Abstract
High levels of pathogenic microorganisms have been documented previously in waters of the Lower Passaic River in northern New Jersey. The purpose of this study was to characterize the microbial contamination of river sediments near combined sewer overflows (CSOs), a known source of pathogens. Concentrations of fecal coliform, total coliform, fecal Streptococcus, fecal Enterococcus, Pseudomonas aeruginosa, Staphylococcus aureus, Giardia lamblia, and Cryptosporidium parvum organisms were measured in 16 samples from three mudflat locations along the Lower Passaic River, as well as from an upstream location. Selected samples were also analyzed for antibiotic resistance. All of the samples contained high concentrations of total coliform, fecal coliform, fecal Streptococcus, and fecal Enterococcus organisms. Analysis of isolates of Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli from several samples indicated that each strain was resistant to at least one antibiotic typically used in clinical settings. Eight of 16 samples contained Giardia, and one sample contained Cryptosporidium. With these sampling data, a quantitative microbial risk assessment was conducted to evaluate the probability of infection or illness resulting from incidental ingestion of contaminated sediments over a 1-year period. Three potential exposure scenarios were considered: visitor, recreator, and homeless person. Single-event risk was first evaluated for the three individual exposure scenarios; overall risk was then determined over a 1-year period using Monte Carlo techniques to characterize uncertainty. For fecal Streptococcus and Enterococcus, annualized risk estimates for gastrointestinal illness ranged from approximately 0.42 to 0.53 for recreators, 0.07 to 0.10 for visitors, and 0.62 to 0.72 for homeless individuals across the three sampling locations. Annualized risk of Giardia infection ranged from 0.14 to 0.64 for recreators, 0.01 to 0.1 for visitors, and 0.30 to 0.87 for homeless individuals, across all locations where detected. Cryptosporidium was detected at one location, and the corresponding annualized risk of infection was 0.32, 0.05, and 0.51 for recreators, visitors, and homeless individuals, respectively. This risk assessment suggests that pathogen-contaminated sediments near areas of CSO discharge in the Lower Passaic River could pose a health risk to individuals coming into contact with sediments in the mudflat areas.
The Lower Passaic River is a 17-mile tidal stretch that extends from the Dundee Dam to the mouth of the river where it enters Newark Bay in northern New Jersey. This section of the river, as well as the surrounding area, has been highly urbanized since the 1800s. Centuries of industrial activity have resulted in degraded water quality, sediment contamination, loss of wetlands, and abandoned or underutilized properties along the shore (38). Recently, numerous efforts to restore the area have been initiated. Most notably, the U.S. Environmental Protection Agency (USEPA) formed a partnership with several other agencies in 2003 to establish the Lower Passaic River Restoration Project; the goals of the Lower Passaic River Restoration Project include remediation of contamination in the river to reduce human health and ecological risks, improvement of water quality, and improvement and creation of the aquatic habitat, as well as reduction of contaminant loading in the Passaic and Hudson-Raritan Estuary (38, 61). In conjunction with these efforts, concerns have also been raised regarding potential contamination of the river due to pathogens.
In June of 2000, the Passaic Valley Sewerage Commissioners began monitoring levels of indicator bacteria in waters of the Lower Passaic River. In their most recent report (which included samples collected in June 2000 through October 2001), the Passaic Valley Sewerage Commissioners reported that water samples from the Lower Passaic River watershed had an average fecal coliform level of 1,120 CFU/100 ml. This value, derived from sampling data from many locations along different segments of the Lower Passaic River, is well above New Jersey Department of Environmental Protection (NJDEP) surface water quality standards for primary- and secondary-contact recreational usage (200 counts/100 ml and 770 counts/100 ml, respectively) (39). In 2003, the Interstate Environmental Commission measured fecal coliform, total coliform, fecal Streptococcus, and Enterococcus levels on six different days (under both wet and dry conditions) at six locations along the Lower Passaic River. Levels of fecal coliforms and Enterococcus exceeded NJDEP surface water quality standards in 35 of the 36 samples collected (28).
The areas surrounding the Lower Passaic River are served by combined sewer systems (CSSs), which can introduce pathogenic microorganisms into surface water via the release of untreated or partially treated sewage from combined sewer overflows (CSOs) or from sanitary sewer outfalls (SSOs). In CSSs, storm water runoff and sanitary sewage are transported in the same system. During precipitation events, storm water is discharged directly to surface water bodies through CSOs to prevent exceeding of the publicly owned treatment works treatment capacity. While the combination of storm water and raw sewage in CSOs will dilute to some degree the concentration of pathogens that otherwise would be present in effluent comprised solely of raw sewage, typical CSO concentrations for total coliforms have been reported to range between 105 and 107 most probable number/100 ml (36). In several recent studies, levels of Giardia lamblia and Cryptosporidium parvum were measured in CSOs in urban areas during overflow events (6, 51; National Risk Management Research Laboratory, USEPA, poster presented at the EPA Science Forum 2003, Washington, DC). A number of viruses, including poliovirus, infectious hepatitis virus, and coxsackie virus, have also been detected in CSOs during overflow events (22, 71). Currently, there are 73 CSOs that discharge directly into the Lower Passaic River.
In recent years, the USEPA has identified CSOs as a significant source of pathogens and other pollutants and contaminants in surface water bodies and has been actively working to reduce reliance on CSSs (59, 65, 71, 72). In 1994, USEPA published its CSO control policy, which was intended to establish a consistent national approach for controlling discharges from CSOs through its National Pollutant Discharge Elimination System (23). Shortly thereafter, USEPA also developed guidelines to facilitate implementation of this policy (59) and estimated that it would cost over $40 billion to control CSOs (71, 72). USEPA has also initiated enforcement litigation against municipalities across the United States that are alleged to have inadequately controlled CSO or SSO sewage discharges into surface water bodies. Despite the significant costs associated with improving antiquated sewer systems, communities are expected to develop long-term CSO control plans for attaining water quality standards in compliance with the Clean Water Act (65). For example, New York City, which has over 400 CSO outfalls, plans to spend over $2 billion on a CSO abatement program that will include over 30 citywide projects designed to optimize the operation of the sewer collection system, pumping stations, and treatment plants during wet weather events (35). Analogous efforts of this scale have been conducted around the United States over the past 10 to 15 years (73). Recently, the U.S. House of Representatives passed the Water Quality Investment Act of 2007, which authorized the appropriation of $1.8 billion over the 2008 to 2012 period for USEPA to provide grants to municipalities and states to control sewage overflows during wet weather events (55).
The potential contribution of CSOs to the pathogen load in the Lower Passaic River was addressed in a small-scale risk assessment conducted in 2003 (22). In this study, surface water samples were collected from a discharging CSO (immediately following a rainstorm), as well as from the river 10 feet downstream of the CSO (referred to as the Saybrook Place CSO, which is located in Newark, NJ). Both samples were found to contain many bacterial species that are associated with human fecal matter (at levels several orders of magnitude greater than water quality standards), as well as Giardia and several viral pathogens. The corresponding risk estimates for gastrointestinal illness were significantly elevated for the exposure scenarios considered: recreator (e.g., swimmer), visitor (e.g., angler or picnicker), and a homeless person living along or otherwise using the shoreline. These specific exposure scenarios were intended to characterize different potential uses of the river. While the exposure scenarios included in this assessment were focused primarily upon direct and/or indirect ingestion of water during a variety of activities (e.g., boating, picnicking, fishing, and swimming), many of these activities would also involve direct contact with sediments as well as the water. As sediments may act as a reservoir for pathogens (16, 18, 40, 45, 46, 48, 80), it is important that they, too, be evaluated to determine if they pose a potential risk to human health.
To date, the potential health risks associated with exposure to pathogens in sediments have not been well studied. Many of the available studies have focused on risks associated with pathogen exposures in sand at ocean beaches or the Great Lakes (15, 80, 81). Pathogens present in surface water often migrate or settle into the underlying sediment, where they have an enhanced potential for survival due to lower amounts of light energy, lower salinity, elevated levels of nutrients and organic matter, and lower temperatures (10, 11, 47, 53). Given that the available data indicate that the Lower Passaic River is contaminated with pathogens (which, in part, is due to CSO discharges) and that pathogens can migrate to and survive in sediment, an accurate human health assessment should consider exposures resulting from contact with sediment in addition to contact with water. The analysis described in this paper uses a quantitative microbial risk assessment approach to (i) characterize the types and levels of pathogens present in river sediments near CSO outfalls along the Lower Passaic River (hazard identification), (ii) develop exposure scenarios and estimate the duration and intensity of exposure to sediment (exposure assessment), (iii) extrapolate dose-response relationships for pathogens in sediment based upon well-established dose-response data for water (dose-response assessment), and (iv) evaluate risk for expected users of the Lower Passaic River in sediment areas that can be accessed by the public (risk characterization).
MATERIALS AND METHODS
Sampling design.
