Abstract
Novel thermophilic crenarchaea have been observed in Fe(III) oxide microbial mats of Yellowstone National Park (YNP); however, no definitive work has identified specific microorganisms responsible for the oxidation of Fe(II). The objectives of the current study were to isolate and characterize an Fe(II)-oxidizing member of the Sulfolobales observed in previous 16S rRNA gene surveys and to determine the abundance and distribution of close relatives of this organism in acidic geothermal springs containing high concentrations of dissolved Fe(II). Here we report the isolation and characterization of the novel, Fe(II)-oxidizing, thermophilic, acidophilic organism Metallosphaera sp. strain MK1 obtained from a well-characterized acid-sulfate-chloride geothermal spring in Norris Geyser Basin, YNP. Full-length 16S rRNA gene sequence analysis revealed that strain MK1 exhibits only 94.9 to 96.1% sequence similarity to other known Metallosphaera spp. and less than 89.1% similarity to known Sulfolobus spp. Strain MK1 is a facultative chemolithoautotroph with an optimum pH range of 2.0 to 3.0 and an optimum temperature range of 65 to 75°C. Strain MK1 grows optimally on pyrite or Fe(II) sorbed onto ferrihydrite, exhibiting doubling times between 10 and 11 h under aerobic conditions (65°C). The distribution and relative abundance of MK1-like 16S rRNA gene sequences in 14 acidic geothermal springs containing Fe(III) oxide microbial mats were evaluated. Highly related MK1-like 16S rRNA gene sequences (>99% sequence similarity) were consistently observed in Fe(III) oxide mats at temperatures ranging from 55 to 80°C. Quantitative PCR using Metallosphaera-specific primers confirmed that organisms highly similar to strain MK1 comprised up to 40% of the total archaeal community at selected sites. The broad distribution of highly related MK1-like 16S rRNA gene sequences in acidic Fe(III) oxide microbial mats is consistent with the observed characteristics and growth optima of Metallosphaera-like strain MK1 and emphasizes the importance of this newly described taxon in Fe(II) chemolithotrophy in acidic high-temperature environments of YNP.
Thermophilic, acidophilic chemolithoautotrophs are thought to be important microorganisms in the evolutionary history of Earth (37). Their ability to thrive in high-temperature environments with minimal requirements other than carbon dioxide and inorganic constituents suggests that these organisms are important primary producers in extreme environments. Acid-tolerant thermophiles have been found in a variety of geothermal springs, mud pots, and deep-sea vents (10, 25, 29, 37). Many of these organisms utilize reduced inorganic compounds, such as hydrogen, sulfide, elemental sulfur, thiosulfate, methane, or ferrous iron, as energy sources for metabolism (2, 7). Although the 16S rRNA gene sequences detected in extreme thermophilic habitats (13, 15, 16, 31) provide important clues regarding microbial processes in situ, the phylogenetic similarity to cultivated organisms is not sufficient to confirm the physiologies of these novel microorganisms. Cultivation efforts, therefore, are of critical importance for implicating specific microorganisms in biogeochemical processes occurring in situ and for interpreting the role of these populations in community ecology.
Yellowstone National Park (YNP) is a pristine location for studying thermophilic acid-tolerant microorganisms and their role in mediating geochemical processes. Recent studies have provided a foundation for understanding potential linkages between specific microbial populations and oxidation-reduction reactions controlling the behavior of As, S, and Fe in acid-sulfate-chloride (ASC) springs of YNP (14, 15, 23, 24). Previous characterization of aqueous and solid-phase geochemistry combined with analysis of 16S rRNA gene sequence distribution has provided data supporting the hypothesis that microbial oxidation of Fe(II) results in the formation of extensive ferric oxide and/or jarositic mats in acidic springs of YNP.
Hydrous ferric oxide (HFO) microbial mats are found in numerous environments, including geothermal springs, hydrothermal vents, wetland seeps, and acid mine drainage (3, 5, 14, 19, 24). The mechanism of Fe(II) oxidation may include both chemical and biological contributions (21), but it is widely recognized that the rate of aqueous Fe(II) oxidation is quite low at pH values less than 4 (26, 35) and that acidophilic microorganisms are often important in mediating the oxidation of Fe(II) in these environments. The HFO phases found in ASC springs (pH ∼3) of Norris Geyser Basin, YNP, form as a result of microbial processes, and oxide phases often occur as 1- to 2-μm-thick encrustations around rod-shaped and filamentous microorganisms (14, 24). Several novel microorganisms (based on 16S rRNA gene phylogeny) have been identified in these HFO mats and may contribute to Fe(II) oxidation and subsequent formation of arsenate-rich HFO phases (14, 24). These organisms are represented by bacterial 16S rRNA gene sequences related to Hydrogenobaculum acidophilum (98 to 99% similarity), Thermaerobacter subterraneus (91%), Acidimicrobium ferrooxidans (98%), and Meiothermus silvanus (92%), as well as archaeal sequences related to Metallosphaera prunae (96%), Sulfolobus solfataricus (97%), Vulcanisaeta distributa (98%), and Sulfolobus islandicus (99%). Additional 16S rRNA gene sequences less than 90% similar to known cultivated organisms have also been consistently observed in these environments (e.g., sequences highly similar to clones YNPFFA85 [99%], YNPFFA108 [99%], SK305 [99%], and YNPFFA23 [99%]) (14). However, there is no direct evidence supporting the potential role of these organisms in Fe cycling in geothermal systems.
Efforts to cultivate relevant Fe(II)-oxidizing microorganisms from acidic geothermal springs in YNP have been limited, yet the metabolism of these thermophiles is responsible for an array of orange, red, and maroon Fe(III) oxide phases in numerous geothermal outflow channels of YNP (see Fig. S1 in the supplemental material). Previous 16S rRNA gene sequence characterization of acidic Fe mats in Norris Geyser Basin suggested that Metallosphaera-like organisms are important in the oxidation of Fe(II) and the subsequent formation of copious amounts of Fe(III) oxides within geothermal outflow channels (14, 24). There are currently four characterized Metallosphaera spp., all of which were isolated from high-temperature acidic environments (9, 11, 22, 39). In most cases, these chemolithoautotrophs are capable of growth on sulfidic ores and/or elemental sulfur. However, the importance of Fe(II) as an electron donor in this understudied genus is not clear. Peeples and Kelly (28) have shown that M. prunae may oxidize Fe(II) as a sole energy source, and several studies have noted that Metallosphaera spp. are effective in accelerating the dissolution of pyrite and the release of metals in biomining applications (27, 30, 32).
