Abstract
Here, we established a system for displaying heterologous protein to the C terminus of the peptidoglycan-binding domain (cA domain) of AcmA (a major autolysin from Lactococcus lactis). Western blot and flow cytometric analyses revealed that the fusion proteins (cA-AmyA) of the cA domain and α-amylase from Streptococcus bovis 148 (AmyA) are efficiently expressed and successfully displayed on the surfaces of L. lactis cells. AmyA was also displayed on the cell surface while retaining its activity. Moreover, with an increase in the number of cA domains, the quantity of cA-AmyA fusion proteins displayed on the cell surface increased. When three repeats of the cA domain were used as an anchor protein, 82% of α-amylase activity was detected on the cells. The raw starch-degrading activity of AmyA was significantly higher when AmyA was fused to the C terminus of the cA domain than when it was fused to the N terminus. In addition, cA-AmyA fusion proteins were successfully displayed on the cell surfaces of Lactobacillus plantarum and Lactobacillus casei.
The development of cell surface display systems of heterologous proteins or peptides has recently become an active research area. By utilizing naturally occurring surface proteins as scaffolds, functional proteins can be displayed on various cell surfaces (8). In these systems, cells displaying functional proteins can be easily separated and have been used in a wide variety of biotechnological applications, such as the delivery of live vaccines (6, 12, 20, 21, 22), bioconversion (11, 17), bioremediation of heavy metals (24), and screening of peptide libraries (6).
Anchor proteins expressed on the cell surface have been employed as scaffolds for displaying heterologous functional proteins on the cell surface. The system from gram-positive bacteria, which consists of anchor proteins that contain the LPXTG motif on their C termini, has been well established (8, 12, 22, 31). This anchoring motif covalently binds to the peptidoglycan of the bacterial cell wall following sortase processing (15, 27). The C terminus of the target protein is genetically fused to the N terminus of the anchor protein, and efficient expression of the target proteins on the bacterial cell surface is thus achieved.
One of the important factors determining whether heterologous proteins are displayed without loss of their function is the site of the target protein/anchor protein fusion (i.e., N or C terminus). In yeast (Saccharomyces cerevisiae) cell surface display systems, the functional display of cellulases (5) and glucoamylase (28) was successfully demonstrated when their C termini were fused to the N terminus of the anchor protein, the C-terminal half of α-agglutinin. On the other hand, α-amylase from Streptococcus bovis 148 (AmyA) (26) and Rhizopus oryzae lipase showed high activity after Flo1, which displays the target protein on its C terminus, was used as the anchor protein (14, 28). However, these proteins showed poor activity when they were fused to the N terminus of an α-agglutinin anchor protein (28, 33). Previously, we reported a cell surface display system that uses PgsA from Bacillus subtilis (1) as an anchor protein (17). Since PgsA has a transmembrane region at its N terminus (17), foreign proteins are introduced into the C terminus of PgsA. Likewise, a system in which functional proteins bind to the C terminus of an anchor protein from a gram-positive bacterium has been reported for Bacillus anthracis (16).
In the present study, to establish cell surface display systems that enable the display of target proteins at both the N and C termini of an anchor protein, we engineered the gene coding for the major autolysin AcmA from Lactococcus lactis (4) as a potent anchor protein. AcmA consists of two domains, an N-terminal catalytic domain and a C-terminal cA peptidoglycan-binding domain. This anchoring cA domain has three 45-amino-acid-long regions that are highly homologous (50 to 63% amino acid identity) LysM repeats (4), which bind proteins to the cell walls of a broad range of gram-positive bacteria (3, 21, 30). Functional proteins fused to the N terminus of the cA domain can thus be displayed on the cell surface (3, 13, 21, 22, 30). Herein, we report ways to increase the functional availability of the cA domain. We report the cell surface display of heterologous protein on the C terminus of the cA domain by using AmyA as a model protein and an increase in binding capacity by use of tandem repeats of the cA domain.
MATERIALS AND METHODS
Bacterial strains and media.
Escherichia coli NovaBlue {endA1 hsdR17(rK12− mK12+) supE44 thi-1 recA1 gyrA96 relA1 lac[F′ proAB+ lacIqZΔM15::Tn10 (Tetr)]} (Novagen, Inc., Madison, WI) was used to construct plasmids. It was grown in Luria-Bertani medium containing 250 μg/ml erythromycin at 37°C. L. lactis IL 1403 was grown in M17 broth (Difco Laboratories, Detroit, MI) containing 5 μg/ml erythromycin supplemented with 0.5% (wt/vol) glucose (GM17) at 30°C. Lactobacillus plantarum NCIMB 8826 and Lactobacillus casei BLSJ 03135 were grown in MRS broth (Difco Laboratories) containing 25 μg/ml erythromycin at 37°C. For solid media, 1.5% (wt/vol) agar was added to the media described above.
Plasmid construction.