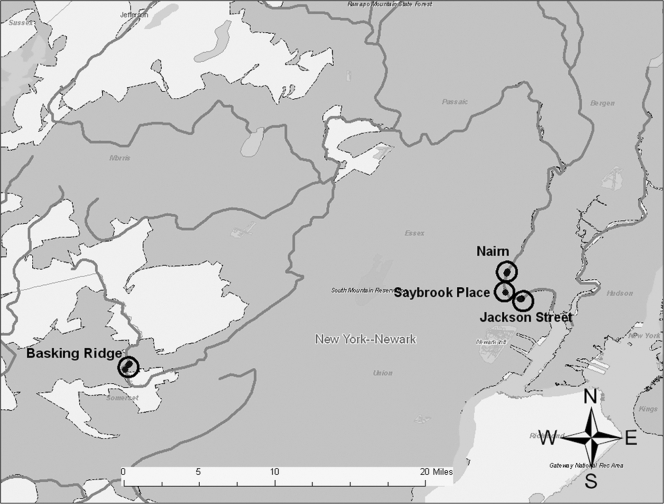
Sediment sampling locations were selected along the Lower Passaic River (the Study Area) based upon accessibility and/or proximity to CSOs and whether the mudflat was clearly visible and accessible during low tide. The boundaries of the mudflat were either the river water, the shore, or any barrier (e.g., cement wall) running along the edge of the mudflat. Each site was assigned a name according to site-specific identifiers (e.g., Jackson Street, Nairn Avenue, and Saybrook Place). In addition to the Study Area mudflat locations, samples were collected from areas along the Upper Passaic River in Basking Ridge, NJ. This location was selected as it was many miles upstream from the other sampling locations and was not subject to tidal influence; it was intended to serve as a control based on the belief at the time that it was not near a major source of pathogenic contamination.
Sediment samples were collected in accordance with the systematic sampling design described below. Global positioning system coordinates and the time of sample collection were noted at each collection site, in addition to any site-specific information (such as observable CSO discharge or observed human activities on the mudflat, etc.). Because it is known that human exposure to sediment on the mudflats occurs during activities such as collecting trash, samples were collected sufficiently close to shore such that it was reasonable to assume that persons collecting trash or conducting other activities would traverse the area. Sediment sampling locations along the Nairn Avenue and Jackson Street mudflats were determined by first measuring the length (running parallel to the river) of the mudflat and dividing it into five segments. A random location on one end of the mudflat was selected by sampling personnel as the initial sample collection site. Subsequent samples were collected at increasing distances from the initial sample location in the remaining segments of the mudflat (one sample per segment). Rather than using a fixed distance from the shore, sediment samples were collected by taking one or two steps into the mudflat and reaching forward toward the river, similar to what has been observed with respect to persons collecting trash along the shore. Sampling sites at the Saybrook Place mudflat were collected by using a sampling container affixed to a long stick; the sampling personnel collected the sample from the top of a cement barrier that was present along the length of the mudflat. Basking Ridge samples were collected on the sandy and rocky shore of the river near the water/sediment interface.
Sample collection procedures.
The procedure used for collection of sediment samples for pathogenic analyses was based upon guidance in the NJDEP Field Sampling Procedures Manual (32) and the NJDEP Guidance for Sediment Quality Evaluations (33). Sediment was collected directly into the sampling container by scooping along the sediment surface (at a depth no more than 6 in. below the surface). Replicate samples (“a” and “b”) were collected at each sampling site: the “a” subsample was collected using a disposable metal trowel and was placed in a 100-ml sterile container provided by the laboratory for bacterial analyses; the “b” subsample was collected using the same disposable metal trowel and was placed in a 250-ml sterile container provided by the laboratory for protozoan analyses. Following collection of the samples, the outside of each sample container was cleaned and the container was sealed in an individual plastic bag, placed in a cooler with ice, and maintained at 1 to 4°C in the dark until analyzed. Handling and shipping of samples were conducted in accordance with U.S. Geological Survey and NJDEP guidance (32, 76, 77); bacterial samples were processed within 6 h, while protozoan samples were processed within 96 h.
Sample collection.
The mudflat sampling locations were accessible only during low tide. Based on tidal schedules, low tide was predicted to occur in the mid- to late afternoon on 10 and 11 July 2006 (31); accordingly, samples were collected between approximately 1400 and 1700 Eastern Standard Time on each sampling day. The National Climatic Data Center service station at the Newark Liberty International Airport reported average temperatures of 75°F and 82°F on 10 and 11 July, respectively. No rainfall was recorded on these days; however, significant rainfall (1.8 in.) had been reported at the airport on 6 July. On 10 July, samples were collected from the Nairn Avenue (n = 5) and the Saybrook Place (n = 2) locations. The remaining samples were collected on the following afternoon at the Jackson Avenue mudflat (n = 6) and at Basking Ridge (n = 3) (Fig. 1).
FIG. 1.
Sediment sampling sites along the Passaic River.
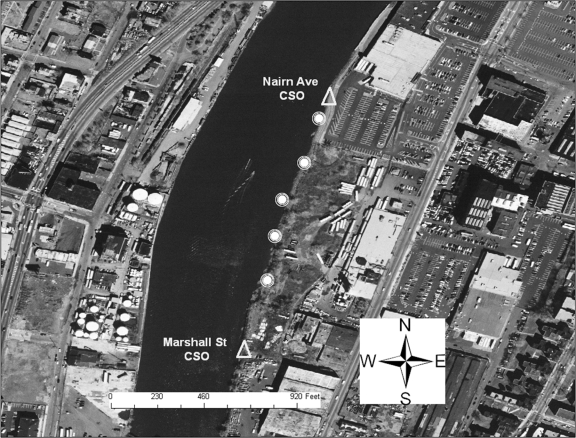
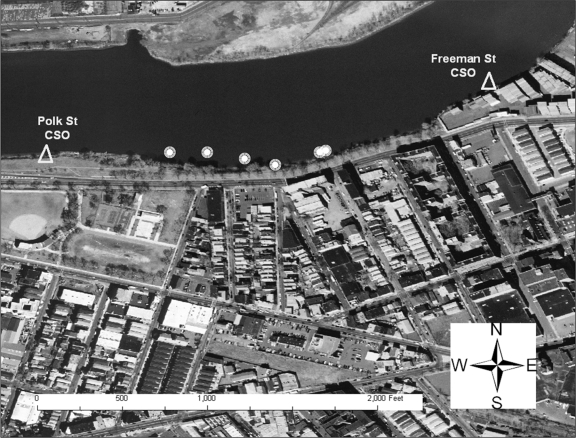
The Nairn Avenue mudflat was easily accessible from a retail store parking lot and is flanked by two CSOs (Fig. 2). Local residents were observed fishing behind the adjacent retail buildings on the day prior to sampling. The Jackson Street mudflat, also flanked by two CSOs, was accessible from a streetside park area via several highly traveled dirt paths (Fig. 3). The shoreline was heavily littered with trash and debris, and several homeless individuals were observed by the sampling team in the area during the sampling event. One individual was washing himself in water discharging into the mudflat from a large concrete pipe (later identified as the Mott Street storm sewer), while another was later seen washing in and sipping the discharge from the same pipe. The Saybrook Place mudflat differed somewhat from the Nairn and Jackson mudflats in that it was triangular; one side ran along the river whereas the other two sides were flanked by large concrete structures approximately 10 feet in height (Fig. 4). Although this mudflat was less accessible due to the concrete walls, several homeless persons live above and around the Saybrook Place CSO and therefore have potential for exposure to sediments in this area. In addition, this area clearly is observed to accumulate a significant amount of trash that could be the focus of local cleanup efforts. Finally, the Basking Ridge samples were collected along the riverbanks in a residential area. No individuals were observed in the Basking Ridge area during the sampling event.
FIG. 2.
Sediment sampling locations on the Nairn Avenue mudflat.
FIG. 3.
Sediment sampling locations on the Jackson Street mudflat.
FIG. 4.
Sediment sampling locations on the Saybrook Place mudflat.
Laboratory analyses.
In accordance with U.S. Geological Survey guidance, bacterial analyses were conducted within 6 h of collection, and protozoan analyses were conducted within 96 h. Bacterial analyses were conducted by EMSL Analytical in Westmont, NJ, using standard analytical methods for the following bacteria: total coliforms, fecal coliforms, Escherichia coli, fecal Streptococcus spp., Enterococcus spp., Pseudomonas aeruginosa, and Staphylococcus aureus (Table 1). In two samples (collected at the Nairn and Jackson locations), isolates of fecal Streptococcus spp., Pseudomonas aeruginosa, and Enterococcus spp. were tested further for antibiotic resistance using the Kirby-Bauer method. Each isolate was subjected to four antibiotics that typically are used in clinical treatment of infections caused by each organism. Protozoan (Giardia and Cryptosporidium) analyses were conducted by Analytical Services, Inc., in Williston, VT, using USEPA method 1623.
TABLE 1.
Analytical methods used to characterize pathogens in Lower Passaic River
| Target pathogen (analyst) | Standard method(s) (SM) of analysis | Method of analyzing antibiotic resistance (antibiotics to which resistance was found)a |
|---|---|---|
| Bacteria (EMSL Analytical) | ||
| Total coliform, fecal coliform, E. coli, fecal Streptococcus | SM 9222B, 9222D, 9222G, 9230C | |
| Pseudomonas aeruginosa | SM 9213E | Kirby-Bauer (G, C, T, N) |
| Staphylococcus aureus | SM 9213B | Kirby-Bauer (V, QD, G, E) |
| Enterococcus | SM 9230C | Kirby-Bauer (V, QD, G, T) |
| Protozoa (Analytical Services, Inc.) | ||
| Giardia and Cryptosporidium | SM 1623 | NT |
Abbreviations: G, gentamicin; C, ciprofloxacin; T, tetracycline; N, nitrofurantoin; V, vancomycin; QD, quinupristin-dalfopristin; E, erythromycin; NT, not tested.
Risk assessment.
