Abstract
Noroviruses, which are members of the Caliciviridae family, represent the leading cause of nonbacterial gastroenteritis in developed countries; such norovirus infections result in high economic costs for health protection. Person-to-person contact, contaminated water, and foods, especially raw shellfish, vegetables, and fruits, can transmit noroviruses. We inactivated feline calicivirus, a surrogate for the nonculturable norovirus, in cell culture medium and mineral water by heat and high hydrostatic pressure. Incubation at ambient pressure and 75°C for 2 min as well as treatment at 450 MPa and 15°C for 1 min inactivated more than 7 log10 PFU of calicivirus per ml in cell culture medium or mineral water. The heat and pressure time-inactivation curves obtained with the calicivirus showed tailing in the logarithmic scale. Modeling by nth-order kinetics of the virus inactivation was successful in predicting the inactivation of the infective virus particles. The developed model enables the prediction of the calicivirus reduction in response to pressures up to 500 MPa, temperatures ranging from 5 to 75°C, and various treatment times. We suggest high pressure for processing of foods to reduce the health threat posed by noroviruses.
The former Norwalk, Norwalk-like, and small-round-structured viruses have been placed together with Snow Mountain viruses in the family of Caliciviridae as the genus Norovirus. Noroviruses are nonenveloped, round particles with a diameter of 27 to 40 nm. Their genome consists of a single-stranded, positive-sense RNA of 7,300 to 8,300 bp surrounded by a protein capsid. The virus genome contains three open reading frames, with at least one encoding a polyprotein that is proteolytically processed in the host cell to mature viral proteins necessary for replication and packaging of the viral genome (18, 25, 42, 46).
The Norwalk virus was first recognized in a school outbreak in Norwalk, Ohio, in 1968 in which water was suspected but not proven as the source (1). During the fall of 2001, a waterborne outbreak of norovirus gastroenteritis occurred in the state of Wyoming. Sewage-contaminated ground water was the source of the human caliciviruses, affecting around 84 victims (42). One of the largest nationwide foodborne outbreaks affected more than 2,000 people in Australia as a result of the consumption of raw oysters contaminated by norovirus (39). Other norovirus outbreaks have been traced to fruits, vegetables, lettuce, potato salad, sandwiches, pastas, ice, cold cooked ham, and raw shellfish (2, 9, 17, 46). Ill or asymptomatic infected food handlers were found to be the source of several norovirus outbreaks. The virus has been detected in uncarbonated mineral water (7) and raw shellfish (34). Noroviruses are now the leading cause of gastroenteritis in the United States, with an estimated 23 million cases per year (11).
Norovirus gastroenteritis is characterized by a sudden onset of vomiting and/or diarrhea, more frequently affecting people older than 69 years and infants up to 5 years of age than other age groups. The average incubation period is 24 to 48 h, and the duration of illness ranges from 12 to 60 h. The most common symptoms include nausea, vomiting, abdominal cramps, and diarrhea. Norovirus infections are self-limiting and usually cause no complications. However, it has been estimated that approximately 6.9% (37) to 11% (49) of all food-related deaths are caused by this virus. Most outbreak cases occurred in closed community situations, predominantly in fall and winter, such as hospitals, nursing homes, kindergartens, schools, hotels, and cruise ships. High attack rates of 50% to 90% and rapid secondary person-to-person spread can prolong these outbreaks, together with the low infectious dose of 10 to 100 virus particles (10, 25).
Noroviruses are related to sapoviruses, formerly described as the “Sapporo-like viruses,” which form the second of four genera of the Caliciviridae family. Sapoviruses cause gastroenteritis but rarely an epidemic of the kind seen with noroviruses (24). In comparison to noroviruses, sapoviruses are rarely transmitted by contaminated food (40).