The oligonucleotides and the plasmids used in this study are summarized in Table 1. PCR was carried out using KOD-Plus-polymerase (Toyobo Co. Ltd., Osaka, Japan). The gene fragment encoding the cA domain of AcmA (C-terminal 669-bp region) (Fig. 1a) from L. lactis IL 1403 was amplified by PCR (95°C for 30 seconds, 58°C for 30 seconds, and 68°C for 2 min for 30 cycles) from the genome of L. lactis IL 1403 by use of oligonucleotide primers cA_F and cA_R. The resulting fragment was digested with BamHI and SphI and then inserted into the BamHI and SphI sites of plasmid pUC19 (Takara Bio Inc., Shiga, Japan) digested with both enzymes. The resulting plasmid was designated pUC19-CA. To construct recombinant genes expressing two and three cA repeat domains, the gene encoding the cA domain was amplified by PCR and digested with BamHI and XhoI and subsequently inserted into the BamHI and SalI sites of plasmid pUC19-CA. The resulting plasmid was designated pUC19-CA2. A procedure similar to that used to construct plasmid pUC19-CA2 was used to construct pUC19-CA3, using pUC19-CA2 plasmid DNA as the starting material. The plasmids for AmyA secretion or for AmyA display were constructed as follows. The mature region of amyA (corresponding to amino acids 40 to 742) was amplified by PCR using oligonucleotide primers amyA_F2 and amyA_R2 or amyA_F3 and amyA_R from pCUSαA (18) and digested with EcoRV and XhoI or XhoI and HindIII, followed by insertion into the EcoRV and XhoI or XhoI and HindIII sites of the secretion vector pCUS (18). The resulting AmyA secretion plasmids, which possess multiple cloning sites (MCS) in different positions, were designated pAmyA-MCS and pMCS-AmyA. Subsequently, the genes encoding the cA domain or cA repeat domains were obtained from pUC19-CA, pUC19-CA2, and pUC19-CA3 by digestion with BamHI and XhoI. They were subsequently inserted into the BamHI and XhoI sites on pMCS-AmyA. The resulting plasmids for displaying AmyA on the C terminus of the cA domain were designated pCA-AmyA, pCA2-AmyA, and pCA3-AmyA, respectively. The gene encoding the three cA repeat domains was also inserted into the BamHI and XhoI sites on pAmyA-MCS. The resulting plasmid for displaying AmyA on the N terminus of the cA domain was designated pAmyA-CA3.
TABLE 1.
Plasmids and oligonucleotide primers used in this study
| Plasmid or primer | Phenotype or sequencea | Source or reference |
|---|---|---|
| Plasmids | ||
| pSECE1 | Wide-host-range vector, erythromycin resistance marker | 25 |
| pCUS | Secretion vector containing signal sequence of amyA gene, derivative of pSECE1 | 18 |
| pCUSαA | Vector for secreting α-amylase, derivative of pCUS | 18 |
| pAmyA-MCS | Vector for secreting α-amylase, cloning sites BamHI-XhoI-HindIII downstream of amyA gene, derivative of pCUS | This study |
| pMCS-AmyA | Vector for secreting α-amylase, cloning sites BamHI-SpeI-XbaI-XhoI upstream of amyA gene, derivative of pCUS | This study |
| pUC19 | Cloning vector, ampicillin resistance marker | Takara Bio |
| pUC19-CA | Vector containing cA from acmA, derivative of pUC19 | This study |
| pUC19-CA2 | Vector containing two cA repeats, derivative of pUC19-CA | This study |
| pUC19-CA3 | Vector containing three cA repeats, derivative of pUC19-CA2 | This study |
| pCA-AmyA | Vector for display of α-amylase to C terminus of cA, derivative of pMCS-AmyA | This study |
| pCA2-AmyA | Vector for display of α-amylase to C terminus of two cA repeats, derivative of pMCS-AmyA | This study |
| pCA3-AmyA | Vector for display of α-amylase to C terminus of three cA repeats, derivative of pMCS-AmyA | This study |
| pAmyA-CA3 | Vector for display of α-amylase to N terminus of three cA repeats, derivative of pAmyA-MCS | This study |
| Oligonucleotide primers | ||
| amyA_F2 | 5′-CCCGATATCGATGAACAAGTGTCAATGAAAGATGGTA−3′ | |
| amyA_F3 | 5′-CCGCTCGAGGATGAACAAGTGTCAATGAAAGATGGTACG−3′ | |
| amyA_R | 5′-CCCAAGCTTATTTTAGCCCATCTTTATTATAGTTTCCAG−3′ | |
| amyA_R2 | 5′-CCGCTCGAGTTAGGATCCTTTTAGCCCATCTTTATTATAGTTTCCAG-3′ | |
| cA_F | 5′-CGCGGATCCACTAGTGTCGACTCTTCTGCTGGTACTTCTAATTCCGGTGG−3′ | |
| cA_R | 5′-ACATGCATGCCTCGAGTTTAATACGAAGATATTGACCAATTAAAATGG−3′ |
For primers, restriction enzyme sites are underlined.
FIG. 1.
(a) Schematic illustration of the AcmA protein. The region comprising the cA domains is highlighted. S.S., signal sequence. (b) Schematic illustration of expressing proteins corresponds to α-amylase secretion or display plasmids.
All plasmids were introduced into L. lactis IL 1403 by electroporation using the method described previously (9). The resulting transformants were designated 1403/pCUS, 1403/pMCS-AmyA, 1403/pCA-AmyA, 1403/pCA2-AmyA, 1403/pCA3-AmyA, and 1403/pAmyA-CA3. The plasmids pCUS and pCA3-AmyA were also introduced into L. plantarum NCIMB 8826 and L. casei BLSJ 03135, respectively, as previously described (17). The resulting transformants were designated 8826/pCUS, 8826/pCA3-AmyA, 03135/pCUS, and 03135/pCA3-AmyA, respectively.