A risk assessment was conducted to evaluate the potential human health risks associated with exposure to pathogens in the sediment of the Lower Passaic River. Three exposure scenarios were evaluated: (i) recreator, (ii) visitor, and (iii) homeless person. These exposure scenarios reflect reasonably expected uses of this water body based upon the designated uses of the river and upon the observations of the sampling team. (The NJDEP has previously identified the entire Lower Passaic River as a possible recreational water body, labeling several areas of the river as having potential for primary-contact recreation, while secondary-contact recreation is likely in other areas of the river [34].) These scenarios are also consistent with the scenarios utilized in the USEPA's recent human and ecological risk assessment for the Lower Passaic River (7).
The current risk assessment is based upon dose-response relationships in which risk is expressed as the probability of infection or illness resulting from multiple exposures to the contaminated sediments over a 1-year period. Specifically, single-event risk was first evaluated for three individual exposure scenarios; overall risk was then determined over a 1-year period, with the assumption that a recreator, visitor, or homeless person would have multiple exposure events over the year. Site-specific reference values were not available to characterize the exposure frequency or the sediment ingestion rates; as such, these parameters were based on a distribution drawn from a survey of values previously utilized in regulatory assessments and other peer-reviewed literature (Table 2). Because the range of sediment ingestion rates, as well as the range of exposure frequencies, varied significantly, overall risk was calculated using Monte Carlo techniques in an effort to characterize uncertainty in the assumptions used in the risk calculations. The distribution of values for sediment ingestion rates and exposure frequencies varied for each exposure scenario. For example, the distribution of sediment ingestion rates and exposure frequencies for the visitor scenario was based on lower values for these parameters reported in Table 2 whereas the distribution of values for the homeless scenario was based on the higher values in Table 2. The specific distributions utilized in each evaluation are discussed below and are summarized in Table 3.
TABLE 2.
Survey of recent risk assessment parameter selections utilized for risk assessment of incidental ingestion resulting from direct contact with sediment
| Reference | Type of guidance or assessment | Location and medium(-a) | Scenario | Sediment ingestion rate (mg/day) | Reference(s) for ingestion rate | Exposure
|
|
|---|---|---|---|---|---|---|---|
| Duration (yrs) | Frequency (days/yr) | ||||||
| Generic guidance | |||||||
| 74 | Risk assessment guidance for Superfund default scenarios | Sediment and soil | Resident/recreator | 50 (adult; commercial/industrial); 100 (adult; residential/agricultural); 100 (child; mean); 200 (child; upper boundary of estimate of mean) | 63, 68 | 9 (50th percentile tenure); 30 (90th percentile tenure); 70 (lifetime) | 365 days/yr multiplied by fraction ingested from contaminated source (residential); 7 days/yr or estimated based on local meteorological factors (recreational swimming [74]); 12 days/yr (recreational swimming [63]) |
| 52 | Human health protective concentration level | Sediment | Recreator | 191 (child); 100 (adult) | 63 | 6 (child); 24 (adult) | 39 |
| 79 | Guidance for State of Virginia (also cited by USEPA) | Sediment | Recreator/trespasser | 25 (child); 10 (adult) | 66 | 6 (child); 24 (adult) | 24 (trespasser); 95 (recreator) |
| 24 | Peer-reviewed recommended distributions | Soil | Resident | 21 (child; mean); 110 (child; 95th percentile) | 12 | Function of age | |
| 37 | Guidance for State of Oregon | Soil | Resident | Lognormal (mean = 60, SD = 67) (child); lognormal (mean = 57, SD = 18) (adult) | 12, 13, 19 | Function of age | Triangular (mode = 5, maximum = 150) (recreational swimming) |
| Site-specific assessment | |||||||
| 3 | Agency for Toxic Substances and Disease Registry health consultation | Calcasieu Estuary in Calcasieu Parish, LA (sediment) | Recreator | 100 (adult); 200 (child) | 68 | Adult, 18 to 70 yrs old; child, 6 to 17 yrs old | 184 |
| 2 | Agency for Toxic Substances and Disease Registry health consultation | Former wood treatment facility in Texarkana, Bowie County, TX (sediment) | Trespasser | 100 (adult); 150 (child, elementary-school age); 200 (child, preschool age) | 68 | 6 (child); 30 (adult) | 350 |
| 4 | Peer-reviewed risk assessment | Meuse River, The Netherlands (sediment) | Recreator | 1,000 (child); 350 (adult) | 9 | 30 | |
| 25 | Peer-reviewed risk assessment | Little Bay, Queens, NY (sediment) | Future child and adult recreators | 50 (child); 12.5 (adult) | 63 | 6 (child); 30 (adult) | 52 |
TABLE 3.
Assumptions used in risk calculations
| Parameter | Symbol | Unit | Distribution | Reference |
|---|---|---|---|---|
| Fraction of organisms ingested that initiate infection (r value) | ||||
| Giardia | rg | Unitless | Empirical (2.5th = 0.009798; 12.5th = 0.01336; 25th = 0.01584; 50th = 0.0198; 75th = 0.02466; 87.5th = 0.02845; 97.5th = 0.03582) | 44; calculated based on likelihood confidence intervals |
| Cryptosporidium | rc | Unitless | Empirical (5th = 0.0074; 25th = 0.0227; 50th = 0.0539; 75th = 0.1197; 95th = 0.3044) | 62 |
| Incidental sediment ingestion rate | IRsed | mg/day | Triangular | Selected based on a |
| Recreator | Minimum = 25, mode = 100, maximum = 200 | review of sediment | ||
| Visitor | Minimum = 25, mode = 50, maximum = 95 | ingestion rates | ||
| Homeless person | Minimum = 25, mode = 200, maximum = 350 | provided in regulatory guidance and recent health assessments | ||
| Exposure frequency | EF | Days/yr | Triangular | Selected based on a |
| Recreator | Minimum = 1, mode = 12, maximum = 95 | review of sediment | ||
| Visitor | Minimum = 1, mode = 2, maximum = 12 | ingestion rates | ||
| Homeless person | Minimum = 1, mode = 24, maximum = 150 | provided in regulatory guidance and recent health assessments. Maximum number of days limited by meteorological factors such as precipitation and temperature | ||
| Incidental water ingestion rate, swimming (for bacterial calculations) | IRswim | ml/day | Triangular (minimum = 10, mode = 16, maximum = 100) | 20 |
Determination of representative sediment concentrations.
The pathogen data used in the risk calculations are derived from the sediment samples collected on 10 and 11 July 2006. Mean values for each mudflat were calculated for fecal coliforms, fecal Streptococcus, and Enterococcus. The mean concentrations for fecal Streptococcus and Enterococcus were used to generate risk estimates.
For Giardia and Cryptosporidium, the numbers of discrete organisms per gram of wet sediment were considerably lower than those reported for bacteria. As such, it was necessary to consider the statistics of their distribution throughout the sediment (27). For this assessment, it was assumed that the representative concentration of the protozoa at each sampling location could be characterized by a Poisson distribution. This assumes that the protozoa are distributed randomly and that in a given volume of sediment (V), the probability that a sample (x) will contain N organisms (including N = 0) which have a constant density (μ) is expressed in the following equation:
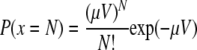 |
Estimates of the mean value were calculated for each location (i.e., expected value). Confidence limits were also derived as described in the work of Haas et al. (27).
Exposure scenarios. (i) Recreator.
The recreator scenario is intended to quantify potential risks among individuals who may come into contact with sediment during recreational activities such as swimming. For all pathogens, the route of exposure was assumed to be incidental ingestion of sediment. This approach is consistent with a recent USEPA risk assessment of the Lower Passaic River in which incidental ingestion of sediment was considered to be a complete pathway for recreators (7). For this scenario, it was assumed that the ingestion rate (milligrams/day) distribution was triangular (minimum = 25, mode = 100, maximum = 200). These values were based on default USEPA soil ingestion value for adolescents and adults of 100 mg (75). These values are also consistent with default values for sediment ingestion along a shoreline used in the Multimedia Environmental Pollutant Assessment System risk model developed for the U.S. Department of Energy (56) as well as with default values used by the Agency for Toxic Substances and Disease Registry in a sediment risk assessment conducted in Louisiana (1). In addition, a triangular distribution was also assumed for exposure frequency (days/year) for recreators over the period of 1 year (minimum = 1, mode = 12, maximum = 95).
(ii) Visitor.
The visitor exposure scenario is intended to quantify potential risks among individuals who may engage in activities with minimal sediment contact, such as anglers, picnickers, or people collecting trash from the shoreline. It is known that angling occurs at several points along the Lower Passaic River on a regular basis (29, 41, 42), and USEPA considered this to be a complete pathway in their draft risk assessment of the Lower Passaic River (7). Again, a triangular distribution was assumed for incidental ingestion (mg/day) for adults and adolescents (minimum = 25, mode = 50, maximum = 95). The use of lower values for visitors than for recreators was based on the assumption that a visitor would likely have less opportunity for sediment contact than a recreator. A triangular distribution was also assumed for exposure frequency (days/year) over the period of 1 year (minimum = 1, mode = 2, maximum = 12).
(iii) Homeless person.
