Abstract
The production by filamentous fungi of therapeutic glycoproteins intended for use in mammals is held back by the inherent difference in protein N-glycosylation and by the inability of the fungal cell to modify proteins with mammalian glycosylation structures. Here, we report protein N-glycan engineering in two Aspergillus species. We functionally expressed in the fungal hosts heterologous chimeric fusion proteins containing different localization peptides and catalytic domains. This strategy allowed the isolation of a strain with a functional α-1,2-mannosidase producing increased amounts of N-glycans of the Man5GlcNAc2 type. This strain was further engineered by the introduction of a functional GlcNAc transferase I construct yielding GlcNAcMan5GlcNac2 N-glycans. Additionally, we deleted algC genes coding for an enzyme involved in an early step of the fungal glycosylation pathway yielding Man3GlcNAc2 N-glycans. This modification of fungal glycosylation is a step toward the ability to produce humanized complex N-glycans on therapeutic proteins in filamentous fungi.
Filamentous fungi belonging to the Aspergillus group are well-established production hosts for extracellular enzymes of industrial importance, such as amylases, glucoamylases, pectinases, phytases, cellulases, and ligninases. (37). Glucoamylase (1,4-β-d-glucan glucohydrolase; EC 3.2.1.3) is one of the most prominent thermostable industrial enzymes (18) and is mainly produced by Aspergillus niger fermentation. Typical fermentation yields by A. niger are up to 25 g/liter of glucoamylase (3).
In addition to homologous protein production, Aspergillus nidulans, A. niger, and Aspergillus oryzae have offered an attractive alternative to Escherichia coli and yeasts, such as Saccharomyces cerevisiae, Kluyveromyces lactis, or Pichia pastoris, for the expression of recombinant, heterologous proteins, and there have been a number of attempts to program filamentous fungi for the production of such heterologous proteins (13, 14, 20, 27, 29).
Whereas posttranslational modifications of secreted heterologous proteins have been shown to influence protein stability, yield, and function, the production by filamentous fungi of therapeutic glycoproteins intended for use in mammals has been hampered by the inherent difference in protein N-glycosylation and by the inability of the fungal cell to modify proteins with mammalian glycosylation structures. While both fungi and mammals attach a specific oligosaccharide to asparagines in the sequence Asn-X-Ser/Thr/Cys (where X represents any amino acid except proline), the subsequent processing of the transferred glycan differs significantly between mammalian and fungal cells (47). The assembly of a lipid-linked oligosaccharide composed of three gluocose (Glc3), nine mannose (Man9), and two N-acetylglucosamine (GlcNAc2) residues (referred to as Glc3Man9GlcNAc2), followed by transfer to the nascent protein and the removal of three glucose residues and one mannose sugar to yield Man8GlcNAc2, is conserved between eukaryotes. The biosynthetic glycosylation pathways diverge between fungi and mammals once a glycoprotein leaves the endoplasmic reticulum (ER) and is shuttled through the Golgi apparatus. Yeasts and other fungi typically produce high-mannose-type N-glycans by adding up to 100 mannose sugars, including beta-linked mannoses and mannosylphosphates, whereas the formation of mammalian glycans generally involves the removal of mannose, followed by the addition of N-acetylglucosamine, galactose, fucose, and sialic acid (8, 9, 25, 31). Recently, glycoengineering in the yeast P. pastoris and the expression of therapeutic glycoproteins with complex “humanized” N-glycosylation structures have shown significant progress (4, 6, 10, 11, 15, 16, 26, 47). Far less progress has been made in glycoengineering of fungal production hosts, such as Aspergillus or Trichoderma species, although the N-glycan structures of several secreted glycoproteins have been elucidated.(1, 2, 17, 24, 28, 29, 34, 39-41, 48) and attempts were made to modify fungal glycosylation structures by the insertion of glycan structure-modifying enzymes (17, 29, 32). Introduction of rabbit N-acetylglucosaminyltransferase I (GnT I) into A. nidulans has resulted in the production of an in vitro active enzyme, but no evidence for in vivo GlcNAc transfer was found (23). When human cDNA encoding GnT I was expressed in Trichoderma reesei, the incorporation of N-acetylglucoseamine into α-1,3-linked mannose of the core oligosaccharide Man5GlcNac2 was demonstrated by 1H nuclear magnetic resonance analysis, but the efficiency of this incorporation was not analyzed (29). In contrast to yeast species, hyperglycosylation is not a typical feature of filamentous fungi, which most often synthesize small high-mannose-type N- and O-glycans (2). So far, no typical mammalian-like complex-type glycans have been found or generated in filamentous fungi (33). Some of the glycans found resemble mammalian high-mannose glycans [Man(6-9)GlcNAc2]. In addition, typical “fungal-type” glycans that are structurally different from the mammalian glycans have been identified on different Aspergillus glycoproteins [Man(5-12)GlcNAc2] (1, 29, 44, 45).
Moreover, growth conditions have been found to influence the glycan modification of some proteins, possibly by modulating the expression of enzymes involved in the fungal glycosylation pathway (12).
In order to approach a solution for this important drawback in the possibility of using fungal heterologous glycoproteins for therapeutic applications (21, 28, 30), we used an approach similar to that described for Pichia pastoris and systematically engineered the glycosylation pathways of two Aspergillus species. Here, we report the introduction of heterologous fusion proteins, such as mannosidases and glycosyltransferases, as well as the deletion of a gene (algC) coding for an enzyme involved in an early step of the fungal glycosylation pathway.
MATERIALS AND METHODS
Strains.
The A. nidulans and A. niger strains used throughout this work are listed in Table 1 and Table 2, respectively. The strain T2 carries an expression plasmid with a reporter protein and argB. This expression plasmid allows the purification of the reporter protein (the C-terminal six-His-tagged K3 domain of human plasminogen, as described in reference 6, and expressed from the A. nidulans gpdA promoter) and and will allow analysis of the glycosylation of the reporter protein in the future. E. coli strain JM 109 was used for routine plasmid propagation.
TABLE 1.
A. nidulans strains used throughout this work
| Strain | Genotypea | Reference |
|---|---|---|
| YR23.5 | pyrG89 argB2 pantoA1 riboB2 | This work |
| YR23.5-BC10 | pyrG89 pantoA1 riboB2 | This work |
| YR23.5-CD28 | pyrG89 pantoA1 riboB2 | This work |
| YR23.5-BC10-coNA15 | pantoA1 riboB2 | This work |
| YR23.5-mnnJ-NA | pantoA1 riboB2 | This work |
| YR23.5-ΔalgC | pyrG89 pantoA1 riboB2 | This work |
pyrG89, pyrimidine requirement, orotidine MP decarboxylase deficient; argB2, arginine requirement, OTCase deficient; pantoA1, pantothenate requirement; riboB2, riboflavin requirement.
TABLE 2.
A. niger strains used throughout this work
| Strain | Genotypea | Reference or source |
|---|---|---|
| A888 | cspA1 argB13 nicA1 | FGSCb |
| T2 (argB+gpdAp-K3) transformant of A888 | nicA1 cspA1 gpdAp-K3 | This work |
| T2-BC10 | nicA1 cspA1 amdS | This work |
| T2-CD28 | nicA1 cspA1 amdS | This work |
| T2-BC10-coNA15 | nicA1 cspA1 amdS hph | This work |
| T2-BC10-mnnJ-NA | nicA1 cspA1 amdS hph | This work |
| T2-ΔalgC | nicA1 cspA1 ΔalgC | This work |
cspA1, morphological phenotype—short conidiophores; nicA1, nicotinic or anthranilic acid requirement; amdS, amide nonutilization, acetamidase deficient; hph, hygromycin B resistance; argB13, arginine requirement, OTCase deficient; ΔalgC, altered glycosilation, Dol-P-Man:Man5GlcNAc2-PP-Dol mannosyltransferase deficient; gpdAp-K3, Kringle-3 domain of human plasminogen.
FGSC, Fungal Genetics Stock Center (www.fgsc.net).
Cloning of pEKgnopat.
