Abstract
Background
In vitro, butyrate inhibits histone deacetylase and down-regulates expression of cyclin D1. We hypothesized that an increased entry rate of butyrate into the cecal lumen would have similar effects in vivo.
Methods
We used frozen cecal tissue and data from previous studies, one showing that lactulose supplementation caused an increased rate of cecal synthesis of butyrate and decreased cecal cell proliferation and density of clostridia and the other showing that cecal cell proliferation was increased by an exogenous cecal butyrate infusion at a comparable rate. The ratio of acetylated to total histones (AH ratio) and cyclin D1 mRNA expression were measured in cecal tissue.
Results
Lactulose supplementation caused a 189% increase in the AH ratio (p = .004), which inversely correlated with cecal cell proliferation (r = −0.782; p = .008). With cecal butyrate infusion, we observed a significant decrease in histone acetylation (p = .02), which also inversely correlated with cecal cell proliferation (r = −0.797; p = .002). Cyclin D1 expression was increased 6.5-fold by lactulose feeding (p = .02) but decreased 50% with cecal butyrate infusion (p = .004).
Conclusions
The effects on histone acetylation of increased “endogenous” butyrate production produced by lactulose feeding, but not exogenous cecal infusion of butyrate, mirror those in vitro. Thus, bacterial production and exogenous infusion of butyrate have opposite effects on histone acetylation and cyclin D1 expression, suggesting that the composition of bacterial flora may play a role in butyrate’s in vivo effects on the cell cycle.
Histone acetylation and deacetylation play a major role in the mitotic cell cycle and in regulating the transcription of individual genes, as well as the entire genome.1–4 Histone deacetylation predominates during the early phases of mitosis, but histone hyperacetylation causes “relaxation” of the chromatin structure, making the DNA more accessible to transcription factors, thus facilitating gene transcription, characteristic of differentiated cells.1–5 Butyrate is a well-known inhibitor of histone deacetylase.1 The cell cycle also is regulated by 3 families of proteins: cyclins, cyclin-dependent kinases (cdk), and inhibitors of the cdk.5 Particular cyclins activate the cdk.5 Butyrate has been shown to down-regulate mRNA but not protein expression of cyclin D1 in human intestinal cells, and down-regulate protein expression of cyclin D1 in fibroblasts; this may occur via a butyrate response element found in the promoter of the gene.1,6,7
Using frozen cecal tissue collected from piglets during 2 previous experiments,8,9 we tested the hypothesis that an increase in the in vivo supply of butyrate would increase histone acetylation and down-regulate mRNA expression of cyclin D1.
We recently observed that lactulose feeding in piglets caused decreased cecal cell proliferation, increased butyrate synthesis within the cecal lumen, and an approximate 16-fold decrease in the cecal density of clostridia.8,10 However, when we then infused butyrate at a rate equal to the increase in butyrate synthesis measured in the lactulosefed piglets, cell proliferation in the cecum and other intestinal tissues was approximately doubled.9 We wondered if assessing certain molecular effects of butyrate, previously observed in cultured colon cells, might provide more insight into our observations. This manuscript contains new data related to histone acetylation and cyclin D1 gene expression in cecal tissue obtained from the piglets in these 2 previous studies, as well as new statistical analysis related to these 2 measurements. Other measurements used in our data analysis were previously reported, including those related to cell proliferation, the rate of cecal luminal synthesis of butyrate, and the cecal concentration of butyrate.8,9
MATERIALS AND METHODS
Animals, Feedings, and Design: Experiments 1 and 2
Details concerning these studies have been published,8,9 and therefore, only an outline of the studies will be given here.