Homeless people have previously been observed living along the banks of the Passaic River in temporary makeshift shelters (8). These individuals have been observed by members of the sampling team using discharge from pipes emptying into the river for bathing or washing their belongings. One homeless person was barefoot, and his feet were in direct contact with the sediment. Because fluid was discharging from the pipes, the sediment had a muddy consistency and thereby would have the potential to adhere to the skin. As such, there are ample opportunities for homeless people to come into contact with sediment in the area around the CSOs. This scenario is consistent with the approach utilized in the USEPA's risk assessment of the Lower Passaic River (7) in which a homeless resident was considered a complete exposure pathway. For this scenario, the ingestion rate (mg/day) was assumed to have a triangular distribution (minimum = 25, mode = 200, maximum = 350). These values were extrapolated from the recreator intake rate based on the assumption that homeless people would be exposed to greater amounts of sediment. Much of the distribution lies below the soil ingestion value of 330 mg/day cited by the U.S. Department of Energy Oak Ridge Operations Environmental Management Program for an on-site construction worker/excavation scenario (57). Based on the assumption that a homeless person would have a greater intake rate than a recreator but a lower intake rate than a construction worker actively working and digging in soil, the mode value of 200 mg/day was considered to be reasonable. A triangular distribution was also assumed for exposure frequency (days/year) over the period of 1 year (minimum = 1, mode = 24, maximum = 150).
Dose response. (i) Fecal Streptococcus/Enterococcus.
The dose-response relationships for indicator bacteria considered in this analysis were based upon the number of reported gastrointestinal illnesses among swimmers following contact with water containing a specific concentration of indicator bacteria. Because this relationship is expressed as an epidemiological relationship which reflects an implicit incidental water ingestion volume for swimmers, the dose-response relationship was related to sediment ingestion using an estimate of the swimming ingestion value likely to be true during the development of the regression models and the scenario-specific sediment ingestion value. Calculated annualized risks were insensitive (i.e., contributing less than 10% of variance in risk) to the specific swimming incidental ingestion rate assumption used to scale the epidemiologic relationship based on an uncertainty analysis completed for the three scenarios.
The endpoint for fecal Streptococcus or Enterococcus exposure is gastrointestinal illness, and the dose-response relationship is based on the USEPA's 1986 standard of 104 fecal Streptococcus or Enterococcus organisms/100 ml causing 19 illnesses per 1,000 swimmers (58). The dose-response relationship is described with the following mathematical equation:
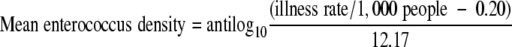 |
Accounting for the implicit water ingestion rate in the above equation and a scenario-specific sediment ingestion rate, the single exposure and annualized illness rates are calculated as follows:
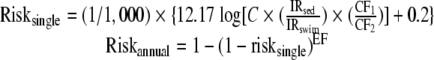 |
where riskannual = annualized risk, risksingle = single-event risk, EF = exposure frequency (days/year), C = arithmetic mean concentration (CFU/g), IRsed = incidental ingestion rate of sediment for scenario (mg/day), IRswim = incidental ingestion rate of water for dose-response regression (ml/day), CF1 = conversion factor = 100 ml/(100 ml), and CF2 = conversion factor = 1,000 mg/g. The concentration term is based on the arithmetic mean such that the single-event risk properly characterizes cumulative risk (26).
(ii) Giardia.
For this risk assessment, the health endpoint is Giardia infection. The USEPA's water quality criteria document for Giardia (64) describes a human health risk assessment methodology using a dose-response model developed by Rose et al. (44): Psingle = 1 − exp(−rN) and Pannual = 1 − (1 − Psingle)EF. The variables in this model are defined as follows: Psingle = probability of infection for a single event, Pannual = annualized probability of infection, r = fraction of organisms ingested that initiate infection, and N = average number of ingested organisms. The value for r developed by Rose et al. was 0.01982 (95% confidence interval, 0.009798 to 0.03582). This dose-response model was based on the experimental data of Rendtorff (43), wherein doses of Giardia ranging from 1 to 106 cysts were ingested by human volunteers (44). The r value was also included in the Monte Carlo uncertainty analysis, which assumed an empirical distribution based on likelihood confidence intervals developed by Rose et al. This exponential model assumes that the microorganisms are distributed randomly in a given environmental medium (e.g., sediment) and follow the Poisson distribution (27). It also assumes that the probability of infection per ingested organism does not vary.
(iii) Cryptosporidium.
For Cryptosporidium, the health endpoint of concern is infection with Cryptosporidium. In many cases, infection with Cryptosporidium can result in a clinical disease known as cryptosporidiosis. USEPA's water quality criteria document for Cryptosporidium (60) describes a human health risk assessment methodology that relies upon the same dose-response model developed by Rose et al. (44) for Giardia. Because the value of r in the dose-response model is known to vary by Cryptosporidium strain, a mean value representative of a distribution (i.e., 0.09) was used in this assessment; this value was selected following a review of the literature presented in USEPA's 2006 Long Term 2 Enhanced Surface Water Treatment Rule (62). The r value was also incorporated into the uncertainty analysis. Similarly to the Giardia model, it was assumed that the microorganisms are distributed randomly in the sediment in a manner consistent with a Poisson distribution (27). This model also assumes that the probability of infection per ingested organism does not vary.
RESULTS
Microbial concentrations in the sediment.
Fecal coliform, fecal Streptococcus, and fecal Enterococcus bacteria were detected in every sediment sample at every location. Mean concentrations are presented in Table 4. Individual results for total coliform, fecal coliform, E. coli, Pseudomonas aeruginosa, and Staphylococcus aureus are also presented, although risk estimates were not generated for these pathogens (due to the lack of a defined dose-response relationship or the unavailability of precise laboratory results for some bacteria, such as E. coli). It is notable that Basking Ridge, which was originally intended to serve as a “control” location, had bacterial concentrations similar to those measured at the Nairn, Jackson, and Saybrook locations. A detailed reconnaissance to investigate the high concentrations of the Basking Ridge sampling site later revealed the presence of a pumping or booster station believed to be owned by a private utility. It was noted that associated water lines and sanitary sewers were leaking into the river approximately 10 feet from one of the sampling locations. Therefore, due to the presence of an alternative point source of pathogens, the Basking Ridge results were not expressed as “controls,” although sampling results and risk estimates are provided for completeness.
TABLE 4.
Concentrations of bacteria in sediment samples
| Sample site and no. | Concn (CFU/g of wet sediment) or presence of bacteria
|
||||||
|---|---|---|---|---|---|---|---|
| Fecal Streptococcus | Total coliform | E. coli | Fecal coliform | Enterococcus | Pseudomonas aeruginosa | Staphylococcus aureus | |
| Nairn | |||||||
| 1 | 100 | 8,450 | Present | 1,200 | 150 | 150 | <50 |
| 2 | 150 | 10,550 | Present | 1,750 | 50 | 50 | <50 |
| 3 | 1,100 | Confluent | Present | 12,100 | 150 | <50 | <50 |
| 4 | 100 | 8,850 | Present | 350 | 50 | <50 | <50 |
| 5 | 350 | Confluent | Present | 1,250 | 50 | <50 | <50 |
| Mean | 360 | 3,330 | 90 | ||||
| Saybrook Place | |||||||
| 6 | 450 | Confluent | Present | 1,150 | 100 | <50 | <50 |
| 7 | 100 | Confluent | Present | 950 | 100 | <50 | <50 |
| Mean | 275 | 1,050 | 100 | ||||
| Jackson Street | |||||||
| 11 | 680 | Confluent | Present | 760 | 540 | 60 | <20 |
| 12 | 400 | Confluent | Present | 660 | 40 | <20 | <20 |
| 13 | 360 | Confluent | Present | 1,400 | 80 | <20 | <20 |
| 14 | 180 | Confluent | Present | 660 | <20 | 80 | <50 |
| 15 | 500 | Confluent | Present | 1,000 | <20 | <20 | <20 |
| 16 | <20 | Confluent | Present | 80 | <20 | <20 | <20 |
| Meana | 355 | 760 | 115 | ||||
| Basking Ridge | |||||||
| 8 | 480 | Confluent | Present | 2,600 | 160 | <20 | <20 |
| 9 | 1,000 | Confluent | Present | 420 | 40 | <20 | <20 |
| 10 | 280 | Confluent | Present | 300 | 60 | <20 | <20 |
| Mean | 587 | 1,107 | 87 | ||||
Value of one-half the limit of detection used for fecal Streptococcus and Enterococcus results, reported as <20.
Average concentrations of Giardia and Cryptosporidium were expressed as the expected values based on the assumption that the distribution of protozoa in the sediment was characterized by a Poisson distribution. Expected values for Giardia and Cryptosporidium are presented in Table 5. Giardia was present in three of five (60%) of the samples at Nairn Avenue, both of the samples at Saybrook (100%), and in three of six (50%) of the samples at Jackson Street. None of the samples collected at Basking Ridge contained Giardia cysts.
TABLE 5.
Protozoan results
| Sample site and no. | No. of cysts/g wet sediment
|
|
|---|---|---|
| Giardia | Cryptosporidium | |
| Nairn | ||
| 1 | 8.2 | 0 |
| 2 | 9.2 | 0 |
| 3 | 0 | 0 |
| 4 | 0 | 0 |
| 5 | 5.8 | 0 |
| Expected valuea | 4.64 (range, 0-8b) | 0 |
| Saybrook | ||
| 6 | 3.9 | 0 |
| 7 | 32.8 | 0 |
| Expected valuea | 18.35 (range, 7-23b) | 0 |
| Jackson | ||
| 11 | 4.5 | 0 |
| 12 | 0 | 0 |
| 13 | 0 | 0 |
| 14 | 2.6 | 10 |
| 15 | 0 | 0 |
| 16 | 2.6 | 0 |
| Expected valuea | 1.62 (range, 0-5b) | 1.67 (range, 0-5b) |
| Basking Ridge | ||
| 8 | 0 | 0 |
| 9 | 0 | 0 |
| 10 | 0 | 0 |
| Expected valuea | 0 | 0 |
Due to the relatively small sample size, it was not possible to fully characterize the distribution of the protozoan data. Therefore, it was assumed that the data came from a Poisson distribution.
