Abstract
Intergeneric coaggregation of drinking water bacteria was tested. Acinetobacter calcoaceticus was found not only to autoaggregate but also to coaggregate with four of the five other isolates (Burkholderia cepacia, Methylobacterium sp., Mycobacterium mucogenicum, Sphingomonas capsulata, and Staphylococcus sp.). In its absence, no coaggregation was found. Interactions were lectin-saccharide mediated. The putative bridging function of A. calcoaceticus was evidenced by multispecies biofilm studies, through a strain exclusion process.
The development of microbial biofilm communities results from a series of processes, including initial surface association and adherence, subsequent multiplication of the constituent organisms, the adherence of other species, and the production of extracellular polymeric substances (17). Many of these events are well described (14, 17). The bacterial surface properties coaggregation and coadhesion, along with interspecies relationships, are believed to play a determinant role in the formation of multispecies biofilms in drinking water distribution systems (11, 13). Nevertheless, the function of coaggregation in the initial development of biofilms still remains unclear. This adhesion mechanism is highly specific and conveys advantages to microorganisms, including the transfer of chemical signals, exchange of genetic information, protection from adverse environmental conditions, and metabolic cooperation between different species as well as cell differentiation in some populations (18). Coaggregation is mediated by protein-saccharide interactions and blocked by simple sugars (1, 2). Coaggregation interactions contribute to the development of biofilms via the specific recognition and adhesion of single suspended cells to genetically distinct bacteria in a developing biofilm and/or by the subsequent adhesion of previously coaggregated secondary colonizers to the developing biofilm. In both cases, bacterial cells in suspension specifically adhere to those within biofilms through a coadhesion process (12). This study describes the intergeneric coaggregation of six heterotrophic bacteria isolated from drinking water and investigates the roles of surface proteins and saccharides in the coaggregation process. Acinetobacter calcoaceticus was investigated as a bridging organism in drinking water biofilms.
Coaggregation assays were performed with six representative drinking water bacteria, Acinetobacter calcoaceticus, Burkholderia cepacia, Methylobacterium sp., Mycobacterium mucogenicum, Sphingomonas capsulata, and Staphylococcus sp. The bacteria were isolated, identified by 16S rRNA gene sequencing, and cultivated according to the method of Simões et al. (13). The stationary phase of growth was selected for coaggregation studies (9), and cells from planktonic batch cultures were harvested by centrifugation (20 min at 13,000 × g), washed three times, and resuspended in sterile tap water. A visual coaggregation assay, with some modifications from the method of Cisar et al. (2), was used. Briefly, bacterial suspensions at an optical density at 640 nm of 1.5 were mixed together in pairs by putting equal volumes (2 ml) of each cell suspension at room temperature (23 ± 2°C) in 10-ml rolled glass tubes. The mixtures were then vortexed for 10 s, and the tubes were rolled gently for 30 s. The degree of coaggregation between each pair was assessed visually in a semiquantitative assay, following the scoring scheme originally described by Cisar et al. (2). If specific cell-to-cell recognition occurs, cells coaggregate and settle out. The scoring criteria were as follows: 0, no visible coaggregates in the cell suspension; 1, very small uniform coaggregates in a turbid suspension; 2, easily visible small coaggregates in a turbid suspension; 3, clearly visible coaggregates which settle, leaving a clear supernatant; and 4, very large flocs of coaggregates that settle almost instantaneously, leaving a clear supernatant. Control tubes of each isolate on their own were also included to assess autoaggregation and scored by the same criteria. The coaggregation and autoaggregation scores were evaluated over time (0, 2, 24, and 48 h). Coaggregation was considered to be present when the score in the reaction mixtures was greater than the autoaggregation score of either strain. Bacterial coaggregates were also observed (2 and 24 h) by epifluorescence microscopy using a DNA binding stain, 4,6-diamino-2-phenylindole (DAPI). Aliquots (15 μl) of bacterial autoaggregates and coaggregates were fixed using 2% (vol/vol) formaldehyde (Merck, Germany) and then filtrated through a 25-mm black Nuclepore polycarbonate membrane with a pore size of 0.2 μm (Whatman, United Kingdom). After filtration, bacterial aggregates were stained with 100 μg/ml DAPI (Sigma) for 5 min and preparations were stored at 4°C in the dark until visualization. Bacterial coaggregates were observed according to the procedure described previously by Simões et al. (14).