In experiment 1,8 we used tissue collected at the time of killing in 7 piglets fed control formula and in 8 piglets supplemented with lactulose. The Yorkshire/Hampshire piglets were studied at the University of Texas Medical Branch, whose Institutional Animal Care and Use Committee approved the research protocol. On approximately day 12 of life, the piglets were transported from the pig farm to the laboratory. The piglets then were fed orally a sow milk replacer formula (SMR; SPF Lac, sterile milk replacer; PetAG Inc, Hampshire, IL). After 6 days of feeding, the piglets underwent a surgical procedure, under general anesthesia using isoflurane, for insertion of catheters into the stomach or duodenum for feeding, portal vein for blood drawing during tracer studies, and a cannula into the cecum for tracer infusion. Lactulose (66 g/L) was introduced into the diet on day 2 or 3 after surgery. At 6–7 days postoperatively, we conducted a [1-13C]-butyrate tracer study to determine the rate of production of butyric acid within the cecum lumen.8 On the day after the tracer study, the piglets were injected with bromodeoxyuridine (25 mg/kg), and cecal tissue was removed 2 hours later.8
In experiment 2, we used tissue collected at the time of killing in 12 Yorkshire/Hampshire piglets studied at the University of Vermont, whose Institutional Animal Care and Use Committee approved the research protocol.9 On approximately day 12 of life, the piglets were transported from the pig farm to the laboratory. For 6 days, the piglets then were fed SMR. After 6 days of feeding, the piglets underwent a surgical procedure for insertion of a cannula into the cecum. For the next 4 days, 6 piglets then received a cecal infusion of sodium butyrate (1 mL/h; butyrate group) and 6 piglets received a cecal infusion of phosphate-buffered saline (PBS) infusion (1 mL/h; control group). The rate of cecal infusion of butyrate averaged 2.30 μmol/kg/min; this rate of infusion of butyrate is comparable to the average rate of cecal synthesis (grand mean) of 2.2 ± 0.3 μmol/kg/min in experiment 1 and thus corresponds to a physiologically relevant rate of entry of butyrate into the cecum.8 After 4 days of cecal infusion, bromodeoxyuridine (25mg/kg) was injected into an ear vein, and under general anesthesia, tissue was biopsied from the cecum.9
Measurement of Histone Acetylation in Cecal Tissue
Acetylated H3 to total H3 histone ratios (AH ratio) were determined to estimate the inhibition of histone deacetylase activity, in vivo, by butyrate. Nuclei from pig intestine tissues were isolated by homogenization of frozen tissue in NP-40 buffer (10 mmol/L Tris-HCl, pH 7.5, 1.5 mmol/L MgCl2, 0.65% NP-40, 1 mmol/L PMSF) in the presence of 10 mmol/L Na butyrate to prevent deacetylation of histones. Regarding the standard use of butyrate in this procedure, please note that after the lysis of cells, the acetyl CoA pools are diluted, which effectively inactivates the histone acetyltransferases; hence, the addition of acetyl groups to histones is stopped. However, histone deacetylation can still occur, and, thus, histone deacetylase inhibitors, such as butyrate, are usually added to the isolation buffers. Nuclei were then resuspended in reticulocyte standard buffer (10 mmol/L NaCl, 10 mmol/L Tris-HCl, pH 7.5, 3 mmol/L MgCl2, 1 mmol/L PMSF) containing 10 mmol/L Na butyrate, and histones were extracted with 0.2 M H2SO4 as previously described.11 Histones were separated by SDS-PAGE. In the first experiment, the histones were immunoblotted with polyclonal antibodies to H3 (Cell Signaling Technology, Danvers, MA) or diacetylated (K9, K14) H3 (Upstate/Millipore, Charlottesville, VA).12 Total H3 and acetylated H3 immunoblot signals were quantified using the Storm 860 Gel and Blot Imaging System (GE Healthcare, England), and the AH ratio was estimated for the chemiluminescent signals obtained with antibodies bound to acetylated H3 relative to those to total H3.
In the second experiment, total H4 protein was used as the loading control. The gels were stained using SYPRO Ruby gel stain (Molecular Probes/Invitrogen, Carlsbad, CA). Detection of total H4 protein was with the VersaDoc 1000 imaging system (BioRad Laboratories, Hercules, CA) to provide band intensity.
Measurement of Cyclin D1 Gene Expression in Mixed Cecal Tissue
The mRNA expression of cyclin D1 was measured because this is a critical cell cycle gene possibly regulated by butyrate.5 The mRNA expression was quantified by real-time quantitative PCR using the iQ Multi Color Real Time PCR Detection System (Bio-Rad Laboratories). A 207-bp cyclin D1 product was obtained using 5′-GCAGAAGTGCGAGGAGGAGGTCTT-3′ (forward) and 5′-CGGATGGAGTTGTCGGTGTAGATGC-3′ (reverse) primers. GAPDH expression was quantified using 5′-ACCTGACCTGCCGTCTGGAGAAA-3′ (forward) and 5′-CCAGCCCCAGCATCAAAGGTAGAAG-3′ (reverse) primers, which produced a 170-bp product. The relative expression of cyclin D1 was normalized to GAPDH and quantified using the 2−ΔΔCt method as described13 (not shown).
Cell Proliferation Index
Unstained, paraffin-embedded slides were deparaf-finized and then stained for bromodeoxyuridine labeling (Cell Proliferation Kit from Amersham Life Science, No. RPN 20, Piscataway, NJ). Then, as previously described,8,9 we assessed fractional proliferation index for the total cecal crypt.
