Abstract
In this study, we investigated the clonal emergence of daptomycin-resistant Enterococcus faecium strains isolated from a patient with leukocyte adhesion deficiency syndrome. The resistance mechanism in these strains is independent of either equivalent point mutations previously described for Staphylococcus aureus or daptomycin inactivation mechanisms identified in soil bacteria.
Daptomycin is a cyclic lipopeptide with potent antimicrobial activity against gram-positive bacteria (2). The currently intended use of daptomycin is the treatment of complicated skin infections caused by gram-positive bacteria and S. aureus endocarditis (Cubicin label information; Food and Drug Administration new drug application no. 021572). Daptomycin is also a useful alternative for the treatment of infections caused by vancomycin-resistant enterococci (16, 26). The antimicrobial activity of daptomycin is dependent on concomitant binding of calcium and membrane components with subsequent dissipation of the cytoplasmatic membrane potential (14).
The development of resistance to daptomycin remains rare in gram-positive bacteria (20, 22); however, resistance in Staphylococcus aureus and Enterococcus spp. during prolonged treatment with daptomycin has been reported (8, 13, 15, 18). Several groups have now reported an association between glycopeptide-intermediate S. aureus strains and an absence of susceptibility to daptomycin (4, 19, 21, 23). Although definitive mechanisms have yet to be determined, reduction of diffusion of daptomycin due to thickening of cell walls induced by vancomycin has been proposed (1, 4). There are few studies that attempt to define the mechanisms of daptomycin resistance. Recently, S. aureus-resistant strains were identified as carriers of mutations in the following genes: mprF (a membrane lysylphosphatidylglycerol synthetase), yycG (a histidine kinase), and rpoC and rpoB (subunits of RNA polymerase) (9). How-ever, direct proof of the relationship between these mutations and decreased daptomycin susceptibility remains to be demonstrated. Indeed, the mechanisms of resistance to this antibiotic are not well understood. It was recognized initially that daptomycin was inactivated by deacylation in the presence of a soil actinomycetes species, Actinoplanes utahensis (5, 6). However, this alternative has not been studied using clinically relevant bacteria.
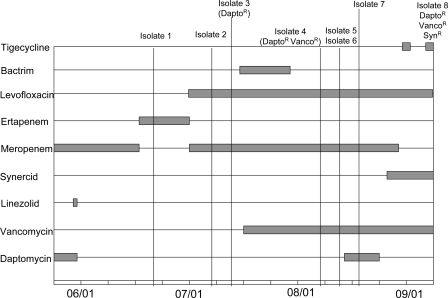
Currently, there are no reports in the literature of studies examining the mechanisms of resistance to daptomycin within Enterococcus species. The present study investigates the mechanism of daptomycin resistance in clonally related susceptible and resistant strains of Enterococcus faecium after extended treatment with vancomycin and short treatment with daptomycin of a patient with severe leukocyte adhesion deficiency syndrome (Fig. 1). These strains were investigated based on the mechanisms of resistance/inactivation previously reported (6, 9).
FIG. 1.
Antimicrobial treatment upon admission for a patient with severe leukocyte adhesion deficiency. The patient was transferred from Duke University Medical Center after a long period of hospitalization and was receiving vancomycin before her admission to the National Institutes of Health.
Over a 3-month period eight clinical E. faecium isolates were obtained from this patient. Initial antimicrobial susceptibility determinations were performed using a MicroScan system (Dade Behring, Deerfield, IL) and Etest for daptomycin (AB Biodisk, Piscataway, NJ) (Table 1). Resistance to vancomycin or dalfopristin/quinupristin was confirmed by Etest.
TABLE 1.
Molecular typing and susceptibility patterns of Enterococcus faecium isolates
| Strain | Source | Date (mo/day) | Antibiogram result (MIC [μg/ml])a
|
PFGE molecular analysisb | ||
|---|---|---|---|---|---|---|
| Vanco | Dapto | Syn | ||||
| 1 | Wound (right groin) | 06/19 | S (<2) | S (4) | S (<1) | S1 |
| 2 | Skin lesion | 07/06 | S (<2) | S (4) | S (<1) | S1 |
| 3 | Wound (abdomen) | 07/10 | S (<2) | R (16) | S (<1) | S1 |
| 4 | Wound (lower extremity) | 08/06 | R (>16) | R (16) | S (<1) | S2 |
| 5 | Blood | 08/11 | R (>16) | R (8) | S (<1) | S2 |
| 6 | Wound (thigh) | 08/11 | R (>16) | R (16) | S (<1) | S2 |
| 7 | Wound (abdomen) | 08/17 | R (>16) | R (>16) | S (<1) | S2 |
| 8 | Wound (right abdomen) | 09/05 | R (>16) | R (16) | R (>2) | S3 |
Interpretation of susceptibility patterns was performed following the current guidelines of CLSI (3) using MicroScan plates (Dade Behring, Deerfield, IL) and Etest strips to confirm resistance to vancomycin (Vanco), daptomycin (Dapto), or dalfopristin/quinupristin (Syn) (AB Biodisk, Piscataway, NJ). S, susceptible; R, resistant.