Given the potential importance of Metallosphaera-like organisms in geothermal environments and their possible utility in controlled bioleaching applications, the objectives of the current study were to (i) isolate and characterize an Fe(II)-oxidizing Metallosphaera-like organism from acidic Fe mats in YNP and (ii) determine the abundance and distribution of similar Metallosphaera-like 16S rRNA gene sequences in a variety of different Fe(III) depositional mats common in acidic geothermal springs of YNP.
MATERIALS AND METHODS
Initial isolation protocols.
Approximately 2 g of an HFO microbial mat from the outflow channel of an ASC geothermal spring was placed in a sealed 60-ml serum bottle, which was filled with native spring water (62 to 63°C). The site is unofficially named Beowulf Spring and is located in the One Hundred Springs Plain of Norris Geyser Basin, YNP (44°43′53.4″N, 110°42′40.9″W; YNP Thermal Inventory NHSP35). After 4 h, 1 ml of the slurry was transferred into each of three 20-ml serum bottles containing 15 ml of filtered (pore size, 0.2 μm) spring water, 2% (wt/wt) pyrite-enriched mine tailings, and a 5-ml gas headspace composed of 25% O2, 50% CO2, and 25% air. The pyrite-enriched mine tailings were obtained from a previous study using density separation techniques and contained ∼50% pyrite (18). The cultures were incubated at 65°C for 10 days and then subjected to several rounds of dilution to extinction. The progress of all cultures was monitored by extracting DNA from the serum bottles, followed by PCR amplification of 16S rRNA genes using universal bacterial and archaeal primers (14, 24) and separation and visualization of the PCR products using denaturing gradient gel electrophoresis (DGGE) (24). The purity of cultures was also confirmed by cloning and sequencing of longer 16S rRNA gene fragments (see below). Pure cultures from serum bottles were centrifuged at 1,000 × g for 10 min and preserved in a 25% glycerol-75% synthetic growth medium. The glycerol stocks were stored at −80°C after they were frozen in liquid nitrogen.
Growth media, conditions, and substrates.
Pure cultures were maintained as described above, but spring water was replaced by a synthetic medium designed so that its aqueous geochemistry matched the spring water aqueous geochemistry. The synthetic growth medium contained 11 mM Na+, 10.1 mM Cl−, 1.5 mM K+, 1.2 mM SO42−, 0.7 mM H3BO3, 0.4 mM NO3−, 0.25 mM Ca2+, 0.1 mM PO43−, 0.2 mM Al3+, 0.1 mM NH4+, 0.1 mM H4SiO4, and 0.1 mM Mg2+. Unless stated otherwise, 20 mM FeSO4 (pH 2.5), vitamins, and trace metals (12) were added to the growth medium, and the pH was adjusted to 2.5 to 3.0 with H2SO4, followed by filter sterilization (pore size, 0.2 μm). Medium specific for Metallosphaera sedula (11) containing 0.1% yeast extract was also used to check for growth enhancement on pyrite. Chemolithotrophic growth of the isolate was evaluated in 20-ml serum bottles containing 15 ml synthetic medium using a variety of possible electron donors, including 0.5 and 10 mM sodium tetrathionate and 1 and 20 mM Fe(II)SO4 (pH 2.5), as well as different solid phases, including elemental S0, Fe(II) sorbed onto ferrihydrite, and various sulfide minerals (Ward's Natural Science, Rochester, NY). Research-grade pyrite (FeS2), chalcopyrite (FeCuS), sphalerite (ZnS), and covellite (CuS) were ground with an agate mortar and pestle, separated by sieving, and sonified in acetone for 15 min to remove fine particles created during grinding. The final mineral fractions were autoclaved twice at 132°C prior to use in growth experiments. Two-line ferrihydrite was synthesized using the method outlined by Schwertmann and Cornell (34) and then washed three times with 0.05 M FeSO4 for 30 min in the dark and filtered (pore size, 0.2 μm). This substrate, containing Fe(II) sorbed to ferrihydrite, is referred to below as Fe(II) ferrihydrite. The amount of the solid phase added to each of the serum bottles was 0.5 g of the <0.15-μm size fraction. Anaerobic growth was tested with pyrite and Fe(II) sorbed to ferrihydrite using 10 mM NO3− as the electron acceptor. Anaerobic growth was also tested in the presence of elemental sulfur and ferrihydrite. All anaerobic samples were purged for 30 min with N2 gas, and anaerobic conditions were verified using 1 mg/liter resazurin.
Detailed growth curves were obtained under several geochemical conditions, including (i) pyrite and synthetic medium, (ii) pyrite with 0.005% yeast extract, (iii) Fe(II) sorbed to ferrihydrite with 0.005% yeast extract, and (iv) elemental sulfur (S0) with 0.005% yeast extract. Temperature and pH optima were determined in serum bottles containing pyrite and synthetic medium using 1 ml of a diluted inoculum (500 to 1,000 cells/ml). Cultures were grown in a solid Al block incubator at 15 temperatures ranging from 40 to 90°C. The pH was adjusted by addition of either HCl or NaOH, and the isolate was cultivated at pH 0.75, 1.0, 2.0, 2.5, 3.0, 3.5, 4.5, 5.0, 5.5, and 6.0. The pH of the growth medium was determined daily and adjusted with acid or base when necessary. Growth was quantified after 11 days using total DNA extracted from serum bottles in triplicate for each treatment after filtration of the contents onto 0.22-μm polycarbonate filters (FastDNA SPIN kit for soil; Q-Biogene, Irvine, CA). DNA was measured with a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE) after a blank consisting of a noninoculated pyrite control sample was examined. Additional pyrite-containing serum bottles were spiked with 100 ng/μl DNA (100-bp DNA ladder; catalog no. G2101; Promega) to evaluate the extraction efficiency. Cell growth for selected treatments was also quantified directly using 0.2-ml samples treated with 4′,6′-diamidino-2-phenylindole (DAPI) and counted in a Hausser Scientific counting chamber (Horsham, PA) using a Zeiss epifluorescence microscope (Zeiss Axioskop 2 plus; Zeiss, Oberkochen, Germany).
Chemical analyses.
Concentrations of SO42−, S2O32−, NO3−, and PO43− in serum bottles were determined as a function of time using anion chromatography (model DX500 with an AS-16 4-mm column; Dionex Corp., Sunnyvale, CA). Total soluble Fe and Fe(II) contents were measured using the ferrozine method (40).