Ranges reflect upper and lower confidence intervals of the expected values.
Cryptosporidium was detected in only one sediment sample from the Jackson Street location. It is common for Cryptosporidium organisms to “cluster” together, resulting in uneven distribution throughout the environmental medium (27). In addition, as discussed previously, sediment samples were analyzed for Cryptosporidium using USEPA method 1623 (69). USEPA generally requires at least a 13% recovery of a matrix spike sample during routine use of the method (70). Of the 16 sediment samples, only three had recovery percentages greater than 13%. These particular samples were all from the Nairn location. Because of the low recovery percentages observed for the majority of the sediment samples, it is possible that Cryptosporidium spores were present but were not detected due to analytical issues. This suggests that the presence of Cryptosporidium at the other locations cannot be ruled out.
Antibiotic resistance in bacteria from selected samples.
Antibiotic-resistant bacteria were found in both of the samples analyzed for resistance (Table 6). Gentamicin-resistant fecal Streptococcus and tetracycline-resistant Enterococcus were found in the sediment from the Nairn Avenue mudflat. Tetracycline- and nitrofurantoin-resistant Pseudomonas and tetracycline-resistant Enterococcus were found in the sediments collected from the Jackson Street mudflat. These results suggest that if a human were to become infected with these resistant bacteria, antibiotic treatment with tetracycline and/or nitrofurantoin would likely be ineffective in addressing the infection.
TABLE 6.
Summary of antibiotic resistance testing results
| Pathogen | Antimicrobial agent | Result by sample location
|
|
|---|---|---|---|
| Nairn | Jackson | ||
| Fecal | Vancomycin | Susceptible | Susceptible |
| Streptococcus | Quinupristin-dalfopristin | Susceptible | Susceptible |
| Gentamicin | Resistant | Susceptible | |
| Erythromycin | Susceptible | Susceptible | |
| Pseudomonas | Gentamicin | Susceptible | Susceptible |
| aeruginosa | Ciprofloxacin | Susceptible | Susceptible |
| Tetracycline | Susceptible | Resistant | |
| Nitrofurantoin | Susceptible | Resistant | |
| Enterococcus | Gentamicin | Susceptible | Susceptible |
| Quinupristin-dalfopristin | Susceptible | Susceptible | |
| Tetracycline | Resistant | Resistant | |
| Vancomycin | Susceptible | Susceptible | |
Risk estimates for exposure to fecal Streptococcus and Enterococcus.
In general, risk estimates for Streptococcus and Enterococcus were similar across all locations. For fecal Streptococcus, annualized risk estimates ranged from 0.51 to 0.53 for recreators, from 0.09 to 0.10 for visitors, and from 0.70 to 0.72 for homeless individuals. Risk estimates for Enterococcus were slightly lower, ranging from 0.42 to 0.45 for recreators, approximately 0.07 for visitors, and ranging from 0.62 to 0.64 for the homeless scenario (Table 7).
TABLE 7.
Annualized risk estimates for bacteria and protozoa
| Site and scenario | Risk estimate (90% prediction interval)
|
|||
|---|---|---|---|---|
| Bacteria
|
Protozoa
|
|||
| Fecal Streptococcus | Enterococcus | Giardiaa | Cryptosporidium | |
| Nairn | ||||
| Recreator | 0.53 (0.18-0.85) | 0.42 (0.12-0.75) | 0.29 (0.05-0.69) | NDb |
| Visitor | 0.1 (0.04-0.19) | 0.07 (0.02-0.14) | 0.03 (0-0.08) | ND |
| Homeless | 0.72 (0.32-0.97) | 0.62 (0.24-0.92) | 0.56 (0.12-0.97) | ND |
| Jackson Street | ||||
| Recreator | 0.53 (0.18-0.85) | 0.45 (0.13-0.77) | 0.14 (0-0.42) | 0.32 (0-0.95) |
| Visitor | 0.1 (0.04-0.19) | 0.07 (0.02-0.15) | 0.01 (0-0.04) | 0.05 (0-0.19) |
| Homeless | 0.72 (0.32-0.97) | 0.64 (0.25-0.93) | 0.3 (0-0.79) | 0.51 (0-1.0) |
| Saybrook Place | ||||
| Recreator | 0.51 (0.17-0.84) | 0.43 (0.13-0.76) | 0.64 (0.19-0.98) | ND |
| Visitor | 0.09 (0.03-0.18) | 0.07 (0.02-0.14) | 0.1 (0.02-0.23) | ND |
| Homeless | 0.7 (0.3-0.96) | 0.63 (0.24-0.93) | 0.87 (0.44-1.0) | ND |
| Basking Ridge | ||||
| Recreator | 0.49 (0.1-0.86) | 0.42 (0.12-0.74) | ND | ND |
| Visitor | 0.06 (0.008-0.14) | 0.07 (0.02-0.13) | ND | ND |
| Homeless | 0.66 (0.21-0.97) | 0.62 (0.24-0.92) | ND | ND |
Due to relatively small sample size, it was not possible to fully characterize the distribution of Giardia data. Expected values are based on best estimate assuming Poisson distribution.
ND, not detected.
Risk estimates for exposure to Giardia.
Using the model developed by Rose et al. (44) described above, risk estimates were developed for Giardia infection. In contrast to the analysis for fecal streptococci/enterococci, which characterizes the risk of gastrointestinal illness, the risk estimates presented for Giardia specifically address the probability of infection. As already discussed, Giardia was detected at the Nairn, Saybrook, and Jackson mudflats but was not found to be present in any of the samples collected at the Basking Ridge control location. The results are presented in Table 7.
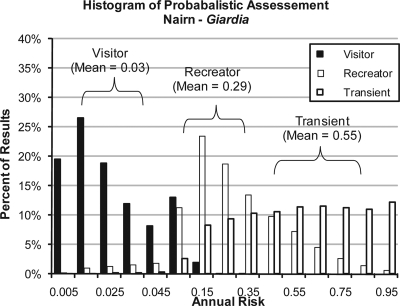
Based upon best estimates of the average concentration of Giardia in the Nairn, Saybrook, and Jackson mudflats, annualized risk estimates for Giardia infection among recreators were 0.29, 0.64, and 0.14, respectively. For visitors, annualized risk estimates for Giardia infection were 0.03, 0.10, and 0.01 for Nairn, Saybrook, and Jackson, respectively, based upon average concentrations of Giardia. The homeless person scenario presented the highest Giardia risks. Average Giardia concentrations of 4.64 cysts/g and 18.35 cysts/g at Nairn and Saybrook, respectively, resulted in annualized risk estimates of 0.56 and 0.87, respectively. At Jackson Street, exposure to an average concentration of 1.62 cysts/g was associated with an annualized probability of infection of 0.30. An example showing the results of 10,000 Monte Carlo iterations of the risk model for the input distribution in Table 3 is provided in Fig. 5.
FIG. 5.
Distribution of annualized risk of infection via incidental ingestion of Giardia.
Taken together, the risk estimates are orders of magnitude above USEPA's recommended public health goal of no more than one Giardia infection per 10,000 persons (i.e., corresponding to a risk of ≤0.0001) from drinking water exposures (64). An equivalent metric does not exist for sediment.
Risk estimates for exposure to Cryptosporidium.
The estimated risks of Cryptosporidium infection for the three exposure scenarios are presented in Table 7. Cryptosporidium was detected in one of five (20%) samples at the Jackson Street sampling location. The annualized risk estimate based upon the estimate of central tendency of 1.67 cysts/g was 0.32 for recreators, 0.05 for visitors, and 0.51 for homeless individuals.
Quantitative uncertainty analysis. (i) Fecal Streptococcus/Enterococcus.
For fecal Streptococcus, exposure frequency contributed to 92 to 97% of the total uncertainty across all locations and for all three exposure scenarios. The contribution of ingestion rate for swimmers (IRswim) used to scale the 1986 USEPA dose-response relationship was similar for the homeless and recreators (2 to 3% across all locations), while for visitors this parameter contributed between 4.7% and 5.4%. The contribution of ingestion rate for sediment was relatively minor, between 1.2% and 1.6% for all scenarios.
For Enterococcus, exposure frequency contributed over 90% of the total uncertainty for visitors, recreators, and homeless individuals across all scenarios. Depending on the scenario, the ingestion rate for swimmers (IRswim) contributed 3.3 to 11% of total uncertainty across all locations, while the ingestion rate of sediment (IRsed) ranged from 2.2% to 2.7% across all locations.
(ii) Giardia.
For Giardia, the parameter that significantly contributed to the uncertainty in the risk estimates was exposure frequency. Across all locations and scenarios, exposure frequency contributed approximately 25 to 65% to the total uncertainty. The relative contribution of the other factors appeared to be somewhat dependent upon the Giardia concentration. For Saybrook Place, which had the highest expected value, the concentration parameter contributed the least to overall uncertainty (range, 6.7 to 10%, across all scenarios). In contrast, for Jackson Street, which had the lowest expected Giardia value, the concentration parameter contributed between 55.3% and 62.4% of the total uncertainty.
(iii) Cryptosporidium.
For all scenarios, concentration and the r values, which reflect the wide range of infectivity of the various strains, contributed the most to total uncertainty of the risk estimates. For all scenarios, the contribution of the r value for Cryptosporidium contributed 40.9 to 46%. The expected value for Cryptosporidium concentration at Jackson contributed 41.8 to 42.5%, exposure frequency contributed 9.6 to 12.9%, and ingestion rate for sediment contributed 1.6 to 4.9%.