From plasmid pAN52-1 (38), the ATG of the A. nidulans gpd promoter, as well as the NcoI site, was deleted by PCR using primers pAN52-SalF (5′-CGCAGACCGGGAACACAAGC-3′) and pAN52-BamR (5′-TAACGTTAAGTGGATCCAAGCTGATGTCTGC-3′). The resulting PCR fragment was cloned as a SalI-BamHI fragment into the SalI/BamHI-cut pAN52-1 vector. The NotI site of the vector pAN52-1 was deleted by partial digestion with NotI, filling in of the protruding ends, and subsequent ligation. Cloning sites consisting of NcoI, SwaI, AscI, and SbfI sites, as well as a PacI site, were introduced by cloning the annealed oligonucleotides Not Pac F New (5′-GATCCGCGGCCGCATTTAAATGGCGCGCCCCTGCAGGTTAATTAAG-3′) and Not Pac R New(5′-GATCCTTAATTAACCTGCAGGGGCGCGCCATTTAAATGCGGCCGCG-3′) as BamHI fragments into the unique BamHI site of pAN52-1. Additionally, AsiSI, FseI, and PmeI restriction sites were introduced by cloning the annealed oligonucleotides pAN52 Marker F (5′-TATGGCGATCGCGGCCGGCCGTTTAAACCA-3′) and pAN52 Marker R (5′-TATGGTTTAAACGGCCGGCCGCGATCGCCA-3′) as NdeI fragments into the unique NdeI site of the vector, yielding the expression vector pEKgnopat.
Cloning of the A. niger mnnJ leader sequence.
The putative MnnJ protein sequence was identified by a similarity search against the A. niger sequence database (Integrated Genomics, Chicago, IL) using S. cerevisiae Mnn10p (accession number YDR245W) as a query sequence. Using the mnnJ sequence information from the similarity search, the mnnJ leader sequence was amplified in a 5′ rapid amplification of cDNA ends experiment with the specific primer RT MNN10 (5′-CTTCTCCCAGCTCTCGCGCCATTCG-3′) and cloned into pGEMT-easy for sequencing. The final leader sequence was amplified from the pGEMT-easy plasmid using the forward primer An-MNN10-5 (5′GCGGCCGCCACCATGCTATTCCTCTTCCGTCGTTTCGAGCTG-3′), creating a NotI restriction site (underlined), and the reverse primer An-MNN10-3 (5′-GGCGCGCCCCCATCGTTCCACATAATTCTTCTTGTTCCA-3′), creating an AscI site (underlined). The NotI-AscI fragments were used in subsequent cloning steps.
Cloning of α-1,2-mannosidase genes into pEKgnopat.
The α-1,2-mannosidase catalytic domain-leader fusions were cloned into the NotI-PacI-digested pEKgnopat as NotI-PacI fragments. In the subsequent cloning step, the A. niger leader sequence was cloned as NotI-AscI fragments into the NotI-AscI-digested vector.
Cloning of GlcNAc-transferase I genes into pEKgnopat.
The GlcNAc-transferase I catalytic domain-leader fusions NA15 and coNA15 were obtained as a gift from GlycoFi, Inc., in vectors pPB104 and pPB104-CO. coNA15 is a codon-optimized sequence for expression in P. pastoris. To achieve this, the human codons have been changed to the optimal P. pastoris codons following the codon usage table available at http://www.kazusa.or.jp/codon/. The plasmids were constructed by fusion of sequences coding for amino acids 1 to 40 of ScMNN9 to sequences coding for amino acids 38 to 445 of HsGNTI (Table 3) using the nucleotide sequences GGGCGCGCC and GGAAGAGCA, respectively, as fusion linkers in the process, adding the three amino acids GlyArgAla. In the case of pPB104, this fusion was accomplished by using the AscI site created by the additional nucleotides, and in the case of pPB104, the complete gene fusion sequence was assembled using oligonucleotides and fusion PCR. Both fusions were then released by NotI-PacI digestion and cloned into the NotI-PacI-digested pEKgnopat. Additionally, A. niger leader sequences were introduced into the pEKgnopat-NA15 plasmid by removing the MNN9 (no. 15) leader with a NotI-AscI digestion and cloning the A. niger leader as NotI-AscI fragments into the vector.
TABLE 3.
Functional-leader-catalytic domain fusion constructs
| Construct | Leader (amino acid coordinates) | Organism (accession no.) | Catalytic (amino acid coordinates) | Organism (accession no.) |
|---|---|---|---|---|
| BC10 | PpSEC12 (334-363) | P. pastoris (AF216960.1) | α-1,2-Mannosidase (81-541) | C. elegans (NM 059715.3) |
| CD28 | ScMNN10 (1-121) | S. cerevisiae (YDR245W) | α-1,2-Mannosidase (187-667) | D. melanogaster (X82640) |
| NA 15 and coNA15 | ScMNN9 (1-40) | S. cerevisiae (YPL050C) | GnTI (38-445) | H. sapiens (NM_002406)a |
| mnnJ-NA | mnnJ (1-250) | A. niger (DQ841153) | GnTI (38-445) | H. sapiens (NM_002406) |
Codon optimized with P. pastoris codon bias in coNA.
algC knockout constructs.
The putative algC protein sequence was identified by a similarity search using S. cerevisiae Alg3p (accession number YBL082C) as a query against the A. nidulans sequence database at Broad Institute (http://www.broad.mit.edu) and the A. niger sequence database (Integrated Genomics, Chicago, IL). Additional 3′ and 5′ sequence information on the algC gene of A. niger was obtained by probing with the algC fragment a minilibrary based on restriction site analysis of A. niger strain 888. A colony lift and subsequent hybridization experiment with the algC PCR product as a probe revealed two clones that yielded additional 3′ sequence information. algC was knocked out by a DNA fragment obtained by chimeric PCR. 3′ and 5′ fragments of the algC gene were amplified from chromosomal A. niger DNA with the primer pairs alg3 F (5′-GTCACGGTCAACGTCCTCCCTCT-3′) plus alg3_5primeR (5′-CACAACCACCAGCGTGATCAAGTAGAG-3′) and alg3_3primeF (5′-GTTGCGATTAAGATGACACTGTTGCTG-3′) plus alg3_3prime_R_new (5′-GGAGGTGAATTAGCTTGAGCGGAATA-3′). An hph fragment with algC overhangs was amplified from the vector pAN7 (38) using primers HmB_chimericF (5′-CTCTACTTGATCACGCTGGTGGTTGTGTACACAGGCTCAAATCAATAAGAAGAACG-3′) and HmB_chimericR (5′-CAGCAACAGTGTCATCTTAATCGCAACTAGATGTGGAGTGGGCGCTTACAC-3′) and a long-run PCR kit (MBI Fermentas). The three fragments were purified via gel excision and assembled in a subsequent PCR, in which the overhangs of the hph fragment functioned as a primer. Amplification of the chimeric PCR product was performed with the primers alg3 F (5′-GTCACGGTCAACGTCCTCCCTCT-3′) and alg3_3prime_R_new (5′-GGAGGTGAATTAGCTTGAGCGGAATA-3′). Fifteen microliters of the PCR product was used directly for transformation.
To knock out the algC gene in A. nidulans, a recombinant DNA fragment was constructed via chimeric PCR. 5′ and 3′ fragments of the algC gene were amplified from chromosomal A. nidulans DNA with the primer pairs ANalgC5′_fwd (5′-GCTGTTGGAGGCTCTGGATAGAAA-3′) plus ANalgC5′_rev (5′-TGCCATTGTAGTTGATGAAGAAGAAGA-3′) and ANalgC3′_fwd (5′-GGACAACGTACATGCAACAGGTCA-3′) plus ANalgC3′_rev (5′-CCCGCGCAATTCCTTCTTTAG-3′). An argB fragment with algC overhangs was amplified from the vector pFB39 (43) using the primers chimeric-argB_fwd (5′-CTCTTCTTCTTCATCAACTACAATGGCATATTTCGCGGTTTTTTGGGGTAGT-3′) and chimeric-argB_rev (5′-TGACCTGTTGCATGTACGTTGTCCTAGCCATTGCGAAACCTCAGAAG-3′) and a long-run PCR kit (Roche). The three fragments were purified and assembled in a subsequent PCR, in which the overhangs of the argB fragment functioned as primers, and amplification of the chimeric nested PCR was performed with the primers ANalgCnested_fwd (5′-GTGTATGGAAACGAGACGATCATCTTC-3′) and ANalgCnested_rev (5′-GCAGCAGATATTCCATTCAACCAAAG-3′) in one PCR; 15 μl of the PCR product was used directly for transformation.
Aspergillus transformation.
Transformation protocols basically followed the procedures published by Tilburn and colleagues (42). Transformants were selected according to the selection marker used (Tables 1 and 2). The transformants were then checked by PCR and/or dot blot hybridization, as well as by Southern blot analysis. Replacement of A. nidulans algC by the marker gene was verified by PCR, using primers ANalgC5′_fwd (5′-GCTGTTGGAGGCTCTGGATAGAAA-3′) and ANalgC3′_rev (5′-GGACAACGTACATGCAACAGGTCA-3′), which anneal to algC 5′ and algC 3′ sequences not present on the knockout construct, giving rise to a larger PCR product (4,881 bp) than an intact algC gene (2,923 bp).