Numbers indicate PFGE patterns that differed by more than four bands.
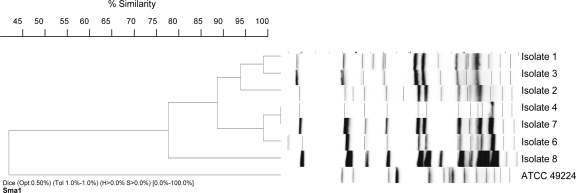
Repetitive extragenic palindromic-PCR was used initially to establish whether the isolates were clonally related. By use of a Diversilab fingerprinting kit (Bacterial Barcodes, Athens, GA) and previously described protocols (12), a high degree of relatedness (similarity index of >97%) was observed for all isolates except isolate 8 (Fig. 2). Pulsed-field gel electrophoresis (PFGE) was performed using digested genomic DNA to corroborate this result. Genomic DNA was extracted in 1.6% InCert agarose plugs (Cambrex Corp., East Rutherford, NJ) following standard methods (17). DNA was digested with SmaI (New England Biolabs, Ipswich, MA) followed by PFGE performed using a CHEF DR III system (Bio-Rad Laboratories, Hercules, CA) as described previously (12). PFGE fingerprint profiles were interpreted according to the guidelines proposed by Tenover et al. (25) and by the use of the program BioNumerics (Applied Maths, Belgium). Strains with differences of ≤3 bands were considered to represent closely related strains. Based on our results (Fig. 2), the group of isolates can be subdivided in three closely related groups: group S1 (isolates 1, 2, and 3), group S2 (isolates 4, 5, 6, and 7), and group S3 (isolate 8), with group S3 a variant of group S2. Daptomycin resistance was observed in related susceptible strains within the S1 group.
FIG. 2.
PFGE analysis of E. faecium isolates evaluated in this study. Analysis was performed using BioNumerics software. Dendrograms were generated using the unweighted-pair group method using average linkages and band-based Dice similarity coefficients. The optimization parameter for this evaluation was set to 0.5 and band position tolerance to 1.0%. Isolate 5 results are not shown in this figure, but that isolate exhibited a restriction pattern identical to that of isolate 4.
Enterococcus spp. display a variety of mechanisms to achieve acquired or intrinsic resistance to several antimicrobial drugs. Acquired resistance among Enterococcus spp. is mediated by transferable transposons or plasmids encoding resistance cassettes (24). On the basis of the modifications of the restriction patterns observed in our PFGE experiments, we investigated the presence of extrachromosomal elements. Using a Qiaprep spin kit (Qiagen, Valencia CA) for plasmid extraction and undigested PFGE plugs, we were unable to identify low- and high-molecular weight plasmids. Therefore, it is unlikely that extrachromosomal elements are associated with the daptomycin-resistant phenotype of our strains. However, using the method described by Depardieu et al. (7), we were able to identify the presence of VanA in the vancomycin-resistant Enterococcus isolates (isolates 4 to 8). This finding resembles data obtained for transposon-mediated resistance mechanisms previously reported in a study of E. faecium (11).
To investigate the intrinsic determinants of resistance to daptomycin, we explored two possibilities described in the literature: (i) point mutations reported for daptomycin-resistant S. aureus strains and (ii) inactivation of daptomycin. Sequencing was performed as previously described (10) using PCR products from the closest homologs in E. faecium to regions within rpoC, rpoB, mprF, and yycG associated with daptomycin-resistant S. aureus strains (9). Relevant regions of rpoB (GenBank accession number ZP_00603961; amino acids [aa] 905 to 1078) and rpoC (ZP_00603960; aa 610 to 755 and aa 940 to 1084) and the full reading frames of mprF (ZP_00604896.1) and yycG (ZP_00604399) were sequenced from isolates 1 (daptomycin susceptible) and 8 (daptomycin resistant). Sequences from these two strains were identical to each other and to the genome sequence of E. faecium DO (ATCC BAA-472), suggesting an alternative resistance mechanism. However, the possibility of additional mutations at other regions within rpoC and rpoB cannot be excluded.