Estimate of the Rate of Luminal Synthesis (Production) of Butyrate in Experiment 1
We used a previously described single-isotope-dilution model for assessing the rate of synthesis (production) of butyrate in the colonic lumen.8,14,15
Analysis of the Concentration of Butyrate in Cecal Fluid
The cecal concentration of butyrate was measured by gas chromatography as described.8,16
Data Analysis and Statistics
All results are expressed as mean ± SEM. The t-test was initially used to compare the AH ratio in the control and lactulose groups (experiment 1) and the control and butyrate infusion groups (experiment 2; SPSS Base 10.0; SPSS Inc, Chicago, IL), but we used the Mann-Whitney test when variance in the 2 groups appeared unequal or the existence of outliers suggested that normal distribution statistics may not apply.
Because of the vagaries of available sample material for measurement of AH ratio and the mRNA expression of cyclin D1, there were modest differences in the sample sizes (and thus p values) for each group in the studies reported here compared with the previous reports.
We also used both linear (Pearson) and rank (Spearman) correlation techniques to examine interrelationships among the AH ratio, the rate of production of butyrate or the cecal concentration of butyrate, and cell proliferation.
RESULTS
Effects of the Rate of Entry of Butyrate Into the Cecal Lumen on Histone Acetylation
Experiment 1
As previously reported,8 lactulose caused an approximate 120% increase in the rate of entry of butyrate into the cecal lumen (μmol/min). Figure 1 shows that the AH ratio was 189% increased in the lactulose group (p = .03, t-test; p = .004, Mann-Whitney test). As previously noted, this diet treatment also caused a significant decrease in cell proliferation.8 Figure 2 demonstrates the inverse correlation between cecal cell proliferation and the AH ratio in the cecal homogenate (Pearson r = −0.756, p = .011; Spearman r = −0.782, p = .008).
Figure 1.
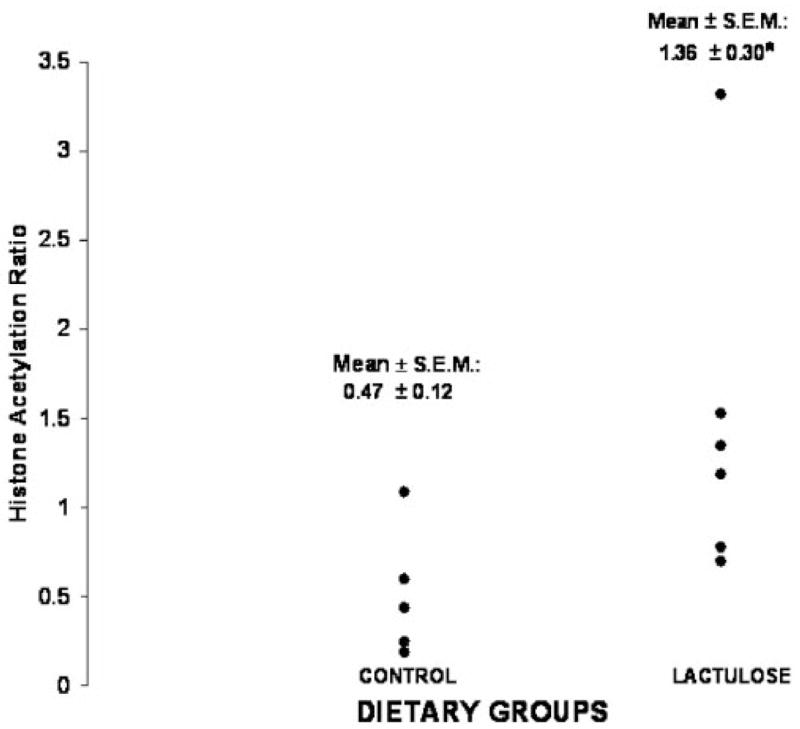
Effects of lactulose feeding on the relative degree of histone acetylation, measured by quantifying acetylated H3 to total H3 histone ratios (AH ratio; experiment 1). *Lactulose vs control groups: p = .03, t-test; p = .004, Mann-Whitney test.
Figure 2.
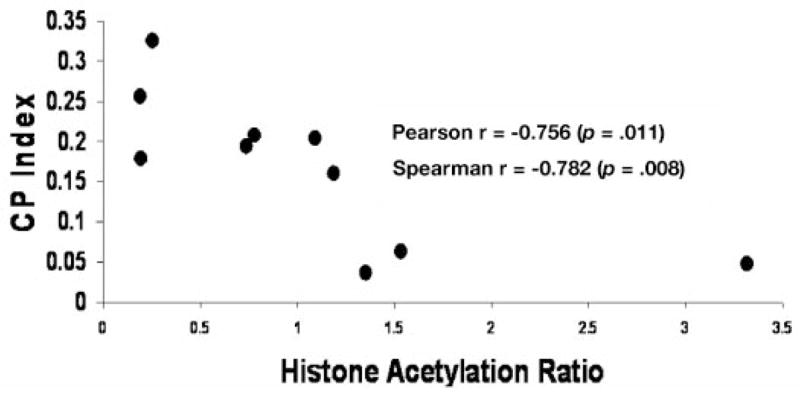
Scattergram depicting the relationship between histone acetylation and cell proliferation (CP index) in the cecum in experiment 1. Pearson r = −0.756 (p = .011) and Spearman r = −0.782 (p = .008).