Replacement of A. niger algC by the marker gene used a similar strategy, employing the algC 5′primers alg3_KO_PCR_F (5′-CGCCGCATCTACATCCCCG-3′) and alg3_KO_gpdA_R (5′-TTGGGACGATGCAAGATATAAACGAA-3′), which anneal to the gpdA promoter of the selection marker on the knockout construct. Additionally, the absence of algC coding sequence was verified by PCR with specific primers and by Southern analysis using an algC open reading frame fragment of either A. nidulans or A. niger.
Culture conditions.
Strains were grown for 20 h at 37°C on 250 ml LB medium containing supplements according to the auxotrophy markers of the strain and ammonium tartrate (10 mM) as an additional nitrogen source. The mycelium was harvested by filtration, washed with ∼500 ml of tap water, and ground to fine powder under liquid nitrogen, and the powder was subsequently lyophilized. The lyophilized biomass was used in glycan digestion and subsequent mass spectrometry (MS) analysis to determine the whole-cell glycosylation.
Glucoamylase production.
Strains were grown on Aspergillus complete medium containing supplements according to the auxotrophy markers of the strain and ammonium tartrate (10 mM) as an additional nitrogen source for 50 h at 200 rpm and 37°C. The mycelium was harvested by filtration and washed with sterile water. Subsequently, the mycelium was transferred to fresh expression medium (70 g/liter trisodium citrate dihydrate, 20 ml/liter Aspergillus salt solution, and 10 g/liter maltodextrin, plus the respective supplements and ammonium tartrate [10 mM] as a nitrogen source) and cultivated for about 52 h at 200 rpm and 30°C. The culture supernatant (containing glucoamylase) was harvested and stored at −80°C. Glucoamylase was purified from the culture supernatant by size exclusion chromatography (PD-10 columns; Amersham Biosciences), using Tris, pH 8.0, according to the manufacturer's manual. The eluate was concentrated via ultrafiltration (Vivaspin 20-ml concentrator; 5,000 MWCO PES; VivaScience) and further purified via strong basic anion-exchange chromatography (Vivapure Q Mini H; VivaScience), using 25 mM Tris, pH 8.0, for equilibration and 1 M NaCl-25 mM Tris, pH 8.0 for elution. The purified protein was lyophilized.
Total cellular protein extraction and release of N-linked glycan.
Total cellular protein was extracted by dissolving 50 mg of lyophilized and ground cell pellet in 0.5 ml RCM buffer (8 M urea, 360 mM Tris, and 3.2 mM EDTA, pH 8.6) at room temperature. Cell debris was removed by centrifugation for 15 min at 2,500 rpm in a Beckmann Coulter Allegra 6 centrifuge. The protein content of the supernatant was determined by protein assay (Bio-Rad, Hercules, CA). The glycans were released and separated from the glycoproteins by a modification of previously reported methods (4, 35). Briefly, 50 μg of solubilized proteins of various samples were adsorbed onto a polyvinylidene difluoride membrane in a 96-well MultiScreen IP plate (Millipore, Bedford, MA). After the proteins were reduced and carboxymethylated and the membranes were blocked, the wells were washed three times with water. The proteins were deglycosylated by the addition of 22 μl of 10 mM NH4HCO3, pH 8.3, containing 1 mU PNGase F (New England Biolabs). After 16 h at 37°C, the solution containing the glycans was removed to a clean 96-well plate by centrifugation and evaporated to dryness.
MS analysis.
The molecular weights of the glycans were determined using a Voyager DE PRO linear matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometer (Applied Biosciences) as described previously (6). All spectra were generated with the instrument in the positive ion mode. In this MALDI-TOF ion mode, it is possible that phosphorylated glycans could not be detected, and the influence of the expression of α-1,2-mannosidase on phosphorylated fungal glycans has not been studied here. All of the masses obtained in MS analysis are summarized in Table 4.
TABLE 4.
Molecular weights of glycans detected by MALDI-TOFa
| Proposed structure | Composition | Theoretical mol wtb | Mol wt detected by MALDI-TOFc |
|---|---|---|---|
| Hex3 | GlcNAc2 Hex3 | 933 (949) | 934 (950)-936 (952) |
| Hex4 | GlcNAc2 Hex4 | 1,095 (1,111) | 1,096 (1,112)-1,098 (1,114) |
| Hex5 | GlcNAc2 Hex5 | 1,257 (1,273) | 1,258 (1,274)-1,261 (1,277) |
| Hex6 | GlcNAc2 Hex6 | 1,419 (1,435) | 1,419 (1,435)-1,425 (1,441) |
| Hex | GlcNAc2 Hex7 | 1,581 (1,597) | 1,581 (1,597)-1,586 (1,602) |
| Hex8 | GlcNAc2 Hex8 | 1,743 (1,759) | 1,743 (1,759)-1,749 (1,765) |
| Hex9 | GlcNAc2 Hex9 | 1,905 (1,921) | 1,905 (1,921)-1,912 (1,928) |
| GlcNAcM5 | GlcNAc2Man5 GlcNAc | 1,460 (1,476) | 1,461 (1,477)-1,465 (1,481) |
Molecular weight masses hexose, 180 (162, loss of H2O); GlcNAc, 221 (203, loss of H2O).
Molecular weight with Na adduct (K adduct in parentheses).
Molecular weight range from multiple spectra.
In vitro α-1,2-mannosidase digestion.
T. reesei α-1,2-mannosidase (a gift from R. Contreras, Unit of Fundamental and Applied Molecular Biology, Department of Molecular Biology, Ghent University, Ghent, Belgium) digestions were performed as described previously (4).
RNA isolation.
Strains were grown overnight on minimal medium containing the appropriate supplements at 37°C and 180 rpm. The mycelium was harvested and frozen in liquid nitrogen. RNA was isolated using Trizol according to the manufacturer's instructions (Gibco BRL).
qRT-PCR.
Quantitative reverse transcription-PCR (qRT-PCR) for gene expression was performed with Platinum Sybr green qPCR SuperMix-UDG (Invitrogen) and a SuperScript III Platinum Two-Step qRT-PCR kit with Sybr green (Invitrogen) and was carried out on an iCycler thermal cycler with a MyiQ single-color real-time PCR detection system (Bio-Rad). Reactions were run in duplicate, and a mean value of the two samples was calculated. The results were calculated as the relative amount of product adjusted for the level of internal control amplification (actin mRNA) obtained for the sample. The primers used for quantification of BC10 in A. nidulans and A. niger were BC10fwd (5′-ACTGCCGGATACTCTGGAATC-3′) and trpCrev (5′-GATTTCAGTAACGTTAAGTGGATCC-3′), those for CD28 in A. nidulans and A. niger were CD28fwd (5′-TCGCCGAAACGCTTAAGTAC-3′) and trpCrev, those for coNA15 in A. nidulans and A. niger were coNA15 F (5′-CAAAACACAATGTCACTTTCTCTTGTATCCTAC-3′) and trpCrev, and those for mnnJ-NA in A. nidulans and A. niger were GNT F (5′-CTTGTATCGTACCGCCTAAGAAAGAACC-3′) and trpCrev. For amplification of the A. niger actin gene (accession no. AAU11333), the primers actNigR (5′-TATCTGAGGGTGAGGATACCACG-3′) and actNigerF (5′-GACAATGGTTCGGGTATGTGC-3′) were used. For amplification of the A. nidulans actin gene (accession no. AN6542.3), the primers actin F (5′-GATCGGTATGGGTCAGAAGGA-3′) and actin R (5′-CGATGTTGCCGTACAGATCC-3′) were used.
Nucleotide sequence accession numbers.
The mnnJ leader sequence has been deposited with NCBI under accession number DQ841153. The A. niger algC sequence has been deposited under accession no. DQ841152. The accession number for A. nidulans algC is AN0104.3.
RESULTS AND DISCUSSION
Expression of functional α-1,2-mannosidase fusion proteins results in trimming of terminal mannose residues to putative Man5GlcNAc2 structures.