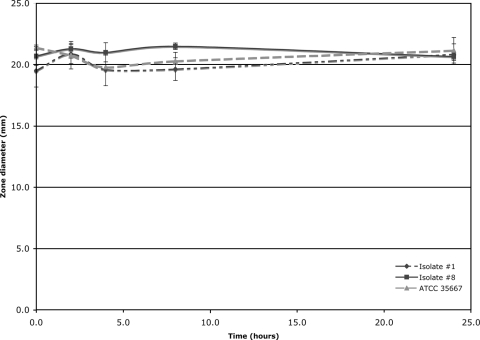
To evaluate the inactivation of daptomycin in the presence of susceptible and resistant strains, a sterile sodium saline solution with 5 mg/ml daptomycin and 100 μg/ml CaCl2 was added to an equal volume of tryptic soy broth cultures of 106 CFU/ml adjusted with the MicroScan turbidimeter (Dade Behring). These mixtures were incubated at 37°C for 2, 4, 8, and 24 h followed by 10 min of centrifugation at 16,000 × g. Reduction of antimicrobial activity of the filtered solutions was evaluated by a modified bioassay (6) by adding 10 μl (25 μg) of the filtered solution to tryptic soy agar plates with 5% sheep blood previously inoculated with S. aureus ATCC 29213 (daptomycin MIC, 0.25 μg/ml). These plates were then incubated overnight at 37°C. Inhibition of growth on plates indicated antimicrobial activity of the filtered solution. When isolates were tested in triplicate (Fig. 3), no daptomycin inactivation was observed after 2, 4, 8, or 24 h of incubation with isolates 1 and 8 or E. faecium ATCC 35667.
FIG. 3.
Diameter of zone of inhibition as a function of incubation time with different E. faecium strains. No significant difference was observed between the strains tested and the control solution with daptomycin and tryptic soy broth. For isolate 1, the daptomycin MIC was 4 μg/ml; for isolate 8, the MIC was 16 μg/ml; and for E. faecium ATCC 35667, the MIC was 1 μg/ml).
Based on our results and the current literature, it is apparent that the mechanism of resistance observed in our strains is different from those represented by the mutations or inactivation mechanisms previously reported (5, 6, 9). However, strains requiring increases in the daptomycin MIC independent of MprF mutations in S. aureus after treatment with vancomycin have been reported (21, 23). Future experiments using comparative genomic hybridization, transcriptional microarray analysis, and genomic libraries might be helpful to identify an as-yet-unknown mechanism of resistance associated with this antibiotic.
Footnotes
Published ahead of print on 7 January 2008.
REFERENCES
- 1.Boucher, H. W., and G. Sakoulas. 2007. Perspectives on daptomycin resistance, with emphasis on resistance in aureus. Clin. Infect. Dis. 45:601-608. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter, C. F., and H. F. Chambers. 2004. Daptomycin: another novel agent for treating infections due to drug-resistant gram-positive pathogens. Clin. Infect. Dis. 38:994-1000. [DOI] [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial susceptibility testing; 16th informational supplement M100-216, vol. 26. CLSI, Wayne, PA.
- 4.Cui, L. Z., E. Tominaga, H. M. Neoh, and K. Hiramatsu. 2006. Correlation between reduced daptomycin susceptibility and vancomycin resistance in vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 50:1079-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Costa, V. M., K. M. McGrann, D. W. Hughes, and G. D. Wright. 2006. Sampling the antibiotic resistome. Science 311:374-377. [DOI] [PubMed] [Google Scholar]
- 6.Debono, M., B. J. Abbott, R. M. Molloy, D. S. Fukuda, A. H. Hunt, V. M. Daupert, F. T. Counter, J. L. Ott, C. B. Carrell, L. C. Howard, et al. 1988. Enzymatic and chemical modifications of lipopeptide antibiotic A21978C: the synthesis and evaluation of daptomycin (LY146032). J. Antibiot. (Tokyo) 41:1093-1105. (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 7.Depardieu, F., B. Perichon, and P. Courvalin. 2004. Detection of the van alphabet and identification of enterococci and staphylococci at the species level by multiplex PCR. J. Clin. Microbiol. 42:5857-5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fowler, V. G., Jr., H. W. Boucher, G. R. Corey, E. Abrutyn, A. W. Karchmer, M. E. Rupp, D. P. Levine, H. F. Chambers, F. P. Tally, G. A. Vigliani, C. H. Cabell, A. S. Link, I. DeMeyer, S. G. Filler, M. Zervos, P. Cook, J. Parsonnet, J. M. Bernstein, C. S. Price, G. N. Forrest, G. Fatkenheuer, M. Gareca, S. J. Rehm, H. R. Brodt, A. Tice, and S. E. Cosgrove. 2006. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N. Engl. J. Med. 355:653-665. [DOI] [PubMed] [Google Scholar]