Cultivation using a 2.0-liter bioreactor.
All cultivation experiments were performed in a 2.0-liter bioreactor (Able & Biott, Tokyo, Japan) with a 1.0-liter working volume. The fermentor containing liquid M17 or modified MRS medium (MRS without glucose and Tween 80 for L. plantarum NCIMB 8826, and MRS without glucose for L. casei BLSJ 03135) was heat sterilized (121°C, 15 min). Next, erythromycin (concentrations indicated above) and heat-sterilized glucose solution (to a final concentration of 20 g/liter) were added to the fermentor. Subsequently, 10 M H2SO4 was added to the medium to adjust the pH to 6.0 (for L. lactis IL 1403) or 5.5 (for L. plantarum NCIMB 8826 and L. casei BLSJ 03135). The 10 ml of inoculum (adjusted to an optical density at 600 nm [OD600] of 3.0 with sterile distilled water) was added to the fermentor. Prior to this addition, the inoculum was grown in GM17 or MRS and subcultured at regular intervals (12 h for L. lactis IL 1403 and L. plantarum NCIMB 8826, and 18 h for L. casei BLSJ 03135) to stabilize the growth rate. The temperature was maintained at 30°C or 37°C, homogeneity was maintained by gentle stirring (100 rpm), and the pH was kept at approximately 6.0 or 5.5 (± 0.03) by the automatic addition of 5 M NaOH solution for L. casei BLSJ 03135 or 10 M NH3 solution for other strains. After incubation (10 h for L. lactis IL 1403, 12 h for L. plantarum NCIMB 8826, and 20 h for L. casei BLSJ 03135), the culture was harvested and used for the following analyses.
Western blot analysis.
The culture of each transformant (L. lactis IL 1403 cells harboring pCUS, pMCS-AmyA, pCA-AmyA, pCA2-AmyA, and pCA3-AmyA) was adjusted to an OD600 of 3.0 with 20 mM Tris-HCl (pH 8.0) and centrifuged at 3,500 × g at 4°C for 5 min, and then supernatants were obtained as supernatant fractions. Cell pellets were washed with 20 mM Tris-HCl (pH 8.0) twice. The cell wall fraction of each transformant was extracted according to the method described by Riepe et al. (23). Briefly, each pellet was treated with 4% sodium dodecyl sulfate (SDS) in 20 mM Tris-HCl (pH 8.0) for 90 min at 37°C, followed by 10 min at 100°C, and centrifuged at 3,500 × g at 4°C for 5 min. SDS-extracted cell wall fractions were then recovered as supernatants. Each fraction was mixed with equivalent volume of sample buffer (125 mM Tris-HCl [pH 6.8], 4% [wt/vol] SDS, 10% [vol/vol] 2-mercaptoethanol, 20% [vol/vol] glycerol, and 0.01% [wt/vol] bromophenol blue), and all samples were boiled for 5 min, cooled on ice for 5 min, and subjected to SDS-polyacrylamide gel electrophoresis on 8% acrylamide gels, followed by Western blot analysis as described previously (17). For the detection of AmyA, rabbit immunoglobulin G (IgG) against AmyA and goat anti-rabbit IgG alkaline phosphatase-conjugated antibody (Promega, Madison, WI) were used.
Immunostaining of transformants and flow cytometric analysis.
Immunostaining was performed as described previously (17) with some modifications. The culture of each transformant was collected by centrifugation (3,500 × g at 4°C for 5 min) and washed twice with phosphate-buffered saline (PBS; 50 mM phosphate, 150 mM sodium chloride, pH 7.2). Cells were then resuspended to an OD600 of 10 in PBS containing 1% (wt/vol) bovine serum albumin. The antibodies were preadsorbed for 30 min with cells harboring pCUS to remove nonspecific antibodies against bacterial cells. The cell suspension was incubated with preadsorbed rabbit IgG against AmyA at room temperature for 1.5 h, centrifuged, and washed twice with PBS. After being resuspended in PBS, cells were incubated at room temperature for 1.5 h with preadsorbed goat anti-rabbit IgG antibody conjugated with Alexa Fluor 488 (Molecular Probes, Inc., Eugene, OR). The stained cells were analyzed by flow cytometry as described previously (17).
Measurement of α-amylase activity.
α-Amylase activity was measured using an α-amylase measurement kit, which contained 2-chloro-4-nitrophenyl 65-azido-65-deoxy-β-maltopentaoside (N3-G5-β-CNP) as a substrate (Kikkoman Co., Chiba, Japan). Samples of culture suspensions were periodically withdrawn from the fermentor and centrifuged at 6,000 × g for 5 min. The resulting cell pellets were washed with buffer solution (20 mM citrate-Na2HPO4 buffer, pH 6.0) twice and resuspended in buffer solution. The supernatant and cell suspension were adjusted to an appropriate concentration using buffer solution and used as a sample solution. One unit of α-amylase activity was defined as the amount of enzyme required to release 1 μmol of CNP from N3-G5-β-CNP per minute at 37°C. To determine the activity on cells, the unit of α-amylase activity was interconverted from U/ml culture to U/g dry cell weight by use of a correlation between the OD600 of the culture suspension and dry cell weight.
Measurement of starch-degrading activity.