The surface-associated molecules involved in coaggregation were investigated by heat and protease treatment and sugar reversal tests. The inhibition or reversal of coaggregation was determined as a reduction in the coaggregation score. The inhibition of coaggregation by heat pretreatment of members of coaggregating pairs was performed using a method modified from that of Kolenbrander et al. (5). Heat-treated (80°C, 30 min) and untreated bacterial cells were combined in reciprocal pairs, and the capacity for the cells to coaggregate was assessed by the visual coaggregation assay. The protease sensitivity of the polymers mediating coaggregation on each element of the coaggregating pair was assessed using a modification of the method used by Cookson et al. (3). Protease type XIV from Streptomyces griseus (P5147; Sigma) was added to the cell suspension to a final concentration of 2 mg/ml. Protease pretreatment of bacteria was carried out at 37°C, and cells were harvested after 2 h by centrifugation and washed three times with sterile tap water. The bacterial suspensions were then readjusted to an optical density at 640 nm of 1.5. Protease-treated and untreated cells were mixed, and their abilities to coaggregate were determined using the visual assay. Filter-sterilized solutions of simple sugar [d(+)-galactose, N-acetyl-d-glucosamine, d(+)-fucose, and d(+)-lactose] were added independently to coaggregating pairs to a final concentration of 50 mM. Mixtures were then vortexed and analyzed by the visual coaggregation assay.
Mixed biofilm formation was performed with the six isolates in seven different combinations, one mixture of all six bacteria and six combinations with mixtures of five distinct bacteria, through a strain exclusion process (biofilm formation in the absence of a specific strain, obtaining distinct species combinations). Biofilms were developed according to the modified microtiter plate test proposed by Stepanović et al. (16) using R2A broth as growth medium. For each condition, at least 16 wells of a sterile 96-well flat tissue culture plate (polystyrene; Orange Scientific) were filled under aseptic conditions with 200 μl of a cell suspension mixture (108 cells/ml). Biofilms were developed with equal initial cell densities of each isolate. To promote biofilm formation, plates were incubated aerobically on an orbital shaker at 150 rpm and room temperature for 24, 48, and 72 h. The growth medium was discarded and freshly added every 24 h. Negative controls were obtained by incubating the wells with R2A broth alone. After each biofilm formation period, biofilm mass was quantified using spectrophotometry at 570 nm and crystal violet according to the method of Simões et al. (13). The relative biofilm formation percentage was assessed by comparing biofilms formed by the strain exclusion process relative to biofilms formed by the mixture of all strains. All experiments were performed in triplicate, with three repeats. The data were analyzed by the nonparametric Wilcoxon test based on a confidence level of ≥95%.
