Abstract
The sfp gene cluster, unique to sorbitol-fermenting (SF) enterohemorrhagic Escherichia coli (EHEC) O157:NM strains, encodes fimbriae that mediate mannose-resistant hemagglutination in laboratory E. coli strains but are not expressed in wild-type SF EHEC O157:NM strains under standard laboratory conditions. We investigated whether Sfp fimbriae are expressed under conditions that mimic the intestinal environment and whether they contribute to the adherence of SF EHEC O157:NM strains to human intestinal epithelial cells. The transcription of sfpA (encoding the major fimbrial subunit) was upregulated in all strains investigated, and all expressed SfpA and possessed fimbriae that reacted with an anti-SfpA antibody when the strains were grown on solid media under anaerobic conditions. Sfp expression was absent under aerobic conditions and in liquid media. Sfp upregulation under anaerobic conditions was significantly higher on blood agar and a medium simulating the colonic environment than on a medium simulating the ileal environment (P < 0.05). The induction of Sfp fimbriae in SF E. coli O157:NM strains correlates with increased adherence to Caco-2 and HCT-8 cells. Our data indicate that the expression of Sfp fimbriae in SF E. coli O157:NM strains is induced under conditions resembling those of the natural site of infection and that Sfp fimbriae may contribute to the adherence of the organisms to human intestinal epithelium.
Enterohemorrhagic Escherichia coli (EHEC) strains of serogroup O157, which cause hemorrhagic colitis and hemolytic-uremic syndrome (HUS), differ in their capacity to ferment the sugar alcohol sorbitol. Strains that do not ferment sorbitol are mostly serotype O157:H7 (51), whereas sorbitol-fermenting (SF) E. coli O157 strains belong to the class of nonmotile strains (O157:NM) (29). Whereas EHEC O157:H7 strains are distributed throughout the world (30, 51), SF EHEC O157:NM strains have been recovered only in certain geographic regions (5, 7, 17, 22, 30, 35, 40), where they have caused several outbreaks of HUS (2, 3, 44) (“E. coli O157 Infections in the UK” [http://www.eurosurveillance.org/ew/2006/060601.asp#2]).
A variety of loci in E. coli O157:H7 are missing from SF E. coli O157:NM, and vice versa (8, 12, 13, 18, 25, 27). The large plasmids pO157 and pSFO157, present in EHEC O157:H7 and SF EHEC O157:NM, respectively, differ both in size and in content (13, 14). pSFO157 contains a cluster (sfp) of six genes, termed sfpA, sfpH, sfpC, sfpD, sfpJ, and sfpG (12, 13), which encode Sfp fimbriae mediating mannose-resistant hemagglutination (MRHA) (12). Using PCR targeting sfpA, which encodes the major fimbrial subunit, we screened various clinical EHEC O157 and non-O157 isolates, all strains in the ECOR and DEC reference collections, and other Enterobacteriaceae for the presence of this gene (18). sfpA was present in all SF E. coli O157:NM strains but absent from all other strains investigated (18), demonstrating that this gene is a unique marker for SF E. coli O157:NM. On the basis of this finding, we included the sfpA PCR in the diagnostic procedure used in our laboratory to detect and isolate EHEC from human stool specimens (18).
Although the sfp gene cluster is present in all SF E. coli O157:NM strains (9, 18), it is not possible to detect fimbriae in wild-type SF EHEC O157:NM when strains are cultured under standard laboratory conditions (12). Moreover, it was not possible to induce expression of the fimbriae by varying the culture media, temperature, aeration, osmolarity, or pH (12). Here we explored the possibility that the expression of Sfp fimbriae depends on conditions that mimic the natural milieu in the host, and the intestinal environment in particular. We also investigated whether Sfp fimbriae contribute to the adherence of SF E. coli O157:NM strains to human intestinal epithelial cells.
MATERIALS AND METHODS
Bacterial strains.
Recombinant E. coli strain HB101/pSFO157-E11 harbors a 10.9-kb EcoRI fragment of plasmid pSFO157 from SF EHEC O157:NM strain 3072/96 containing the sfp gene cluster which has been cloned into the pBluescript II KS(+) vector (Stratagene, La Jolla, CA) (12). Wild-type SF E. coli O157:NM strains 493/89, 3072/96, and E03/71 were isolated from stool specimens of patients with HUS (9, 12, 29). The first two strains are stx2 positive, whereas the third lacks stx; all strains contain eae, encoding the adhesin intimin, and a complete sfp cluster. E. coli HB101 harboring the pBluescript II KS(+) vector without any insert was used as a control.
Culture conditions.
To investigate the influence of a change in the environmental conditions on the expression of Sfp fimbriae, strains were passaged four times (24 h, 37°C) on Luria-Bertani (LB) agar, Columbia blood agar (Heipha, Heidelberg, Germany), simulated-ileal-environment medium (SIEM), and simulated-colonic-environment medium (SCEM) under standard aerobic and anaerobic (Anaerocult A; Merck, Darmstadt, Germany) conditions. SIEM and SCEM were prepared as described elsewhere (6) with minor modifications. SIEM (pH 7.0) contained 5.7 g of Bacto tryptone (Difco, Hamburg, Germany)/liter, 2.4 g of d-glucose/liter, 6.14 g of NaCl/liter, 0.68 g of KH2PO4/liter, 0.3 g of NaH2PO4/liter, 1.01 g of NaHCO3/liter, 5.6 g of bile salts/liter, 0.2 g of lysozyme/liter, 1,000 U of α-amylase/liter, 110 U of trypsin/liter, and 380 U of chymotrypsin/liter. SCEM (pH 7.0) contained Bacto tryptone at 6.25 g/liter, d-glucose at 2.6 g/liter, NaCl at 0.88 g/liter, KH2PO4 at 0.43 g/liter, NaHCO3 at 1.7 g/liter, KHCO3 at 2.7 g/liter, and bile salts at 4.0 g/liter. Solid media were prepared by adding 1.5% (wt/vol) Bacto agar (Difco). In some experiments, bacteria were also cultured in LB broth or CDMT medium [13 mM K2HPO4, 6 mM KH2PO4, 8 mM (NH4)2SO4, 2 mM sodium citrate, 0.4 mM MgSO4, 0.2% Casamino Acids, 0.2% glucose, 5 μM CaCl2, 0.01% tryptone (pH 7.4)] (12). Ampicillin (100 μg/ml) was added to media used to culture HB101/pSFO157-E11.