α-1,2-Mannosidase hydrolyzes terminal 1,2-linked α-d-mannose residues in the oligomannose oligosaccharide Man9GlcNAc2 in a calcium-dependent manner to produce Man8GlcNAc2 in a first step and Man5GlcNAc2 N-glycans on glycosylated proteins in a second step (19). Generation of Man5GlcNAc2 is one of the critical steps in obtaining complex glycoproteins because this substrate is the precursor for all subsequent downstream processing steps (11). In an attempt to express functional α-1,2-mannosidase in Aspergillus, cDNAs coding for leader sequences from enzymes known to target the protein to the ER or Golgi apparatus from S. cerevisiae, P. pastoris, or A. niger were combined in the pEKgnopat Aspergillus expression vector (see Materials and Methods) with different catalytic domains derived from Caenorhabditis elegans, Drosophila melanogaster, Mus musculus, and T. reesei. Leader sequences from putative A. niger orthologues of S. cerevisiae Gls1p, Mns1p, Mnn10p, Mnn11p, and Anp1p were identified and cloned by similarity searches against an A. niger sequence database (data not shown). Among all putative A. niger leaders, only the Mnn10p orthologue was found to be functional in combination with heterologous catalytic domains (see below). The combinatorial fusion constructs were individually transformed into A. niger strain T2 and A. nidulans strain YR23.5 (in A. nidulans, only constructs BC10 and CD28 were transformed), and the integration events were verified by PCR and Southern blotting (results not shown). The copy numbers of constructs integrated into the genome varied from one copy to several copies in our Southern analysis, potentially influencing the expression levels of the integrated genes. We determined the expression levels in at least two single-copy strains of each transformation event in Aspergillus complete medium (36) by qRT-PCR. Expression levels were monitored by a combination of α-1,2-mannosidase-specific and trpC terminator-specific primers, and the transcript levels obtained were normalized to the A. nidulans or A. niger actin gene acnA (see Materials and Methods). Additionally, the actin-normalized expression levels of the transgenes were compared with the actin-normalized mRNA levels of the endogenous gpdA genes of A. niger or A. nidulans. In single-copy transformants, similar mRNA levels (with differences of less than 20%) were recorded (data not shown). Such strains were selected for further analysis, for which the cultures were processed as described in Materials and Methods, and whole-cell glycans were released by PNGase digestion and analyzed by MALDI-TOF analysis.
Structure assignments were based on the masses (summarized in Table 4) and sensitivity to α-1,2-mannosidase. However, we cannot rule out the possibility of other hexoses. For example, in A. niger N-linked glycans, the presence of galactose in a galactofuranose conformation has been established (44, 46). In this work, we did not analyze for their appearance by digestion with alternative mannosidases, such as an α-1,2/3/6-mannosidase.
Therefore, the masses of N-linked glycans released by PNGase F digestion of proteins obtained by MALDI-TOF are referred to as hexoses. For example, in the figures, we refer to a GlcNAc2Hex6 structure as Hex6.
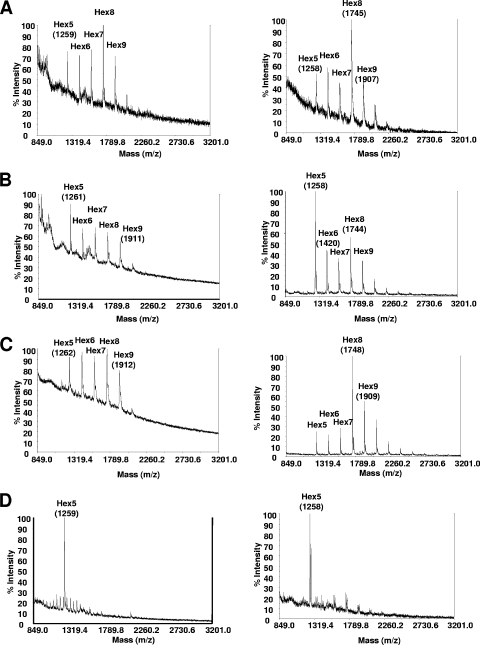
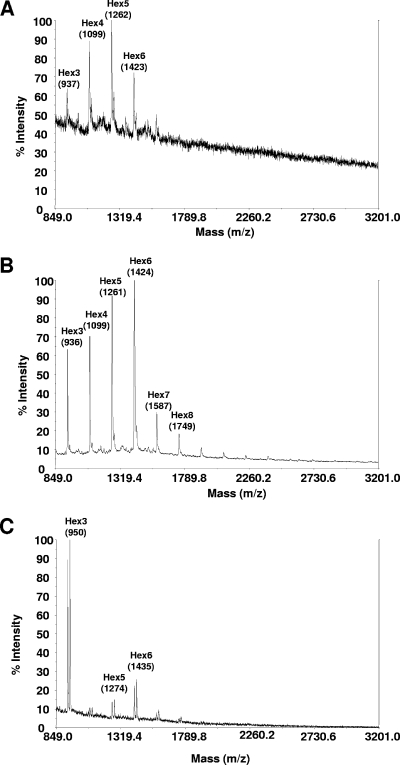
The BC10 construct (a P. pastoris SEC12 leader fused to a C. elegans α-1,2-mannosidase lacking a transmembrane domain [Table 3]) was the only one found to be functional in A. niger and resulted in a considerable shift of glycan composition from mainly Man8GlcNAc2 (Fig. 1A, right) to strongly elevated amounts of Man5GlcNAc2 (Fig. 1B, right). All other constructs were not functional in modifying the N-glycan masses; one example is shown in Fig. 1C, right, for the CD28 fusion construct (an S. cerevisiae MNN10 leader fused to a D. melanogaster α-1,2-mannosidase lacking a transmembrane domain). Of these two fusion proteins, only the expression of BC10 resulted in a different glycosylation pattern at the level of whole-cell glycans in A. nidulans. Figure 1, left, compares the glycan masses obtained by MALDI-TOF analysis for the A. nidulans recipient strain YR23.5 (Fig. 1A) with the spectra obtained from the strain expressing the BC10 construct (Fig. 1B) and an example of a typical spectrum obtained from a strain carrying a nonfunctional fusion construct (CD28) (Fig. 1C). The mass spectrum of BC10 clearly shows elevated masses consistent with Man5GlcNAc2 structures. Ions appearing with low abundance between Hex6 and Hex7 that have masses similar to GlcNAcMan5GlcNAc2 in Fig. 1A and B are probably a matrix artifact and not N-linked glycans. The glycan patterns of A. nidulans (Fig. 1D, left) and A. niger (Fig. 1D, right), which were obtained after in vitro digestions using purified α-1,2-mannosidase on whole-cell extracts, show an almost complete reduction of any N-glycan other than Man5GlcNAc2, and this result shows that the in vivo functionality of the α-1,2-mannosidase fusion constructs is only partial.
FIG. 1.
Whole-cell N-glycan analysis of Aspergillus strains expressing engineered α-1,2-mannosidases. The images on the left compare whole-cell N-glycans from A. nidulans recipient strain YR23.5 (A) with strain YR23.5-BC10 (B) and strain YR23.5-CD28 (C). The images on the right show whole-cell N-glycan spectra obtained from A. niger recipient strain T2 (A), strain T2-BC10 (B), and strain T2-CD28 (C). (D) N-glycan spectra obtained after in vitro PNGase F digestion of A. nidulans (left) or A. niger (right) whole-cell extracts with α-1,2-mannosidase. Preparation and analysis of N-glycans was performed as described in Materials and Methods. Structure assignments in this figure and all subsequent figures are based on MALDI-TOF masses (m/z) of individual PNGase F-released ions sensitive to α-1,2-mannosidase, and the relative intensities of the masses are shown (% intensity).
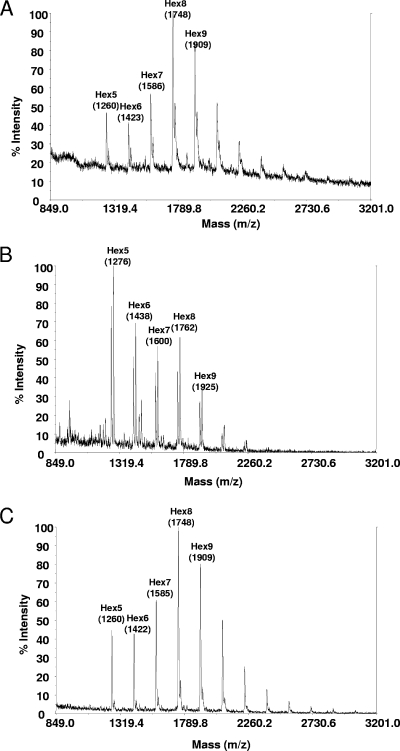
In addition to whole-cell glycan analysis, the N-glycans of a prominent secreted A. niger protein were analyzed. Glucoamylase, an enzyme secreted under starch or maltodextrin induction, was purified (see Materials and Methods) and analyzed for glycan composition. The glycan pattern of glucoamylase was comparable to that of the whole-cell glycans, with a shift from Man8GlcNAc2 to high levels of Man5GlcNAc2 (Fig. 2).