Starch-degrading activity was measured using soluble starch and raw corn starch (Wako Pure Chemical Industries, Ltd., Osaka, Japan) as substrates. The culture of each transformant was collected by centrifugation (3,500 × g, 4°C, 5 min). The cell pellets were washed with 20 mM sodium acetate buffer (pH 5.6) twice and then lyophilized. The lyophilized cells were suspended in 20 mM sodium acetate buffer (pH 5.6) and mixed with an equivalent volume of 1.2% soluble starch or raw corn starch in 20 mM sodium acetate buffer (pH 5.6). The reaction was performed at 150 oscillations per minute at 45°C in a water bath. The amount of reducing sugar released from the substrate was determined by measuring the number of glucose equivalents by use of the Somogyi-Nelson method (34). One unit of starch-degrading activity was defined as the amount of enzyme required to release 1 μmol of reducing sugar (as the glucose equivalent) from the substrate per minute at 45°C.
RESULTS
Construction of AmyA-secreting and AmyA-displaying plasmids.
Construction of AmyA-secreting and AmyA-displaying plasmids was carried out. As shown in Fig. 1b, the plasmid pMCS-AmyA was constructed for the secretion of AmyA. The plasmids pCA-AmyA, pCA2-AmyA, and pCA3-AmyA were constructed for the display of AmyA by use of cA or cA repeat domains; for these constructions, AmyA was fused to the C terminus of the cA domain. Meanwhile, the plasmid pAmyA-CA3 was constructed for the display of AmyA by use of three cA repeat domains; for this construction, AmyA was fused to the N terminus of the cA domain.
Expression of cA-AmyA fusion proteins on the cell surface.
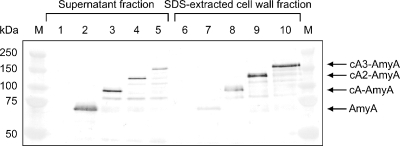
Expression of fusion proteins (cA-AmyA) of the cA domain and AmyA were confirmed by Western blot analysis using anti-AmyA serum. Since the cA domain is known to noncovalently bind to peptidoglycan (30) and to be extracted by SDS (23), cA-AmyA fusion proteins were extracted by SDS after separating the cells from culture. As shown in Fig. 2, AmyA produced by 1403/pMCS-AmyA cells was observed mainly in the supernatant fraction (lanes 2 and 7), whereas cA-AmyA fusion proteins were expressed in the cell wall fraction (lanes 8 to 10) rather than in the supernatant fraction (lanes 3 to 5). Judging from intensity of each band, the amount of cA-AmyA fusion proteins decreased in supernatant fractions and increased in cell wall fractions as the number of cA domains increased (lanes 3 to 5 and 8 to 10).
FIG. 2.
Western blot analysis of AmyA and cA-AmyA fusion proteins from the supernatant fraction (lanes 1 to 5) and the SDS-extracted cell wall fraction (lanes 6 to 10) of genetically modified L. lactis IL 1403 cells. Lanes: M, marker proteins with molecular masses indicated; 1 and 6, cells harboring pCUS; 2 and 7, cells harboring pMCS-AmyA; 3 and 8, cells harboring pCA-AmyA; 4 and 9, cells harboring pCA2-AmyA; 5 and 10, cells harboring pCA3-AmyA. The samples were separated by SDS-polyacrylamide gel electrophoresis with an 8% gel and stained with rabbit anti-AmyA serum, followed by secondary goat anti-rabbit IgG conjugated with alkaline phosphatase.
Flow cytometric analysis.
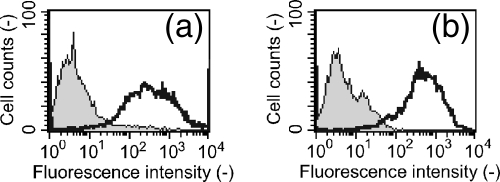
To confirm that AmyA was displayed on the cell surfaces of L. lactis IL 1403, immunofluorescence staining was performed using anti-AmyA serum. The stained cells were analyzed by flow cytometry. As shown in Fig. 3a, the fluorescence intensity was only slightly higher for 1403/pMCS-AmyA cells than for control cells. This may be explained by the small amount of AmyA on the cell surfaces (Fig. 2, lane 7). However, the fluorescence intensity was markedly higher for 1403/pCA-AmyA, 1403/pCA2-AmyA, and 1403/pCA3-AmyA cells (producing cA-AmyA fusion proteins) than for control cells (Fig. 3b, c, and d) and increased significantly as the number of repeat cA domains increased (Fig. 3b, c, and d). This finding suggests that the amount of displayed cA-AmyA increased in parallel with the number of repeat cA domains, which is consistent with the results shown in Fig. 2. These results confirmed that AmyA fused to the C terminus of cA was successfully displayed on the cell surfaces of L. lactis IL 1403 and suggested that the amount of displayed protein depends on the number of cA domains.
FIG. 3.
Flow cytometric analysis of recombinant L. lactis IL 1403 cells. In all figures, gray histograms indicate results for control cells harboring pCUS, and white histograms indicate results for L. lactis IL 1403 cells harboring pMCS-AmyA (a), pCA-AmyA (b), pCA2-AmyA (c), pCA3-AmyA (d), and pAmyA-CA3 (e). Cells were labeled with primary rabbit anti-AmyA serum, followed by secondary goat anti-rabbit IgG conjugated with Alexa Fluor 488. For each experiment, 10,000 cells were analyzed.