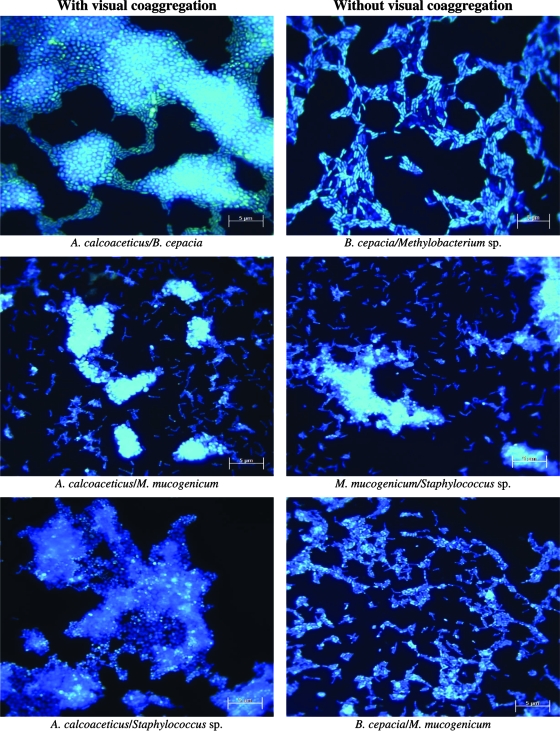
A. calcoaceticus coaggregated with four of the five other bacteria, the exception being Methylobacterium sp. (Table 1). The other bacteria did not coaggregate in the absence of A. calcoaceticus. Coaggregation, after immediate bacterial association, was higher for A. calcoaceticus with Staphylococcus sp., with an invariable score throughout the 48 h of the experiment. A. calcoaceticus with B. cepacia was the only interaction that decreased the coaggregation score after incubation. All other interactions increased coaggregation scores over time. A. calcoaceticus was the only bacterium that autoaggregated, with visible small aggregates (score, 2). Microscopic analysis (Fig. 1) revealed a higher degree of interaction than did the visual assay. This feature was evident for all the interactions, even for autoaggregation. According to Buswell et al. (1), low visual coaggregation scores are not necessarily indicators of weak interaction between cells. The scores detected with this assay are not accurate measures of the relative interaction strength between individual ligands on different cells. Furthermore, these authors proposed that visual coaggregation scores will depend on the relative sizes and morphologies of the bacteria involved and may depend on the densities of interacting ligands on the bacterial surface. A lack of sensitivity associated with the visual assay was also proposed by Elliott et al. (4). Nevertheless, the rapid and simple visual assay provided reproducible results with enough sensitivity to detect significant interactions (1).
TABLE 1.
Coaggregation scores over time of drinking water bacteria by the visual assay
| Time (h) of assay for A. calcoaceticus and indicated partner strain | Coaggregation score for bacteriuma
|
|||||
|---|---|---|---|---|---|---|
| S. capsulata | B. cepacia | M. mucogenicum | Methylobacterium sp. | A. calcoaceticus | Staphylococcus sp. | |
| 0 | 2/3 | 3 | 2/3 | 1/2 | 2 | 3/4 |
| 2 | 3/4 | 2/3 | 3 | 2 | 2 | 3/4 |
| 24 | 4 | 2 | 3 | 2 | 2 | 3/4 |
| 48 | 4 | 2 | 3 | 2 | 2 | 3/4 |
Bold numbers indicate the bacterial interactions with effective coaggregation. Values separated by slashes indicate an intermediate value between the two scores.
FIG. 1.
Microscopy visualizations by epifluorescence microscopy of the distinct interacting drinking water bacteria with and without visual coaggregation. Magnification, ×1,320; bar = 5 μm.
Coaggregation is a highly specific process involving interaction between bacterial surface molecules that act as adhesins and complementary receptors, including proteins and carbohydrates. Heat and protease treatment, when applied to both partners, led to complete coaggregation inhibition (score, 0) for all coaggregation partnerships, except that of A. calcoaceticus and S. capsulata, with a partial inhibition for heat treatment (score, 1). When only one partner was treated, and if it was A. calcoaceticus, the results were similar to those observed when both partners were treated. No disaggregation was verified if the treated partner was one of the other bacteria, except A. calcoaceticus with S. capsulata and A. calcoaceticus with M. mucogenicum (heat and protease) and A. calcoaceticus with B. cepacia (protease). For these cases, the interaction score decreased lightly, translated in a partial disaggregation. The interactions between the tested coaggregation partnerships are apparently mediated by heat- and protease-sensitive adhesins of A. calcoaceticus and heat- and protease-stable interactive sites on the surface of the other bacterium. However, for A. calcoaceticus with S. capsulata and A. calcoaceticus with M. mucogenicum, the results suggest the existence of other types of interactions between heat- and protease-stable receptors in A. calcoaceticus and heat- and protease-sensitive adhesins in S. capsulata and M. mucogenicum.