FIG. 2.
MALDI-TOF spectra of N-glycans derived from purified A. niger glucoamylase of the recipient strain T2 (A), strain T2-BC10 (B), or strain T2-CD28 (C).
Interestingly, only one construct (BC10) was shown to be functional in both Aspergillus species at the level of whole-cell glycans (A. nidulans and A. niger) and on secreted glucoamylase (A. niger). As in P. pastoris, the catalytic domain of C. elegans worked best in both Aspergillus species. In P. pastoris, when combined with the S. cerevisiae leaders MNS1 and MNN10, the catalytic domain of C. elegans produced predominantly Man5GlcNAc2 on the Pichia reporter protein K3 (6). A slightly lower rate, as estimated from MALDI-TOF analysis, was observed in A. niger. Other constructs shown to be functional in Pichia were not successful in Aspergillus. Even though previous studies established a correlation between certain domains and targeting events, there is currently no tool available that allows one to predict the behavior of a given targeting peptide across different hosts. Accordingly, we were not able to predict from comparison of the primary protein sequences of functional versus nonfunctional mannosidases which of the constructs tested would work in Aspergillus.
Human β-1,2-N-acetylglucosaminyltransferase I (GNT I) is functional in vivo.
The transfer of N-acetyl-d-glucosamine to Man5GlcNAc2 substrates generated by the previous Man8GlcNAc2-to-Man5GlcNAc2 trimming step was investigated. The GNT I enzyme localizes to the luminal face of the Golgi apparatus, allowing the sequential processing of glycoproteins as they are shuttled through the secretory pathway. In one construct, the catalytic domain of human GNT I (abbreviated as NA) was fused to the MNN9 leader (abbreviated as 15) from S. cerevisiae to yield construct NA15. In a second construct, a fungal codon-optimized form (coNA; see Materials and Methods for details) was combined with the same MNN9 leader to yield construct coNA15. Yet another construct used a leader sequence from a putative A. niger gene with similarity to the S. cerevisiae MNN10 gene, designated mnnJ according to the Aspergillus nomenclature (for the mnnJ accession number and all construct details, see Materials and Methods and Table 3). Fusion of the mnnJ leader sequence with NA yielded mnnJ-NA, which was, like all the other fusion constructs, subsequently integrated into the same expression vector backbone. These expression vectors were stably introduced by transformation into A. nidulans and A. niger strains already carrying the BC10 α-1,2-mannosidase construct, which showed the highest in vivo activity. Integration events were verified by PCR and Southern analysis, and expression of the constructs was tested by qRT-PCR (see Materials and Methods).
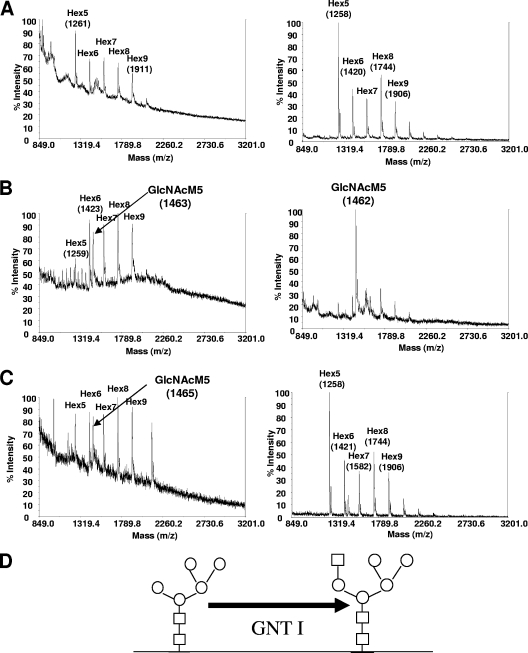
Whole-cell N-glycan analysis of A. niger BC10-transformed strains revealed that expression of the fusion construct coNA15 (Table 3) resulted in efficient transformation of Man5GlcNAc2 to GlcNAcMan5GlcNAc2 N-glycans, as seen from mass analysis (Fig. 3, right). It is intriguing that in the A. niger T2-BC10 recipient strain a considerable portion of higher-mannose N-glycans, such as Man6GlcNAc2, Man7GlcNAc2, and Man8GlcNAc2, still existed in parallel with a large amount of Man5GlcNAc2 (Fig. 3A, right). In the strain additionally expressing the GNT I enzyme (Fig. 3B, right), these higher-mannose glycans were significantly reduced.
FIG. 3.
Whole-cell N-glycan analysis of Aspergillus strains expressing engineered GNT I. (A, B, and C) The images on the left compare whole-cell N-glycans from A. nidulans strain YR23.5-BC10, which served as a recipient for the GNT I constructs (A) (shown in Fig. 1B, left, and repeated here for clarity), with strain YR23.5-BC10-coNA15 (B) and strain YR23.5-BC10-mnnJ-NA (C). The images on the right show whole-cell glycan spectra for A. niger strain T2-BC10, which served as a recipient strain for the GNT I constructs (A), compared to N-glycans obtained from strain T2-BC10-coNA15 (B) and strain T2-BC10-mnnJ-NA (C). (D) Schematic diagram representing the function of GNT I, which generates GlcNAcMan5GlcNAc2 structures by the addition of one GlcNAc moiety on Man5GlcNAc2. GlcNAc residues are represented by squares and mannose (hexose) residues by circles.
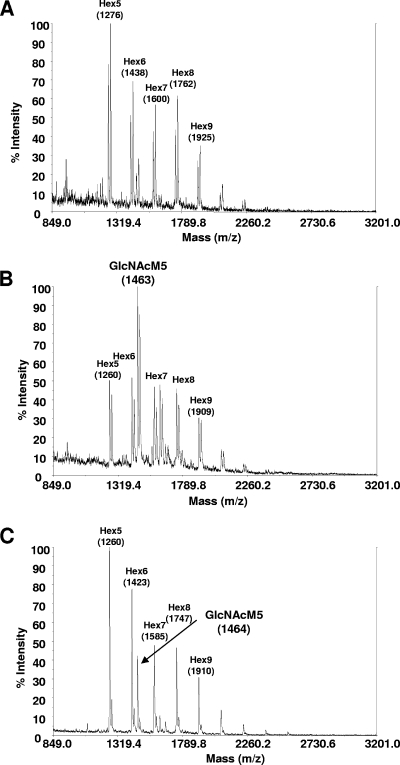
The N-glycans released from the purified A. niger glucoamylase were subsequently analyzed in strains T2-BC10-coNA15 und T2-BC10-mnnJ-NA. The glycan pattern of glucoamylase was comparable to that of the whole-cell glycans, with a shift from Man5GlcNAc2 to GlcNAcMan5GlcNAc2 (Fig. 4). Whereas at the level of whole-cell glycans, the construct mnnJ-NA was not functional at all (Fig. 3C, right), some GlcNAc transfer was detected on glucoamylase (Fig. 4C).
FIG. 4.
N-glycan analysis of purified A. niger glucoamylase from strain T2-BC10, which served as a recipient for the GNT I constructs (A) (shown in Fig. 2B and repeated here for clarity), compared to strains T2-BC10-coNA15 (B) and T2-BC10-mnnJ-NA (C).
On glucoamylase of A. niger strain T2-BC10-coNA15, predominantly masses corresponding to GlcNAcMan5GlcNAc2 were found, but (Man5-Man9)GlcNAc2 and a mass consistent with GlcNAcMan6GlcNAc2 were also detected. It remains to be determined whether introduction of a GlcNAc transporter would shift the glycosylation of secreted protein in A. niger toward GlcNAcMan5GlcNAc2, as was reported for P. pastoris (6).
Whole-cell glycan analysis of the transformed strains, along with the A. nidulans BC10 recipient strain (Fig. 3A, left), was carried out by mass spectrometry following glycan digestions. Of all transformants tested, the strain carrying construct coNA15 showed a new peak corresponding to the mass of GlcNAcMan5GlcNAc2 structures (Fig. 3B, left). Interestingly, the construct mnnJ-NA showed some activity in A. nidulans (Fig. 3C, left) but no activity in A. niger.
