Abstract
Dibenzothiophene (DBT) and its derivatives can be microbially desulfurized by enzymes DszC, DszA, and DszB, which are encoded by the operon dszABC and contribute to the conversion in tandem. We investigated the expression characteristics of the dsz operon. Our results revealed that the levels of transcription and translation of dszA, dszB, and dszC decreased according to the positions of the genes in the dsz operon. Furthermore, the translation of dszB was repressed by an overlapping structure in the dsz operon. In order to get better and steady expression of the Dsz enzymes and optimize the metabolic flux of DBT, we rearranged the dsz operon according to the catalytic capabilities of the Dsz enzymes and expressed the rearranged dsz operon, dszBCA, in Rhodococcus erythropolis. After rearrangement, the ratio of dszA, dszB, and dszC mRNAs in the cells was changed, from 11:3.3:1 to 1:16:5. Western blot analysis revealed that the levels of expression of dszB and dszC had been enhanced but that the expression of dszA had decreased. The desulfurization activity of resting cells prepared from R. erythropolis DRB, which carried the rearranged dsz operon, was about 12-fold higher than that of resting cells of R. erythropolis DRA, which carried the original operon in a similarly constructed vector.
The combustion of sulfur-containing fossil fuels contributes to environmental pollution. Steady increases in the average sulfur content of petroleum and stricter environmental regulations concerning the sulfur content and CO2 emissions have promoted studies of biodesulfurization (BDS) to upgrade fossil fuels. BDS can potentially provide a solution to the need for improved and expanded fuel-upgrading processes worldwide, since BDS does not require hydrogen and produces far less CO2 than the traditional thermochemical processes (7). Dibenzothiophene (DBT) and its derivatives constitute a broad range of sulfur heterocyclic compounds found in petroleum that are recalcitrant to desulfurization via the traditionally applied hydrodesulfurization method but can easily be desulfurized via BDS (5).
Most of the reported strains can remove sulfur from DBT and its derivatives in a sulfur-specific manner without affecting the carbon skeleton by following the 4S pathway (3, 5, 7, 11). The 4S pathway proceeds via two cytoplasmic monooxygenases (DszC and DszA) and a desulfinase (DszB), which are encoded by an operon (dszABC). The pathway is supported by flavin reductase (DszD). DBT monooxygenase (DszC) catalyzes the sequential conversion of DBT into DBT sulfoxide and DBT sulfone. DBT sulfone monooxygenase (DszA) catalyzes the transformation of DBT sulfone into DBT sulfinate with a reaction rate 5- to 10-fold higher than that of the conversion catalyzed by DszC. DszB, an aromatic sulfinic acid hydrolase, effects a nucleophilic attack of a base-activated water molecule on the sulfinate sulfur to form 2-hydroxybiphenyl (2-HBP), with a reaction rate about 20% of that of the reaction catalyzed by DszC (13, 4).
The main goal of BDS research is to develop a commercial process for petroleum desulfurization, and it has been estimated that a successful commercial process would require a biocatalyst with a desulfurization activity of 1.2 to 3 mmol of DBT/g (dry weight) of cells/h (7). However, the desulfurization activity of naturally occurring bacterial cultures is low in comparison to these estimated requirements. In order to achieve higher desulfurization rates, genetic manipulations have been used to increase the levels of expression of the dsz genes. These approaches include measures such as supplying multiple copies of the dsz genes in Rhodococcus erythropolis KA 2-5-1 (6, 8), placing the genes under the control of alternative promoters (2, 12, 14), enhancing the level of expression of dszB by mutating its 5′ untranslated region, and removing the gene overlap regions in the dsz operon (9). The desulfurization rate can also be increased using directed-evolution methods such as DNA shuffling and gene rearrangement. The first approach was applied to alter the dszC gene by a new in vitro DNA recombination method called random chimeragenesis on transient templates (1), by which both the rate and the substrate utilization extent of catalysis by DszC were improved. However, all the efforts discussed above have achieved a maximum metabolic flow of only about 250 μmol of DBT/g (dry weight) of cells/h, which is still low in comparison to the requirements of a commercial process.