- 9.Friedman, L., J. D. Alder, and J. A. Silverman. 2006. Genetic changes that correlate with reduced susceptibility to daptomycin in Staphylococcus aureus. Antimicrob. Agents Chemother. 50:2137-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenberg, D. E., L. Ding, A. M. Zelazny, F. Stock, A. Wong, V. L. Anderson, G. Miller, D. E. Kleiner, A. R. Tenorio, L. Brinster, D. W. Dorward, P. R. Murray, and S. M. Holland. 2006. A novel bacterium associated with lymphadenitis in a patient with chronic granulomatous disease. PloS Pathog. 2:260-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Handwerger, S., and J. Skoble. 1995. Identification of chromosomal mobile element conferring high-level vancomycin resistance in Enterococcus faecium. Antimicrob. Agents Chemother. 39:2446-2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrington, S. M., F. Stock, A. L. Kominsky, J. D. Campbell, J. C. Hormzabal, S. Livio, L. Rao, K. L. Kotloff, S. O. Sow, and P. R. Murray. 2007. Genotypic analysis of invasive Streptococcus pneumoniae from Mali, Africa, by semiautomated repetitive-element PCR and pulsed-field gel electrophoresis. J. Clin. Microbiol. 45:707-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayden, M. K., K. Rezai, R. A. Hayes, K. Lolans, J. P. Quinn, and R. A. Weinstein. 2005. Development of daptomycin resistance in vivo in methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 43:5285-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung, D., A. Rozek, M. Okon, and R. E. Hancock. 2004. Structural transitions as determinants of the action of the calcium-dependent antibiotic daptomycin. Chem. Biol. 11:949-957. [DOI] [PubMed] [Google Scholar]
- 15.Lewis, J. S., 2nd, A. Owens, J. Cadena, K. Sabol, J. E. Patterson, and J. H. Jorgensen. 2005. Emergence of daptomycin resistance in Enterococcus faecium during daptomycin therapy. Antimicrob. Agents Chemother. 49:1664-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mangili, A., I. Bica, D. R. Snydman, and D. H. Hamer. 2005. Daptomycin-resistant, methicillin-resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 40:1058-1060. [DOI] [PubMed] [Google Scholar]
- 17.Miranda, A. G., K. V. Singh, and B. E. Murray. 1991. DNA fingerprinting of Enterococcus faecium by pulsed-field gel electrophoresis may be a useful epidemiologic tool. J. Clin. Microbiol. 29:2752-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munoz-Price, L. S., K. Lolans, and J. P. Quinn. 2005. Emergence of resistance to daptomycin during treatment of vancomycin-resistant Enterococcus faecalis infection. Clin. Infect. Dis. 41:565-566. [DOI] [PubMed] [Google Scholar]
- 19.Patel, J. B., L. A. Jevitt, J. Hageman, L. C. McDonald, and F. C. Tenover. 2006. An association between reduced susceptibility to daptomycin and reduced susceptibility to vancomycin in Staphylococcus aureus. Clin. Infect. Dis. 42:1652-1653. [DOI] [PubMed] [Google Scholar]
- 20.Pfaller, M. A., H. S. Sader, and R. N. Jones. 2007. Evaluation of the in vitro activity of daptomycin against 19615 clinical isolates of Gram-positive cocci collected in North American hospitals (2002-2005). Diagn. Microbiol. Infect. Dis. 57:459-465. [DOI] [PubMed] [Google Scholar]
- 21.Pillai, S. K., H. S. Gold, G. Sakoulas, C. Wennersten, R. C. Moellering, and G. M. Eliopoulos. 2007. Daptomycin nonsusceptibility in Staphylococcus aureus with reduced vancomycin susceptibility is independent of alterations in MprF. Antimicrob. Agents Chemother. 51:2223-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sader, H. S., J. M. Streit, T. R. Fritsche, and R. N. Jones. 2006. Antimicrobial susceptibility of gram-positive bacteria isolated from European medical centres: results of the Daptomycin Surveillance Programme (2002-2004). Clin. Microbiol. Infect. 12:844-852. [DOI] [PubMed] [Google Scholar]
- 23.Sakoulas, G., J. Alder, C. Thauvin-Eliopoulos, R. C. Moellering, Jr., and G. M. Eliopoulos. 2006. Induction of daptomycin heterogeneous susceptibility in Staphylococcus aureus by exposure to vancomycin. Antimicrob. Agents Chemother. 50:1581-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shepard, B. D., and M. S. Gilmore. 2002. Antibiotic-resistant enterococci: the mechanisms and dynamics of drug introduction and resistance. Microbes Infect. 4:215-224. [DOI] [PubMed] [Google Scholar]
- 25.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vouillamoz, J., P. Moreillon, M. Giddey, and J. M. Entenza. 2006. Efficacy of daptomycin in the treatment of experimental endocarditis due to susceptible and multidrug-resistant enterococci. J. Antimicrob. Chemother. 58:1208-1214. [DOI] [PubMed] [Google Scholar]