Isolation of Sfp fimbriae.
Sfp fimbriae were isolated from the HB101/pSFO157-E11 and wild-type SF E. coli O157:NM strains as described elsewhere (12). Briefly, after four passages on blood agar, bacteria were enriched for 20 h in CDMT medium under aerobic and anaerobic (Anaerocult A) conditions, harvested by centrifugation, and resuspended in a 1/10 volume of a solution containing 75 mM NaCl and 0.5 mM Tris-HCl (pH 7.4). After heating (60°C, 90 min), bacteria were pelleted by centrifugation, and the thermoeluted proteins in the supernatant were precipitated with trichloroacetic acid and dissolved in Laemmli sample buffer (34).
SDS-PAGE and immunoblotting.
The Sfp preparations were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (34) and a 15% SDS gel in a minislab gel apparatus (Bio-Rad, Munich, Germany). Gels were stained with Coomassie blue, or unstained proteins were transferred to a nitrocellulose membrane (Roth, Karlsruhe, Germany) for immunoblot analysis. Immunoblotting used a rabbit antibody against SfpA (12) diluted 1:2,000 and an alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G antibody (Dianova, Hamburg, Germany) diluted 1:2,000.
Quantitative RT-PCR assay.
Total RNA was isolated with the RNeasy minikit (Qiagen, Hilden, Germany). One-step quantitative reverse transcription-PCR (RT-PCR), performed with the LightCycler system (Roche, Mannheim, Germany) and the QuantiTect Sybr green RT-PCR kit (Qiagen), was used to determine the relative expression levels of sfpA mRNA. The PCRs were performed in 10 μl containing 1 μl of total RNA (20 ng), 5 μl of 2× QuantiTect Sybr green RT-PCR master mix, 0.1 μl of QuantiTect RT mix, 4 mM MgCl2, and 0.5 μM each upstream and downstream primer. Primers sfpA-1 (5′-TTTTGGATTATGTTCTGCGGT-3′) and sfpA-2 (5′-CACAGAAATACCGTTAGCCTCC-3′) were used to amplify sfpA. gapA (encoding d-glyceraldehyde-3-phosphate dehydrogenase A) was amplified with primers GapA_forward and GapA_reverse (10). One-step RT-PCR included a reverse transcription step at 50°C for 20 min and preliminary denaturation at 95°C for 15 min. This was followed by 40 cycles of denaturation (94°C, 10 s), annealing (52°C, 10 s), and extension (72°C, 20 s). For each mRNA assayed, relative quantification was achieved using an external standard (purified gapA and the respective sfpA PCR products). Data were analyzed using the fit point method with LightCycler (version 3.5) software. sfpA mRNAs were normalized to gapA mRNA. Control reactions were performed without reverse transcriptase to confirm that the target detected was RNA.
Hemagglutination assay.
Medium-sized colonies from cultures grown aerobically and anaerobically on solid medium (LB agar, Columbia blood agar, SIEM, or SCEM) or 15 μl of cultures grown in liquid medium (LB broth, CDMT, SIEM, or SCEM) was mixed with 15 μl of a 10% suspension of human erythrocytes with or without 0.5% d-mannose (Roth). Agglutination of erythrocytes was examined visually after a short period (up to 1 min) of rocking at room temperature.
Cell cultures and adherence assay.
The human intestinal epithelial cell lines Caco-2 (colonic carcinoma cell line; German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany; ACC 169) and HCT-8 (ileocecal adenocarcinoma cell line; ATCC CCL-244) were cultured as described previously (1). For the adherence assay, cells were grown to ∼70% confluence on coverslips placed in 6-well plates (Corning Inc., Corning, NY) which were seeded with 2 × 105 (Caco-2) or 2.5 × 105 (HCT-8) cells in antibiotic-free medium. After the cells were washed and fresh medium containing 0.5% d-mannose was added, cultures were infected with bacteria (1 × 108 CFU) that had been passaged four times (24 h, 37°C) under aerobic or anaerobic (Anaerocult A) conditions either in liquid medium (CDMT, SIEM, or SCEM) or on solid medium (Columbia blood agar, SIEM, or SCEM). The cells were incubated with bacteria (37°C, 5% CO2) for 2 h (Caco-2) or 4.5 h (HCT-8); these incubation periods corresponded to the time intervals, determined in preliminary experiments, over which the vector control strain showed no adhesion. The cells were then washed thoroughly with phosphate-buffered saline (PBS) (Cambrex Bioscience, Verviers, Belgium), fixed with 70% ethanol, and stained with 10% Giemsa stain (Merck, Darmstadt, Germany). The cover slides were mounted using Glycergel medium (DakoCytomation, Hamburg, Germany), and bacterial adherence was examined using light microscopy (Axio Imager A1; Zeiss, Jena, Germany) and photographed (AxioCam MRm camera; Zeiss).
Electron microscopy.
A single bacterial colony, passaged four times on blood agar under aerobic and anaerobic conditions, was applied to Formvar-coated copper grids. Bacteria were allowed to sediment for 5 min and were then negatively contrasted with 1% phosphotungstic acid. For immunogold staining, bacteria were incubated for 2 h with an anti-SfpA antibody diluted 1:20 in Dulbecco's PBS (D-PBS) containing 10% bovine serum albumin (Sigma). After three washes with D-PBS, immunogold labeling was performed for 45 min using a goat anti-rabbit antibody absorbed with 12-nm-diameter gold particles (Dianova, Hamburg, Germany) diluted 1:50 in D-PBS. After washes with D-PBS and then with distilled water, the bacteria were contrasted with 1% phosphotungstic acid in distilled water for 45 to 50 s. The samples were analyzed using a Tecnai G2 Spirit Twin electron microscope (FEI Company, Hillsboro, OR) (magnification, ×21,000 to ×49,000).
MALDI-TOF MS.
Matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) analysis of the 18-kDa band (putative SfpA protein) from the SDS-PAGE gel was performed as previously described (38). Briefly, in-gel digestion of the Coomassie blue-stained gel band was achieved after reduction and alkylation in an overnight incubation at 37°C using either sequencing-grade trypsin alone or both trypsin and endoproteinase Glu-C (protease V8) from Staphylococcus aureus (Roche Diagnostics GmbH, Mannheim, Germany). Peptide extracts were vacuum dried, desalted with reverse-phase ZipTip C18 pipette tips (Millipore, Bedford, MA), and redissolved in 10 μl of 50% acetonitrile (vol/vol). One microliter was mixed with an equal volume of 2,5-dihydroxybenzoic acid (10 mg/ml in acetonitrile-H2O at a 7/3 [vol/vol] ratio) as a UV-absorbing matrix, spotted onto a stainless-steel target, and dried under a stream of air. Positive-ion MALDI-TOF mass spectra were acquired in the reflectron mode using a Bruker Reflex III instrument (Bruker Daltonik, Bremen, Germany). The SfpA protein (UniProt/TrEMBL entry Q7B2V9) (12) was theoretically digested by the ProteinProspector tool, version 4.27.1 (University of California, San Francisco), and the calculated peptide fragments obtained from the database were compared with the experimentally acquired fragments. Matching of the two approaches results in the amino acid sequence coverage of the putative protein, expressed as a percentage.
Statistical analysis.
Statistical analysis was performed using the paired Student t test and Origin 7.0 software (OriginLab Corp., Northampton, MA). P values of ≤0.05 were considered significant.
RESULTS
Effects of culture conditions on MRHA caused by SF E. coli O157:NM.
When grown on LB agar, blood agar, or a solid medium simulating the ileal (SIEM) or colonic (SCEM) environment under aerobic conditions, none of the wild-type SF E. coli O157:NM strains 493/89, 3072/96, and E03/71 caused MRHA (Table 1). However, the MRHA phenotype could be induced in all strains by passaging on the respective media under anaerobic conditions (Table 1). The ability to cause MRHA became apparent after the second passage and reached a maximum in all strains and with all media after four passages. The intensity of the MRHA was greater when the bacteria were grown on blood agar than when they were grown on either LB agar, SIEM, or SCEM (Table 1). The MRHA phenotypes of cultures grown on SCEM were greater than those observed following growth on SIEM, and this was particularly evident for strain 493/89 (Table 1). MRHA was not observed for wild-type SF E. coli O157:NM strains grown in liquid media, including LB broth and CDMT, under either aerobic or anaerobic conditions (data not shown); repeated passages (as many as six) had no effect on this result. The MRHA abilities of liquid cultures grown in SIEM or SCEM could not be determined, because these cultures rapidly hemolyzed erythrocytes. The sfp-positive strain HB101/pSFO157-E11 caused strong MRHA after a single passage regardless of the medium and culture conditions used, but MRHA was not observed with the vector control strain (Table 1).
TABLE 1.
Influence of anaerobic conditions on the expression of Sfp fimbriae in wild-type SF E. coli O157:NM strains and the sfp-positive HB101/pSFO157-E11 clone in culture on solid media
| Strain and culture conditionsa | MRHAb on:
|
SfpA immunoblot resultc | Presence of Sfp fimbriaed | |||
|---|---|---|---|---|---|---|
| LB agar | BA | SIEM | SCEM | |||
| 493/89 | ||||||
| Aerobic | − | − | − | − | − | − |
| Anaerobic | + | +++ | + | ++ | + | + |
| 3072/96 | ||||||
| Aerobic | − | − | − | − | − | − |
| Anaerobic | + | ++ | + | +/++ | + | + |
| E03/71 | ||||||
| Aerobic | − | − | − | − | − | NP |
| Anaerobic | + | ++ | NP | NP | + | NP |
| HB101/pSFO157-E11 sfp+ clone | ||||||
| Aerobic | ++++ | ++++ | ++++ | ++++ | ++ | +++ |
| Anaerobic | ++++ | ++++ | ++++ | ++++ | ++ | +++ |
| Vector control [HB101/pBluescript II KS(+)] | ||||||
| Aerobic | − | − | − | − | − | − |
| Anaerobic | − | − | − | − | − | − |
Anaerocult A (Merck) was used for anaerobic culture.
Observed after four passages. BA, Columbia blood agar. The intensities of the MRHA reactions were classified as follows: ++++, immediate, complete, very strong HA (large aggregates involving all erythrocytes); +++, immediate, complete, strong HA (smaller aggregates involving all erythrocytes); ++, immediate, incomplete HA (smaller aggregates involving most but not all erythrocytes), +, delayed (ca. 1 min), incomplete HA (small aggregates involving only a subset of erythrocytes); −, no HA observed; NP, not performed.
Expressed as the binding of an anti-SfpA antibody to an 18-kDa band of Sfp fimbrial preparations from cultures passaged four times on blood agar. ++, strong binding; +, weaker binding; −, no binding.
As determined by electron microscopy of bacteria passaged four times on blood agar using negative staining and immunogold staining. +++, numerous fimbriae detected on the surfaces of bacteria (examples in Fig. 2A and B, panels 1); +, less frequent fimbriae detected on the surfaces of bacteria (examples in Fig. 2A and B, panels 3); −, no fimbriae detected (examples in Fig. 2A and B, panels 2 and 4).
Quantitative RT-PCR analysis of sfpA mRNA in SF E. coli O157:NM strains grown under aerobic and anaerobic conditions.
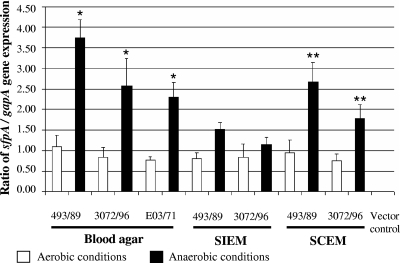
Strains 493/89, 3072/96, and E03/71, passaged four times using blood agar, SIEM, and SCEM under anaerobic conditions, showed increases in sfpA transcription of 1.9- to 3.4-fold, 1.4- to 3.1-fold, and 3.0-fold, respectively, over that observed under aerobic conditions (Fig. 1). These differences were significant (P < 0.05) for cultures on blood agar and SCEM (Fig. 1). The upregulation of sfpA transcription in strains 493/89 and 3072/96, which was examined using all three media, was most pronounced in cultures grown on blood agar, intermediate in cultures on SCEM, and lowest in cultures on SIEM (Fig. 1). Whereas the upregulation of sfpA transcription on blood agar and SCEM did not differ significantly, the transcription of sfpA with these two media was greater than that with SIEM (P < 0.05) (Fig. 1). sfpA transcription in HB101/pSFO157-E11 was constitutive under the conditions (media and culture atmosphere) used and was 57-fold to 187-fold higher than that in the wild-type SF E. coli O157:NM strains (data not shown). No sfpA transcripts were elicited in the vector control strain (Fig. 1).