The rate of an enzyme catalytic reaction is determined by the catalytic activity and the quantity of the enzyme and the substrate concentration. In prokaryotes, several functionally related genes are often clustered and transcribed into polycistronic mRNAs with different lengths, and the transcription will potentially terminate at any termination codons or secondary structures in the polycistronic mRNAs that are unfavorable to transcription. Therefore, the levels of transcription of these genes usually decrease with increasing distance from the regulatory elements. This phenomenon is known as polar transcription. Gene expression is controlled first and foremost at the level of transcription for the simultaneous transcription and translation and the very short half-life of the mRNA in prokaryotes. Higher levels of mRNA are the precondition for higher levels of the encoded protein. Therefore, rearranging these genes according to the catalytic capabilities of the enzymes and their reaction orders could not only balance the catalytic capabilities but also increase the substrate concentrations for the enzymes. In this paper, we introduce a genetic rearrangement strategy for optimizing the metabolic pathway of DBT. By using recombinant PCR, the dsz operon of R. erythropolis DS-3 was rearranged according to the catalytic capabilities of the Dsz enzymes and their reaction orders in the 4S pathway. The rearranged dsz operon can also be recombined into its native position by a double crossover in any subsequent work.
MATERIALS AND METHODS
Chemicals.
DBT, 2-hydroxybiphenyl-2-sulfinic acid (HBPS), and 2-HBP were purchased from Acoros (NJ). All other reagents were of analytical grade and were obtained commercially.
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. R. erythropolis DS-3, a DBT- and DBT derivative-desulfurizing strain isolated from soil (16) that carries the same dsz operon as Rhodococcus erythropolis IGTS8, was used throughout the study. R. erythropolis CGMCC 4.1491 (dsz null), which normally is not able to desulfurize DBT, was purchased from the China General Microbiological Culture Collection Center. Escherichia coli strains were cultured in Luria-Bertani medium. Antibiotics were added in order to select plasmids, as follows: ampicillin, 100 μg/ml, and kanamycin, 34 μg/ml.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Characteristics | Source or reference |
|---|---|---|
| Strains | ||
| R. erythropolis 4.1491 | Wild type; dsz null | CGMCC |
| R. erythropolis DS-3 | Wild type; dsz+ Cmr | 16 |
| E. coli BL21(DE3) | F−ompT hsdSB(rB− rB−) gal dcm (DE3) | Novagen |
| E. coli DH5α | hsdR17(rK− mK+) recA1 endA1 (lac-proAB) gyrA96 thi-1 relA1 supE44 [F′ traD36 proAB lacIqZΔM15] | Takara |
| Plasmids | ||
| pMD 18-T simple vector | Clone vector carrying lac promoter and f1 ori; Ampr | Takara |
| pRHK1 | Shuttle vector carrying arabinose promoter and p15A ori; Kanr | 6 |
| pRABC | Carries dsz operon (dszABC) from R. erythropolis DS-3 on pRHK1; Kanr | This study |
| pRBCA | Carries reconstructed dsz operon (dszBCA) from dsz operon on pRHK1; Kanr | This study |
Rearrangement of the dsz operon by overlap PCR.
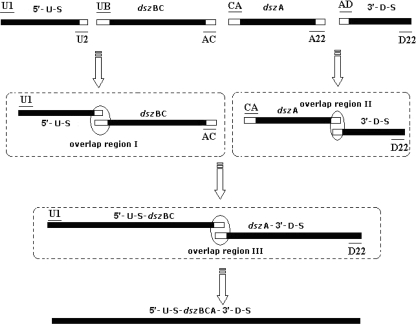
The rearranged dsz operon was created by three steps of PCR amplification (Fig. 1). In the first round, fragments with overlapping regions, the 400-bp 5′ upstream segment and the 400-bp 3′ downstream segment of dszABC and the dszA and dszBC segments, were produced by PCR with primers U1 and U2, CA and A22, UB and AC, and AD and D22 (Table 2). Each reaction mixture contained 15 pmol of each deoxynucleoside triphosphate, 25 μl of GC buffer (Takara, Otsu, Shiga, Japan), 20 pmol of each primer, 2.5 U of PrimeSTAR hot-start DNA polymerase, and 15 ng of the R. erythropolis DS-3 genome. A hot start was initiated with 5 min of denaturation at 95°C, followed by 30 amplification cycles (95°C for 30 s, 56°C for 45 s, and 72°C for 3 min). PCR products from each reaction were gel purified with an agarose gel DNA purification kit (Takara, Otsu, Shiga, Japan) and used in subsequent amplifications. The second-round PCR mixture was the same as that for the first round, except that 10 ng of each segment of the 5′ upstream and dszBC segments and 10 ng of the dszA and 3′ downstream segments from the first-round PCR, together with 20 pmol of primers U1 and AC or CA and D22, were included. PCR conditions were the same as those for the first round, except that the extension time was 4 min at 72°C. The third-round PCR mixture was the same as that for the first round, except that 10 ng of the ligated 5′ upstream-dszBC segment and 10 ng of the ligated dszA-3′ downstream segment from the second-round PCR, together with 20 pmol of primers U1 and D22, were added. PCR conditions were the same as those for the first round, except that the extension time was 5 min at 72°C. The native dsz operon was also created by PCR amplification with primers U1 and D22. The PCR products were ligated into pMD 18-T simple vector for sequence analysis.