Electron microscopy.
Cultures were filtered through 0.22-μm polycarbonate filters after incubation for 10 days, and the resulting pyrite suspensions were immediately fixed with 3% glutaraldehyde. Samples were then imbedded in 2% Noble agar, refixed in glutaraldehyde, dehydrated in an ethanol series, and treated with propylene oxide before infiltration with Spurr's epoxy resin (36). Thin sections were placed on 300-mesh copper grids and analyzed by transmission electron microscopy using a LEO 912AB (LEO Electron Microscopy Inc., Thornwood, NY) equipped with an in-column OMEGA-type imaging spectrometer and controlled with Soft Imaging System software (Lakewood, CO).
Characterization of geothermal sites in YNP.
Numerous acidic Fe mats from three geothermal basins in YNP (Norris Geyser Basin, Rainbow Springs, and Joseph's Coat Springs) were sampled and used for aqueous and solid-phase geochemical analysis and for molecular characterization (16S rRNA gene sequencing). The following 14 distinct springs were analyzed in detail (see Fig. S1 and Table S1 in the supplemental material): nine sites in Norris Geyser Basin, including sites NGB-BE (unofficially referred to as Beowulf-East), NGB-BW (Beowulf-West), NGB-DS (unofficially referred to as Dragon Spring), NGB-OSP (unnamed feature in the One Hundred Spring Plain), NGB-WG (Whirlygig Geyser), NGB-TC (unnamed spring along Tantalus Creek), NGB-EG (Echinus Geyser), NGB-PB (unnamed spring in Porcelain Basin), and NGB-GAP (unnamed spring west of Ragged Hills); two unnamed springs at Joseph's Coat Springs (sites JC1 and JC2); and three unnamed springs at Rainbow Springs (sites RS1, RS2, and RS3). Samples were obtained from each spring at multiple locations along a transect down the primary flow channel, including both S0 and Fe oxide depositional zones. A thorough geochemical characterization of spring water samples obtained directly above the Fe microbial mats was conducted for all sites (see Table S1 in the supplemental material).
DNA extraction and 16S rRNA gene sequencing.
The distribution and relative abundance of Metallosphaera-like 16S rRNA gene sequences were examined for all 14 geothermal sites described above. In the majority of cases, the sites were sampled several times over a 3-year period (2003 to 2005). Microbial mat samples were obtained using sterile tools and collection tubes and were immediately placed on dry ice for transport to a −80°C freezer. Total DNA was extracted from the samples (or pure cultures) using a FastDNA SPIN kit for soil (Q-Biogene, Irvine, CA). The nearly full-length PCR primers targeting 16S rRNA genes were the Bacteria-specific primer Bac8f (5′-AGAGTTTGATCCTGGCTCAG-3′) coupled with the universal primer Univ1392r (5′-ACGGGCGGTGTGTAC-3′) and the Archaea-specific primer Arc2f (5′-TTCCGGTTGATCCYGCCGGA-3′) also coupled with the universal primer Univ1392r. Briefly, each 50-μl PCR mixture contained 10 mM Tris-HCl (pH 8), 50 mM KCl, 0.1% Triton X-100, 4.0 mM MgCl2, each deoxynucleoside triphosphate at a concentration of 800 μM, 0.5 μl of each primer, 1.25 U of Taq DNA polymerase (Promega, Madison, WI), and 1 to 5 μl of template DNA (2 to 20 ng). The thermal cycler protocol was 94°C for 6 min, 25 to 35 cycles of 94°C for 45 s, 54°C for 45 s, and 72°C for 110 s, and a final 7-min extension at 72°C. Negative control reactions (no template) were routinely performed to ensure purity. The purified PCR products were cloned using the pGEM-T vector system from Promega Corp. (Madison, WI), and the inserts were amplified and sequenced (TGEN, Phoenix, AZ, and the Ohio State Plant Genomics Facility, Columbus, OH).
Isolate-specific 16S rRNA primer.
An 18-bp primer specific for MK1-like organisms was designed around 16S rRNA class I and II sites (4) of strain MK1, as well as highly related (>99% similarity) sequences from Fe mats described in this study. The primer (5′-AGAAGGACTGCCGGTGTT-3′) had no homology to 16S rRNA gene sequences of other organisms and was analyzed further using IDT ScitTools OligoAnalyzer 3.0 (Integrated DNA Technologies, Coralville, IA) to determine possible hairpins, dimers, and the melting temperature. The MK1 primer was used as a reverse primer with the Archaea-specific primer Arc2f (total fragment length, ∼1,000 bp) to verify and test cultures and spring locations for MK1-like sequences. The PCR conditions were the same as those described above except that the annealing temperature was 55°C and there was 90 s of extension at 72°C. Control reactions with no template were routinely performed to ensure purity. The specificity of the primer set was tested and confirmed by sequencing 25 clones after amplification of DNA extracts from ASC springs (NGB-BE, NGB-DS, and NGB-OSP).
Quantitative PCR and fluorescent in situ hybridization.
Quantitative PCR analysis was performed with a Rotor-Gene RG-3000 (Corbett Life Science, Sydney, Australia). The standards used for the calibration curve (e.g., initial DNA concentration versus cycle threshold number) were created using pooled DNA extracts from subject Fe mats from ASC springs. Extracted DNA was amplified with each of the following primer sets using the PCR protocol described above: Arch931f/Univ1392r, Bac1070f/Univ1392r, and MET-MK1/Univ1392r. The resultant ∼300- to 430-bp products (the product size depended on the primer set) were purified with a QIAquick PCR cleanup kit (Qiagen, Valencia, CA) and were quantified using UV spectroscopy (NanoDrop ND-1000) and/or agarose gel electrophoresis. Standards for each primer set were then serially diluted with master mixture to create six quantitative PCR working standards ranging from 1:103 to 1:108. The master reaction mixture was prepared as described above with addition of 1× SYBR green I dye (Applied Biosystems, Foster City, CA). Environmental samples were obtained from a 55°C Beowulf HFO mat, a 62.5°C Beowulf HFO mat, and a 55°C Dragon Spring HFO mat, and the DNA was extracted as described above. Extracted DNA was diluted 1:10 with the master mixture and analyzed along with the standards, a blank and a positive control consisting of DNA extracted from strain MK1.