Measurement of α-amylase activity.
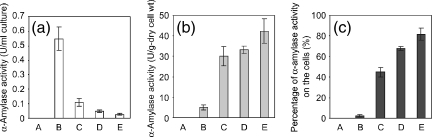
To evaluate the α-amylase activity of the displayed proteins on the surfaces of recombinant L. lactis cells, α-amylase activities in the supernatant and on the cells were compared. To evaluate the activity of α-amylase as a whole-cell biocatalyst, α-amylase activity on the cells was evaluated in U/g dry cell weight. As shown in Fig. 4a and b, no activity was detected for the control cells (1403/pCUS). In the case of the α-amylase-secreting cells (1403/pMCS-AmyA), most activity was in the supernatant (0.55 U/ml) rather than on the cells (4.9 U/g dry cell weight). In contrast, 1403/pCA-AmyA showed α-amylase activity both in the supernatant (0.11 U/ml) and on the cells (30 U/g dry cell weight). For 1403/pCA2-AmyA and 1403/pCA3-AmyA, activity increased further on the cells (33 and 42 U/g dry cell weight, respectively) and decreased further in the supernatant (0.05 and 0.03 U/ml, respectively), suggesting that the cA domain (used as a scaffold) permitted expression of fully active α-amylase on the cell surface and that increasing the number of cA domains increased the number of displayed enzyme molecules. These observations were corroborated by the results from Western blot analysis (Fig. 2) and flow cytometric analysis (Fig. 3). As an indicator of the amount of cA-AmyA fusion proteins bound to L. lactis cells, the α-amylase activity on the cells in relation to total α-amylase activity (given as the sum of activities in supernatant and on the cells in U/ml culture) was calculated. As shown in Fig. 4c, the relative activity retained for the cells was only 45% for 1403/pCA-AmyA but 82% for 1403/pCA3-AmyA cells.
FIG. 4.
Measurement of α-amylase activity by use of L. lactis IL 1403 harboring pCUS (A), pMCS-AmyA (B), pCA-AmyA (C), pCA2-AmyA (D), and pCA3-AmyA (E). (a) α-Amylase activity in culture supernatant. (b) α-Amylase activity on the cell surfaces of recombinant L. lactis IL 1403. (c) The ratio of α-amylase activity on the cells to the total α-amylase activity. The data points represent the averages and standard deviations from three independent experiments.
Effect of the site of the cA domain-AmyA fusion.
To determine the effect of the fusion site, the quantities of proteins displayed by flow cytometry and the enzyme activities of the AmyA-displaying cells toward several substrates were compared for 1403/pCA3-AmyA and 1403/pAmyA-CA3 cells. For 1403/pCA3-AmyA cells, AmyA was fused to the C terminus of the cA domain, while for 1403/pAmyA-CA3 cells, it was fused to the N terminus. As shown in Fig. 3d and e, both types of cells displayed approximately the same quantities of AmyA. α- Amylase and starch-degrading activities were measured in lyophilized cells. Any loss of activity was not observed by lyophilization. As shown in Table 2, α-amylase activity in 1403/pAmyA-CA3 was 84% of that in 1403/pCA3-AmyA when the substrate was N3-G5-β-CNP (50 U versus 60 U/g dry cell weight) or soluble starch (69 U versus 82 U/g dry cell weight). In contrast, when raw starch was used as a substrate, the activity was significantly higher in 1403/pCA3-AmyA (0.11 U/g dry cell weight) than in 1403/pAmyA-CA3 (0.0070 U/g dry cell weight).
TABLE 2.
Effect of fusion site on enzyme activity
| Substrate | Enzyme activity (U/g dry cell wt) for:
|
|
|---|---|---|
| 1403/pAmyA-CA3 | 1403/pCA3-AmyA | |
| N3-G5-β-CNP | 50 | 60 |
| Soluble starch | 69 | 82 |
| Raw corn starch | 0.0070 | 0.11 |
Cell surface display on other lactic acid bacteria.
To determine whether the cA domain will serve as an anchor protein in other lactic acid bacteria, the cell surface display of the cA-AmyA fusion protein was examined for L. plantarum NCIMB 8826 and L. casei BLSJ 03135. Since three repeat cA domains resulted in a high binding capacity to L. lactis IL 1403 (Fig. 4c), pCA3-AmyA was introduced into L. plantarum and L. casei. As shown in Fig. 5, the intensities of fluorescence signals were greater in both 8826/pCA3-AmyA and 03135/pCA3-AmyA cells than in 8826/pCUS and 03135/pCUS (the corresponding control cells). In addition, in both L. plantarum and L. casei, the displayed proteins retained their α-amylase activity (59 and 133 U/g dry cell weight, respectively).
FIG. 5.
Flow cytometric analysis of recombinant lactic acid bacteria. In both figures, gray histograms indicate the results for control cells harboring pCUS, and white histograms indicate the results for L. plantarum NCIMB 8826 cells harboring pCA3-AmyA (a) and L. casei BLSJ 03135 cells harboring pCA3-AmyA (b). Cells were labeled with primary rabbit anti-AmyA serum, followed by secondary goat anti-rabbit IgG conjugated with Alexa Fluor 488. For each experiment, 10,000 cells were analyzed.