FIG. 1.
Rearrangement of the dsz operon by overlap PCR. Fragments of the 400-bp 5′ upstream segment (5′-U-S) and the 400-bp 3′ downstream segment (3′-D-S) of dszABC and the dszA and dszBC segments, including the overlap regions, were yielded by PCR, then the ligated 5′ upstream-dszBC segment (5′-U-S-dszBC) and the ligated dszA-3′ downstream segment (dszA-3′-D-S) were produced by overlap PCR via their overlap regions, and finally, the 5′ upstream-dszBC segment and the dszA-3′ downstream segment were linked together by overlap PCR via their overlap region to yield the reconstructed dsz operon. Black bars represent genes, and white bars represent overlap regions.
TABLE 2.
Specific primers used in this study
| Primer | Gene | Sequence |
|---|---|---|
| Specific PCR primers used for overlap PCR | ||
| U1 | 5′ upstream segment | 5′-GACGAATTCTAACTGACTAGACGTGTGTGGTG-3′ |
| U2 | 5′ upstream segment | 5′-TCCTAAATGTCAATCATGGCTAGA-3′ |
| CA | dszA | 5′-CCCGGCTTCACCTCCTAGGAACATCCGCATGACTCAACAACGACAAATGC-3′ |
| A22 | dszA | 5′-CCTCATGAAGGTTGTCCTTGCA-3′ |
| UB | dszBC | 5′-TCTAGCCATGATTGACATTTAGGAACATCCTCATGACAAGCCGCGTCGAC-3′ |
| AC | dszBC | 5′-GCATTTGTCGTTGTTGAGTCATGCGGATGTTCCTAGGAGGTGAAGCCGGG-3′ |
| AD | 3′ downstream segment | 5′-TGCAAGGACAACCTTCATGAGGATCTGAGGCGCTGATCG-3′ |
| D22 | 3′ downstream segment | 5′-GGTACCCTCTAGAGCACTGGATCCACTTG-3′ |
| Specific PCR primers used for quantitative real-time PCR | ||
| A1 | dszA | 5′-GCGCGGCAAGTTCGATCTGT-3′ |
| A2 | dszA | 5′-TCCCGCAGGATGTCCTTGATC-3′ |
| B1 | dszB | 5′-GCAGGGCACGGTTCATTTCAC-3′ |
| B2 | dszB | 5′-ACGGGTTCCTGCAGCAAGTTG-3′ |
| C1 | dszC | 5′-TCGGCTCGCAGGAACAAGAAG-3′ |
| C2 | dszC | 5′-GTCGGCGATCTTGTAGGACACC-3′ |
| N1 | NADH gene | 5′-TGGGCGGATTGCTCGAAGGTA-3′ |
| N2 | NADH gene | 5′-GCGCCACCGTCGACGTAGATG-3′ |
Transformation of Rhodococcus by electroporation.