Although we observed greater histone acetylation in the lactulose group, which also manifested a higher butyrate entry rate into the cecum (“production”), butyrate production and the AH ratio did not significantly correlate among the piglets in both treatment groups. We also examined the relationship between the AH ratio and the cecal concentration of butyrate. The cecal concentration of butyrate (mmol/L) was significantly lower in the lactulose group compared with the control group (p = .03, t-test),8 and there was a highly significant, inverse correlation of cecal concentration of butyrate (mmol/L) with the AH ratio (Spearman r = −0.71, p = .015). There also was a borderline significant positive correlation of butyrate concentration with cecal cell proliferation (Pearson r = 0.82, p = .04; Spearman r = 0.77, p = .07).
Experiment 2
Infusion of butyrate, at a rate equivalent to the average increment in butyrate production caused by lactulose feeding caused an approximate 120% increase in cecal cell proliferation.9 Butyrate infusion also caused a modest (30%) but significant decrease in histone acetylation (p = .03, t-test; p = .02, Mann-Whitney test; Figure 3). Figure 4 depicts the inverse relationship between cecal cell proliferation and histone acetylation in experiment 2 (Pearson r = − 0.744, p = .006; Spearman r = −0.797, p = .002).
Figure 3.
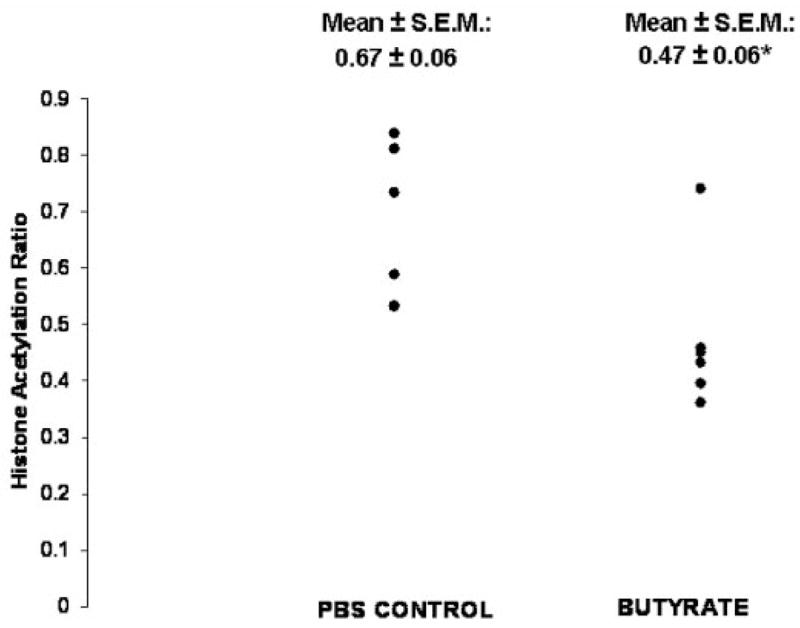
Effects of cecal butyrate infusion on relative histone acetylation in experiment 2. The AH ratio was estimated from the acetylated H3 to total H4 ratio. *Butyrate infusion vs control infusion with phosphate-buffered saline: p = .03, t-test; p = .02, Mann-Whitney test.
Figure 4.