DISCUSSION
The aim of this study was to establish a novel cell surface display system which enables the display of target proteins fused to the N and/or C terminus of the anchor protein. We have focused on the cA domain of AcmA from Lactococcus lactis. The cA domain is noncovalently attached to the cell wall after secretion into the supernatant (3), suggesting that both the N and C termini of the cA domain are exposed outside the cell wall. Additionally, unlike AcmA (2, 32), some LysM motif-containing proteins have functional domains located at the C terminus of the LysM, which may be exposed outside the cell wall. These reports (2, 3, 32) prompted us to determine whether heterologous functional proteins bound to the C terminus of the cA domain are displayed and exposed on cell surfaces. Western blot analysis (Fig. 2) and flow cytometric analysis (Fig. 3) clearly showed successful expression and display of cA-AmyA fusion proteins on the cell surfaces of L. lactis IL 1403. Moreover, as shown in Fig. 4, the exposed AmyA retained its activity. These results demonstrated that AmyA on the C terminus of the cA domain could be successfully displayed on the cell surface without loss of its function. In a similar way, we confirmed that AmyA on the N terminus of the cA domain was also exposed on the cell surface (Fig. 3e).
To investigate the effect of the site of the AmyA-cA domain fusion (i.e., N- or C-terminal fusion of the target protein to the anchor protein) on the function of the target protein, cA domain is a significantly useful and attractive anchor protein. As shown in Fig. 3d and e, the quantities of proteins displayed were approximately the same whether the fusion site was on the N or the C terminus. However, as shown in Table 2, when AmyA was fused to the N terminus of the cA domain, 1403/pAmyA-CA3 cells degraded very little raw starch (0.0070 U/g dry cell weight), although AmyA has been shown to degrade raw starch (26). In contrast, 1403/pCA3-AmyA cells displaying AmyA bound to the C terminus of the cA domain could degrade more raw starch (0.11 U/g dry cell weight). These findings suggest that the site of fusion to the target protein significantly affects its function and that the use of the cA domain allows an evaluation of the most suitable position of the fusion protein.
LysM is a common domain of various enzymes located in bacterial cell walls (2). The cA domain has three LysM motifs and binds to various kinds of bacterial cell walls (3, 21, 30). To determine whether cA-assisted cell surface display was functional on other lactic acid bacteria, we expressed the cA-AmyA fusion protein on L. plantarum and L. casei. Figure 5 showed that AmyA with three repeat cA domains was successfully displayed on the cell surfaces of these two strains. Thus, the cA domain is a very useful scaffold to achieve on various lactic acid bacteria, via binding to anchor proteins, the cell surface display of functional heterologous proteins as both N-terminal (3, 13, 21, 22, 30) and C-terminal fusions.
A high capacity of binding to the cell surface of target cells is a particularly desirable feature of anchor proteins that noncovalently bind to the cell wall. For example, Steen et al. (29) reported that the number of LysM repeats in AcmA is an important factor to increase the binding ability of AcmA. Moreover, LysM deletion derivatives of AcmA exhibit significantly reduced binding activity and cell wall-degrading activity (29). In addition, a large quantity of protein is released into culture supernatants even when the three-LysM-repeat form of the cA domain is used (3, 29, 30). This inefficient binding of the cA domain to cell walls has been attributed to cell wall-associated molecules that are known to hinder binding of the cA domain, e.g., lipoteichoic acid. Treatment with trichloroacetic acid and with several other acids has been shown to efficiently remove such cell wall-associated molecules and as a result to increase the binding ratio of the cA domain to the cell wall (3, 30). However, this harsh treatment causes bacterial cell death (3).
To improve the binding capacity of living cells, we proposed the use of cA repeat domains. Ito et al. (10) reported that the cellulose binding ratio and the cellulose-degrading activity of a chimeric protein (repeated cellulose binding domain fused with endoglucanase) were improved by increasing the number of cellulose binding domains. Similar results were reported in the case of starch binding domains (7). From these results, we considered that the substrate binding activity of such binding modules was additive or synergistic. Therefore, we used the cA domain as a basic unit and constructed three types of cA-AmyA fusion proteins (Fig. 1b). One, two, or three cA repeat domains were fused to the N terminus of AmyA and expressed on the cell surface of L. lactis. Figure 4c shows that 82% of the α-amylase activity was retained on the surfaces of L. lactis cells when 1403/pCA3-AmyA (three cA repeat domains) was used, while only 45% of this activity was retained when 1403/pCA-AmyA (one cA domain) was used. These results clearly show that the number of the cA repeat domains is critical to the binding capacity of the cell wall and that the use of tandem cA repeat domains is a significantly advantageous procedure for the applied use of living cells.
Western blot analysis showed the existence of some degraded products of cA-AmyA fusion proteins (Fig. 2). These are likely degradation products resulting from the activity of endogenous surface housekeeping proteases, such as HtrA (19). HtrA degrades AcmA (19) and this breakdown is not observed for htrA-deficient strains (3, 29). To further improve binding capacity, htrA-deficient strains thus represent possible solutions.