FIG. 1.
Influence of anaerobiosis on sfpA transcription in SF E. coli O157:NM strains. Strains were passaged four times on the media indicated under aerobic or anaerobic (Anaerocult A) conditions, and sfpA mRNA was quantified using RT-PCR. *, sfpA transcription under anaerobic conditions was significantly higher than that under aerobic conditions on blood agar for strains 493/89 (P = 0.015), 3072/96 (P = 0.038), and E03/71 (P = 0.038). **, sfpA transcription under anaerobic conditions was significantly higher than that under aerobic conditions on SCEM for strains 493/89 (P = 0.020) and 3072/96 (P = 0.038). The upregulation of sfpA mRNA was significantly higher on blood agar and SCEM than on SIEM for strain 493/89 (P, 0.020 and 0.038, respectively) and significantly higher on blood agar than on SIEM for strain 3072/96 (P = 0.038). All data are means from three independent experiments. Error bars, standard deviations.
Immunoblot detection of SfpA in SF E. coli O157:NM strains grown under aerobic and anaerobic conditions.
The SfpA protein was not detected in Sfp preparations of any of the wild-type strains grown aerobically (Table 1). In contrast, in all strains grown anaerobically, an 18-kDa band in the Sfp fimbrial preparations, corresponding in size to SfpA (12), was detected by the SfpA antibody (Table 1). In accordance with the lower sfpA transcription in the wild-type SF E. coli O157:NM strains than in HB101/pSFO157-E11 (see above), the signals elicited from the wild-type strains were substantially weaker than that from the clone (Table 1). For the HB101/pSFO157-E11 clone, in which the transcription of sfpA was constitutive, the intensities of the SfpA immunoblot signals for cultures grown aerobically and anaerobically were similar (Table 1).
MALDI-TOF MS analysis of SfpA protein.
The identification of the 18-kDa SDS-PAGE band that reacted with the anti-SfpA antibody in HB101/pSFO157-E11 and in wild-type SF E. coli O157:NM strains grown anaerobically as the SfpA protein was verified by analyzing the fragments of the enzymatic cleavage of this band using MALDI-TOF MS. Combined data from tryptic digestion and simultaneous digestion with trypsin and Glu-C demonstrated that the amino acid sequence of the band corresponded (with 81% sequence coverage) to that predicted for the SfpA protein from HB101/pSFO157-E11 (UniProt/TrEMBL entry Q7B2V9).
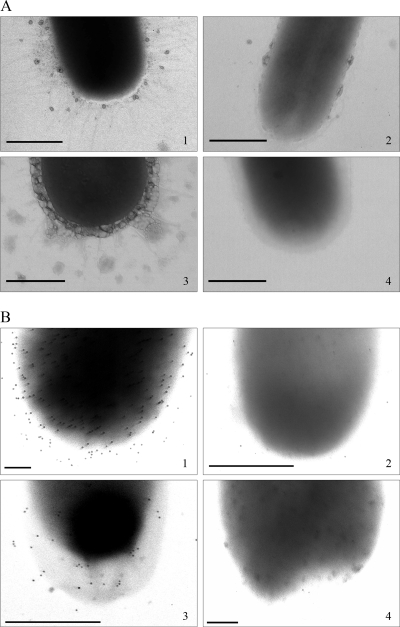
Electron microscopic evidence of Sfp fimbriae in wild-type SF E. coli O157:NM strains.
After negative staining, numerous fimbriae with diameters of ∼3 nm were observed for HB101/pSFO157-E11 in both anaerobic (Fig. 2A, panel 1) and aerobic (electron microscopic data not shown) cultures (Table 1). Very fine fimbriae, with diameters similar to those in HB101/pSFO157-E11 but much less numerous, were also present in strain 493/89 when the strain was cultured anaerobically (Fig. 2A, panel 3). However, no fimbriae could be demonstrated in strain 493/89 grown aerobically (Fig. 2A, panel 4). Fimbriae were also absent from the vector control strain when grown anaerobically (Fig. 2A, panel 2) and aerobically (electron microscopic data not shown) (Table 1).
FIG. 2.
Visualization of Sfp fimbriae in HB101/pSFO157-E11 and in wild-type SF EHEC O157:NM strain 493/89 using electron microscopy after negative staining (A) and immunogold staining with an anti-SfpA antibody (B). (Panels 1) HB101/pSFO157-E11; (panels 2) vector control strain HB101/pBluescript II KS(+); (panels 3) strain 493/89 grown under anaerobic conditions; (panels 4) strain 493/89 grown under aerobic conditions. Bars represent 500 nm (A, panels 1 to 4, and B, panels 2 and 3) or 150 nm (B, panels 1 and 4).
Confirmation that the structures expressed by HB101/pSFO157-E11 and strain 493/89 were Sfp fimbriae was obtained by immunogold staining with an anti-SfpA antibody (Fig. 2B, panels 1 and 3, respectively). The difference in the intensity of the gold labeling between the clone (Fig. 2B, panel 1) and strain 493/89 (Fig. 2B, panel 3) corresponded to the differences in the number of fimbriae after negative staining (Fig. 2A, panels 1 and 3, respectively). Strain 493/89 cultured aerobically showed no gold staining (Fig. 2B, panel 4), in accordance with the absence of fimbriae in the negatively stained strain grown under the same conditions (Fig. 2A, panel 4). Results similar to those obtained with strain 493/89 were obtained with SF E. coli O157:NM strain 3072/96 (Table 1), but the expression of Sfp fimbriae under anaerobic conditions was less than that in strain 493/89. Immunogold staining was absent from the vector control strain under both anaerobic (Fig. 2B, panel 2) and aerobic (data not shown) conditions.
Correlation between expression of Sfp fimbriae and adherence to intestinal epithelium.