To obtain electrocompetent cells of R. erythropolis 4.1491 (dsz null), 100 ml of basal salt medium (BSM) supplemented with 2.5% (wt/vol) glucose and 0.5% yeast extract in a 250-ml baffled Erlenmeyer flask was inoculated with 4 ml of precultured broth and the culture was grown at 30°C to an optical density of 0.6 at 600 nm. The cells were harvested, washed twice with 0.3 mol of ice-cold sucrose/liter, and concentrated 40-fold in 0.5 mol of ice-cold sucrose/liter. Competent cells were either used directly for electroporation or stored at −70°C. The native dsz operon and the rearranged dsz operon fragment were ligated into the EcoRI and XbaI sites of pRHK1 to yield pRABC and pRBCA. Plasmids were introduced into R. erythropolis 4.1491 (dsz null) by electroporation using a Micropulser electroporator (Bio-Rad, CA) as described previously (6) to yield R. erythropolis DRA and R. erythropolis DRB.
Quantitative analysis of transcription of the natural and rearranged dsz operons by real-time PCR.
The total RNA of R. erythropolis DS-3, R. erythropolis DRA, and R. erythropolis DRB was prepared with a UNIQ-10 spin column total RNA isolation kit (Sangon, Shanghai, China). RNA samples were treated with DNase I (Takara, Otsu, Shiga, Japan) to eliminate any genomic DNA contamination. First-strand cDNA was synthesized from R. erythropolis DS-3, R. erythropolis DRA, or R. erythropolis DRB total RNA by using SuperScriptII RNase H− reverse transcriptase (Invitrogen, Carlsbad, CA) with primers A1 and A2, B1 and B2, and C1 and C2 for 30 min at 42°C in an incubation mixture containing 1 mg of total RNA, 10 pmol of primers, 200 U of SuperScriptII RNase H− reverse transcriptase, 1× reverse transcriptase buffer, and 10 mmol of each deoxynucleoside triphosphate. The quality of single-stranded cDNA was monitored by the reverse transcriptase PCR method, as described by Michalski and Weil (10). Real-time PCRs were carried out with an Opticon2 continuous fluorescence detection system (MJ Research, Waltham, MA) with primers A1 and A2, B1 and B2, and C1 and C2 and SYBR green I master mix (PE Biosystems, Foster City, CA). The PCR conditions were 5 min of denaturation at 95°C, followed by 40 amplification cycles (95°C for 30 s, 52°C for 30 s, and 72°C for 1 min). Fluorescence was detected at the end of every 72°C extension phase. cDNA primers were designed from dsz gene sequences from DS-3 with Primer Express software (version 1.0; PE Applied Biosystems, Foster City, CA). All primer sets had a calculated annealing temperature of ≥58°C (by the nearest-neighbor method). The primer sequences for internal controls are shown in Table 2.
Western blot analysis.
Western blot analysis was done as described previously (15). Antibodies against DszA, DszB, and DszC of R. erythropolis DS-3 were raised in rabbits by subcutaneous injection of purified DszA, DszB, and DszC, respectively. The source of DszA, DszB, and DszC was recombinant E. coli BL21, which carries the recombinant plasmid pET 28a-dszA, pET 28a-dszB, or pET 28a-dszC. This work was done by Jingmei Biotechnology, Tianjin, China.
Desulfurization by resting cells.
Recombinant R. erythropolis strains were cultured in BSM with shaking for 48 h with DBT as the sole sulfur source. The resting-cell suspensions were prepared according to the method described previously (17). Samples of 25 ml of the suspension and an equal volume of n-hexadecane containing 0.5 mmol of DBT/liter were transferred into 250-ml flasks with baffles. Resting-cell reactions were performed with shaking (160 rpm) at 30°C.
Substrate and product analysis.
Before analysis, DBT and intermediates in the aqueous phase were extracted with a volume of ethyl acetate equal to that of the extracts. Products in resting-cell reaction systems were centrifuged (8,000 × g; 10 min), and the oil-phase supernatants were collected for analysis. DBT, HPBS, and 2-HBP were analyzed by high-performance liquid chromatography (HPLC) according to the method described previously (18).
RESULTS
Transcriptional characteristics of the native dsz operon.