MnnJ is the putative orthologue of S. cerevisiae Mnn10p, which functions in a complex with MNN9p in a subunit of the yeast Golgi apparatus mannosyltransferase complex, also containing Anp1p, Mnn11p, and Hoc1p, that mediates elongation of the polysaccharide mannan backbone (22). Interestingly, the MnnJ leader sequence was the only fungal leader with significant activity out of five putative proteins derived from the Golgi apparatus mannosyltransferase complex.
One GNT I construct was shown to be particularly active in A. niger and to shift the equilibrium of the enzymatic reaction toward GlcNAcMan5GlcNAc2. Interestingly, in the strain expressing only α-1,2-mannosidase from the BC10 construct, a considerable number of Man7GlcNAc2 and Man8GlcNAc2 glycan structures still existed in parallel with the large amount of Man5GlcNAc2 (Fig. 3A, right), but in the strain additionally expressing the GNT I enzyme (Fig. 3B, right), these higher-mannose structures were almost completely lost. These results suggest that removal of Man5GlcNAc2, produced by α-1,2-mannosidase, by GNT I, which uses Man5GlcNAc2 as a substrate, shifts the substrate-product equilibrium for α-1,2-mannosidase, which in turn results in a more active α-1,2-mannosidase enzyme along the glycosylation line. Additionally or alternatively, removal of Man5GlcNAc2 could also remove the substrate for alternative resident glycosyltransferases. Moreover, in a GNT I strain expressing a nonfunctional α-1,2-mannosidase fusion construct (e.g., T2-CD28), no GlcNAcMan5GlcNAc2 was detected, and therefore, it seems that the endogenous A. niger α-1,2-mannosidase is not sufficient to provide enough substrate for a functional GNT I enzyme. As already seen with the α-1,2-mannosidase fusion construct BC10, the GNT I fusion construct already shown to be functional in Pichia pastoris also worked best in Aspergillus. In contrast to the α-1,2-mannosidase, which showed only partial activity in Aspergillus, the GNT I construct led almost exclusively to GlcNAcMan5GlcNAc2, as reported for P. pastoris (6). Our results on whole-cell glycan structures also suggest that the α-1,2-mannosidase fusion construct BC10 is correctly localized in Aspergillus and that α-1,2-mannosidase trimming occurs in the ER and early Golgi apparatus and not through secreted α-1,2-mannosidase in the medium. This was of concern, since leakage of α-1,2-mannosidase into the medium was reported by other groups (5, 6).
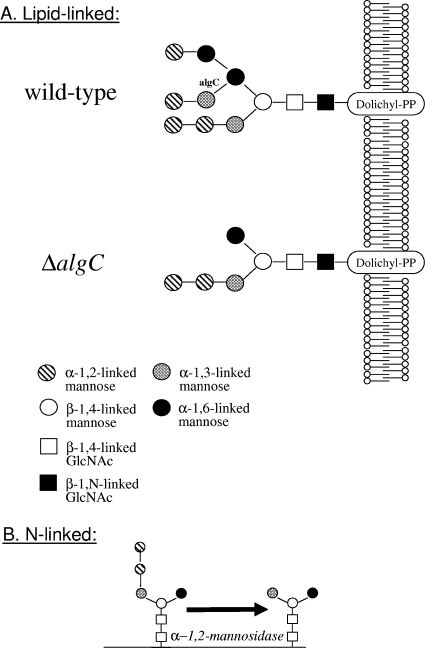
Generation of Man3GlcNAc2 structures by knockout of the algC gene.
The formation of N-glycosidic linkages of glycoproteins involves the ordered assembly of the common Glc3Man9 GlcNAc2 core oligosaccharide on the lipid carrier dolichyl pyrophosphate. Whereas early mannosylation steps occur on the cytoplasmic side of the ER with GDP-Man as the donor, the final reactions from Man5GlcNAc2-PP-Dol to Man9GlcNAc2-PP-Dol on the luminal side use Dol-P-Man. The ALG3 gene encodes the Dol-P-Man:Man5GlcNAc2-PP-Dol mannosyltransferase, which converts Man5GlcNAc2-Dol-PP to Man6GlcNAc2-Dol-PP. Knockout of ALG3 in S. cerevisiae and P. pastoris leads to specific Man5GlcNAc2 structures, which can be trimmed to Man3GlcNAc2 by α-1,2-mannosidase (Fig. 5). We have previously shown that the distinct Δalg3 Man5GlcNAc2 can be trimmed by α-1,2-mannosidases to obtain paucimannose Man3GlcnNAc2. Paucimannose then can serve as a substrate for GnT I. (7).
FIG. 5.
The function of Alg3p. (A) The yeast ALG3 gene encodes the Dol-P-Man:Man5GlcNAc2-PP-Dol mannosyltransferase, which converts Man5GlcNAc2-Dol-PP to Man6GlcNAc2-Dol-PP. (B) Knockout of ALG3 leads to Man5GlcNAc2 structures, which can be trimmed to Man3GlcNAc2 by α-1,2-mannosidase.
We cloned the ALG3 orthologues of both, A. nidulans and A. niger (see Materials and Methods) and designated the corresponding genes algC. Alignments of the A. nidulans (AN0104.2), A. niger (DQ841152), and A. terreus (EAU37037) AlgC proteins with S. cerevisiae Alg3p (YBL082C) showed high similarity among the three proteins only in the N-terminal part. The A. nidulans protein is around 200 amino acids smaller than the yeast Alg3p and showed a similarity score of only 8e-33 in a BLAST search against the A. nidulans genome database (http://www.broad.mit.edu) using S.c.Alg3p as the in silico probe. High overall similarity can be observed only among the putative AlgC proteins of filamentous fungi (e.g., A. oryzae, Aspergillus fumigatus, and Neurospora crassa), but yeasts and basidiomycetes show only very limited overall similarity in their putative Dol-P-Man:Man5GlcNac2-PP-Dol mannosyltransferases (not shown).
Using roughly 1 kb of the nucleotide sequence upstream and downstream of the algC open reading frame, we generated by hybrid PCR two sets of deletion constructs. One construct carried between the upstream and downstream sequences the auxotrophic marker gene argB (for A. nidulans), while the other harbored the dominant hygromycin resistance marker gene hygB (for A. niger). We then generated algC knockout strains (ΔalgC) for both organisms. Morphologically, A. nidulans strains YR23.5 and YR23.5-ΔalgC did not show any significant differences. The knockout strain did not show any growth defects on minimal or complete medium and sporulated as well as the recipient and other algC+ strains (not shown). Analysis of the whole-cell glycans of the A. nidulans strain YR23.5-ΔalgC showed a shift of the whole-cell glycan pattern to lower-mannose-type glycosylation. Additional masses, such as Man3GlcNAc2 and Man4GlcNAc2, previously not seen in the parental strain were observed (Fig. 6A). Structures larger than GlcNAcMan3GlcNAc2 could be the result of endogenous glycosyltransferase activity capping the free α-1,6-mannose arm. However, knockout of algC efficiently inhibited core oligosaccharide assembly in A. nidulans, confirming that indeed we had for the first time cloned and deleted an ALG3 homologue of a filamentous fungus. We then tested whole-cell glycans in the A. niger T2-ΔalgC knockout strain. As in A. nidulans, the lack of AlgC activity led to a shift of the whole-cell glycan pattern to lower-mannose-type glycosylation, like Man3GlcNAc2 and Man4GlcNAc2 (not shown). In vitro digestion of N-glycans released from the A. niger ΔalgC strain with purified α-1,2-mannosidase led to an almost complete trimming of the Man4GlcNAc2, Man5GlcNAc2, and Man6GlcNAc2 structures to Man3GlcNAc2 (Fig. 6C). When N-glycans released from glucoamylase purified from this strain were analyzed (not shown), the mass spectra showed a peak composition similar to that seen for A. niger whole-cell glycans, i.e., a loss of higher masses and the appearance of masses corresponding to Man3GlcNAc2 to Man6GlcNAc2 (Fig. 6B).
FIG. 6.
Whole-cell N-glycan analysis of Aspergillus strains lacking AlgC activity. (A and B) Whole-cell N-glycan structures of the A. nidulans strain YR23.5-ΔalgC (A) and A. niger strain T2-ΔalgC (B). (C) N-glycan spectrum obtained after in vitro digestion of A. niger T2-ΔalgC whole-cell extracts with purified α-1,2-mannosidase.