The Norwalk virus was not affected by 3.75 ppm chlorine in drinking water, as shown in a volunteer study (31). However, noroviruses appear to be heat sensitive and so cooking inactivates them (45), since heating at 60°C for 30 min was insufficient to inactivate a norovirus completely (14). Detailed conditions of heat and/or high-pressure inactivation of human noroviruses remain unknown because of the inability of noroviruses infecting humans to be grown in tissue culture and the lack of a susceptible animal model. Immune electron microscopy, enzyme-linked immunoassays, and reverse transcription-PCR, currently the available detection methods, do not verify the infectivity of norovirus particles. However, related caliciviruses, especially feline caliciviruses, are culturable, share the genome organization of human noroviruses, and exhibit sequence similarities (29). Therefore, feline calicivirus was examined as a model system for human caliciviruses. Doultree et al. (15) studied the heat sensitivity of feline calicivirus and achieved a significant population reduction of this virus at and above 70°C.
High hydrostatic pressure, a nonthermal, additive-free preservation method, can be used to inactivate viruses while foods retain organoleptic quality almost identical to that of the raw product (22). Nonenveloped viruses show a wider range of sensitivities in response to high hydrostatic pressure than enveloped viruses (22). Although inactivation of human norovirus cannot be determined by examining its propagation, Chen et al. (12) described the related feline calicivirus as barosensitive when treated at 200 MPa. Recently, Kingsley et al. (33) found that a murine norovirus is inactivated in oysters when exposed to pressures higher than 300 MPa at refrigeration temperatures. The genogroup of this murine virus strain differs from that of human noroviruses (30).
To our knowledge, this report represents the first description of a predictive model for the inactivation of a calicivirus by a combination of high hydrostatic pressure and temperature. The inactivation of feline calicivirus in a cell culture medium and still mineral water by high hydrostatic pressure and heat is predicted by an empirically approximated nth-order reaction model.
MATERIALS AND METHODS
Propagation of feline calicivirus.
Uwe Truyen (Universität Leipzig, Leipzig, Germany) kindly provided FCV KS20 and Crandell-Reese feline kidney (CRFK) cells (13) for its propagation. The cells were grown in Dulbecco's modified Eagle medium (Invitrogen, Karlsruhe, Germany) supplemented with nonessential amino acids (Invitrogen), 44 mM sodium bicarbonate, 10% fetal calf serum (Biochrom, Berlin, Germany), 100,000 IU penicillin G (Grünenthal, Aachen, Germany), and 100 mg streptomycin sulfate (Sigma, Deisenhofen, Germany) per liter, which is referred to as cell culture medium in the paper. The CRFK cell culture was incubated in a 175 cm2 tissue culture flask (Sarstedt, Nümbrecht, Germany) at 37°C in a 5% CO2 humidified atmosphere. The spent supernatant was poured off, and the cells were rinsed three times with phosphate-buffered saline (PBS) when the cells were at 70% confluence.
One milliliter of the virus suspension, containing 109 to 1010 PFU, was added to CRFK cells in 75 ml sterile cell culture medium. The cells detached after incubation for 24 h. Subsequently, the cell debris was separated by centrifugation for 10 min at 800 rpm. The supernatant was stored at −80°C and used as virus stock.
Thermal inactivation of feline calicivirus.
Isothermal inactivation of strain FCV KS20 at ambient pressure was carried out in 0.2 ml reaction tubes containing 90 μl of preheated media and 10 μl of the virus suspension in a thermocycler (Biometra, Göttingen, Germany). A thermocouple wire (Conrad, Hirschau, Germany) (0.8 mm) was used to measure the temperature of the suspension. Before treatment, the time required to achieve the desired temperature was ascertained. Two different suspension media were used for heat and high-pressure inactivation studies: (i) cell culture medium and (ii) sterile filtered still mineral water sourced from a local retail market. For heat and high-pressure treatments, the virus-containing suspensions (107 to 108 PFU ml−1) were immediately mixed with 9 volumes of prewarmed sterile cell culture medium or sterile filtered uncarbonated mineral water.
After preset time intervals during the thermal inactivation experiments, the samples were withdrawn and immediately cooled in ice water. In order to obtain kinetics at close to isothermal conditions the initial titer (N0) was defined as the number of the viruses found when heating a sample to target temperature in approximately 20 s and immediately cooling it in ice water. Thermal inactivation of strain FCV KS20 was examined from 50 to 75°C.