DISCUSSION
The results of this study clearly demonstrate that the sediment samples collected from the Lower Passaic River mudflats near CSOs contained concentrations of bacteria capable of causing gastrointestinal illness. Additionally, Giardia was present in about half of the samples and an additional sample was found to contain Cryptosporidium. The human health risk assessment indicated that there was substantial risk (defined as risk greater than 1 in 10,000) associated with exposure to the Giardia-contaminated sediments at each location. To date, assessments of health risks associated with exposure to pathogens in sediment have been conducted only rarely, the focus appearing to be more on beachgoers (e.g., those visiting the ocean or Great Lakes). Sediment exposure among recreators at and visitors to rivers or smaller lakes has not been described well in the scientific literature (14, 17, 54, 78). In particular, the risks faced by homeless individuals (who likely have the greatest potential for exposure and who may often rely upon local rivers or lakes for their daily needs) are poorly characterized. Thus, this analysis was intended to determine pathogen concentrations in the sediment of the Lower Passaic River and to estimate health risks associated with exposure via several probable and previously observed exposure scenarios.
Due to the absence of standard approaches for evaluating risk associated with exposure to pathogen-contaminated sediments, a number of assumptions were made which in turn contribute to the overall uncertainty in the analysis. Sources of uncertainty in this analysis include reliance on dose-response relationships derived from media other than sediment, temporal variability of pathogens in sediment, utilization of generic exposure frequencies (rather than site-specific ones), and considerations for human variability in the toxicological response to pathogenic organisms. As discussed previously, exposure frequency was found to be one of the primary contributors to the uncertainty of the risk estimates, particularly for fecal Streptococcus and Enterococcus. While there is evidence that recreational activities such as boating or fishing occur on a fairly regular basis on the river, the impact of factors such as seasonality or tourism has not been well studied in this area. Similarly, although it is likely that homeless individuals would have repeated contact with the river water and sediment, such contact has not been well characterized to date.
This risk assessment also relied on the conversion of dose-response relationships established for pathogen contact or ingestion associated with water or soil. Consequently, many of the primary sources of uncertainty stem from the lack of readily available dose-response or ingestion parameters specific to sediment. However, the approach taken in this risk assessment is consistent with USEPA's guidance for Superfund risk assessments, wherein it is noted that contact with sediment can occur via direct contact, which includes dermal contact and incidental ingestion of sediment, and it is recommended that soil ingestion rates can serve as an acceptable surrogate for sediment ingestion rates when medium-specific ingestion rates are not available (74).
The temporal variability of pathogen concentrations in the sediment also may contribute to the uncertainty in this assessment. Although the sediment environment (including the population of microorganisms present at any given time) is sensitive to weather incidents or other factors that could significantly alter the flow of water over the sediment, sampling of each mudflat itself was conducted on a single day. It has been estimated that rainfall intensities as low as 0.04 in./h can trigger CSO discharges into the Lower Passaic River area (21). The National Climatic Data Center reports that rainfall intensities were equal to or exceeded 0.04 in./h on four of the seven days in the week preceding sampling (30). Five days prior to sampling, rainfall intensities reached 1.17 in./h. It is therefore very likely that CSOs near the Nairn, Saybrook Place, and Jackson Street sampling areas had discharged prior to sampling. Although precipitation events are relatively common during the summer months (when recreators and/or visitors are more likely to be present), it is expected that the overall pathogen load in the sediment and overlying water column attributable to CSOs would vary over time.
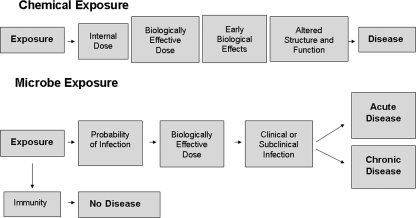
Finally, an uncertainty inherent in any microbial risk assessment is the human variability in the response to pathogenic organisms. The factors that ultimately determine whether an individual will experience illness due to infection are complex and depend on both the host (infected person) and the pathogen (Fig. 6). The occurrence and severity of the health endpoints associated with exposure to pathogenic bacteria and/or protozoa are directly related to the immune status of the exposed individual (50). It is therefore difficult to accurately predict adverse responses following exposure to pathogenic organisms given that there are a number of immune factors that play a role in determining whether there is any clinical manifestation of disease. In this study, the predicted risk for bacterial exposure, which was based upon a dose-response relationship derived from observed illness rates among swimmers, could potentially be underestimated if the exposed individual is particularly susceptible or immunocompromised. For protozoa, the risk estimate was expressed as a probability of infection. Infection is defined as the colonization of the microorganism in the body and is considered an initial step in the microbial disease process (27). It is important to emphasize that a high probability of infection may not always lead to illness due to factors such as immune status of the exposed individual or the virulence of the pathogen.
FIG. 6.
Chemical and microbial exposure-to-disease paradigm.
The health outcome generally considered in this risk assessment was gastrointestinal illness, characterized by abdominal pain, diarrhea, vomiting, and in some cases hospitalization. In healthy individuals, symptoms usually last from days to weeks, depending on the organism. However, several of the pathogens addressed in this analysis are capable of causing more serious diseases or developing into chronic conditions. For example, E. coli O157:H7 can also cause hemolytic-uremic syndrome, which is characterized by microangiopathic hemolytic anemia, thrombocytopenia, and renal failure (82). For some sensitive populations, such as young children, infection with this strain of E. coli can be fatal. Symptoms of Giardia infection among healthy individuals can include severe diarrhea, loose or watery stool, stomach cramps, bloating, and upset stomach, in some cases lasting for weeks. However, giardiasis can also become a chronic disease, with recurring symptoms in addition to malabsorption (82). Chronic giardiasis can also shorten the life span of immunocompromised individuals. Similarly, Cryptosporidium has been associated with more persistent symptoms and serious illness among immunocompromised persons, in particular those with human immunodeficiency virus, primary immunodeficiency, X-linked hyper-immunoglobulin M syndrome, severe combined immunodeficiency, and selective immunoglobulin A deficiency; those undergoing solid-organ transplantation; and children who are undernourished. Among the immunocompromised, cryptosporidiosis can last until death and in many cases is considered incurable (82).
In a 2002 reassessment of bacterial dose-response data for recreational waters, USEPA also noted that the increasing presence of antibiotic resistance in bacteria may cause a greater number of illnesses to occur at a given concentration than would be predicted using the established dose-response equations (67). Antibiotic resistance was observed in both samples analyzed in this study. Infections caused by resistant bacteria can be particularly serious for immunocompromised people, children, and the elderly. Resistant strains of pathogenic bacteria ultimately lead to higher health care costs as treatment may often require more-expensive antibiotics in combination with longer hospital stays. While approximately 70% of bacteria causing infections that require people to be hospitalized are resistant to at least one drug (5), 100% of the samples analyzed in this study were resistant to at least one drug. Therefore, the presence of resistant strains of these common pathogens associated with sewage should not be overlooked.
When considering pathogen-related risk in sediment as distinguished from water, an additional pathway that warrants discussion in the context of this assessment is dermal exposure. In this scenario, pathogen exposure could occur if wounds or cuts on the skin come into direct contact with or are submerged in contaminated sediment. This could occur in two ways: (i) an individual (recreator, visitor, or homeless person) received a fresh cut (potentially exposing the blood vessels) while the skin was in contact with the sediment or (ii) a preexisting cut, open wound, or skin abrasion was exposed to contaminated sediment. In either scenario, the exposure route could result in a direct contamination of the skin or a possible systemic infection through the blood. For these health endpoints, pathogens such as group A streptococci would present the greatest hazard (as opposed to the bacteria considered in the quantitative risk assessment, which are primarily associated with gastrointestinal disease). Although Staphylococcus aureus was not detected in this analysis, the presence of Pseudomonas aeruginosa, a microbe capable of causing systemic disease if transmitted into the bloodstream, is notable.
The potential for illness via a dermal pathway could be significant and warrants further study in a separate assessment. An analysis of dermal exposure to pathogens would require a detailed and specific dose-response assessment. The ability of pathogens to penetrate skin and cause infection probably would vary considerably depending on the type and location of the wound and the type of pathogen. During the 10 to 11 July sampling trip, a considerable amount of debris (e.g., metal and broken glass) was noted in the mudflats. It is plausible that an individual could receive a cut from walking barefoot or putting his or her hands into the sediment. With a substantial sediment pathogen load, the risks of infecting that cut could be considerable. Furthermore, homeless individuals living along the shores of the Lower Passaic could have persistent wounds that could be consistently exposed to high concentrations of pathogens.
Lastly, the samples considered in this analysis were collected in mudflats located in close proximity to CSOs (with the exception of samples collected at Basking Ridge). High concentrations of microorganisms that are typically associated with sewage were observed in this analysis, but it is difficult to definitively relate sediment concentrations to CSO discharge events without additional data. Previous studies have demonstrated that CSOs can and do impact the pathogen load in the sediment near the outfall (15, 16, 46, 48, 49, 80). One important difference between these studies and this analysis is that prior studies usually addressed a single CSO. In contrast, there are 73 CSOs in the Lower Passaic River, and it is likely that a mudflat could be impacted by discharges from multiple CSOs. Recent advances in microbiology and sampling methods, such as DNA fingerprinting techniques, may be useful in addressing this issue in future studies in which samples are collected and compared from both a discharging CSO and the surrounding sediment.