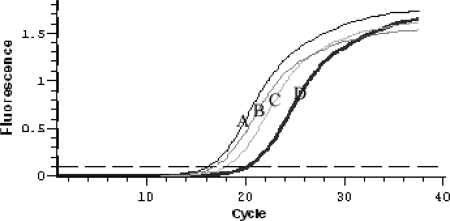
We investigated the transcriptional characteristics of the native dsz operon by analyzing the quantities of mRNA from the transcription of dszA, dszB, and dszC in cells of R. erythropolis DS-3 by a real-time quantitative PCR assay. The analysis fragment of each gene was about 800 bp. The amplification efficiencies of the primers used in real-time quantitative PCR were detected with the dsz operon by real-time PCR assays. The results indicated that each pair of primers had equivalent amplification efficiencies. The mRNA quantitative analysis revealed that the ratio of dszA, dszB, and dszC mRNAs was about 11:3.3:1, based on the threshold cycle (CT) values of each gene (Fig. 2). The results indicated that the transcription levels of the desulfurization enzyme genes decreased according to the positions of the genes in the dsz operon. This finding indicated that we could change the transcriptional levels of the dsz genes by changing their positions in the dsz operon.
FIG. 2.
Plot depicting the amplification of the native dsz operon. The plot shows the accumulation of dszA, dszB, and dszC PCR products in real-time PCR as detected by the MJ Opticon2 continuous fluorescence detection system. dszA, dszB, and dszC cDNAs were used as the template. The threshold line for the calculation of the CT was set at 0.1. A, dszA; B, internal control gene (NADH gene); C, dszB; D, dszC.
Rearrangement of the dsz operon.
The rate of desulfurization is limited by the last enzyme in the 4S pathway, DszB, due to its low expression level and low level of activity. In our previous work, we enhanced the level of expression of DszB and increased the DBT metabolic velocity by reconstructing the ribosome binding site of dszB (9). However, the DBT metabolic rate still did not reach the optimal rate because the activity of DszA was not maximal due to low substrate concentrations. In order to enhance the substrate concentration for DszA, the catalytic capabilities of DszB and DszC must be increased simultaneously. An alternative method to accomplish the same goal is increasing the specific production of DszB and DszC in the cell. In the present work, we rearranged the order of the dsz genes in the operon to generate operon dszBCA. We also reconstructed the ribosome binding site of dszB by replacing it with the 13-bp string of nucleotides that was located between dszB and dszC. The reconstructed dsz operon was generated by overlap PCR, as described in Materials and Methods. To ensure the absence of insertions or mutations, any fragment generated by PCR was ligated into the pMD 18-T simple vector for sequence analysis.
Expression characteristics of the dsz operon.
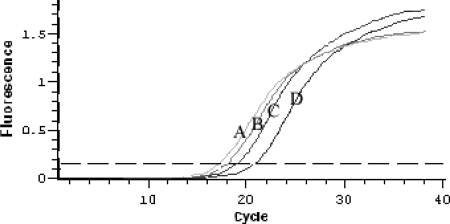
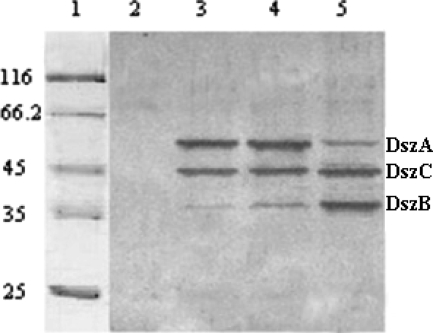
The Rhodococcus-E. coli shuttle vector was used to express the reconstructed dsz and native dsz operons in R. erythropolis CGMCC 4.1491 (dsz null). The native and reconstructed dsz operons were inserted separately into the EcoRI and XbaI sites of the pRHK1 vector to construct expression plasmids pRABC and pRBCA, respectively. These plasmids were then introduced into R. erythropolis 4.1491 by electroporation to yield R. erythropolis DRA and R. erythropolis DRB, respectively. A real-time quantitative PCR assay indicated that the quantities of dszA, dszB, and dszC mRNAs in R. erythropolis DRA were not greatly increased compared to the quantities detected in R. erythropolis DS-3. However, the quantities of dszB and dszC mRNAs in R. erythropolis DRB were considerably higher than those in DS-3, and the quantity of dszA mRNA decreased; the ratio of these mRNAs was about 1:16:5 (dszA mRNA to dszB mRNA to dszC mRNA) (Fig. 3). Western blot analysis revealed that R. erythropolis DRB produced more DszB and DszC than did R. erythropolis DRA and R. erythropolis DS-3, whereas the production of DszA in R. erythropolis DRB decreased sharply compared to that in R. erythropolis DRA and R. erythropolis DS-3 (Fig. 4).