Knockout of algC (AN0104.4) in A. nidulans led to some Man3GlcNAc2, in addition to Man4GlcNAc2 and Man5GlcNAc2, which was the dominant glycan, as well as Man6GlcNAc2. In A. niger, the knockout of algC also led to the generation of masses consistent with Man3GlcNAc2 and Man4GlcNAc2, structures not present in the parent strain (compare Fig. 1). Similarly to A. nidulans, Man5GlcNAc2 and Man6GlcNAc2 were additional glycans, in this case dominated by Man6GlcNAc2. In vitro digestion of T2-ΔalgC whole-cell glycans led almost exclusively to Man3GlcNAc2 paucimannose glycans. Previously observed larger glycan masses recalcitrant to in vitro α-1,2-mannosidase treatment in a P. pastoris Δalg3 Δoch1 strain (7) were not detected in A. niger. Such larger structures can be formed by endogenous glycosyltransferases, which may add hexoses to various glycan structures (such as the unusual ΔalgC glycan created here) in vivo. Digestion of N-linked glycans derived from the ΔalgC strain with purified α-1,2-mannosidase led to an almost complete trimming of the masses consistent with Man4GlcNAc2, Man5GlcNAc2, and Man6GlcNAc2 structures to Man3GlcNAc2. Similar larger-size oligosaccharides were observed after the cloning and deletion of the ALG3 gene in the methylotrophic yeast P. pastoris. This finding implies the existence of endogenous glycosyltransferases capable of transferring sugars to the canonical alg3 Man5 N-glycan (7). Subsequent experiments, such as the analysis of lipid-linked oligosaccharides, should allow the elucidation of these novel structures and will in turn provide a deeper understanding of the substrate specificity of glycosyltransferases residing in the secretory pathway in Aspergillus.
In sum, the findings presented here suggest, as also previously demonstrated for the methylotrophic yeast P. pastoris (4), that the deletion of algC can indeed provide a promising route in Aspergillus for the addition of one additional GlcNAc residue to produce GlcNAcMan3GlcNAc2, and hence, to drive further the production of humanized complex glycoproteins in filamentous fungi.
Acknowledgments
We acknowledge receipt of plasmid pAN52 from Peter Punt, TNO, NL, and are grateful for receiving purified α-1,2 mannosidase from R. Contreras’ laboratory.
Footnotes
Published ahead of print on 14 December 2007.
REFERENCES
- 1.Aleshin, A. E., L. M. Firsov, and R. B. Honzatko. 1994. Refined structure for the complex of acarbose with glucoamylase from Aspergillus awamori var. X100 to 2.4- Å resolution. J. Biol. Chem. 269:15631-9. [PubMed] [Google Scholar]
- 2.Archer, D. B., and J. F. Peberdy. 1997. The molecular biology of secreted enzyme production by fungi. Crit. Rev. Biotechnol. 17:273-306. [DOI] [PubMed] [Google Scholar]
- 3.Aunstrup, K. 1977. Production of industrial enzymes, p. 151-171. In J. Meyrath and J. D. Bu'Lock (ed.), Biotechnology and fungal differentiation. FEMS Symposium vol. 4. Academic Press, New York, NY. [Google Scholar]
- 4.Bobrowicz, P., R. C. Davidson, H. Li, T. I. Potgieter, J. H. Nett, S. R. Hamilton, T. A. Stadheim, R. G. Miele, B. Bobrowicz, T. Mitchell, S. Rausch, E. Renfer, and S. Wildt. 2004. Engineering of an artificial glycosylation pathway blocked in core oligosaccharide assembly in the yeast Pichia pastoris: production of complex humanized glycoproteins with terminal galactose. Glycobiology 14:757-766. [DOI] [PubMed] [Google Scholar]
- 5.Callewaert, N., W. Laroy, H. Cadirgi, S. Geysens, X. Saelens, W. Min Jou, and R. Contreras. 2001. Use of HDEL-tagged Trichoderma reesei mannosyl oligosaccharide 1,2-alpha-d-mannosidase for N-glycan engineering in Pichia pastoris. FEBS Lett. 503:173-178. [DOI] [PubMed] [Google Scholar]
- 6.Choi, B. K., P. Bobrowicz, R. C. Davidson, S. R. Hamilton, D. H. Kung, H. Li, R. G. Miele, J. H. Nett, S. Wildt, and T. U. Gerngross. 2003. Use of combinatorial genetic libraries to humanize N-linked glycosylation in the yeast Pichia pastoris. Proc. Natl. Acad. Sci. USA 100:5022-5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson, R. C., J. H. Nett, E. Renfer, H. Li, T. A. Stadheim, B. J. Miller, R. G. Miele, S. R. Hamilton, B. K. Choi, T. I. Mitchell, and S. Wildt. 2004. Functional analysis of the ALG3 gene encoding the Dol-P-Man: Man5GlcNAc2-PP-Dol mannosyltransferase enzyme of P. pastoris. Glycobiology 14:399-407. [DOI] [PubMed] [Google Scholar]
- 8.Dean, N. 1999. Asparagine-linked glycosylation in the yeast Golgi. Biochim. Biophys. Acta 1426:309-322. [DOI] [PubMed] [Google Scholar]
- 9.Gemmill, T. R., and R. B. Trimble. 1999. Overview of N- and O-linked oligosaccharide structures found in various yeast species. Biochim. Biophys. Acta 1426:227-237. [DOI] [PubMed] [Google Scholar]
- 10.Gerngross, T. 2005. Production of complex human glycoproteins in yeast. Adv. Exp. Med. Biol. 564:139. [DOI] [PubMed] [Google Scholar]
- 11.Gerngross, T. U. 2004. Advances in the production of human therapeutic proteins in yeasts and filamentous fungi. Nat. Biotechnol. 22:1409-1414. [DOI] [PubMed] [Google Scholar]
- 12.Goochee, C. F., and T. Monica. 1990. Environmental effects on protein glycosylation. Biotechnology 8:421-427. [DOI] [PubMed] [Google Scholar]
- 13.Gouka, R. J., P. J. Punt, and C. A. van den Hondel. 1997. Efficient production of secreted proteins by Aspergillus: progress, limitations and prospects. Appl. Microbiol. Biotechnol. 47:1-11. [DOI] [PubMed] [Google Scholar]
- 14.Gwynne, D. I., and M. Devchand. 1992. Expression of foreign proteins in the genus Aspergillus. Biotechnology 23:203-214. [PubMed] [Google Scholar]
- 15.Hamilton, S. R., P. Bobrowicz, B. Bobrowicz, R. C. Davidson, H. Li, T. Mitchell, J. H. Nett, S. Rausch, T. A. Stadheim, H. Wischnewski, S. Wildt, and T. U. Gerngross. 2003. Production of complex human glycoproteins in yeast. Science 301:1244-1246. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton, S. R., R. C. Davidson, N. Sethuraman, J. H. Nett, Y. Jiang, S. Rios, P. Bobrowicz, T. A. Stadheim, H. Li, B. K. Choi, D. Hopkins, H. Wischnewski, J. Roser, T. Mitchell, R. R. Strawbridge, J. Hoopes, S. Wildt, and T. U. Gerngross. 2006. Humanization of yeast to produce complex terminally sialylated glycoproteins. Science 313:1441-1443. [DOI] [PubMed] [Google Scholar]
- 17.Harrison, M. J., A. S. Nouwens, D. R. Jardine, N. E. Zachara, A. A. Gooley, H. Nevalainen, and N. H. Packer. 1998. Modified glycosylation of cellobiohydrolase I from a high cellulase-producing mutant strain of Trichoderma reesei. Eur. J. Biochem. 256:119-127. [DOI] [PubMed] [Google Scholar]
- 18.Harvey, L., and B. McNeil. 1994. Liquid fermentation systems and product recovery of Aspergillus. In J. Smith (ed.), Aspergillus. Biotechnology handbooks. Plenum Press, New York, NY.