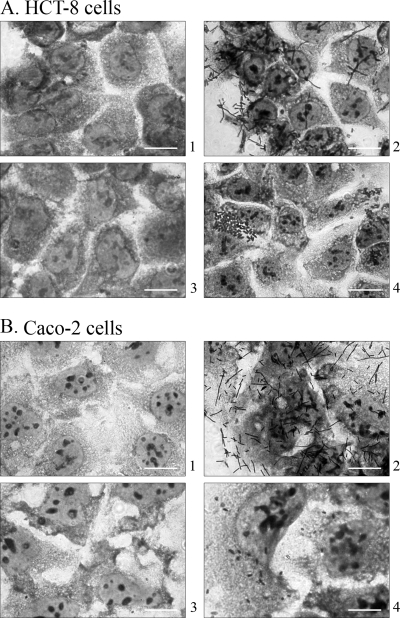
To investigate if Sfp fimbriae contribute to the adherence of SF E. coli O157:NM to human intestinal epithelial cells, strains 493/89, 3072/96, and E03/71, grown under conditions that did or did not induce expression of Sfp fimbriae, were tested for their capacities to adhere to Caco-2 and HCT-8 cells. None of the strains grown aerobically on solid medium adhered (Table 2), whereas all wild-type strains in which the expression of Sfp fimbriae had been induced by four passages under anaerobic conditions (as demonstrated by RT-PCR, immunoblotting, and electron microscopy) adhered to HCT-8 cells (Table 2; Fig. 3A, panel 4). As predicted by the quantitative RT-PCR analysis of sfpA mRNA (Fig. 1), cultures from SIEM adhered less intensively to HCT-8 cells than those from blood agar and SCEM, and they did not adhere at all to Caco-2 cells (Table 2). The intensity of the adherence of the wild-type strains was substantially less (Fig. 3A and B, panels 4) than that of HB101/pSFO157-E11 (Fig. 3A and B, panels 2), which adhered strongly to both cell lines when grown anaerobically or aerobically (Table 2). The vector control strain HB101 harboring pBluescript II KS(+) did not adhere to any cell line under any conditions used.
TABLE 2.
Influence of culture conditions on the adherence of wild-type SF E. coli O157:NM strains and the sfp-positive HB101/pSFO157-E11 clone to human intestinal epithelial cell lines
| Strain and culture conditionsa | Adherence of bacteriab grown on the indicated medium to the following cell line (exposure timec):
|
|||||
|---|---|---|---|---|---|---|
| Caco-2 (2 h)
|
HCT-8 (4.5 h)
|
|||||
| BA | SIEM | SCEM | BA | SIEM | SCEM | |
| 493/89 | ||||||
| Aerobic | − | − | − | − | − | − |
| Anaerobic | + | − | + | ++ | + | ++ |
| 3072/96 | ||||||
| Aerobic | − | − | − | − | − | − |
| Anaerobic | + | − | + | ++ | + | ++ |
| E03/71 | ||||||
| Aerobic | − | − | − | − | − | − |
| Anaerobic | + | − | + | ++ | + | ++ |
| HB101/pSFO157-E11 sfp+ clone | ||||||
| Aerobic | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
| Anaerobic | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
| Vector control | ||||||
| Aerobic | − | − | − | − | − | − |
| Anaerobic | − | − | − | − | − | − |
Anaerocult A (Merck) was used for anaerobic culture.
After four passages on solid medium. BA, Columbia blood agar. The intensity of the adherence was classified as follows: ++++, numerous single bacteria (>100) and/or clusters (5 to 10) in each microscopic field; ++, several single bacteria (10 to 20) and/or bacterial clusters (2 to 3) in each field; +, single bacteria (5 to 20) in each field; −, no adhering bacteria.
Duration of exposure of cells to bacteria. The longest time intervals for which no adherence of the vector control strain was observed were used.
FIG. 3.
Adherence of SF EHEC O157:NM strain 493/89 grown aerobically (panels 3) or anaerobically (panels 4) to HCT-8 (A) and Caco-2 (B) cells compared with the adherence of HB101/pSFO157-E11 (panels 2) and the vector control strain HB101/pBluescript II KS(+) (panels 1). Bars represent 10 μm.
In accordance with the lack of expression of Sfp fimbriae in liquid media, none of the wild-type SF E. coli O157:NM strains adhered to any intestinal epithelial cell lines when grown aerobically or anaerobically, whether CDMT medium, liquid SIEM, or liquid SCEM was used (data not shown).
DISCUSSION
SF EHEC O157:NM strains are the second most common cause of sporadic HUS in Germany (9, 22) and are emerging pathogens in other countries (5, 7, 17, 30, 35, 40; http://www.eurosurveillance.org/ew/2006/060601.asp#2). Multiple differences in the features of infections involving SF EHEC O157:NM and EHEC O157:H7 (22, 30) point to differences in the nature of the reservoirs, vehicles for transmission, and virulence factors. Currently known differences in putative virulence factors include the presence in SF EHEC O157:NM of a complete efa1 gene (25), which encodes the EHEC factor for adherence (Efa1) (39) or lymphostatin (32), but the absence of the gene clusters encoding tellurite resistance (ter) (8, 42) and urease (ure) (19, 41). Moreover, SF EHEC O157:NM possesses a mosaic genomic island composed of segments of the Shigella resistance locus and the E. coli O157:H7 strain EDL933 genome (27), which is absent from EHEC O157:H7 (27). Also, most SF EHEC O157:NM strains possess the cdt-V cluster, encoding a novel member of the cytolethal distending toxin family (26), which is only rarely found in E. coli O157:H7 (20). The large plasmid of SF EHEC O157:NM lacks espP and katP (13), which encode the serine protease EspP and catalase-peroxidase, respectively, on pO157 of EHEC O157:H7 (14), but contains a unique sfp gene cluster that mediates the expression of fimbriae when cloned into an E. coli laboratory strain (12). However, Sfp fimbriae have not been demonstrated in wild-type SF EHEC O157:NM (12).
Our data demonstrate a strong influence of growth conditions on the expression of Sfp fimbriae. The anaerobic induction of Sfp fimbriae in wild-type SF E. coli O157:NM strains correlated closely with an increased ability to adhere to Caco-2 and HCT-8 cells, suggesting that Sfp fimbriae may contribute to the adherence of these pathogens to human colonic epithelial cells during infection. The finding of anaerobic upregulation of Sfp expression on media simulating the environments of the colon and ileum, where EHEC presumably adheres during human infection (15), supports this conclusion. Taken together, our data show that in SF E. coli O157:NM the expression of Sfp fimbriae responds to the needs of the pathogen at the site of the infection. We have also confirmed the findings of Brunder et al. (12) that Sfp fimbriae in such strains are not expressed under standard laboratory conditions. Nevertheless, the inducibility of expression of Sfp fimbriae under particular environmental conditions demonstrates that the sfp cluster in wild-type SF E. coli O157:NM strains is functional, in contrast to several fimbrial gene clusters in EHEC O157:H7 strains that apparently do not encode functional fimbriae (36, 48).
