased pentamidine cytotoxicity. J. Biol. Chem. 279:11798-11813. [DOI] [PubMed] [Google Scholar]
- 29.Miller, P. G., and R. A. Klein. 1980. Effects of oligomycin on glucose utilization and calcium transport in African trypanosomes. J. Gen. Microbiol. 116:391-396. [DOI] [PubMed] [Google Scholar]
- 30.Minagawa, N., Y. Yabu, K. Kita, K. Nagai, N. Ohta, K. Meguro, S. Sakajo, and A. Yoshimoto. 1997. An antibiotic, ascofuranone, specifically inhibits respiration and in vitro growth of long slender bloodstream forms of Trypanosoma brucei brucei. Mol. Biochem. Parasitol. 84:271-280. [DOI] [PubMed] [Google Scholar]
- 31.Moreno, S. N. 1996. Pentamidine is an uncoupler of oxidative phosphorylation in rat liver mitochondria. Arch. Biochem. Biophys. 326:15-20. [DOI] [PubMed] [Google Scholar]
- 32.Moreno, S. N., and R. Docampo. 2003. Calcium regulation in protozoan parasites. Curr. Opin. Microbiol. 6:359-364. [DOI] [PubMed] [Google Scholar]
- 33.Njogu, R. M., C. J. Whittaker, and G. C. Hill. 1980. Evidence for a branched electron transport chain in Trypanosoma brucei. Mol. Biochem. Parasitol. 1:13-29. [DOI] [PubMed] [Google Scholar]
- 34.Nok, A. J. 2003. Arsenicals (melarsoprol), pentamidine and suramin in the treatment of human African trypanosomiasis. Parasitol. Res. 90:71-79. [DOI] [PubMed] [Google Scholar]
- 35.Nolan, D. P., and H. P. Voorheis. 1991. The distribution of permeant ions demonstrates the presence of at least two distinct electrical gradients in bloodstream forms of Trypanosoma brucei. Eur. J. Biochem. 202:411-420. [DOI] [PubMed] [Google Scholar]
- 36.Nolan, D. P., and H. P. Voorheis. 2000. Hydrogen ion gradients across the mitochondrial, endosomal and plasma membranes in bloodstream forms of Trypanosoma brucei solving the three-compartment problem. Eur. J. Biochem. 267:4601-4614. [DOI] [PubMed] [Google Scholar]
- 37.Nolan, D. P., and H. P. Voorheis. 1992. The mitochondrion in bloodstream forms of Trypanosoma brucei is energized by the electrogenic pumping of protons catalysed by the F1F0-ATPase. Eur. J. Biochem. 209:207-216. [DOI] [PubMed] [Google Scholar]
- 38.Priest, J. W., and S. L. Hajduk. 1994. Developmental regulation of mitochondrial biogenesis in Trypanosoma brucei. J. Bioenerg. Biomembr. 26:179-191. [DOI] [PubMed] [Google Scholar]
- 39.Rogerson, G. W., and W. E. Gutteridge. 1979. Oxidative metabolism in mammalian and culture forms of Trypanosoma cruzi. Int. J. Biochem. 10:1019-1023. [DOI] [PubMed] [Google Scholar]
- 40.Schnaufer, A., G. J. Domingo, and K. Stuart. 2002. Natural and induced dyskinetoplastic trypanosomatids: how to live without mitochondrial DNA. Int. J. Parasitol. 32:1071-1084. [DOI] [PubMed] [Google Scholar]
- 41.Schneider, A. 2001. Unique aspects of mitochondrial biogenesis in trypanosomatids. Int. J. Parasitol. 31:1403-1415. [DOI] [PubMed] [Google Scholar]
- 42.ter Kuile, B. H. 1994. Membrane-related processes and overall energy metabolism in Trypanosoma brucei and other kinetoplastid species. J. Bioenerg. Biomembr. 26:167-172. [DOI] [PubMed] [Google Scholar]
- 43.Vassella, E., K. Straesser, and M. Boshart. 1997. A mitochondrion-specific dye for multicolour fluorescent imaging of Trypanosoma brucei. Mol. Biochem. Parasitol. 90:381-385. [DOI] [PubMed] [Google Scholar]
- 44.Vercesi, A. E., and R. Docampo. 1992. Ca2+ transport by digitonin-permeabilized Leishmania donovani. Effects of Ca2+, pentamidine and WR-6026 on mitochondrial membrane potential in situ. Biochem. J. 284(Pt 2):463-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vercesi, A. E., R. Docampo, and S. N. Moreno. 1992. Energization-dependent Ca2+ accumulation in Trypanosoma brucei bloodstream and procyclic trypomastigotes mitochondria. Mol. Biochem. Parasitol. 56:251-257. [DOI] [PubMed] [Google Scholar]
- 46.Werbovetz, K. 2006. Diamidines as antitrypanosomal, antileishmanial and antimalarial agents. Curr. Opin. Investig. Drugs 7:147-157. [PubMed] [Google Scholar]
- 47.Wilkes, J. M., W. Mulugeta, C. Wells, and A. S. Peregrine. 1997. Modulation of mitochondrial electrical potential: a candidate mechanism for drug resistance in African trypanosomes. Biochem. J. 326(Pt 3):755-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams, N., and P. H. Frank. 1990. The mitochondrial ATP synthase of Trypanosoma brucei: isolation and characterization of the intact F1 moiety. Mol. Biochem. Parasitol. 43:125-132. [DOI] [PubMed] [Google Scholar]
- 49.Williams, S., L. Saha, U. K. Singha, and M. Chaudhuri. 15 October 2007, posting date. Trypanosoma brucei: differential requirement of membrane potential for import of proteins into mitochondria in two developmental stages. Exp. Parasitol. [Epub ahead of print.] [DOI] [PMC free article] [PubMed]
- 50.Zhou, L., K. Lee, D. R. Thakker, D. W. Boykin, R. R. Tiwell, and J. E. Hall. 2002. Enhanced permeability of the antimicrobial agent 2,5-bis(4-amidinophenyl)furan across Caco-2 cell monolayers via its methylamidoidme prodrug. Pharm. Res. 19:1689-1695. [DOI] [PubMed] [Google Scholar]
- 51.Zhou, L., D. R. Thakker, R. D. Voyksner, M. Anbazhagan, D. W. Boykin, J. E. Hall, and R. R. Tidwell. 2004. Metabolites of an orally active antimicrobial prodrug, 2,5-bis(4-amidinophenyl)furan-bis-O-methylamidoxime, identified by liquid chromatography/tandem mass spectrometry. J. Mass Spectrom. 39:351-360. [DOI] [PubMed] [Google Scholar]