- 19.Herscovics, A. 2001. Structure and function of class I alpha 1,2-mannosidases involved in glycoprotein synthesis and endoplasmic reticulum quality control. Biochimie 83:757-762. [DOI] [PubMed] [Google Scholar]
- 20.Iwashita, K. 2002. Recent studies of protein secretion by filamentous fungi. J. Biosci. Bioeng. 94:530-535. [DOI] [PubMed] [Google Scholar]
- 21.Jenkins, N., R. B. Parekh, and D. C. James. 1996. Getting the glycosylation right: implications for the biotechnology industry. Nat. Biotechnol. 14:975-981. [DOI] [PubMed] [Google Scholar]
- 22.Jungmann, J., and S. Munro. 1998. Multi-protein complexes in the cis Golgi of Saccharomyces cerevisiae with alpha-1,6-mannosyltransferase activity. EMBO J. 17:423-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalsner, I., W. Hintz, L. S. Reid, and H. Schachter. 1995. Insertion into Aspergillus nidulans of functional UDP-GlcNAc: alpha 3-D-mannoside beta-1,2-N-acetylglucosaminyl-transferase I, the enzyme catalysing the first committed step from oligomannose to hybrid and complex N-glycans. Glycoconj J. 12:360-370. [DOI] [PubMed] [Google Scholar]
- 24.Klarskov, K., K. Piens, J. Stahlberg, P. B. Hoj, J. V. Beeumen, and M. Claeyssens. 1997. Cellobiohydrolase I from Trichoderma reesei: identification of an active-site nucleophile and additional information on sequence including the glycosylation pattern of the core protein. Carbohydr. Res. 304:143-154. [DOI] [PubMed] [Google Scholar]
- 25.Kukuruzinska, M. A., and K. Lennon. 1998. Protein N-glycosylation: molecular genetics and functional significance. Crit. Rev. Oral Biol. Med. 9:415-448. [DOI] [PubMed] [Google Scholar]
- 26.Li, H., N. Sethuraman, T. A. Stadheim, D. Zha, B. Prinz, N. Ballew, P. Bobrowicz, B. K. Choi, W. J. Cook, M. Cukan, N. R. Houston-Cummings, R. Davidson, B. Gong, S. R. Hamilton, J. P. Hoopes, Y. Jiang, N. Kim, R. Mansfield, J. H. Nett, S. Rios, R. Strawbridge, S. Wildt, and T. U. Gerngross. 2006. Optimization of humanized IgGs in glycoengineered Pichia pastoris. Nat. Biotechnol. 24:210-215. [DOI] [PubMed] [Google Scholar]
- 27.Lombrana, M., F. J. Moralejo, R. Pinto, and J. F. Martin. 2004. Modulation of Aspergillus awamori thaumatin secretion by modification of bipA gene expression. Appl. Environ. Microbiol. 70:5145-5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maras, M., A. De Bruyn, J. Schraml, P. Herdewijn, M. Claeyssens, W. Fiers, and R. Contreras. 1997. Structural characterization of N-linked oligosaccharides from cellobiohydrolase I secreted by the filamentous fungus Trichoderma reesei RUTC 30. Eur. J. Biochem. 245:617-625. [DOI] [PubMed] [Google Scholar]
- 29.Maras, M., A. De Bruyn, W. Vervecken, J. Uusitalo, M. Penttila, R. Busson, P. Herdewijn, and R. Contreras. 1999. In vivo synthesis of complex N-glycans by expression of human N-acetylglucosaminyltransferase I in the filamentous fungus Trichoderma reesei. FEBS Lett. 452:365-370. [DOI] [PubMed] [Google Scholar]
- 30.Maras, M., X. Saelens, W. Laroy, K. Piens, M. Claeyssens, W. Fiers, and R. Contreras. 1997. In vitro conversion of the carbohydrate moiety of fungal glycoproteins to mammalian-type oligosaccharides—evidence for N-acetylglucosaminyltransferase-I-accepting glycans from Trichoderma reesei. Eur. J. Biochem. 249:701-707. [DOI] [PubMed] [Google Scholar]
- 31.Moremen, K. W., R. B. Trimble, and A. Herscovics. 1994. Glycosidases of the asparagine-linked oligosaccharide processing pathway. Glycobiology 4:113-125. [DOI] [PubMed] [Google Scholar]
- 32.Nevalainen, K. M., V. S. Te'o, and P. L. Bergquist. 2005. Heterologous protein expression in filamentous fungi. Trends Biotechnol. 23:468-474. [DOI] [PubMed] [Google Scholar]
- 33.Palamarczyk, G., M. Maras, R. Contreras, and J. Kruzewska. 1998. Enzymes, biological control and commercial applications, p. 121-133. In G. E. Harman and C. P. Kubicek (ed.), Trichoderma and Gliocladium, vol. 1. Taylor and Francis, Bristol, PA. [Google Scholar]
- 34.Panchal, T., and R. J. Wodzinski. 1998. Comparison of glycosylation patterns of phytase from Aspergillus niger (A. ficuum) NRRL 3135 and recombinant phytase. Prep. Biochem. Biotechnol. 28:201-217. [DOI] [PubMed] [Google Scholar]
- 35.Papac, D. I., J. B. Briggs, E. T. Chin, and A. J. Jones. 1998. A high-throughput microscale method to release N-linked oligosaccharides from glycoproteins for matrix-assisted laser desorption/ionization time-of-flight mass spectrometric analysis. Glycobiology 8:445-454. [DOI] [PubMed] [Google Scholar]
- 36.Pontecorvo, G., J. A. Roper, L. M. Hemmons, K. D. MacDonald, and A. W. J. Bufton. 1953. The genetics of Aspergillus nidulans. Adv. Genet. 5:141-238. [DOI] [PubMed] [Google Scholar]
- 37.Punt, P. J., P. A. Greaves, A. Kuyvenhoven, J. C. van Deutekom, J. R. Kinghorn, P. H. Pouwels, and C. A. van den Hondel. 1991. A twin-reporter vector for simultaneous analysis of expression signals of divergently transcribed, contiguous genes in filamentous fungi. Gene 104:119-122. [DOI] [PubMed] [Google Scholar]
- 38.Punt, P. J., R. P. Oliver, M. A. Dingemanse, P. H. Pouwels, and C. A. van den Hondel. 1987. Transformation of Aspergillus based on the hygromycin B resistance marker from Escherichia coli. Gene 56:117-124. [DOI] [PubMed] [Google Scholar]
- 39.Takayanagi, T., A. Kimura, S. Chiba, and K. Ajisaka. 1994. Novel structures of N-linked high-mannose type oligosaccharides containing alpha-d-galactofuranosyl linkages in Aspergillus niger alpha-d-glucosidase. Carbohydr. Res. 256:149-158. [DOI] [PubMed] [Google Scholar]
- 40.Takayanagi, T., K. Kushida, K. Idonuma, and K. Ajisaka. 1992. Novel N-linked oligo-mannose type oligosaccharides containing an alpha-d-galactofuranosyl linkage found in alpha-d-galactosidase from Aspergillus niger. Glycoconj. J. 9:229-234. [DOI] [PubMed] [Google Scholar]
- 41.Takegawa, K., A. Kondo, H. Iwamoto, K. Fujiwara, Y. Hosokawa, I. Kato, K. Hiromi, and S. Iwahara. 1991. Novel oligomannose-type sugar chains derived from glucose oxidase of Aspergillus niger. Biochem. Int. 25:181-190. [PubMed] [Google Scholar]
- 42.Tilburn, J., C. Scazzocchio, G. G. Taylor, J. H. Zabicky-Zissman, R. A. Lockington, and R. W. Davies. 1983. Transformation by integration in Aspergillus nidulans. Gene 26:205-221. [DOI] [PubMed] [Google Scholar]
- 43.Upshall, A. 1986. Genetic and molecular characterization of argB+ transformants of Aspergillus nidulans. Curr. Genet. 10:593-599. [DOI] [PubMed] [Google Scholar]
- 44.Wallis, G. L., R. L. Easton, K. Jolly, F. W. Hemming, and J. F. Peberdy. 2001. Galactofuranoic-oligomannose N-linked glycans of alpha-galactosidase A from Aspergillus niger. Eur. J. Biochem. 268:4134-4143. [DOI] [PubMed] [Google Scholar]
- 45.Wallis, G. L., F. W. Hemming, and J. F. Peberdy. 1999. Investigation of the glycosyltransferase enzymes involved in the initial stages of the N-linked protein glycosylation pathway in Aspergillus niger. Biochim. Biophys. Acta 1426:91-98. [DOI] [PubMed] [Google Scholar]
- 46.Wallis, G. L., R. J. Swift, F. W. Hemming, A. P. Trinci, and J. F. Peberdy. 1999. Glucoamylase overexpression and secretion in Aspergillus niger: analysis of glycosylation. Biochim. Biophys. Acta 1472:576-586. [DOI] [PubMed] [Google Scholar]
- 47.Wildt, S., and T. U. Gerngross. 2005. The humanization of N-glycosylation pathways in yeast. Nat. Rev. Microbiol. 3:119-128. [DOI] [PubMed] [Google Scholar]
- 48.Yang, Y., C. Bergmann, J. Benen, and R. Orlando. 1997. Identification of the glycosylation site and glycan structures of recombinant endopolygalacturonase II by mass spectrometry. Rapid Commun. Mass Spectrom. 11:1257-1262. [DOI] [PubMed] [Google Scholar]