The MK1 primer sequence (described above) was labeled with a 5′ Cy5 fluorescent tag by Integrated DNA Technologies (Coralville, IA), and probe hybridization was performed as described by Amann et al. (1). Fluorescent images were obtained using a Zeiss epifluorescence microscope (Axioskop 2 plus; Zeiss, Oberkochen, Germany) and a fluorescein isothiocyanate filter.
Nucleotide sequence accession numbers.
Eighty-six Metallosphaera-like 16S rRNA gene sequences (SK, EM, and MK series) from Fe(III) oxide mats in YNP have been deposited in the GenBank database (see Table S2 in the supplemental material for accession numbers and locations of the environmental clones). The nearly full-length 16S rRNA gene sequence of Metallosphaera sp. strain MK1 has been deposited in the GenBank database under accession no. DQ350777.
RESULTS
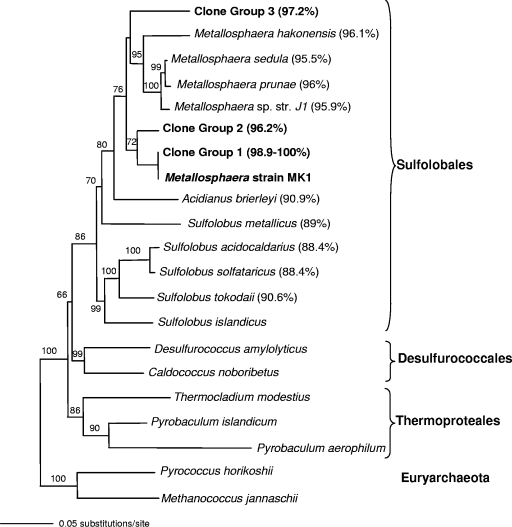
Initial enrichment cultures prepared using an Fe(III) oxide microbial mat inoculum contained three or four thermophilic populations based on DGGE analysis of culture complexity. Subsequent dilution to extinction resulted in isolation of a pure culture, as determined using both DGGE analysis and full-length 16S rRNA gene sequence analysis with universal archaeal and bacterial primer sets. Sequencing results verified that the closest cultivated relatives of the pure culture obtained with this isolation protocol are Metallosphaera spp. in the order Sulfolobales (Fig. 1). The 16S rRNA gene sequence (1,331-bp fragment) of this organism is only 95 to 96% similar to sequences of other Metallosphaera spp. and is less than 89.1% similar to sequences of other Sulfolobus spp. Although this suggests that the strain may be classified as a member of a new genus within the Sulfolobales, we designated this new taxon as Metallosphaera sp. strain MK1.
FIG. 1.
Phylogenetic (16S rRNA gene) tree showing the positions of Metallosphaera sp. strain MK1 described in the current study and three Metallosphaera-like clone groups obtained from 14 different acidic geothermal sites in YNP. A total of 421 Metallosphaera-like clones were in clone groups 1 to 3; however, the majority of these clones (415 clones) were in group 1 and were >98.9% similar to strain MK1. The values in parentheses are the levels of 16S rRNA gene sequence similarity to Metallosphaera sp. strain MK1. The tree is a PAUP neighbor-joining tree constructed with 1,000 bootstraps, using Aquifex pyrophilus as the outgroup.
Growth optima and electron donors.
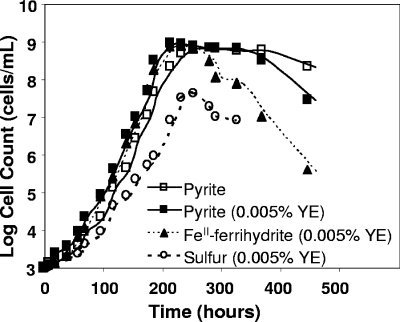
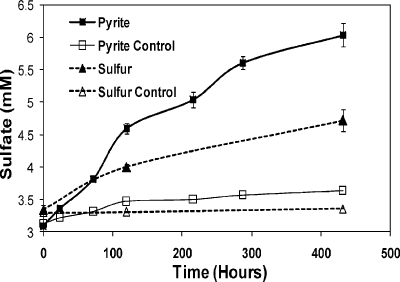
The growth of strain MK1 was evaluated in the presence of pyrite, S0, and Fe(II) ferrihydrite. No significant statistical differences (α = 0.05) in the growth rates were noted in treatments containing pyrite, pyrite with 0.005% yeast extract, or Fe(II) ferrihydrite (Fig. 2), and under these conditions maximum growth rates (2.1 × 107 ± 0.5 × 107 cells ml−1 h−1) were observed either in the presence of pyrite or in the presence of Fe(II) ferrihydrite. The maximum growth rates observed at 65°C corresponded to doubling times of 10.3 ± 0.5 and 11.4 ± 0.5 h (means ± standard errors) in the presence of Fe(II) ferrihydrite and pyrite, respectively. Strain MK1 did not exhibit measurable growth in the presence of 20 mM ferrous sulfate, suggesting that sorbed Fe(II) or the presence of a mineral substrate is an important prerequisite for the growth of this organism. This organism is also capable of growth on elemental S0 (Fig. 2), albeit at a reduced rate (doubling times, 16 ± 2 h) compared to treatments containing Fe(II). The amount of sulfate produced (Fig. 3) during growth in the presence of pyrite (∼3 mM) or elemental sulfur (∼1.3 mM) was consistent with the differential growth rates on these substrates and confirmed that strain MK1 is capable of oxidizing reduced forms of S.
FIG. 2.
Growth curves (log10) of Metallosphaera sp. strain MK1 on specimen-grade pyrite (with and without added yeast extract), Fe(II) sorbed to ferrihydrite, and elemental S. The maximum growth rates in the presence of pyrite or Fe(II) ferrihydrite were 2.1 × 107 ± 0.5 × 107 cells ml−1 h−1. YE, yeast extract.
FIG. 3.
Production of sulfate during growth of Metallosphaera sp. strain MK1 in the presence of pyrite and elemental S0 (pH 3, 65°C, 0.005% yeast extract). The growth medium contained aqueous sulfate at an initial concentration of 3 mM, and controls revealed that there was little change in sulfate in the absence of microbial growth. The error bars indicate standard errors (n = 3).