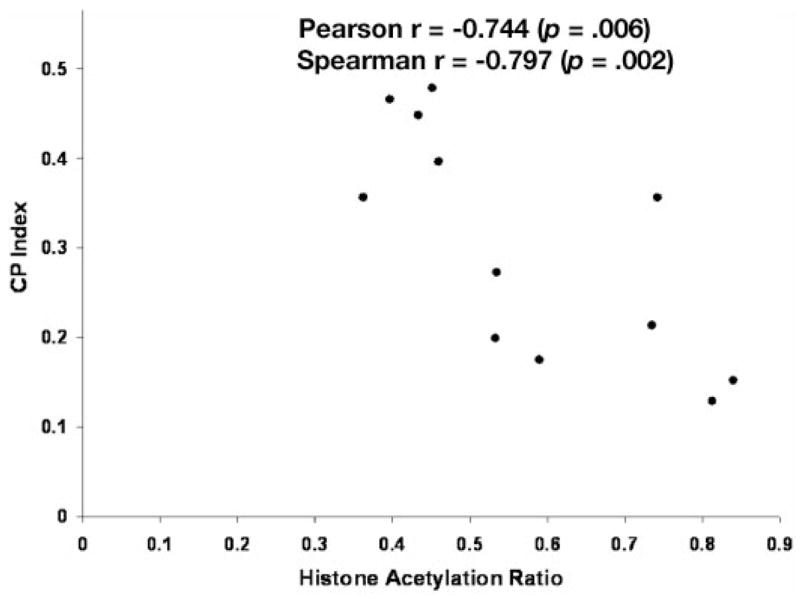
Scattergram depicting the relationship between histone acetylation and cell proliferation (CP index) in the cecum in experiment 2. Pearson r = −0.744, p = .006; Spearman r = −0.797, p = .002.
Effects of Butyrate Entry Rate on mRNA Expression of Cyclin D1 in Homogenized Cecal Mucosa
Experiment 1
In experiment 1, the mRNA expression of cyclin D1 in cecal tissue was increased approximately 6.5-fold by lactulose feeding (5.9 ± 3.4 relative expression units) compared with control (0.9 ± 0.4; p = .02, Mann-Whitney test). There also was a significant rank correlation between cyclin D1 expression and the AH ratio (r = 0.686, p = .005).
Experiment 2
In contrast, in experiment 2, cecal butyrate infusion, which had opposite effects as lactulose on histone acetylation and cell proliferation, lowered cyclin D1 expression by 50% (30.5 ± 2.2 relative expression units) compared with control (59.5 ± 9.0; p = .004, Mann-Whitney test). Cyclin D1 expression did not correlate with the AH ratio in this experiment, however.
DISCUSSION
This was an exploratory study, carried out primarily after the completion of experiments 1 and 2. Thus, we did not have the opportunity to use techniques such as scraping the mucosal cells or laser microdissection so that histone acetylation and cyclin D1 gene expression were measured only in colonocytes.17,18 Nevertheless, it is still of interest that lactulose feeding (increased butyrate production by cecal bacteria) and cecal infusion of butyrate both caused statistically significant changes in histone acetylation and cyclin D1 expression of cecal tissue, albeit in opposite directions. Despite the obvious limitations to inferences that can be made about molecular changes in epithelial cells per se from tissue studies representing a mixed cell composition, both of our experiments show that the degree of cecal cell proliferation in living animals inversely correlates with overall histone acetylation of the tissue. Moreover, treatment with lactulose, which doubled the rate of butyrate production within the cecal lumen, induced an approximate doubling of the degree of histone acetylation, consistent with the effects of butyrate in cultured cells.1 Boffa et al19 studied colonic cell proliferation (using [3H]-thymidine) and histone acetylation of colonocytes separated from intact colonic tissue in response to varying levels of fiber intake in rats. They found that the fiber-free group manifested both the highest rate of cell proliferation and lowest degree of histone acetylation, and mention in their discussion that histone acetylation inversely correlated with cell proliferation.19 From a comparison of our data with that of Boffa et al,19 we believe that our findings would have been similar had we been able to assess histone acetylation only in cecal epithelial cells.
Cyclin D1 expression correlated with the AH ratio in experiment 1, but not in experiment 2. Transcription of cyclin D1 was enhanced in 2 conditions for either experiment where histone acetylation was increased and cell proliferation was relatively decreased: lactulose feeding in experiment 1 and control infusion of PBS in experiment 2. This finding is consistent with the general view that histone acetylation facilitates gene expression,1–4 but only the effects of exogenous butyrate were apparently consistent with the reported effects, in vitro, of butyrate per se on colonocytes.6,7
Although our study substantiates the role of histone deacetylation in cell cycle progression, our major, more original, interest was in determining whether butyrate had similar effects in vivo on histone acetylation and cyclin D1 expression as reported in vitro.1,6,7 In experiment 1, the rate of cecal luminal synthesis of butyrate in the lactulosefed group was, on average, much higher than the controls, and this was associated with both reduced cecal cell proliferation and increased histone acetylation (similar to in vitro effects) as well as increased cyclin D1 expression.8 However, in experiment 2, exogenous butyrate was infused into the cecal lumen at a similar rate, and we observed opposite findings: increased cell proliferation, reduced histone acetylation, and reduced cyclin D1 expression.9 The diametrically opposing effects of in vivo production of butyrate and exogenous butyrate infusion on histone acetylation and cyclin D1 expression perhaps relate in some way to the different effects on cecal cell proliferation of butyrate produced by bacteria vs “exogenous” butyrate. One may then ask if these different effects of butyrate in vivo also are relevant to observed effects of butyrate on cell proliferation in vitro.9 In studies of cultured neoplastic cells of colonic origin,1,20 the medium concentration of butyrate is in the range found in the colon lumen (eg, 0.5–2.0 mmol/L),20 presumably because of the assumption that colonic mucosal uptake of butyrate will be proportional to luminal concentration. However, our previous study suggested that cecal luminal concentration of butyrate does not change in response to the rate of entry of butyrate into the cecum of piglets or the rate of uptake of the butyrate produced in the lumen (virtually complete).8,21 Therefore, luminal butyrate concentration may be merely a secondary consequence of water absorption in the colon and may not reflect the butyrate supply to the colonocytes. Thus, concentrations of butyrate in cell culture media may not necessarily be physiologically relevant, and perhaps in vivo effects of butyrate on histone acetylation cannot be predicted from cell culture studies where butyrate is present at concentrations found in the lumen. However, as noted below, if specific species of bacteria proliferate in response to local concentrations of butyrate, this could have other more indirect effects on molecular regulation of colonocytes.