Several transcription factors control gene expression in response to key cues, such as oxygen availability (28, 31, 43, 46, 47, 54). These factors either act operon-specifically or are global regulators, coordinating the expression of numerous promoters in response to the availability of oxygen (28, 31, 43, 46, 47, 54). Moreover, it has become increasingly clear that intra- and interspecies communication among bacteria in the gastrointestinal tract (traditionally called quorum sensing) is a common mechanism of gene regulation in pathogenic bacteria (49, 50). Studies are under way to determine the mechanisms involved in the regulation of the sfp fimbrial gene cluster.
By responding to environmental signals via global gene regulators, bacteria adapt their phenotypes accordingly to ensure maximal virulence factor induction at the site of the infection (23, 33, 37, 45, 46, 53, 54). E. coli can grow in the presence and absence of oxygen and thus can survive and multiply following excretion from its oxygen-starved colonic niche into an external aerobic environment. The ability to adapt to such changes is controlled by alterations in gene expression (24). Specifically, transitions from aerobic to anaerobic environments, or vice versa, involve changes in a large number of E. coli genes, including those participating in different metabolic pathways, but also virulence genes such as those encoding adhesins, toxins, or the type III secretion system (4, 16, 21, 24, 46). In EHEC O157:H7, the most common EHEC serotype associated with human disease (30, 51), upregulation of numerous putative virulence or fitness genes under anaerobic conditions has been reported (4, 16, 21); these include the stx2 gene, encoding Shiga toxin 2; espP, encoding the extracellular serine protease EspP (11); toxB, encoding the ToxB protein, which is required for full expression of adherence in E. coli O157:H7 (52); genes of the ure and ter clusters, encoding urease and tellurite resistance, respectively (8, 19); and genes of the cluster encoding curli fimbriae, the type III secretion system, lipopolysaccharide, and flagella (4, 16). These regulations of gene expression are though to contribute to enhanced virulence, persistent colonization, or transmission potential (16). The enhanced expression of Sfp fimbriae in SF EHEC O157:NM strains, which might also enhance the virulence of these strains, thus extends the spectrum of EHEC O157 genes that are upregulated under anaerobic conditions. The defining of the conditions for the expression of Sfp fimbriae in wild-type SF EHEC O157:NM strains enables further studies, which will address the role of these fimbriae in the pathogenesis of diseases caused by SF EHEC O157:NM.
Acknowledgments
This study was supported by a grant from the “Deutsche Forschungsgemeinschaft” (DFG)-funded International Graduate School, “Molecular interactions of pathogens with biotic and abiotic surfaces” (GRK1409), and by a grant from the Interdisciplinary Center of Clinical Research (IZKF) Münster (Ka2/061/04).
We thank Philip I. Tarr (Washington University School of Medicine, St. Louis, MO) for fruitful discussions during the preparation of the manuscript.
Footnotes
Published ahead of print on 14 December 2007.
REFERENCES
- 1.Aldick, T., M. Bielaszewska, W. Zhang, J. Brockmeyer, H. Schmidt, A. W. Friedrich, K. S. Kim, M. A. Schmidt, and H. Karch. 2007. Hemolysin from Shiga toxin-negative Escherichia coli O26 strains injures microvascular endothelium. Microbes Infect. 9:282-290. [DOI] [PubMed] [Google Scholar]
- 2.Allison, L., S. Harding, M. Locking, K. Pollock, J. Evans, H. Knight, G. Foster, J. Cowden, and M. Hanson. 2006. Sorbitol-fermenting E. coli O157 in Scotland—laboratory investigation of an outbreak of haemolytic uraemic syndrome, abstr. P01.1.01, p. 4. In Abstracts of the 6th International Symposium on Shiga Toxin (Verocytotoxin)-Producing Escherichia coli Infections (VTEC 2006). Cambridge Publishing, West Leederville, Australia.
- 3.Ammon, A., L. R. Peterson, and H. Karch. 1999. A large outbreak of hemolytic uremic syndrome caused by an unusual sorbitol-fermenting strain of E. coli O157:H−. J. Infect. Dis. 179:1274-1277. [DOI] [PubMed] [Google Scholar]
- 4.Ando, H., H. Abe, N. Sugimoto, and T. Tobe. 2007. Maturation of functional type III secretion machinery by activation of anaerobic respiration in enterohaemorrhagic Escherichia coli. Microbiology 153:464-473. [DOI] [PubMed] [Google Scholar]
- 5.Bettelheim, K. A., M. Whipp, S. P. Djordjevic, and V. Ramachandran. 2002. First isolation outside Europe of sorbitol-fermenting verocytotoxigenic Escherichia coli (VTEC) belonging to O group O157. J. Med. Microbiol. 51:713-714. [DOI] [PubMed] [Google Scholar]
- 6.Beumer, R. R., J. de Vries, and F. M. Rombouts. 1992. Campylobacter jejuni non-culturable coccoid cells. Int. J. Food Microbiol. 15:153-163. [DOI] [PubMed] [Google Scholar]
- 7.Bielaszewska, M., H. Schmidt, M. A. Karmali, R. Khakhria, J. Janda, K. Blahova, and H. Karch. 1998. Isolation and characterization of sorbitol-fermenting Shiga toxin (verocytotoxin)-producing Escherichia coli O157:H− strains in the Czech Republic. J. Clin. Microbiol. 36:2135-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bielaszewska, M., P. I. Tarr, H. Karch, W. Zhang, and W. Mathys. 2005. Phenotypic and molecular analysis of tellurite resistance among enterohemorrhagic Escherichia coli O157:H7 and sorbitol-fermenting O157:NM clinical isolates. J. Clin. Microbiol. 43:452-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bielaszewska, M., R. Köck, A. W. Friedrich, C. von Eiff, L. B. Zimmerhackl, H. Karch, and A. Mellmann. 2007. Shiga toxin-mediated hemolytic uremic syndrome: time to change the diagnostic paradigm? PLoS ONE 2:e1024. doi: 10.1371/journal.pone.0001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blumer, C., A. Kleefeld, D. Lehnen, M. Heintz, U. Dobrindt, G. Nagy, K. Michaelis, L. Emödy, T. Polen, R. Rachel, V. F. Wendisch, and G. Unden. 2005. Regulation of type 1 fimbriae synthesis and biofilm formation by the transcriptional regulator LrhA of Escherichia coli. Microbiology 151:3287-3298. [DOI] [PubMed] [Google Scholar]