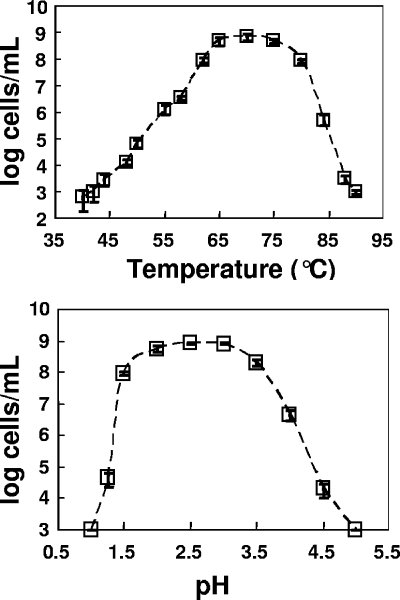
The optimal pH and temperature for growth of strain MK1 were determined in the presence of 2% pyrite. This organism exhibited optimal growth at pH 2 to 3 and at 65 to 75°C (Fig. 4); however, it could survive over a reasonably broad temperature range (45 to 85°C). The pH and temperature optima for strain MK1 are similar to the geothermal site conditions (pH 3.1, 65°C) of the Fe mat used as the original inoculum.
FIG. 4.
Temperature and pH growth optima of Metallosphaera sp. strain MK1 grown on pyrite plus 0.005% yeast extract. The temperature experiment was performed at a constant pH, pH 3, while the pH experiment was performed at a constant temperature, 65°C. Maximum growth rates were observed at pH 2.5 and 75°C. The error bars indicate standard errors (n = 3) but are generally within the symbols; the y axis is a log10 axis.
Autotrophic growth was also significant in the presence of pyrite-enriched mine tailings, pyrrhotite (FeS), and chalcopyrite (CuFeS2), as determined by evaluating DNA yields after 11 days of growth (Table 1). Growth was modest on S0 plus 0.005% yeast extract. Interestingly, growth on S0 was enhanced considerably after the addition of 10 mM Fe(II)SO4, despite the fact that strain MK1 did not grow in media containing 20 mM aqueous Fe(II)SO4. Poor or modest growth was observed on other sulfide minerals, including sphalerite (ZnS) and covellite (CuS); however, no growth was observed on aqueous sulfide, thiosulfate, or tetrathionate or on the reduced Fe minerals magnetite (Fe3O4) and siderite (FeCO3). Anaerobic growth was not detected using S0 as an electron donor and ferric iron (as ferrihydrite) as an electron acceptor. In addition, no growth was observed on pyrite, Fe(II) ferrihydrite, or S0 under anaerobic conditions when NO3− was present as a potential electron acceptor.
TABLE 1.
Growth of Metallosphaera sp. strain MK1 in the presence of different minerals, substrates, or aqueous electron donors (pH 3, 65°C)a
| Culture growth conditions | Growth | Peak amt of DNA (ng) (SE) |
|---|---|---|
| Pyrite-enriched mine tailings | +++ | 3,610 (255) |
| Fe(II) sorbed to ferrihydrite | +++ | 3,277 (53) |
| Pyrite (FeS2)+ yeast extract (DSMZ 485 medium) | +++ | 3,244 (110) |
| Pyrrhotite (FeS) | +++ | 2,779 (280) |
| Pyrite (FeS2) + 20 mM Fe(II) | +++ | 2,592 (60) |
| Elemental sulfur + 20 mM Fe(II) | +++ | 2,576 (313) |
| Chalcopyrite (CuFeS2) | ++ | 2,140 (95) |
| Pyrite (FeS2) + 20 mM Fe(II) | ++ | 1,807 (58) |
| Elemental sulfur + 0.02% yeast extract | ++ | 1,609 (133) |
| Covellite (CuS) | + | 1,000 (92) |
| Sphalerite (ZnS) | + | 335 (54) |
| Siderite (FeCO3) | − | NDb |
| Magnetite (Fe3O4) | − | ND |
| FeSO4 (20 mM) | − | NDc |
| Tetrathionate (20 mM) | − | ND |
| Na2S (5 μM) | − | ND |
| Yeast extract (0.02%) in synthetic growth medium | − | ND |
| Anaerobic (sulfur/ferrihydrite) | − | ND |
| Anaerobic (pyrite/10 mM NO3−) | − | ND |
| Anaerobic [Fe(II) ferrihydrite/10 mM NO3−) | − | ND |
Growth was assessed visually based on oxidation products (+++, substantial Fe(III) oxide production; −, no growth) and quantitatively after 11 days using total extractable DNA from 20-ml growth vessels (n = 3).
ND, not detected.
Abiotic oxidation of aqueous Fe(II) for >2 weeks resulted in the presence of Fe(III) oxide plus aqueous Fe(II), which could support growth of strain MK1.
Isolate morphology and surface association.
Samples from actively growing pyrite cultures were examined using transmission electron microscopy. Cells of strain MK1 have lobed coccus morphology (diameter, 0.9 to 1.2 μm), as well as a plasma membrane and an S layer approximately 50 nm thick, both of which are typical of other members of the Sulfolobales (6, 17, 41) (see Fig. S2 in the supplemental material). Optical, DAPI, fluorescent in situ hybridization, and transmission electron microscopy images also confirmed that strain MK1 is closely associated with pyrite and/or Fe(III) oxide particles formed as a result of Fe(II) oxidation (see Fig. S2 in the supplemental material).
Distribution of Metallosphaera-like 16S rRNA gene sequences.
A total of 883 full-length 16S rRNA gene sequences from 14 different geothermal Fe mat samples were characterized, and 419 of these sequences (∼46% of the total archaeal clones) were highly related (∼99 to 100%) to Metallosphaera sp. strain MK1. The majority of Metallosphaera-like clones (404 clones) were highly related to one another (>99% similarity) and were represented in the phylogenetic tree as a single entry designated group 1 clones (Fig. 1). This phylogenetic group also contained the 16S rRNA gene sequence of Metallosphaera sp. strain MK1, which exhibited 98.9 to 100% sequence similarity to group 1 clones (Fig. 1). Two additional Metallosphaera-like clone groups (groups 2 and 3) were identified in this study, and each group contained two entries from sites in Norris Geyser Basin (sites NGB-WG, NGB-OSP, and NGB-EG). Compared to group 1 clones, these less frequent clones were more distantly related to strain MK1, with similarity values ranging from 96 to 97% (Fig. 1). All Metallosphaera-like 16S rRNA gene sequences from these sites were less than 96% similar to previously cultured Metallosphaera spp. Finally, based on the 14 geothermal sites investigated in the current study, there was no obvious separation or grouping of Metallosphaera-like 16S rRNA sequences by location. For example, group 1 contained representatives from all 14 sites.