In conclusion, we have first succeeded in demonstrating the cell surface display of heterologous protein fused to the C terminus of the cA domain without loss of its function and established a system that enables the display of target proteins regardless of whether attachment is achieved at the N or the C terminus of the cA domain. Additionally, the binding capacity of the heterologous protein was significantly improved by increasing the number of cA domains. Remarkably, the cell surface display system reported here could in principle be used to enhance the immunogenic response by displaying two different antigenic determinants in a live oral vaccine and to perform a sequential enzymatic reaction by displaying two enzymes (one at the C terminus and one at the N terminus) in an enzymatic chain.
Acknowledgments
This work was supported in part by the 2003 Regional Innovative Consortium Project of the Ministry of Economy, Trade and Industry of Japan.
We are grateful to Meiji Dairies Corporation for supplying the pSECE1 plasmid.
Footnotes
Published ahead of print on 21 December 2007.
REFERENCES
- 1.Ashiuchi, M., K. Soda, and H. Misono. 1999. A poly-γ-glutamate synthetic system of Bacillus subtilis IFO 3336: gene cloning and biochemical analysis of poly-γ-glutamate produced by Escherichia coli clone cells. Biochem. Biophys. Res. Commun. 263:6-12. [DOI] [PubMed] [Google Scholar]
- 2.Bateman, A., and M. Bycroft. 2000. The structure of a LysM domain from E. coli membrane-bound lytic murein transglycosylase D (MltD). J. Mol. Biol. 289:1113-1119. [DOI] [PubMed] [Google Scholar]
- 3.Bosma, T., R. Kanninga, J. Neef, S. A. L. Audouy, M. L. van Roosmalen, A. Steen, G. Buist, J. Kok, O. P. Kuipers, G. Robillard, and K. Leenhouts. 2006. Novel surface display system for proteins on non-genetically modified gram-positive bacteria. Appl. Environ. Microbiol. 72:880-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buist, G., J. Kok, K. J. Leenhouts, M. Dabrowska, G. Venema, and A. J. Haandrikman. 1995. Molecular cloning and nucleotide sequence of the gene encoding the major peptidoglycan hydrolase of Lactococcus lactis, a muramidase needed for cell separation. J. Bacteriol. 177:1554-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujita, Y., J. Ito, M. Ueda, H. Fukuda, and A. Kondo. 2004. Synergistic saccharification, and direct fermentation to ethanol, of amorphous cellulose by use of an engineered yeast strain codisplaying three types of cellulolytic enzyme. Appl. Environ. Microbiol. 70:1207-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Georgiou, G., C. Stathopoulos, P. S. Daugherty, A. R. Nayak, B. L. lverson, and R. Curtis III. 1997. Display of heterologous proteins on the surface of microorganisms: from the screening of combinatorial libraries to live recombinant vaccines. Nat. Biotechnol. 15:29-34. [DOI] [PubMed] [Google Scholar]
- 7.Guillén, D., M. Santiago, L. Linares, R. Pérez, J. Morlon, B. Ruiz, S. Sánchez, and R. Rodríguez-Sanoja. 2007. Alpha-amylase starch binding domains: cooperative effects of binding to starch granules of multiple tandemly arranged domains. Appl. Environ. Microbiol. 73:3833-3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansson, M., P. Samuelson, E. Gunneriusson, and S. Ståhl. 2001. Surface display on gram positive bacteria. Comb. Chem. High Throughput Screen. 4:171-184. [DOI] [PubMed] [Google Scholar]
- 9.Holo, H., and I. F. Nes. 1989. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl. Environ. Microbiol. 55:3119-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito, J., Y. Fujita, M. Ueda, H. Fukuda, and A. Kondo. 2004. Improvement of cellulose-degrading ability of a yeast strain displaying Trichoderma reesei endoglucanase II by recombination of cellulose-binding domains. Biotechnol. Prog. 20:688-691. [DOI] [PubMed] [Google Scholar]
- 11.Lee, S. H., J.-I. Choi, S. J. Park, S. Y. Lee, and B. C. Park. 2004. Display of bacterial lipase on the Escherichia coli cell surface by using FadL as an anchoring motif and use of the enzyme in enantioselective biocatalysis. Appl. Environ. Microbiol. 70:5074-5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liljeqvist, S., P. Samuelson, M. Hansson, T. N. Nguyen, H. Binz, and S. Ståhl. 1997. Surface display of the cholera toxin B subunit on Staphylococcus xylosus and Staphylococcus carnosus. Appl. Environ. Microbiol. 63:2481-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindholm, A., A. Smeds, and A. Palva. 2004. Receptor binding domain of Escherichia coli F18 fimbrial adhesin FedF can be both efficiently secreted and surface displayed in a functional form in Lactococcus lactis. Appl. Environ. Microbiol. 70:2061-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsumoto, T., H. Fukuda, M. Ueda, A. Tanaka, and A. Kondo. 2002. Construction of yeast strains with high cell surface lipase activity by using novel display systems based on the Flo1p flocculation functional domain. Appl. Environ. Microbiol. 68:4517-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazmanian, S. K., G. Liu, E. R. Jensen, E. Lenoy, and O. Schneewind. 