- 11.Brockmeyer, J., M. Bielaszewska, A. Fruth, M.-L. Bonn, A. Mellmann, H.-U. Humpf, and H. Karch. 2007. Subtypes of the plasmid-encoded serine protease EspP in Shiga toxin-producing Escherichia coli: distribution, secretion, and proteolytic activity. Appl. Environ. Microbiol. 73:6351-6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunder, W., A. S. Khan, J. Hacker, and H. Karch. 2001. Novel type of fimbriae encoded by the large plasmid of sorbitol-fermenting enterohemorrhagic Escherichia coli O157:H−. Infect. Immun. 69:4447-4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunder, W., H. Karch, and H. Schmidt. 2006. Complete sequence of the large virulence plasmid pSFO157 of the sorbitol-fermenting enterohemorrhagic Escherichia coli O157:H− strain 3072/96. Int. J. Med. Microbiol. 296:467-474. [DOI] [PubMed] [Google Scholar]
- 14.Burland, V., Y. Shao, N. T. Perna, G. Plunkett, H. J. Sofia, and F. R. Blattner. 1998. The complete DNA sequence and analysis of the large virulence plasmid of Escherichia coli O157:H7. Nucleic Acids Res. 26:4196-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chong, Y., R. Fitzhenry, R Heuschkel, F. Torrente, G. Frankel, and A. D. Phillips. 2007. Human intestinal tissue tropism in Escherichia coli O157:H7—initial colonization of terminal ileum and Peyer's patches and minimal colonic adhesion ex vivo. Microbiology 153:794-802. [DOI] [PubMed] [Google Scholar]
- 16.Dowd, S. E., and H. Ishizaki. 2006. Microarray based comparison of two Escherichia coli O157:H7 lineages. BMC Microbiol. 6:30. doi: 10.1186/1471-2180-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eklund, M., M. Bielaszewska, U. Nakari, H. Karch, and A. Siitonen. 2006. Molecular and phenotypic profiling of sorbitol-fermenting Escherichia coli O157:H− human isolates from Finland. Clin. Microbiol. Infect. 12:634-641. [DOI] [PubMed] [Google Scholar]
- 18.Friedrich, A. W., K. V. Nierhoff, M. Bielaszewska, A. Mellmann, and H. Karch. 2004. Phylogeny, clinical associations, and diagnostic utility of the pilin subunit gene (sfpA) of sorbitol-fermenting, enterohemorrhagic Escherichia coli O157:H−. J. Clin. Microbiol. 42:4697-4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedrich, A. W., R. Köck, M. Bielaszewska, W. Zhang, H. Karch, and W. Mathys. 2005. Distribution of the urease gene cluster among and urease activities of enterohemorrhagic Escherichia coli O157 isolates from humans. J. Clin. Microbiol. 43:546-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedrich, A. W., S. Lu, M. Bielaszewska, R. Prager, P. Bruns, J. G. Xu, H. Tchäpe, and H. Karch. 2006. Cytolethal distending toxin in Escherichia coli O157:H7: spectrum of conservation, structure, and endothelial toxicity. J. Clin. Microbiol. 44:1844-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garmendia, J., and G. Frankel. 2005. Operon structure and gene expression of the espJ-tccP locus of enterohaemorrhagic Escherichia coli O157:H7. FEMS Microbiol. Lett. 247:137-145. [DOI] [PubMed] [Google Scholar]
- 22.Gerber, A., H. Karch, F. Allerberger, H. M. Verweyen, and L. B. Zimmerhackl. 2002. Clinical course and the role of Shiga toxin-producing Escherichia coli infection in the hemolytic-uremic syndrome in pediatric patients, 1997-2000, in Germany and Austria: a prospective study. J. Infect. Dis. 186:493-500. [DOI] [PubMed] [Google Scholar]
- 23.Guiney, D. G. 1997. Regulation of virulence gene expression by the host environment. J. Clin. Investig. 99:565-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunsalus, R. P., and S. J. Park. 1994. Aerobic-anaerobic gene regulation in Escherichia coli—control by the arcAB and fnr regulons. Res. Microbiol. 145:437-450. [DOI] [PubMed] [Google Scholar]
- 25.Janka, A., M. Bielaszewska, U. Dobrindt, and H. Karch. 2002. Identification and distribution of the enterohemorrhagic Escherichia coli factor for adherence (efa1) gene in sorbitol-fermenting Escherichia coli O157:H−. Int. J. Med. Microbiol. 292:207-214. [DOI] [PubMed] [Google Scholar]
- 26.Janka, A., M. Bielaszewska, U. Dobrindt, L. Greune, M. A. Schmidt, and H. Karch. 2003. The cytolethal distending toxin (cdt) gene cluster in enterohemorrhagic Escherichia coli O157:H− and O157:H7: characterization and evolutionary considerations. Infect. Immun. 71:3634-3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janka, A., G. Becker, A.-K. Sonntag, M. Bielaszewska, U. Dobrindt, and H. Karch. 2005. Presence and characterization of a mosaic genomic island which distinguishes sorbitol-fermenting enterohemorrhagic Escherichia coli O157:H− from E. coli O157:H7. Appl. Environ. Microbiol. 71:4875-4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang, Y., K. D. Weber, Y. Qiu, P. J. Kiley, and F. R. Blattner. 2005. Genome-wide expression analysis indicates that FNR of Escherichia coli K-12 regulates a large number of genes of unknown function. J. Bacteriol. 187:1135-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karch, H., H. Böhm, H. Schmidt, F. Gunzer, S. Aleksic, and J. Heesemann. 1993. Clonal structure and pathogenicity of Shiga-like toxin-producing, sorbitol-fermenting Escherichia coli O157:H−. J. Clin. Microbiol. 31:1200-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karch, H., P. I. Tarr, and M. Bielaszewska. 2005. Enterohaemorrhagic Escherichia coli in human medicine. Int. J. Med. Microbiol. 295:405-418. [DOI] [PubMed] [Google Scholar]