Semiquantitative estimates of numerical relevance.
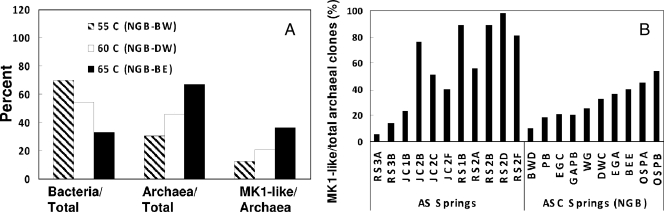
Quantitative PCR analysis was performed with universal bacterial, universal archaeal, and Metallosphaera sp. strain MK1-specific 16S rRNA gene primer sets for three Fe mats from Norris Geyser Basin (sites NGB-BEE, NGB-BWD, and NGB-DC). The results indicate that sequences highly similar to Metallosphaera sp. strain MK1 represented a significant proportion of not only the archaeal species in ASC springs but also the total microbial diversity (Fig. 5A). For example, between 13 and 36% of the total amplifiable archaeal sequences could be attributed to Metallosphaera sp. strain MK1-like sequences in ASC springs. Interestingly, the results showed that there was a decrease in Metallosphaera sp. strain MK1-like sequences from about 36% of total archaeal sequences at 65°C (site NGB-BEE) to 12% at 55°C (site NGB-BWD). Previous work on these springs had suggested that Metallosphaera-like sequences were more common at temperatures above 60°C (14, 24), and this observation is consistent with the temperature optima of strain MK1 (range, 65 to 75°C). In fact, HFO mats that formed at temperatures above 80°C, such as those found at the source of site NGB-GAP (85°C), did not contain clones related to Metallosphaera spp., further suggesting that strain MK1 is ecologically constrained to a temperature range of approximately 55 to 75°C.
FIG. 5.
Distribution and abundance of Metallosphaera-like 16S rRNA gene sequences in acid-sulfate (AS) and ASC geothermal springs in YNP. (A) Quantitative PCR results for three ASC springs, indicating the percentages of bacterial and archaeal 16S rRNA genes relative to the total amount of 16S rRNA genes and the percentage of Metallosphaera sp. strain MK1-like 16S rRNA genes relative to the total amount of archaeal 16S rRNA genes. (B) Relative abundance of Metallosphaera-like clones (total, 421 clones) expressed as a percentage of all of the archaeal clones (883 clones) for different sites.
Estimates of the relative importance of MK1-like organisms obtained from quantitative PCR agreed favorably with the relative abundance of Metallosphaera-like sequences identified in clone libraries from these three sites (ASC springs) (Fig. 5B). Further, environmental clones highly similar to strain MK1 were also obtained from acid-sulfate springs at Rainbow Springs (sites RS1 and RS2) and Joseph's Coat Springs (sites JC1 and JC2) (Fig. 5B). These geothermal systems contain 1 to 2 mM NH4+, 5 to 8 mM SO42−, and very low concentrations of Cl− (see Table S1 in the supplemental material) (www.rcn.montana.edu), which is quite different than the predominant cations and anions in springs of Norris Geyser Basin (e.g., Na+ and Cl−). Despite these differences, the high temperature (65 to 75°C), low pH (pH 2.5 to 2.6), and high ferrous Fe content (∼100 μM) of the Rainbow Springs and Joseph's Coat Springs sites appear to be optimum for Metallosphaera sp. MK1-like organisms. In these habitats, MK1-like clones often dominated the archaeal clone libraries, accounting for 24 to 98% of the sequences detected at the sites.
DISCUSSION
Two primary niche environments for Sulfolobales have been proposed: (i) aerobic geothermal springs and acidic soils usually having ferric Fe deposits and (ii) anaerobic habitats containing sulfide minerals (37). Strain MK1 exhibits growth and morphological characteristics that are generally similar to those of other members of the Sulfolobales; however, strain MK1 is capable of growth using Fe(II) sorbed to ferrihydrite as a sole electron donor in the absence of sulfide minerals, dissolved sulfide, or elemental S. No measurable growth was observed on aqueous ferrous sulfate. Although several Sulfolobus-like 16S rRNA gene sequences were observed in Fe(III) mats of YNP acidic springs, sequences related to Metallosphaera sp. strain MK1 were considerably more abundant. Based on the distribution patterns of MK1-like 16S rRNA gene sequences, as well as the characteristics of strain MK1, these organisms prefer Fe(II) over reduced forms of S and represent an important taxon responsible for Fe(II) chemolithotrophy and biomineralization of Fe(III) solid phases in geothermal systems of YNP (12, 38). This is an important finding because numerous low-pH, high-ferrous Fe geothermal habitats exist in YNP and elsewhere, which do not contain sufficient sulfide or elemental S to support robust growth of S-oxidizing Sulfolobales. Consequently, these environments constitute a separate niche for members of the Sulfolobales capable of Fe(II) oxidization.
Gibbs free energy values calculated based on actual spring conditions revealed that the Fe2+ oxidation reactions forming aqueous Fe3+ or Fe(III) solid phases are exergonic in these systems (Gibbs free energy, ∼−30 to −40 kJ mol electron−1) when O2 serves as the electron acceptor (2, 15). The rate of abiotic Fe2+ oxidation is highly pH dependent, and the abiotic rate is quite low at pH values less than 4 (26, 35). Consequently, the deposition of Fe(III) secondary minerals in high-flow-rate environments (velocity, 0.05 to 0.5 m/s) reflects the importance of microbial oxidation in mineral deposition (14, 24). An important geochemical attribute of these habitats is the combination of high dissolved ferrous Fe concentrations and the presence of Fe(III) solid phases, such as Fe(III) oxyhydroxides as well as jarosite (15). The interesting finding that strain MK1 grows robustly using Fe(II) sorbed to ferrihydrite as an energy source is consistent with the geochemical conditions in these habitats.