FIG. 3.
Plot depicting the amplification of the reconstructed dsz operon. The plot shows the accumulation of dszA, dszB, and dszC PCR products in real-time PCR as detected by the MJ Opticon2 continuous fluorescence detection system. dszA, dszB, and dszC cDNAs were used as the template. The threshold line for the calculation of the CT was set at 0.15. A, dszB; B, internal control gene (NADH gene); C, dszC; D, dszA.
FIG. 4.
Western blot analysis of Dsz enzymes in cells grown with antibody specific to DszA, DszB, and DszC. Lanes: 1, marker; 2, R. erythropolis 4.1491 (dsz null); 3, R. erythropolis DS-3 (dsz+); 4, R. erythropolis DRA; and 5, R. erythropolis DRB. R. erythropolis DS-3, R. erythropolis DRA, and R. erythropolis DRB were cultured in BSM with DBT as the sole source of sulfur, and the control strain R. erythropolis 4.1491 (dsz null) was cultured in BSM with yeast extract as the sulfur source.
Desulfurization by resting cells of recombinant strains.
To test the desulfurization activities of recombinant strains R. erythropolis DRA and R. erythropolis DRB, resting cells (25 ml) were placed into a 250-ml Erlenmeyer flask, together with an equal volume of n-hexadecane containing 0.5 mmol of DBT/liter. The mixture was incubated at 30°C with shaking at 160 rpm. At different intervals, sample aliquots (1 ml) were withdrawn and quenched in an Eppendorf tube with 6 mol of HCl/liter, and the oil phase was separated by centrifugation. When the oil-phase samples were examined by HPLC, no intermediate products were detected, only DBT and 2-HBP. However, the intermediate product HPBS was detected in cell lysates of R. erythropolis DRA and R. erythropolis DS-3 and not in cell lysates of R. erythropolis DRB. The time courses for 2-HBP production in the resting-cell reaction systems catalyzed by the two recombinant strains, R. erythropolis DRA and R. erythropolis DRB, are shown in Fig. 5. As a result of the reactions, 96% of DBT (0.48 mmol of DBT/liter) was desulfurized after 3 h, and the maximum desulfurization rate was 320 μmol of DBT/g (dry weight) of cells/h. The same amount of DBT was desulfurized after 24 h by R. erythropolis DRA resting cells, and the desulfurization rate in this case was only about 26 μmol of DBT/g (dry weight) of cells/h. Thus, the maximum resting-cell desulfurization rate of R. erythropolis DRB was about 12-fold higher than that of R. erythropolis DRA.
FIG. 5.
Time course of the conversion of DBT into 2-HBP by resting cells of R. erythropolis DRA and DRB. Cells were grown to the end of the exponential phase in BSM with DBT as the sole source of sulfur, washed twice with 1.0 liter of 50-mmol/liter potassium phosphate buffer (pH 7.2), and finally resuspended in the same buffer. The suspension was portioned into 25-ml aliquots, and an equal volume of n-hexadecane containing 0.5 mmol of DBT/liter was added. At each time point, aliquots were withdrawn for analysis by HPLC, as described in Materials and Methods. ▴, R. erythropolis DRB; ▪, R. erythropolis DRA.
DISCUSSION
The microbial conversion of DBT into 2-HBP is accomplished by the 4S pathway, consisting of two monooxygenases (DszC and DszA) and one desulfinase (DszB), which are encoded by the dsz operon. Sequence and subcloning analyses revealed that the three genes, dszA, dszB, and dszC, were transcribed and expressed in the same orientation. The termination codon for dszA and the initiation codon for dszB overlap, and there is a 13-bp gap between dszB and dszC. Potential ribosome binding sites are also present upstream of each putative ATG initiation codon (13). We investigated the expression characteristics of the dsz operon in the present work. The dsz genes (dszA, dszB, and dszC) were transcribed as a polar model, and the ratio of dszA, dszB, and dszC mRNAs was about 11:3.3:1 in R. erythropolis DS-3, which carries the original dsz operon. Western blot analysis revealed that the translational levels of DszA, DszB, and DszC appeared to be controlled by the quantities of corresponding mRNAs (Fig. 4, lane 5). However, the expression level of dszB was far lower than that of dszC (Fig. 4, lanes 3 and 4). In our prior work, we have shown that the expression level of dszB is determined by two factors (9). First, due to the location of dszB in the dsz operon, the quantity of dszB mRNA is only about 30% of that of dszA mRNA because of the polar transcription of the dsz operon. Second, there is an overlapping region in the termination codon for dszA and the initiation codon for dszB. The expression level of dszB is lower than that of dszC for this reason, even though the quantity of dszC mRNA is lower than that of dszB mRNA (Fig. 2). This result indicates that there is, in fact, a baffle structure before the initiation codon of dszB.