Heat and protease treatment inhibited A. calcoaceticus autoaggregation. No inhibition was detected when treated cells were mixed 1:1 with untreated cells, (Table 2). This result demonstrates not only that heat- and protease-sensitive proteins (lectins) mediate aggregation between the tested bacteria, but also that other molecules, such as saccharides, that can bind to lectins of untreated cells may be involved. Moreover, this result also suggests that A. calcoaceticus extracellular binding molecules are apparently constituted by lectins and saccharides, therefore increasing the interaction potential with other bacteria (12). In fact, many bacteria have been found to possess proteinaceous adhesins on their surfaces that bind, in a stereochemically specific manner, to complementary molecules/receptors (often saccharides) on the surfaces of other cells of the same or different species (12, 15). The ability of simple sugars to reverse the coaggregation process was not verified for all coaggregating bacterial pairs. For those with reversed coaggregation, interactions were only partially inhibited (Table 3). The addition of simple sugars was expected to reverse lectin-saccharide (protein-carbohydrate)-like interactions. Nevertheless, such interactions are known to be very specific (6). It is possible that neither the selected sugars nor the tested concentrations were appropriate. Kolenbrander et al. (7) found that depending upon the involved bacterial pairs, a varied response to the addition of sugar was observed in the case of potential lectin-saccharide-like coaggregation of oral pathogens. A study by Malik et al. (8) shows that reversibility by simple sugars is not an essential feature of lectin-like interactions. Although the present study could not elucidate the exact nature of the surface molecules involved in coaggregation, the results suggest the possibility of lectin-saccharide-like interactions involvement. This finding is in agreement with previous studies regarding freshwater bacteria (9, 10, 11).
TABLE 2.
Effect of heat and protease treatment on coaggregation scoresa
| A. calcoaceticus treatment type | Coaggregation score for bacterium with indicated partner typeb
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
S. capsulata
|
B. cepacia
|
M. mucogenicum
|
Methylobacterium sp.
|
A. calcoaceticus
|
Staphylococcus sp.
|
|||||||
| UT | T | UT | T | UT | T | UT | T | UT | T | UT | T | |
| Heat | ||||||||||||
| UT | 4 | 3 | 2 | 2 | 3 | 2 | 2 | 2 | 2 | 2 | 3/4 | 3/4 |
| T | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| Protease | ||||||||||||
| UT | 4 | 2 | 2 | 1/2 | 3 | 2 | 2 | 2 | 2 | 2 | 3/4 | 3/4 |
| T | 0/1 | 0 | 0 | 0 | 0/1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
Shown are results for when each partner was pretreated separately with heat and protease and then mixed with either a treated (T) or an untreated (UT) partner.
Values separated by slashes indicate an intermediate value between the two scores.
TABLE 3.
Reversal of coaggregation using simple sugars
| A. calcoaceticus coaggregation partner | Result for sugara
|
|||
|---|---|---|---|---|
| d(+)-Galactose | N-Acetyl-d-glucosamine | d(+)-Fucose | d(+)-Lactose | |
| S. capsulata | + | − | + | + |
| B. cepacia | − | − | − | − |
| M. mucogenicum | − | − | − | − |
| Methylobacterium sp. | + | − | + | + |
| A. calcoaceticus | − | − | − | − |
| Staphylococcus sp. | − | − | − | − |
++, complete disaggregation; +, partial disaggregation; −, no disaggregation.