During the course of the current study, we inventoried a fairly extensive number of acidic springs in YNP, including different Fe(III) oxide mats with variable geochemistry, and we concluded that organisms highly related to Metallosphaera sp. strain MK1 are found primarily in low-pH (pH 2 to 4), high-ferrous Fe springs associated with the biomineralization of Fe(III) solid phases at temperatures ranging from 55 to 85°C. Organisms highly related to strain MK1 were especially prevalent in aerobic outflow channel positions of the acid-sulfate systems at Rainbow Springs (sites RS1 and RS2) and at Joseph's Coat Springs (site JC2) and are important members of the archaeal community in the ASC springs of Norris Geyser Basin (Fig. 5). Conversely, strain MK1-like 16S rRNA sequences were not commonly observed in locations where dissolved sulfide (DS) concentrations were greater than approximately 10 μM or where dissolved oxygen concentrations were near or below the limit of detection (1 to 3 μM) (15, 28). In several of the acidic springs studied (e.g., sites NGB-BE, NGB-DW, and RS2), the concentrations of DS were significant at the point of discharge (10 to 150 μM) and Metallosphaera-like sequences were generally not observed until the DS concentration had dropped to ∼5 to 10 μM, where Fe(III) oxide mats began to form.
Future progress in characterization of regulatory mechanisms of Fe versus S oxidation in strain MK1 and other novel Sulfolobales should help workers develop more definitive linkages between habitat parameters [i.e., temperature, elemental S, Fe(III) oxides, dissolved O2] and the distribution of Metallosphaera-like organisms in geothermal habitats. Understanding microbial mechanisms of sulfide mineral oxidation under thermophilic conditions has important implications for bioleaching, metal recovery (20, 27, 30, 32), and coal desulfurization (6, 8, 33). Strain MK1 oxidizes pyrite, pyrite-enriched mine tailings, and other sulfide minerals (Fig. 2 and Table 1) at significant rates under batch culture conditions. Given its broad temperature range (∼50 to 80°C), strain MK1 and/or closely related organisms from YNP may be suitable candidates for enhanced bioleaching under acidic, thermophilic conditions.
Supplementary Material
Acknowledgments
This work was supported by the National Science Foundation Microbial Observatory Program (grant MCB-0132022), the National Aeronautic and Space Administration via funds provided to the Thermal Biology Institute at Montana State University (projects NAG5-8807 and NNG04GR46G), and the Montana Agricultural Experiment Station (project 911398).
We thank S. Jennings for providing the pyrite-enriched mine tailing sample, Y. Odake and S. Brumfield for performing the transmission electron microscopy, and C. Hendrix and T. Olliff for permitting the work in Yellowstone National Park (permit YELL-2007-SCI-5068).
Footnotes
Published ahead of print on 14 December 2007.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Amann, R. I., B. Zarda, D. A. Stahl, and K. H. Schleifer. 1992. Identification of individual prokaryotic cells by using enzyme-labeled, rRNA-targeted oligonucleotide probes. Appl. Environ. Microbiol. 58:3007-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amend, J. P., and E. L. Shock. 2001. Energetics of overall metabolic reactions of thermophilic and hyperthermophilic Archaea and Bacteria. FEMS Microbiol. Rev. 25:175-243. [DOI] [PubMed] [Google Scholar]
- 3.Baker, B. J., and J. F. Banfield. 2003. Microbial communities in acid mine drainage. FEMS Microbiol. Ecol. 44:139-152. [DOI] [PubMed] [Google Scholar]
- 4.Behrens, S., C. Rühland, J. Inácio, H. Huber, A. Fonseca, I. Spencer-Martins, B. Fuchs, and R. Amann. 2003. In situ accessibility of small-subunit rRNA of members of the domains Bacteria, Archaea, and Eucarya to Cy3-labeled oligonucleotide probes. Appl. Environ. Microbiol. 69:1748-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blake, R., and D. B. Johnson. 2000. Phylogenetic and biochemical diversity among acidophilic bacteria that respire on iron, p. 53-78. In L. Derek (ed.), Environmental microbe-metal interactions. ASM Press, Washington, DC.
- 6.Brierley, C. L., and J. A. Brierley. 1973. A chemoautotrophic and thermophilic microorganism isolated from an acid hot spring. Can. J. Microbiol. 19:183-188. [DOI] [PubMed] [Google Scholar]
- 7.Brock, T. D., K. M. Brock, R. T. Belly, and R. L. Weiss. 1972. Sulfolobus: a new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch. Mikrobiol. 84:54-68. [DOI] [PubMed] [Google Scholar]
- 8.Clark, T. R., F. Baldi, and G. J. Olson. 1993. Coal depyritization by the thermophilic archaeon Metallosphaera sedula. Appl. Environ. Microbiol. 59:2375-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuchs, T., H. Huber, K. Teiner, S. Burggraf, and K. O. Stetter. 1995. Metallosphaera prunae, sp. nov., a novel metal-mobilizing, thermoacidophilic Archaeum, isolated from a uranium mine in Germany. Syst. Appl. Microbiol. 18:560-566. [Google Scholar]
- 10.Hallberg, K.B., and D. B. Johnson. 2001. Biodiversity of acidophilic prokaryotes. Adv. Appl. Microbiol. 49:37-84. [DOI] [PubMed] [Google Scholar]
- 11.Huber, G., C. Spinnler, A. Gambacorta, and K. O. Stetter. 1989. Metallosphaera sedula gen. nov. and sp. nov. represents a new genus of aerobic, metal-mobilizing, thermoacidophilic archaebacteria. Syst. Appl. Microbiol. 12:38-47. [Google Scholar]
- 12.Huber, G., and K. O. Stetter. 1991. Sulfolobus metallicus, sp. nov., a novel strictly chemolithoautotrophic thermophilic archaeal species of metal-mobilizers. Syst. Appl. Microbiol. 14:372-378. [Google Scholar]
- 13.Hugenholtz, P., C. Pitulle, K. L. Hershberger, and N. R. Pace. 1998. Novel division-level bacterial diversity in a Yellowstone hot spring. J. Bacteriol. 180:366-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inskeep, W. P., R. E. Macur, G. Harrison, B. C. Bostick, and S. Fendorf. 2004. Biomineralization of As(V)-hydrous ferric oxyhydroxide in microbial mats of an acid-sulfate-chloride geothermal spring, Yellowstone National Park. Geochim. Cosmochim. Acta 68:3141-3155. [Google Scholar]
- 15.Inskeep W. P., G. G. Ackerman, W. P. Taylor, M. Kozubal, S. Korf, and R. E. Macur. 2005. On the energetics of chemolithotrophy in nonequilibrium systems: case studies of geothermal springs in Yellowstone National Park. Geobiology 3:297-320. [Google Scholar]
- 16.Jackson, C. R., H. W. Langner, J. Donahoe-Christiansen, W. P. Inskeep, and T. R. McDermott. 2001. Molecular analysis of microbial community structure in an arsenite-oxidizing acidic thermal spring. Environ. Microbiol. 3:532-542. [DOI] [PubMed] [Google Scholar]
- 17.Jan, R. L., J. Wu, S. M. Chaw, C. W. Tsai, and S. D. Tsen. 1999. A novel species of thermoacidophilic archaeon, Sulfolobus yangmingensis sp. nov. Int. J. Syst. Bacteriol. 49:1809-1816. [DOI] [PubMed] [Google Scholar]
- 18.Jennings, S. R. 1995. Geochemical characterization of sulfide mineral weathering for remediation of acid producing mine wastes. M.S. thesis. Publication no. 9502. Montana State University, Bozeman.