Gray et al. did a kinetic analysis of the DBT desulfurization reactions in the 4S pathway. Their results showed that the ratio of the activities of the dsz enzymes DszA, DszB, and DszC is about 25:1:5 (4). Under conditions in which dszB expression is not baffled by the overlapping structure, the ratio of the catalytic capabilities of the dsz enzymes is about 5:275:3.3, according to an analysis of the quantities of mRNAs and the activities of the dsz enzymes. To enhance the DBT metabolic flux, the intrinsic catalytic properties or the specific production of DszB and DszC in the cell must be improved. Here, we have reported a gene-rearranging strategy that enhanced the expression levels of dszB and dszC. Real-time PCR analysis and Western blot analysis with specific antibodies for the dsz enzymes (Fig. 3 and 4) confirmed that the recombinant strain exhibited higher levels of transcription of dszB and dszC than R. erythropolis DS-3 and R. erythropolis DRA. The levels of translation of dszB and dszC were also enhanced.
Gray et al. proved that HBPS accumulates in the reaction system catalyzed by cell extracts of R. erythropolis IGTS8 (4), which carries the same dsz operon as R. erythropolis DS-3. However, we did not detect any intermediates except for DBT and 2-HBP in the cell lysate of R. erythropolis DRB, whereas HBPS was detected in the cell lysates of R. erythropolis DRA and R. erythropolis DS-3. These results indicate that there are not any intermediates accumulated in the cells of R. erythropolis DRB, which carries the rearranged dsz operon. The desulfurization experiment was conducted using resting cells with 0.5 mmol of DBT/liter in n-hexadecane as a model diesel oil. The DBT desulfurization rate of R. erythropolis DRB was about 12-fold higher than that of R. erythropolis DRA, which carries the native dsz operon. This finding indicates that the enhanced expression levels of DszC and DszB increase the metabolic rate for DBT and HBPS in the cells and contribute to the improved desulfurization rate of R. erythropolis DRB.
Function-related genes are often arranged in tandem in an operon and transcribed in polar models in prokaryotes. The Dsz enzymes have different catalytic activities. Therefore, the rearrangement of the corresponding genes according to the catalytic capabilities of the enzymes can balance these catalytic abilities and breach the metabolic bottleneck. Furthermore, the rearranged dsz operon could easily be recombined into its native organization by a double-crossover event to avoid losing the exogenous gene in the process of subculture.
Acknowledgments
This work was supported by the China Postdoctoral Science Foundation (grant no. 20060400689) and the Tianjin Natural Scientific Foundation, China (grant no. 05YFJMJC00700).
Footnotes
Published ahead of print on 28 December 2007.