Federal regulatory agencies, particularly the USEPA, have clearly demonstrated that reducing the risk of health effects related to CSO contamination of surface waters is a priority. From a public health perspective, it is important to consider all contaminants, including pathogens, impacting water quality in the area. Water samples collected in and around the Lower Passaic River indicate that pathogens are present at levels that far exceed health-based water quality standards. The present analysis indicates that pathogens are also present in sediments at high levels and that significant health risks are associated with contact with these sediments. Individuals who come into contact with the Lower Passaic River (whether they be recreators, visitors, or homeless persons) may not be aware that they can be exposed to pathogens via sediment contact/ingestion and, consequently, may not take adequate precautions (e.g., hand washing) following contact with sediment. In summary, the results of this study indicate that pathogen-contaminated sediments, particularly those near CSOs, in the Lower Passaic River may pose health risks to individuals using the river for any of a variety of different purposes.
Acknowledgments
This study was funded by Tierra Solutions, Inc.
Footnotes
Published ahead of print on 21 December 2007.
REFERENCES
- 1.Agency for Toxic Substances and Disease Registry. 2006. Calcasieu Estuary sediment sample evaluation. EPA facility ID: LA0002368173. Office of Public Health, Louisiana Department of Health and Hospitals, Baton Rouge, LA.
- 2.Agency for Toxic Substances and Disease Registry. 2006. Health consultation: Tronox LLC, Texarkana Facility. Agency for Toxic Substances and Disease Registry, Dallas, TX.
- 3.Agency for Toxic Substances and Disease Registry. 2005. Health consultation: Calcasieu Estuary sediment sample evaluation. EPA facility ID: LA0002368173. Office of Public Health, Louisiana Department of Health and Hospitals, Baton Rouge, LA.
- 4.Albering, H. J., J. P. Rila, E. J. Moonen, J. A. Hoogewerff, and J. C. Kleinjans. 1999. Human health risk assessment in relation to environmental pollution of two artificial freshwater lakes in The Netherlands. Environ. Health Perspect. 107:27-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alliance for the Prudent Use of Antibiotics. 2006. Antibiotic resistance. http://www.tufts.edu/med/apua/Q&A/Q&A_AR.html. Alliance for the Prudent Use of Antibiotics, Boston, MA.
- 6.Arnone, R. D., and J. P. Walling. 2006. Evaluating cryptosporidium and giardia concentrations in combined sewer overflow. J. Water Health 4:157-165. [PubMed] [Google Scholar]
- 7.Battelle. 2007. Lower Passaic River Restoration Project. Draft focused feasibility risk assessment. Appendix C. Contract no. KC-ACE2002-18. Battelle, Columbus, OH.
- 8.Battelle. 2005. Lower Passaic River Restoration Project. Pathway analysis report. Contract no. DACW41-02-D-003. Battelle, Columbus, OH.
- 9.Bockting, G., J. Koolenbrander, and F. Swartjes. 1996. SEDISOIL. Estimation of human exposure to sediment. National Institute of Public Health and the Environment, Bilthoven, The Netherlands. (In Dutch.)
- 10.Bothner, M. H., H. Takeda, I. T. Knight, R. Hill, B. Butman, J. W. Farrington, R. R. Catwell, and J. F. Grassle. 1994. Sewage contamination in sediments beneath a deep-ocean dumpsite off New York. Mar. Environ. Res. 38:43-59. [Google Scholar]
- 11.Burton, G. A., Jr., D. Gunnison, and G. R. Lanza. 1987. Survival of pathogenic bacteria in various freshwater sediments. Appl. Environ. Microbiol. 53:633-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calabrese, E. J., H. Pastides, R. Barnes, C. Edwards, and P. T. Kostecki. 1989. How much soil do young children ingest: an epidemiologic study, p. 363-417. In E. J. Calabrese and P. T. Kostecki (ed.), Petroleum-contaminated soils, vol. 2. Lewis Publishers, Chelsea, MI. [DOI] [PubMed] [Google Scholar]
- 13.Calabrese, E. J., E. J. Stanek, C. E. Gilbert, and R. M. Barnes. 1990. Preliminary adult soil ingestion estimates: results of a pilot study. Regul. Toxicol. Pharmacol. 12:88-95. [DOI] [PubMed] [Google Scholar]
- 14.Clark, A., T. Turner, K. P. Dorothy, J. Goutham, C. Kalavati, and B. Rajanna. 2003. Health hazards due to pollution of waters along the coast of Visakhapatnam, east coast of India. Ecotoxicol. Environ. Saf. 56:390-397. [DOI] [PubMed] [Google Scholar]
- 15.Clean Beaches Council. 2005. 2005 State of the beach report: bacteria and sand. Clean Beaches Council, Washington, DC.
- 16.Crabill, C., R. Donald, J. Snelling, R. Foust, and G. Southam. 1999. The impact of sediment fecal coliform reservoirs on seasonal water quality in Oak Creek, Arizona. Water Res. 33:2163-2171. [Google Scholar]
- 17.Craig, D. L., H. J. Fallowfield, and N. J. Cromar. 2003. Effectiveness of guideline faecal indicator organism values in estimation of exposure risk at recreational coastal sites. Water Sci. Technol. 47:191-198. [PubMed] [Google Scholar]
- 18.Davies, C. M., J. A. Long, M. Donald, and N. J. Ashbolt. 1995. Survival of fecal microorganisms in marine and freshwater sediments. Appl. Environ. Microbiol. 61:1888-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis, S., P. Waller, R. Buschbom, J. Ballou, and P. White. 1990. Quantitative estimates of soil ingestion in normal children between the ages of 2 and 7 years: population-based estimates using aluminum, silicon, and titanium as soil tracer elements. Arch. Environ. Health 45:112-122. [DOI] [PubMed] [Google Scholar]
- 20.Dufour, A. P., O. Evans, T. D. Behymer, and R. Cantu. 2006. Water ingestion during swimming activities in a pool: a pilot study. J. Water Health 4:425-430. [PubMed] [Google Scholar]
- 21.Elson T. Killam Associates. 1976. Report upon overflow analysis to Passaic Valley Sewerage Commissioners, Passaic River overflows. Elson T. Killam Associates, Millburn, NJ.
- 22.Exponent. 2004. Pathogen sampling and preliminary human health risk assessment-Saybrook Place combined sewer overflow, Newark, New Jersey. Exponent, Chicago, IL.
- 23.Federal Register. 1994. Combined sewer overflow (CSO) control policy; notice. Fed. Regist. 59:18688-18698. [Google Scholar]
- 24.Finley, B., D. Proctor, P. Scott, N. Harrington, D. Paustenbach, and P. Price. 1994. Recommended distributions for exposure factors frequently used in health risk assessment. Risk Anal. 14:533-553. [DOI] [PubMed] [Google Scholar]
- 25.Goldblum, D. K., A. Rak, M. D. Ponnapalli, and C. J. Clayton. 2006. The Fort Totten mercury pollution risk assessment: a case history. J. Hazard. Mater. 136:406-417. [DOI] [PubMed] [Google Scholar]
- 26.Haas, C. N. 1996. How to average microbial densities to characterize risk. Water Res. 30:1036-1038. [Google Scholar]
- 27.Haas, C. N., J. B. Rose, and C. P. Gerba. 1999. Quantitative microbial risk assessment. John Wiley & Sons, Inc., New York, NY.
- 28.Interstate Environmental Commission. 2003. Annual report of the Interstate Environmental Commission. Interstate Environmental Commission, New York, NY. http://www.iec-nynjct.org/reports/2004/annualreport_03.pdf.
- 29.Kinnell, J. C., M. F. Bingham, E. A. Hastings, R. Ray, V. Craven, and M. Freeman. 2007. A survey methodology for collecting fish consumption data in urban and industrial water bodies (part 1). J. Toxicol. Environ. Health A 70:477-495.17365602 [Google Scholar]
- 30.National Climatic Data Center. 2007. NOAA TD 3240 hourly precipitation data (1/1/05-9/1/06). U.S. Department of Commerce, Washington, DC.
- 31.National Oceanic and Atmospheric Administration. 2006. Tides and currents online database, managed by the Center for Operational Oceanographic Products and Services. Center for Operational Oceanographic Products and Services, Silver Spring, MD. http://tidesandcurrents.noaa.gov/index.shtml.
- 32.New Jersey Department of Environmental Protection. 2005. Field sampling procedures manual. Site remediation and waste management. New Jersey Department of Environmental Protection, Trenton, NJ.
- 33.New Jersey Department of Environmental Protection. 1998. Guidance for sediment quality evaluations. Site Remediation Program, Bureau of Environmental Evaluation and Risk Assessment, New Jersey Department of Environmental Protection, Trenton, NJ.
- 34.New Jersey Department of Environmental Protection. 2005. Surface water quality standards. N.J.A.C. 7:9B. New Jersey Department of Environmental Protection, Trenton, NJ.
- 35.New York State Department of Environmental Conservation. 2004. State enforcement targets quality of water near New York City. New York State Department of Environmental Conservation, Albany, NY. http://www.dec.state.ny.us/website/environmentdec/2004b/nycwater908.html.
- 36.Novotny, V., K. R. Imhoff, M. Olthof, and P. A. Krenkel. 1989. Karl Imhoff's handbook of urban drainage and wastewater disposal. John Wiley & Sons, Inc., New York, NY.
- 37.Oregon Department of Environmental Quality. 1998. Guidance for use of probabilistic analysis in human health risk assessments. Waste Management and Cleanup Division, Oregon Department of Environmental Quality, Portland, OR.
- 38.ourpassaic.org. 2007. The Lower Passaic River Restoration Project fact sheet. http://www.ourpassaic.org/projectsites/premis_public/DM/index.cfm/fact.pdf?fuseaction=GetDoc&DocId=5026.