- 19.Johnson, D. B., and K. B. Hallberg. 2003. The microbiology of acidic mine waters. Res. Microbiol. 154:466-473. [DOI] [PubMed] [Google Scholar]
- 20.Kappler, U., L. I. Sly, and A. G. McEwan. 2005. Respiratory gene clusters of Metallosphaera sedula—differential expression and transcriptional organization. Microbiology 151:35-43. [DOI] [PubMed] [Google Scholar]
- 21.Konhauser, K. O. 1998. Diversity of bacterial iron mineralization. Earth Sci. Rev. 43:91-121. [Google Scholar]
- 22.Kurosawa, N., Y. H. Itoh, and T. Itoh. 2003. Reclassification of Sulfolobus hakonensis Takayanagi et al. 1996 as Metallosphaera hakonensis comb. nov. based on phylogenetic evidence and DNA G+C content. Int. J. Syst. Evol. Microbiol. 53:1607-1608. [DOI] [PubMed] [Google Scholar]
- 23.Langner, H. W., C. R. Jackson, T. R. McDermott, and W. P. Inskeep. 2001. Rapid oxidation of arsenite in a hot spring ecosystem, Yellowstone National Park. Environ. Sci. Technol. 35:3302-3309. [DOI] [PubMed] [Google Scholar]
- 24.Macur, R. E., H. W. Langner, B. D. Kocar, and W. P. Inskeep. 2004. Linking geochemical processes with microbial community analysis: successional dynamics in an arsenic-rich, acid-sulfate-chloride geothermal spring. Geobiology 2:163-177. [Google Scholar]
- 25.Miroshnichenko, M. L. 2004. Thermophilic microbial communities of deep-sea hydrothermal vents. Microbiology 73:1-13. [PubMed] [Google Scholar]
- 26.Nordstrom, D. K., and G. Southam. 1997. Geomicrobiology of sulfide mineral oxidation. Rev. Mineral. 35:361-390. [Google Scholar]
- 27.Olson, G. J., J. A. Brierley, and C. L. Brierley. 2003. Bioleaching review, part B. Appl. Microbiol. Biotechnol. 63:249-257. [DOI] [PubMed] [Google Scholar]
- 28.Peeples, T. L., and R. M. Kelly. 1995. Bioenergetic response of the extreme thermoacidophile Metallosphaera sedula to thermal and nutritional stresses. Appl. Environ. Microbiol. 61:2314-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prieur, D., M. A. Voytek, C. Jeanthon, and A. L. Reysenbach. 2001. Deep-sea thermophilic prokaryotes, p. 11-22. In A.-L. Reysenbach, M. A. Voytek, and R. Mancinelli (ed.), Thermophiles: biodiversity, ecology and evolution. Kluwer Academic/ Plenum Publishers, New York, NY.
- 30.Rawlings, D. E. 2002. Heavy metal mining using microbes. Annu. Rev. Microbiol. 56:65-91. [DOI] [PubMed] [Google Scholar]
- 31.Reysenbach, A. L., G. S. Wickham, and N. R. Pace. 1994. Phylogenetic analysis of the hyperthermophilic pink filament community in Octopus Spring, Yellowstone National Park. Appl. Environ. Microbiol. 60:2113-2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rohwerder, T., T. Gehrke, K. Kinzler, and W. Sand. 2003. Bioleaching review, part A. Progress in bioleaching: fundamentals and mechanisms of bacterial metal sulfide oxidation. Appl. Microbiol. Biotechnol. 63:239-248. [DOI] [PubMed] [Google Scholar]
- 33.Schippers, A., T. Rohwerder, and W. Sand. 1999. Intermediary sulfur compounds in pyrite oxidation: implications for bioleaching and biodepyritization of coal. Appl. Microbiol. Biotechnol. 52:104-110. [Google Scholar]
- 34.Schwertmann, U., and R. M. Cornell. 2000. Iron oxides in the laboratory, 2nd ed., p. 105-107. VCH, Weinheim, Germany.
- 35.Singer, P. C., and M. W. Stumm. 1970. Acid mine drainage: the rate determining step. Science 167:1121-1123. [DOI] [PubMed] [Google Scholar]
- 36.Spurr, A. R. 1969. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct.Res. 26:31-43. [DOI] [PubMed] [Google Scholar]
- 37.Stetter, K. O. 1999. Extremophiles and their adaptation to hot environments. FEBS Lett. 452:22-25. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki, T., T. Iwasaki, T. Uzawa, K. Hara, N. Nemoto, T. Kon, T. Ueki, A. Yamagishi, and T. Oshima. 2002. Sulfolobus tokodaii sp. nov. (f. Sulfolobus sp. strain 7), a new member of the genus Sulfolobus isolated from Beppu Hot Springs, Japan. Extremophiles 6:39-44. [DOI] [PubMed] [Google Scholar]
- 39.Takayanagi, S., H. Kawasaki, K. Sugimori, T. Yamada, A. Sugai, T. Ito, K. Yamasato, and M. Shioda. 1996. Sulfolobus hakonensis sp. nov., a novel species of acidothermophilic archaeon Int. J. Syst. Bacteriol. 46:377-382. [DOI] [PubMed] [Google Scholar]
- 40.To, T. B., D. K. Nordstrom, K. M. Cunningham, J. W. Ball, and R. B. McClesky. 1999. New method for the direct determination of dissolved Fe(III) concentration in acid mine waters. Environ. Sci. Technol. 33:807-813. [Google Scholar]
- 41.Trevisanato, S. I., N. Larsen, A. H. Segerer, K. O. Stetter, and R. A. Garrett. 1996. Phylogenetic analysis of the archaeal order of Sulfolobales based on sequences of 23S rRNA genes and 16S/23S rDNA spacers. Syst. Appl. Microbiol. 19:61-65. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.