REFERENCES
- 1.Coco, W. M., W. E. Levinson, M. J. Crist, H. J. Hektor, A. Darzins, P. T. Pienkos, C. H. Squires, and D. J. Monticello. 2001. DNA shuffling method for generating highly recombined genes and evolved enzymes. Nat. Biotechnol. 19:354-359. [DOI] [PubMed] [Google Scholar]
- 2.Franchi, E., F. Rodriguez, L. Serbolisca, and F. de Ferra. 2003. Vector development, isolation of new promoters and enhancement of the catalytic activity of the Dsz enzyme complex in Rhodococcus sp. strains. Oil Gas Sci. Technol. Rev. IFP 58:515-520. [Google Scholar]
- 3.Gray, K. A., G. T. Mrachko, and C. H. Squires. 2003. Biodesulfurization of fossil fuels. Curr. Opin. Microbiol. 6:229-235. [DOI] [PubMed] [Google Scholar]
- 4.Gray, K. A., O. S. Pogrebinsky, G. T. Mrachko, L. Xi, D. J. Monticello, and C. H. Squires. 1996. Molecular mechanisms of biocatalytic desulfurization of fossil fuels. Nat. Biotechnol. 16:1705-1709. [DOI] [PubMed] [Google Scholar]
- 5.Gupta, N., P. K. Roychoudhury, and J. K. Deb. 2005. Biotechnology of desulfurization of diesel: prospects and challenges. Appl. Microbiol. Biotechnol. 66:356-366. [DOI] [PubMed] [Google Scholar]
- 6.Hirasawa, K., Y. Ishii, M. Kobayashi, K. Koizumi, and K. Maruhashi. 2001. Improvement of desulfurization activity in Rhodococcus erythropolis KA2-5-1 by genetic engineering. Biosci. Biotechnol. Biochem. 65:239-246. [DOI] [PubMed] [Google Scholar]
- 7.Kilbane, J. J., II. 2006. Microbial biocatalyst developments to upgrade fossil fuels. Curr. Opin. Biotechnol. 17:305-314. [DOI] [PubMed] [Google Scholar]
- 8.Konishi, M., M. Kishimoto, T. Omasa, Y. Katakura, S. Shioya, and H. Ohtake. 2005. Effect of sulfur sources on specific desulfurization activity of Rhodococcus erythropolis KA2-5-1 in exponential fed-batch culture. J. Biosci. Bioeng. 99:259-263. [DOI] [PubMed] [Google Scholar]
- 9.Li, G. Q., T. Ma, S. S. Li, H. Li, F. L. Liang, and R. L. Liu. 2007. Improvement of dibenzothiophene desulfurization activity by removing the gene overlap in the dsz operon. Biosci. Biotechnol. Biochem. 71:849-854. [DOI] [PubMed] [Google Scholar]
- 10.Michalski, M. L., and G. J. Weil. 1999. Gender-specific gene expression in Brugia malayi. Mol. Biochem. Parasitol. 104:247-257. [DOI] [PubMed] [Google Scholar]
- 11.Monticello, D. J. 2000. Biodesulfurization and the upgrading of petroleum distillates. Curr. Opin. Biotechnol. 11:540-546. [DOI] [PubMed] [Google Scholar]
- 12.Noda, K., K. Watanabe, and K. Maruhashi. 2003. Cloning of a rhodococcal promoter using a transposon for dibenzothiophene biodesulfurization. Biotechnol. Lett. 25:1875-1882. [DOI] [PubMed] [Google Scholar]
- 13.Piddington, C. S., B. R. Kovacevich, and J. Rambosek. 1995. Sequence and molecular characterization of a DNA region encoding the dibenzothiophene desulfurization operon of Rhodococcus sp. strain IGTS8. Appl. Environ. Microbiol. 61:468-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reichmuth, D. S., H. W. Blanch, and J. D. Keasling. 2004. Dibenzothiophene biodesulfurization pathway improvement using diagnostic GFP fusions. Biotechnol. Bioeng. 88:94-99. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka, Y., O. Yoshikawa, K. Maruhashi, and R. Kurane. 2002. The cbs mutant strain of Rhodococcus erythropolis KA2-5-1 expresses high levels of Dsz enzymes in the presence of sulfate. Arch. Microbiol. 178:351-357. [DOI] [PubMed] [Google Scholar]
- 16.Ting, M., L. Jian, T. Mingyou, Z. Xinping, and L. Rulin. 2002. Desulfurization of dibenthiophene by Rhodococcus sp. DS-3. Acta Microbiol. Sin. 42:126-131. [Google Scholar]
- 17.Watanabe, K., K. I. Noda, and K. Maruhashi. 2003. Enhanced desulfurization in a transposon-mutant strain of Rhodococcus erythropolis. Biotechnol. Lett. 25:1299-1304. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe, K., K. I. Noda, J. Konishi, and K. Maruhashi. 2003. Desulfurization of 2,4,6,8-tetraethyl dibenzothiophene by recombinant Mycobacterium sp. strain MR65. Biotechnol. Lett. 25:1451-1456. [DOI] [PubMed] [Google Scholar]