Besides the issue of whether colonic luminal concentrations should be the basis for cell culture studies, there is one other pertinent factor which may modify the cellular and molecular effects of butyrate in vivo compared with what is observed in vitro. By potentially altering the bacterial flora, we surmise that feeding the fructooligosaccharide (lactulose), as well as the secondary osmotic diarrhea it causes, may alter or even antagonize the effects of butyrate also produced in response to lactulose feeding.10 Indeed, lactulose feeding was observed to cause an approximate 16-fold decrease in the cecal density of clostridia10; as explained below, this disruption of colonization by clostridia, as well as, potentially, other bacterial species not specifically quantified, could have a complex effect on cell proliferation and histone acetylation, in part by modifying the innate immune response.
Structural components of bacteria (pathogen-associated molecular patterns, PAMP) such as lipopolysaccharide (Gram-negative bacteria), lipoteichoic acid (Gram-positive bacteria), and surface layer proteins (eg, Clostridium difficile) are recognized by various toll-like receptors (TLRs) expressed in macrophages, dendritic cells, and enterocytes in the small intestine and colon cells.22–26 TLRs then initiate a signaling sequence, often via an adaptor molecule (MyD88), which causes activation of NFκB signaling and cytokine production (including interleukin-6 and tumor necrosis factor) but also cyclooxygenase-2 and prostaglandin E2 production. These mammalian signaling pathways facilitate the reparative response to intestinal injury, in part via stimulation of cell proliferation,22–26 but also may be associated with reduced proliferation of enterocytes in the baseline, noninjured state.23 Deficient TLR-4 signaling also causes much more severe injury in both a dextran sulfate colitis model23–25 and in a radiation injury model.23 Histone acetylation potentiates NFκB-dependent inflammatory responses in monocytes (in the presence a phorbol ester)27 or microglia.28 In vitro, butyrate or the supernatant forms a culture of C butyricum, which contains butyrate, down-regulated mRNA expression of TLR-4.29–31 But, the comparable in vivo effects of butyrate are not known. There also are examples in the literature showing that bacteria may induce signal transduction in mammalian cells via histone acetylation.32,33 Thus, bacterial colonization, via effects on TLR signaling, may alter the underlying effects of butyrate, which may affect histone acetylation and TLR transcription as well. Therefore, when butyrate production is stimulated by the administration of a highly fermentable carbohydrate such as lactulose, which causes both changes in bacterial flora composition and osmotic diarrhea,8,10 the ultimate effects on cell proliferation and histone acetylation may be different than when butyrate is used in vitro or administered as a low-dose cecal infusion (which does not obviously affect bowel function).9 Our recent observations8,10 related to lactulose feeding, when considered in the context of our study of cecal butyrate infusion,9 elicit the conjecture that if increased bacterial butyrate production were caused by changes in fermentation pathways involving down-regulation of the TLR-4/PAMP interaction (eg, decreased density of Gram-negative bacteria), there could be synergistic, antagonistic effects of bacterial colonization and butyrate production on the reparative response to injury via the TLR-4 pathway.22–26,29–31 However, we only observed effects of lactulose on the cecal density of clostridia and did not specifically examine cecal densities of Gram-negative bacteria, which may interact specifically with TLR-4.10 At present, we also do not know if exogenous butyrate infusion also could alter the cecal density of specific bacterial species most likely to produce it, namely, clostridia (quorum sensing) or bacteria recognized by TLR-4.10
In conclusion, the “effects” in piglets with severe carbohydrate malabsorption of increased cecal luminal synthesis of butyrate both compare (cell proliferation and histone acetylation) and contrast (cyclin D1 expression) with the previously described in vitro effects of butyrate but may be modified by changes in cecal bacterial flora.1,6,10 The respective effects of a direct cecal infusion of butyrate (experiment 2) in piglets with presumably normal cecal bacterial flora included repression of both cyclin D1 expression and histone acetylation, which also both mirrored and contrasted with the reported in vitro effects of butyrate on these processes.1,6 We speculate that understanding of the physiologic effects of carbohydrate fermentation on small intestinal or colonic cell turnover would be enhanced by an integrated knowledge of how compounds such as butyrate produced by bacteria transcriptionally regulate the TLR signaling mechanism, which appears to have a major effect on intestinal homeostasis.23
Acknowledgments
This research was supported by National Institutes of Health grant No. R01 DK061775 (C.L.K.) and by Canadian Institute of Health Research grant MOP-9186 (J.R.D.) and a Canada Research Chair to J.R.D. We acknowledge the technical assistance of Mary Schmitz-Brown, Travis Solley, Rhonda Maple, and Karen Everingham.