- 39.Passaic Valley Sewerage Commissioners. 2002. Lower Passaic watershed report. Passaic Valley Sewerage Commissioners, Newark, NJ.
- 40.Rao, V. C., K. M. Seidel, S. M. Goyal, T. G. Metcalf, and J. L. Melnick. 1984. Isolation of enteroviruses from water, suspended solids, and sediments from Galveston Bay: survival of poliovirus and rotavirus adsorbed to sediments. Appl. Environ. Microbiol. 48:404-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ray, R., V. Craven, M. Bingham, J. Kinnell, E. Hastings, and B. Finley. 2007. Human health exposure factor estimates based upon a creel/angler survey of the lower Passaic River (part 3). J. Toxicol. Environ. Health A 70:512-528.17365604 [Google Scholar]
- 42.Ray, R., V. Craven, J. Kinnell, M. Bingham, M. Freeman, and B. Finley. 2007. A statistical method for analyzing data collected by a creel/angler survey (part 2). J. Toxicol. Environ. Health A 70:496-511.17365603 [Google Scholar]
- 43.Rendtorff, R. C. 1954. The experimental transmission of human intestinal protozoan parasites. II. Giardia lamblia cysts given in capsules. Am. J. Hyg. 59:209-220. [DOI] [PubMed] [Google Scholar]
- 44.Rose, J. B., C. N. Haas, and S. Regli. 1991. Risk assessment and control of giardiasis. Am. J. Public Health 80:1440-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schaiberger, G. E., T. D. Edmond, and C. P. Gerba. 1982. Distribution of enteroviruses in sediments contiguous with a deep marine sewage outfall. Water Res. 16:1425-1428. [Google Scholar]
- 46.Searcy, K. E., A. I. Packman, E. R. Atwill, and T. Harter. 2006. Deposition of Cryptosporidium oocysts in streambeds. Appl. Environ. Microbiol. 72:1810-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sherer, B. M., R. Miner, J. A. Moore, and J. C. Buckhouse. 1992. Indicator bacteria survival in stream sediments. J. Environ. Qual. 21:591-595. [Google Scholar]
- 48.Shiaris, M. P., A. C. Rex, G. W. Pettibone, K. Keay, P. McManus, M. A. Rex, J. Ebersole, and E. Gallagher. 1987. Distribution of indicator bacteria and Vibrio parahaemolyticus in sewage-polluted intertidal sediments. Appl. Environ. Microbiol. 53:1756-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skanavis, C., and W. A. Yanko. 2001. Clostridium perfringens as a potential indicator for the presence of sewage solids in marine sediments. Mar. Pollut. Bull. 42:31-35. [DOI] [PubMed] [Google Scholar]
- 50.Soller, J. A. 2006. Use of microbial risk assessment to inform the national estimate of acute gastrointestinal illness attributable to microbes in drinking water. J. Water Health 4(Suppl. 2):165-186. [DOI] [PubMed] [Google Scholar]
- 51.States, S., et al. 1997. Protozoa in river water: sources, occurrence, and treatment. J. Am. Water Works Assoc. 89:74-87. [Google Scholar]
- 52.Texas Commission on Environmental Quality. 2007. Page 1, chapter 350—Texas Risk Reduction Program subchapter D: development of protective concentration levels. Public Law March 19, 2007. Texas Commission on Environmental Quality, Austin, TX.
- 53.Thoman, R. V., and J. A. Mueller. 1987. Principles of surface water quality modeling and control. Harper & Row, New York, NY.
- 54.U.S. Congress. 2000. Beaches Environmental Assessment and Coastal Health Act of 2000. Public Law 102-284. 106th U.S. Congress, Washington, DC.
- 55.U.S. Congress. 2007. Water Quality Investment Act of 2007. H.R. 569.I.H. 110th U.S. Congress, Washington, DC.
- 56.U.S. Department of Energy. 2006. Multimedia environmental pollutant assessment system (MEPAS) risk model developed for the U.S. Department of Energy. U.S. Department of Energy, Washington, DC. http://mepas.pnl.gov/mepas/.
- 57.U.S. Department of Energy. 2007. Guidance for conducting risk assessments and related risk activities for the DOE-ORO Environmental Management Program. U.S. Department of Energy-Oak Ridge Operations, Oak Ridge, TN. http://rais.ornl.gov/homepage/tm/for_exc_so.shtml.
- 58.U.S. Environmental Protection Agency. 1986. Ambient water quality criteria for bacteria. 1986 EPA 440-5-84-002. U.S. Environmental Protection Agency, Washington, DC.
- 59.U.S. Environmental Protection Agency. 1995. Combined sewer overflows. Guidance for nine minimum controls. EPA 832-B-95-003. U.S. Environmental Protection Agency, Washington, DC.
- 60.U.S. Environmental Protection Agency. 2001. Cryptosporidium: human health criteria document EPA-822-K-94-001. U.S. Environmental Protection Agency, Washington, DC.
- 61.U.S. Environmental Protection Agency. 2007. Diamond Alkali. U.S. Environmental Protection Agency, Washington, DC. http://www.epa.gov/region02/superfund/npl/diamondalkali/.
- 62.U.S. Environmental Protection Agency. 2006. Economic analysis for the Final Long Term 2 Enhanced Surface Water Treatment Rule. Appendix N EPA 815-R-06-001. U.S. Environmental Protection Agency, Washington, DC.
- 63.U.S. Environmental Protection Agency. 1997. Exposure factors handbook (EFH), volumes I, II and III. Office of Research and Development, U.S. Environmental Protection Agency, Washington, DC.
- 64.U.S. Environmental Protection Agency. 1998. Giardia: human health criteria document. EPA 823-R-002. U.S. Environmental Protection Agency, Washington, DC.
- 65.U.S. Environmental Protection Agency. 2001. Guidance: coordinating CSO long-term planning with water quality standards reviews. EPA-833-R-01-002. U.S. Environmental Protection Agency, Washington, DC.
- 66.U.S. Environmental Protection Agency. 1991. Human health evaluation manual, supplemental guidance: standard default exposure factors. OSWER directive 9285.6-03. Office of Solid Waste and Emergency Response, U.S. Environmental Protection Agency, Washington, DC.
- 67.U.S. Environmental Protection Agency. 2002. Implementation guidance for ambient water quality criteria for bacteria. EPA-823-B-02-003. Office of Water, U.S. Environmental Protection Agency, Washington, DC.
- 68.U.S. Environmental Protection Agency. 1989. Interim final guidance for soil ingestion rates. OSWER Directive 9850.4. Office of Solid Waste and Emergency Response, U.S. Environmental Protection Agency, Washington, DC.
- 69.U.S. Environmental Protection Agency. 2001. Method 1623: Cryptosporidium and Giardia in water by filtration/IMS/FA. EPA-821-R-01-025. U.S. Environmental Protection Agency, Washington, DC.
- 70.U.S. Environmental Protection Agency. 2001. Protocol for developing pathogen TMDLs. EPA 841-R-00-002. Office of Water, U.S. Environmental Protection Agency, Washington, DC.
- 71.U.S. Environmental Protection Agency. 2004. Report to Congress. Impacts and control of CSOs and SSOs. EPA 833-R-04-001. U.S. Environmental Protection Agency, Washington, DC.
- 72.U.S. Environmental Protection Agency. 2001. Report to Congress. Implementation and enforcement of the combined sewer overflow control policy. EPA 833-R-01-003. U.S. Environmental Protection Agency, Washington, DC.
- 73.U.S. Environmental Protection Agency. 2001. Report to Congress. Implementation and enforcement of the combined sewer overflow control policy. Appendix C: CSO community case studies. EPA 833-R-01-003. U.S. Environmental Protection Agency, Washington, DC.
- 74.U.S. Environmental Protection Agency. 1989. Risk assessment guidance for Superfund, vol. I. Human health evaluation manual (part A). EPA/540/1-89/002. U.S. Environmental Protection Agency, Washington, DC.
- 75.U.S. Environmental Protection Agency. 1996. Soil screening guidance. EPA/540/F-95/041. U.S. Environmental Protection Agency, Washington, DC.
- 76.U.S. Geological Survey. 2000. Microbial monitoring for the U.S. Geological Survey National Water Quality Assessment Program Water Resources Investigations. Report 00-4018. U.S. Geological Survey, Reston, VA.
- 77.U.S. Geological Survey. 2002. National field manual for the collection of water-quality data. Techniques of water-resources investigations, book 9. U.S. Geological Survey, Reston, VA.
- 78.van Beelen, P. 2003. A review on the application of microbial toxicity tests for deriving sediment quality guidelines. Chemosphere 53:795-808. [DOI] [PubMed] [Google Scholar]
- 79.Virginia Department of Environmental Quality. 2007. Voluntary remediation program risk assessment guidance. Virginia Department of Environmental Quality, Richmond, VA.
- 80.Wheeler Alm, E., J. Burke, and A. Spain. 2003. Fecal indicator bacteria are abundant in wet sand at freshwater beaches. Water Res. 37:3978-3982. [DOI] [PubMed] [Google Scholar]
- 81.Whitman, R. L., D. A. Shively, H. Pawlik, M. B. Nevers, and M. N. Byappanahalli. 2003. Occurrence of Escherichia coli and enterococci in Cladophora (Chlorophyta) in nearshore water and beach sand of Lake Michigan. Appl. Environ. Microbiol. 69:4714-4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.World Health Organization. 2005. Water recreation and disease. Plausibility of associated infections: acute effects, sequelae and mortality. World Health Organization, Geneva, Switzerland.