2000. Staphylococcus aureus sortase mutants defective in the display of surface proteins and in the pathogenesis of animal infections. Proc. Natl. Acad. Sci. USA 97:5510-5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mesnage, S., M. Weber-Levy, M. Haustant, M. Mock, and A. Fouet. 1999. Cell surface-exposed tetanus toxin fragment C produced by recombinant Bacillus anthracis protects against tetanus toxin. Infect. Immun. 67:4847-4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narita, J., K. Okano, T. Kitao, S. Ishida, T. Sewaki, M.-H. Sung, H. Fukuda, and A. Kondo. 2006. Display of α-amylase on the surface of Lactobacillus casei cells by use of the PgsA anchor protein, and production of lactic acid from starch. Appl. Environ. Microbiol. 72:269-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okano, K., S. Kimura, J. Narita, H. Fukuda, and A. Kondo. 2007. Improvement in lactic acid production from starch using α-amylase-secreting Lactococcus lactis cells adapted to maltose or starch. Appl. Microbiol. Biotechnol. 75:1007-1013. [DOI] [PubMed] [Google Scholar]
- 19.Poquet, I., V. Saint, E. Seznec, N. Simoes, A. Bolotin, and A. Gruss. 2000. HtrA is the unique surface housekeeping protease in Lactococcus lactis and is required for natural protein processing. Mol. Microbiol. 35:1042-1051. [DOI] [PubMed] [Google Scholar]
- 20.Pouwels, P. H., R. J. Leer, M. Shaw, M.-J. Heijne den Bak-Glashouwer, F. D. Tielen, E. Smit, B. Martinez, J. Jore, and P. L. Conway. 1998. Lactic acid bacteria as antigen delivery vehicles for oral immunization purposes. Int. J. Food Microbiol. 41:155-167. [DOI] [PubMed] [Google Scholar]
- 21.Raha, A. R., N. R. S. Varma, K. Yusoff, E. Ross, and H. L. Foo. 2005. Cell surface display system for Lactococcus lactis: a novel development for oral vaccine. Appl. Microbiol. Biotechnol. 68:75-81. [DOI] [PubMed] [Google Scholar]
- 22.Ramasamy, R., S. Yasawardena, A. Zomer, G. Venema, J. Kok, and K. Leenhouts. 2006. Immunogenicity of a malaria parasite antigen displayed by Lactococcus lactis in oral immunisations. Vaccine 24:3900-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riepe, H. R., C. J. Pillidge, P. K. Gopal, and L. L. McKay. 1997. Characterization of the highly autolytic Lactococcus lactis subsp. cremoris strains CO and 2250. Appl. Environ. Microbiol. 63:3757-3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samuelson, P., H. Wernérus, M. Svedberg, and S. Ståhl. 2000. Staphylococcal surface display of metal-binding polyhistidyl peptides. Appl. Environ. Microbiol. 66:1243-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Satoh, E., Y. Ito, Y. Sasaki, and T. Sasaki. 1997. Application of the extracellular α-amylase gene from Streptococcus bovis 148 to construction of a secretion vector for yogurt starter strains. Appl. Environ. Microbiol. 63:4593-4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Satoh, E., Y. Nimura, T. Uchimura, M. Kozaki, and K. Komagata. 1993. Molecular cloning and expression of two α-amylase genes from Streptococcus bovis 148 in Escherichia coli. Appl. Environ. Microbiol. 59:3669-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schneewind, O., A. Fowler, and K. F. Faull. 1995. Structure of the cell wall anchor of surface proteins in Staphylococcus aureus. Science 268:103-106. [DOI] [PubMed] [Google Scholar]
- 28.Shigechi, H., J. Koh, Y. Fujita, T. Matsumoto, Y. Bito, M. Ueda, E. Satoh, H. Fukuda, and A. Kondo. 2004. Direct production of ethanol from raw corn starch via fermentation by use of a novel surface-engineered yeast strain codisplaying glucoamylase and α-amylase. Appl. Environ. Microbiol. 70:5037-5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steen, A., G. Buist, G. J. Horsburgh, G. Venema, O. P. Kuipers, S. J. Foster, and J. Kok. 2005. AcmA of Lactococcus lactis is an N-acetylglucosaminidase with an optimal number of LysM domains for proper functioning. FEBS J. 272:2854-2868. [DOI] [PubMed] [Google Scholar]
- 30.Steen, A., G. Buist, K. J. Leenhouts, M. El Khattabi, F. Grijpstra, A. L. Zomer, G. Venema, O. P. Kuipers, and J. Kok. 2003. Cell wall attachment of a widely distributed peptidoglycan binding domain is hindered by cell wall constituents. J. Biol. Chem. 278:23874-23881. [DOI] [PubMed] [Google Scholar]
- 31.Steidler, L., J. Viaene, W. Fiers, and E. Remaut. 1998. Functional display of a heterologous protein on the surface of Lactococcus lactis by means of the cell wall anchor of Staphylococcus aureus protein A. Appl. Environ. Microbiol. 64:342-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turner, M. S., L. M. Hafner, T. Walsh, and P. M. Giffard. 2004. Identification and characterization of the novel LysM domain-containing surface protein Sep from Lactobacillus fermentum BR11 and its use as a peptide fusion partner in Lactobacillus and Lactococcus. Appl. Environ. Microbiol. 70:3673-3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Washida, M., S. Takahashi, M. Ueda, and A. Tanaka. 2001. Spacer-mediated display of active lipase on the yeast cell surface. Appl. Microbiol. Biotechnol. 56:681-686. [DOI] [PubMed] [Google Scholar]
- 34.Wood, T. M., and K. M. Bhat. 1988. Methods for measuring cellulase activities. Methods Enzymol. 160:87-112. [Google Scholar]