References
- 1.Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr. 2003;133(Suppl 7):2485S–2493S. doi: 10.1093/jn/133.7.2485S. [DOI] [PubMed] [Google Scholar]
- 2.Kruhlak MJ, Hendzel MJ, Fischle W, et al. Regulation of global acetylation in mitosis through loss of histone acetyltransferases and deacetylases from chromatin. J Biol Chem. 2001;276:38307–38319. doi: 10.1074/jbc.M100290200. [DOI] [PubMed] [Google Scholar]
- 3.Vogelauer M, Wu J, Suka N, Grunstein M. Global histone acetylation and deacetylation in yeast. Nature. 2000;408:495–498. doi: 10.1038/35044127. [DOI] [PubMed] [Google Scholar]
- 4.Berger SL. Gene regulation: local or global? Nature. 2000;408:412–415. doi: 10.1038/35044160. [DOI] [PubMed] [Google Scholar]
- 5.Blottiere HM, Buecher B, Galmiche JP, Cherbut C. Molecular analysis of the effect of short-chain fatty acids on intestinal cell proliferation. Proc Nutr Soc. 2003;62:101–106. doi: 10.1079/PNS2002215. [DOI] [PubMed] [Google Scholar]
- 6.Siavoshian S, Segain JP, Kornprobst M, et al. Butyrate and trichostatin A effects on the proliferation/differentiation of human intestinal epithelial cells: induction of cyclin D3 and p21 expression. Gut. 2000;46:507–514. doi: 10.1136/gut.46.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lallemand F, Courilleau D, Sabbah M, Redeuilh G, Mester J. Direct inhibition of the expression of cyclin D1 gene by sodium butyrate. Biochem Biophys Res Commun. 1996;229:163–169. doi: 10.1006/bbrc.1996.1774. [DOI] [PubMed] [Google Scholar]
- 8.Kien CL, Schmitz-Brown M, Solley T, Sun D, Frankel WL. Increased colonic luminal synthesis of butyric acid is associated with lowered colonic cell proliferation in piglets. J Nutr. 2006;136:64–69. doi: 10.1093/jn/136.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kien CL, Blauwiekel R, Bunn JY, Jetton TL, Frankel WL, Holst JJ. Cecal infusion of butyrate increases intestinal cell proliferation in piglets. J Nutr. 2007;137:916–922. doi: 10.1093/jn/137.4.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kien CL, Blauwiekel R, Williams CH, Bunn JY, Buddington RK. Lactulose feeding lowers cecal densities of Clostridia in piglets. JPEN J Parenter Enteral Nutr. 2007;31:194–198. doi: 10.1177/0148607107031003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delcuve GP, Davie JR. Chromatin structure of erythroid-specific genes of immature and mature chicken erythrocytes. Biochem J. 1989;263:179–186. doi: 10.1042/bj2630179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun JM, Chen HY, Davie JR. Effect of estradiol on histone acetylation dynamics in human breast cancer cells. J Biol Chem. 2001;276:49435–49442. doi: 10.1074/jbc.M108364200. [DOI] [PubMed] [Google Scholar]
- 13.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 14.Kien CL, Chang JC, Cooper JR. Quantitation of colonic luminal synthesis of butyric acid in piglets. J Pediatr Gastroenterol Nutr. 2002;35:324–328. doi: 10.1097/00005176-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Kien CL, Chang JC, Cooper JR. Butyric acid is synthesized by piglets. J Nutr. 2000;130:234–237. doi: 10.1093/jn/130.2.234. [DOI] [PubMed] [Google Scholar]
- 16.Deschner EE, Ruperto JF, Lupton JR, Newmark HL. Dietary butyrate (tributyrin) does not enhance AOM-induced colon tumorigenesis. Cancer Lett. 1990;52:79–82. doi: 10.1016/0304-3835(90)90080-h. [DOI] [PubMed] [Google Scholar]
- 17.Manjarrez-Orduno N, Moreno-Garcia ME, Fink K, Santos-Argumedo L. CD38 cross-linking enhances TLR-induced B cell proliferation but decreases IgM plasma cell differentiation. Eur J Immunol. 2007;37:358–367. doi: 10.1002/eji.200636453. [DOI] [PubMed] [Google Scholar]