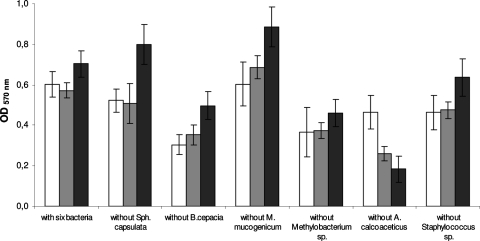
In order to ascertain the putative bridging function of A. calcoaceticus in drinking water bacterial interactions, multispecies biofilm formation was carried out with the six isolates (Fig. 2). Biofilm mass increased over time, except for multispecies biofilms without A. calcoaceticus. Only those without Methylobacterium sp. had similar biomass amounts for the three sampling times (P > 0.05). The combination excluding M. mucogenicum formed the highest biofilm mass (P < 0.05). Bacterial combinations without A. calcoaceticus exhibited the smallest productivity for 48 and 72 h (P < 0.05), while at 24 h it was the bacterial combination without B. cepacia. To better understand the function of each bacterium in multispecies biofilm formation, the relative percentage of biofilm formation by the combination of all six drinking water bacteria was assessed in the strain exclusion tests and compared with the multispecies biofilms formed by the six bacteria (Table 4). M. mucogenicum was the only bacterium that when not present led to a relative increase of biofilm mass over time compared to the level of biofilm formation with all six bacteria. The remaining bacteria reduced biofilm formation. The decrease of biofilm mass formation was less significant (P > 0.05) for biofilms in the absence of S. capsulata (24 and 48 h) and Staphylococcus sp. (72 h) and more significant (P < 0.05) in the absence of B. cepacia (24 h) and A. calcoaceticus (48 and 72 h). Nevertheless, even if the relative biofilm formation decreased for five of the six strain exclusion scenarios, it was only significant (P < 0.05) and decreased over time (P < 0.05) for biofilms without A. calcoaceticus. This result provides additional evidence concerning the role of A. calcoaceticus in drinking water microbial ecosystems. In fact, A. calcoaceticus coaggregated with the other drinking water bacteria tested, suggesting the ability to form multigeneric coaggregates and a potential bridging function, in a manner similar to those of Fusobacterium sp. and Prevotella sp. in dental plaque accretion (13, 15). Similarly, Rickard et al. (10) reported similar findings for Blastomonas natatoria in freshwater bacterial communities. An Acinetobacter johnsonii strain has also been proposed as a bridging bacterium in an activated sludge microflora (8). Such bridging microorganisms are believed to carry complementary receptors recognized by functionally similar adhesins on cells from distinct genera (8).
FIG. 2.
Values of optical density at 570 nm (OD570) as a measure of multispecies biofilm mass for 24 h (□), 48 h (▒), and 72 h (▪). The means ± standard deviations (error bars) for at least three replicates are illustrated.
TABLE 4.
Relative multispecies biofilm formation over time
| Bacteria present in multispecies biofilma | Relative (%) biofilm formation at indicated time (h):
|
||
|---|---|---|---|
| 24 | 48 | 72 | |
| All six bacteria | 100 | 100 | 100 |
| All except: | |||
| S. capsulata | 86.6 | 88.8 | 114 |
| B. cepacia | 50.6 | 61.7 | 70.7 |
| M. mucogenicum | 100 | 120 | 126 |
| Methylobacterium sp. | 60.5 | 65.2 | 65.3 |
| A. calcoaceticus | 76.8 | 45.3 | 26.1 |
| Staphylococcus sp. | 76.8 | 83.0 | 90.5 |
Members of the group of six bacteria studied were S. capsulata, B. cepacia, M. mucogenicum, Methylobacterium sp., A. calcoaceticus, and Staphylococcus sp.
In conclusion, to our knowledge, this is the first report demonstrating that A. calcoaceticus performs a bridging function in drinking water biofilm formation. This bacterium coaggregated with almost all other tested bacteria, and its presence in a multispecies community represents a colonization advantage. This bacterium may facilitate the association of the other species that do not coaggregate directly with each other, increasing the opportunity for metabolic cooperation. The presence or the absence of A. calcoaceticus in multispecies biofilms can therefore enhance or decrease, respectively, biofilm formation by drinking water-isolated bacteria.
Acknowledgments
We acknowledge the financial support provided by the Portuguese Foundation for Science and Technology (SFRH/BD/31661/2006 to Lúcia C. Simões and SFRH/BPD/20582/2004 to Manuel Simões).
Footnotes
Published ahead of print on 21 December 2007.