Combined pressure-temperature inactivation of feline calicivirus.
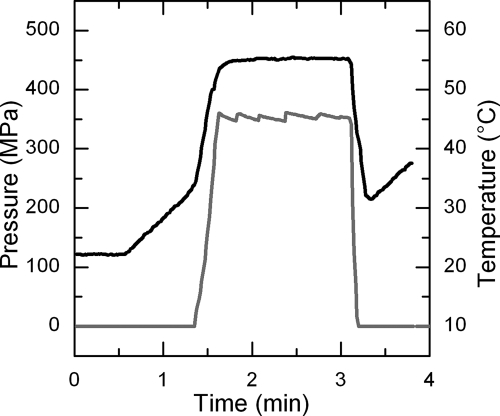
Nearly isothermal conditions (±1°C) were achieved by using laboratory-scale multivessel, high-pressure equipment (catalog no. U111; Unipress, Warsaw, Poland) described elsewhere (3). The target temperature was adjusted between 10 and 65°C, and the pressure ranged from 100 to 500 MPa (Table 1 and 2). Freshly prepared virus suspensions were transferred into 1.65 ml flexible cryotubes (Nunc, Roskilde, Denmark). The samples were placed into the pressure vessels preheated to target temperature. Pressurization was started when the sample reached a temperature level that was calculated to result in the target temperature after adiabatic heating (in aqueous media, approximately 3°C per 100 MPa) during the compression phase. The pressurization rate was standardized at 25 MPa s−1 in order to minimize the loss of virus during the compression phase. The timer for measuring the dwell time was immediately started after commencing the compression to the target pressure-temperature conditions. Figure 1 shows the pressure and temperature profiles of a sample treated at 350 MPa and 55°C for 90 s. The initial titer (N0) was defined as the number of virus particles (in PFU per milliliter) found immediately after pressure buildup and pressure release. As with the approach used for isothermal inactivation kinetic studies, this titer definition was chosen as a means of eliminating the effect of altering pressure conditions during the compression and decompression phases, which do not allow kinetic analysis under isothermal-isobaric conditions.
TABLE 1.
Specific rate constant for combined pressure-temperature inactivation of feline calicivirus FCV KS20 in cell culture medium determined using a reaction order n = 1.2
| MPa |
k′ (10−1 s−1) ± SE of regression at indicated temp (°C)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 10 | 20 | 25 | 30 | 40 | 50 | 55 | 60 | 65 | 70 | 75 | |
| 0.1 | NDa | ND | ND | ND | ND | ND | 0.029 ± 0.004 | 0.343 ± 0.038 | 1.454 ± 0.388 | 4.980 ± 0.599 | 10.297 ± 1.528 | 42.919 ± 2.412 |
| 100 | ND | ND | ND | ND | ND | ND | ND | ND | ND | 9.739 ± 0.397 | ND | ND |
| 200 | 0.467 ± 0.085 | 0.431 ± 0.179 | ND | 0.215 ± 0.028 | ND | 0.268 ± 0.105 | ND | 2.450 ± 0.107 | ND | ND | ND | ND |
| 250 | 2.058 ± 0.328 | 1.497 ± 0.318 | ND | ND | ND | 0.951 ± 0.086 | ND | 4.425 ± 0.407 | ND | ND | ND | ND |
| 300 | ND | 2.445 ± 0.678 | ND | 1.423 ± 0.197 | ND | 4.492 ± 0.397 | ND | 9.066 ± 0.576 | ND | ND | ND | ND |
| 350 | ND | ND | 2.645 ± 0.104 | ND | ND | ND | ND | 31.410 ± 0.465 | ND | ND | ND | ND |
| 400 | ND | ND | 5.729 ± 0.584 | ND | 3.649 ± 0.445 | 10.483 ± 0.889 | ND | ND | ND | ND | ND | ND |
| 450 | ND | ND | 17.644 ± 0.430 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 500 | ND | ND | ND | ND | 43.249 ± 0.240 | ND | ND | ND | ND | ND | ND | ND |
ND, not determined.
TABLE 2.