- 18.Hooper LV. Laser microdissection: exploring host-bacterial encounters at the front lines. Curr Opin Microbiol. 2004;7:290–295. doi: 10.1016/j.mib.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Boffa LD, Lupton JR, Mariani MR, et al. Modulation of colonic epithelial cell proliferation, histone acetylation, and luminal short chain fatty acids by variation of dietary fiber (wheat bran) in rats. Cancer Res. 1992;52:5906–5912. [PubMed] [Google Scholar]
- 20.Singh B, Halestrap A, Paraskeva C. Butyrate can act as a stimulator of growth or inducer of apoptosis in human colonic epithelial cell lines depending on the presence of alternative energy sources. Carcinogen. 1997;18:1265–1270. doi: 10.1093/carcin/18.6.1265. [DOI] [PubMed] [Google Scholar]
- 21.Ruppin H, Bar-Meir S, Soergel KH, Wood CM, Schmitt MG., Jr Absorption of short-chain fatty acids by the colon. Gastroenterology. 1980;78:1500–1507. [PubMed] [Google Scholar]
- 22.Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 23.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Fukata M, Chen A, Klepper A, et al. Cox-2 is regulated by toll-like receptor-4 (TLR4) signaling: role in proliferation and apoptosis in the intestine. Gastroenterology. 2006;131:862–877. doi: 10.1053/j.gastro.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukata M, Michelsen KS, Eri R, et al. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1055–G1065. doi: 10.1152/ajpgi.00328.2004. [DOI] [PubMed] [Google Scholar]
- 26.Ausiello CM, Cerquetti M, Fedele G, et al. Surface layer proteins from Clostridium difficile induce inflammatory and regulatory cytokines in human monocytes and dendritic cells. Microbes Infect. 2006;8:2640–2646. doi: 10.1016/j.micinf.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Lee JY, Kim NA, Sanford A, Sullivan KE. Histone acetylation and chromatin conformation are regulated separately at the TNF-alpha promoter in monocytes and macrophages. J Leukoc Biol. 2003;73:862–871. doi: 10.1189/jlb.1202618. [DOI] [PubMed] [Google Scholar]
- 28.Suuronen T, Huuskonen J, Nuutinen T, Salminen A. Characterization of the pro-inflammatory signaling induced by protein acetylation in microglia. Neurochem Int. 2006;49:610–618. doi: 10.1016/j.neuint.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Kanauchi O, Fukuda M, Matsumoto Y, et al. Eubacterium limosum ameliorates experimental colitis and metabolite of microbe attenuates colonic inflammatory action with increase of mucosal integrity. World J Gastroenterol. 2006;12:1071–1077. doi: 10.3748/wjg.v12.i7.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bocker U, Yezerskyy O, Feick P, et al. Responsiveness of intestinal epithelial cell lines to lipopolysaccharide is correlated with toll-like receptor 4 but not toll-like receptor 2 or CD14 expression. Int J Colorectal Dis. 2003;18:25–32. doi: 10.1007/s00384-002-0415-6. [DOI] [PubMed] [Google Scholar]
- 31.Isono A, Katsuno T, Sato T, et al. Clostridium butyricum TO-A culture supernatant downregulates TLR4 in human colonic epithelial cells. Dig Dis Sci. 2007;52:2963–2971. doi: 10.1007/s10620-006-9593-3. [DOI] [PubMed] [Google Scholar]
- 32.Schmeck B, Beermann W, van Laak V, et al. Listeria monocytogenes induced Rac1-dependent signal transduction in endothelial cells. Biochem Pharmacol. 2006;72:1367–1374. doi: 10.1016/j.bcp.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 33.Slevogt H, Schmeck B, Jonatat C, et al. Moraxella catarrhalis induces inflammatory response of bronchial epithelial cells via MAPK and NF-kappaB activation and histone deacetylase activity reduction. Am J Physiol Lung Cell Mol Physiol. 2006;290:L818–L826. doi: 10.1152/ajplung.00428.2005. [DOI] [PubMed] [Google Scholar]