REFERENCES
- 1.Buswell, C. M., Y. M. Herlihy, P. D. Marsh, C. W. Keevil, and S. A. Leach. 1997. Coaggregation amongst aquatic biofilm bacteria. J. Appl. Microbiol. 83:477-484. [Google Scholar]
- 2.Cisar, J. O., P. E. Kolenbrander, and F. C. McIntire. 1979. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect. Immun. 24:742-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cookson, A. L., P. S. Handley, A. E. Jacob, K. G. Watson, and C. Allison. 1995. Coaggregation between Prevotella nigrescens and Prevotella intermedia with Actinomyces naeslundii strains. FEMS Microbiol. Lett. 132:291-296. [Google Scholar]
- 4.Elliott, R. D., M. Wilson, C. M. F. Buckley, and D. A. Spratt. 2006. Aggregative behaviour of bacteria isolated from canine dental plaque. Appl. Environ. Microbiol. 72:5211-5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolenbrander, P. E., R. N. Andersen, and L. V. Holdeman. 1985. Coaggregation of oral Bacteroides species with other bacteria: central role in coaggregation bridges and competitions. Infect. Immun. 48:741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolenbrander, P. E. 1989. Surface recognition among oral bacteria: multigeneric coaggregations and their mediators. Crit. Rev. Microbiol. 17:137-158. [DOI] [PubMed] [Google Scholar]
- 7.Kolenbrander, P. E., K. D. Parrish, R. N. Andersen, and E. P. Greenberg. 1995. Intergeneric coaggregation of oral Treponema spp. with Fusobacterium spp. and intrageneric coaggregation among Fusobacterium spp. Infect. Immun. 63:4584-4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malik, A., M. Sakamoto, S. Hanazaki, M. Osawa, T. Suzuki, M. Tochigi, and K. Kakii. 2003. Coaggregation among nonflocculating bacteria isolated from activated sludge. Appl. Environ. Microbiol. 69:6056-6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rickard, A. H., A. L. Stephen, C. M. Buswell, N. J. High, and P. S. Handley. 2000. Coaggregation between aquatic bacteria is mediated by specific-growth-dependent lectin-saccharide interactions. Appl. Environ. Microbiol. 66:431-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rickard, A. H., S. A. Leach, L. S. Hall, C. M. Buswell, N. J. High, and P. S. Handley. 2002. Phylogenetic relationships and coaggregation ability of freshwater biofilm bacteria. Appl. Environ. Microbiol. 68:3644-3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rickard, A. H., A. J. McBain, R. G. Ledder, P. S. Handley, and P. Gilbert. 2003. Coaggregation between freshwater bacteria within biofilm and planktonic communities. FEMS Microbiol. Lett. 220:133-140. [DOI] [PubMed] [Google Scholar]
- 12.Rickard, A. H., P. Gilbert, N. J. High, P. E. Kolenbrander, and P. S. Handley. 2003. Bacterial coaggregation: an integral process in the development of multispecies biofilms. Trends Microbiol. 11:94-100. [DOI] [PubMed] [Google Scholar]
- 13.Simões, L. C., M. Simões, and M. J. Vieira. 2007. Biofilm interactions between distinct bacterial genera isolated from drinking water. Appl. Environ. Microbiol. 73:6192-6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simões, M., M. O. Pereira, S. Sillankorva, J. Azeredo, and M. J. Vieira. 2007. The effect of hydrodynamic conditions on the phenotype of Pseudomonas fluorescens biofilms. Biofouling 23:249-258. [DOI] [PubMed] [Google Scholar]
- 15.Skillman, L. C., I. W. Sutherland, and M. V. Jones. 1999. The role of exopolysaccharides in dual species biofilm development. J. Appl. Microbiol. 85:13S-18S. [DOI] [PubMed] [Google Scholar]
- 16.Stepanović, S., D. Vuković, I. Davić, B. Savić, and M. Ŝvabić-Vlahović. 2000. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 40:175-179. [DOI] [PubMed] [Google Scholar]
- 17.Stoodley, P., K. Sauer, D. G. Davies., and J. W. Costerton. 2002. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56:187-209. [DOI] [PubMed] [Google Scholar]
- 18.Wimpenny, J., and R. Colasanti. 2004. A simple automaton model for coaggregation. Biofilms 1:369-375. [Google Scholar]