- 31.Kim, S. O., K. Merchant, R. Nudelman., W. F. Bayer., T. Keng., J. DeAngelo, A. Hausladen, and J. S. Stamler. 2002. OxyR: a molecular code for redox-mediated signalling. Cell 109:383-396. [DOI] [PubMed] [Google Scholar]
- 32.Klapproth, J. M., I. C. Scaletsky, B. P. McNamara, L. C. Lai, C. Malstrom, S. P. James, and M. S. Donnenberg. 2000. A large toxin from pathogenic Escherichia coli strains that inhibits lymphocyte activation. Infect. Immun. 68:2148-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krishnan, H. H., A. Ghosh, K. Paul, and R. Chowdhury. 2004. Effect of anaerobiosis on expression of virulence factors in Vibrio cholerae. Infect. Immun. 72:3961-3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 35.Lee, J. H., and S. J. Choi. 2006. Isolation and characteristics of sorbitol-fermenting Escherichia coli O157 strains from cattle. Microbes Infect. 8:2021-2026. [DOI] [PubMed] [Google Scholar]
- 36.Low, A. S., N. Holden, T. Rosser, A. J. Roe, C. Constantinidou, J. L. Hobman, D. G. Smith, J. C. Low, and D. L. Gally. 2006. Analysis of fimbrial gene clusters and their expression in enterohaemorrhagic Escherichia coli O157:H7. Environ. Microbiol. 8:1033-1047. [DOI] [PubMed] [Google Scholar]
- 37.Mekalanos, J. J. 1992. Environmental signals controlling expression of virulence determinants in bacteria. J. Bacteriol. 174:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Müthing, J., I. Meisen, P. Bulau, M. Langer, K. Witthohn, H. Lentzen, U. Neumann, and J. Peter-Katalinić. 2004. Mistletoe lectin I is a sialic acid-specific lectin with strict preference to gangliosides and glycoproteins with terminal Neu5Acα2-6Galβ1-4GlcNAc residues. Biochemistry 43:2996-3007. [DOI] [PubMed] [Google Scholar]
- 39.Nicholls, L., T. H. Grant, and R. M. Robins-Browne. 2000. Identification of a novel genetic locus that is required for in vitro adhesion of a clinical isolate of enterohaemorrhagic Escherichia coli to epithelial cells. Mol. Microbiol. 35:275-288. [DOI] [PubMed] [Google Scholar]
- 40.Orth, D., K. Grif, M. P. Dierich, and R. Würzner. 2006. Sorbitol-fermenting Shiga toxin-producing Escherichia coli O157: indications for an animal reservoir. Epidemiol. Infect. 134:719-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orth, D., K. Grif, M. P. Dierich, and R. Würzner. 2006. Prevalence, structure and expression of urease genes in Shiga toxin-producing Escherichia coli from humans and the environment. Int. J. Hyg. Environ. Health 209:513-520. [DOI] [PubMed] [Google Scholar]
- 42.Orth, D., K. Grif, M. P. Dierich, and R. Würzner. 2007. Variability in tellurite resistance and the ter gene cluster among Shiga toxin-producing Escherichia coli isolated from humans, animals and food. Res. Microbiol. 158:105-111. [DOI] [PubMed] [Google Scholar]
- 43.Overton, T. W., L. Griffiths, M. D. Patel, J. L. Hobman, C. W. Penn, J. A. Cole, and C. Constantinidou. 2006. Microarray analysis of gene regulation by oxygen, nitrate, nitrite, FNR, NarL and NarP during anaerobic growth of Escherichia coli: new insights into microbial physiology. Biochem. Soc. Trans. 34:104-107. [DOI] [PubMed] [Google Scholar]
- 44.Robert Koch Institute. 2003. Ein HUS-Ausbruch durch sorbitol-fermentierende EHEC des Serovars O157:H−: Untersuchungsergebnisse und Lehren für die Surveillance. Epidemiol. Bull. 22:171-175. [Google Scholar]
- 45.Rollenhagen, C., and D. Bumann. 2006. Salmonella enterica highly expressed genes are disease specific. Infect. Immun. 74:1649-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruiz-Perez, F., J. Sheikh, S. Davis, E. C. Boedeker, and J. P. Nataro. 2004. Use of a continuous-flow anaerobic culture to characterize enteric virulence gene expression. Infect. Immun. 72:3793-3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salmon, K., S. P. Hung, K. Mekjian, P. Baldi, G. W. Hatfield, and R. P. Gunsalus. 2003. Global gene expression profiling in Escherichia coli K12: the effects of oxygen availability and FNR. J. Biol Chem. 278:29837-29855. [DOI] [PubMed] [Google Scholar]
- 48.Shaikh, N., N. J. Holt, J. R. Johnson, and P. I. Tarr. 2007. Fim operon variation in the emergence of enterohemorrhagic Escherichia coli: an evolutionary and functional analysis. FEMS Microbiol. Lett. 273:58-63. [DOI] [PubMed] [Google Scholar]
- 49.Sperandio, V., J. L. Mellies, W. Nguyen, S. Shin, and J. B. Kaper. 1999. Quorum sensing controls expression of the type III secretion gene transcription and protein secretion in enterohemorrhagic and enteropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 96:15196-15201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sperandio, V., A. G. Torres, B. Jarvis, J. P. Nataro, and J. B. Kaper. 2003. Bacteria-host communication: the language of hormones. Proc. Natl. Acad. Sci. USA 100:8951-8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tarr, P. I., C. A. Gordon, and W. L. Chandler. 2005. Shiga toxin-producing Escherichia coli and the haemolytic uraemic syndrome. Lancet 365:1073-1086. [DOI] [PubMed] [Google Scholar]
- 52.Tatsuno, I., M. Horie, H. Abe, T. Miki, K. Makino, H. Shinagawa, H. Taniguchi, S. Kamiya, T. Hayashi, and C. Sasakawa. 2001. toxB gene on pO157 of enterohemorrhagic Escherichia coli O157:H7 is required for full epithelial cell adherence phenotype. Infect. Immun. 69:6660-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Torres, A. G., L. Milflores-Flores, J. G. Garcia-Gallegos, S. D. Patel, A. Best, R. M. La Ragione, Y. Martinez-Laguna, and M. J. Woodward. 2007. Environmental regulation and colonization attributes of the long polar fimbriae of Escherichia coli O157:H7. Int. J. Med. Microbiol. 297:177-185. [DOI] [PubMed] [Google Scholar]
- 54.Vallet-Gely, I., J. S. Sharp, and S. L. Dove. 2007. Local and global regulators linking anaerobiosis to cupA fimbrial gene expression in Pseudomonas aeruginosa. J. Bacteriol. doi: 10.1128/JB.01344-07. [DOI] [PMC free article] [PubMed]