Specific rate constants for combined pressure-temperature inactivation of feline calicivirus FCV KS20 in mineral water determined using a reaction order n = 1.25
| MPa |
k′ (10−1 s−1) ± SE of regression at indicated temp (°C)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 10 | 15 | 25 | 30 | 40 | 50 | 55 | 60 | 65 | 70 | 75 | |
| 0.1 | NDa | ND | ND | ND | ND | ND | 0.076 ± 0.007 | 0.681 ± 0.025 | 2.066 ± 0.296 | 5.607 ± 1.266 | 10.694 ± 1.603 | 29.631 ± 2.335 |
| 100 | 0.006 ± 0.001 | ND | ND | ND | ND | ND | ND | 0.690 ± 0.012 | ND | 20.921 ± 1.766 | ND | ND |
| 200 | 0.908 ± 0.043 | ND | ND | 0.248 ± 0.018 | ND | 0.416 ± 0.065 | ND | 2.710 ± 0.190 | ND | ND | ND | ND |
| 250 | 12.031 ± 2.077 | ND | ND | 2.224 ± 0.229 | ND | 2.104 ± 0.103 | ND | 7.105 ± 1.667 | ND | ND | ND | ND |
| 300 | ND | 33.701 ± 6.780 | 8.001 ± 0.8784 | ND | ND | 10.421 ± 1.497 | ND | 108.164 ± 22.839 | ND | ND | ND | ND |
| 350 | ND | ND | ND | 21.513 ± 1.833 | 18.060 ± 3.606 | ND | ND | ND | ND | ND | ND | ND |
| 400 | ND | ND | ND | ND | 86.423 ± 5.177 | 43.305 ± 7.345 | ND | ND | ND | ND | ND | ND |
ND, not determined.
FIG. 1.
Pressure (gray line) and temperature (black line) profile of a sample treated at 350 MPa and 55°C for 90 s.
After decompression, the sample tubes were stored on ice until quantitative determination of the virus titer was performed. Three untreated samples were used as controls to determine the initial value of the virus titer (in PFU per milliliter).
Quantitative determination of feline calicivirus levels by plaque assay.
Following high pressure or heat treatment, surviving virus particles were quantitated by plaque assays using CRFK cells. The high-pressure- and heat-treated virus samples were serially diluted in the viral dilution solution (phosphate-buffered saline supplemented with 0.1 g magnesium chloride, 0.132 g calcium chloride, 100,000 IU penicillin G, and 0.1 g streptomycin sulfate per liter). After confluent growth of the CRFK cells in six-well plates (Biochrom), all culture medium was removed and 800 μl of the virus dilutions was added. Culture plates were rocked gently after inoculation and incubated for 60 min at room temperature. The supernatant of the culture was removed, and the cells were subsequently covered with 3 ml overlay agar at 45°C. This agar was prepared from 2× modified Eagle medium containing Earle's salts and l-glutamine without sodium bicarbonate (Invitrogen), 1% DEAE dextran (Amersham, Freiburg, Germany), 5% sodium bicarbonate, and 3% purified agar (Oxoid, Wesel, Germany). The overlay agar was carefully removed after 48 to 72 h, and the cells were stained with 2.5 g Coomassie brilliant blue R250 dissolved in 45% deionized water, 45% methanol, and 10% acetic acid for 5 min. The staining solution was removed prior to plaque counting.
Kinetic data analysis and modeling of virus inactivation.
From the analysis of three independent inactivation experiments under nearly isobaric and isothermal conditions, the inactivation of the feline calicivirus was determined by use of an nth-order reaction scheme (equation 1). This allowed an appropriate description of the inactivation kinetics which showed significant deviations from those of the first-order reactions:
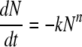 |
(1) |
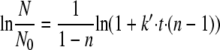 |
(2) |
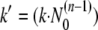 |
(3) |
where t represents time in minutes, N represents the number of survivors at time t in PFU per milliliter, N0 represents the initial virus count in PFU per milliliter, k represents the rate constant per second, and n represents the reaction order.
Integration of equation 1 yields equation 2, introducing the specific inactivation rate constant k′, which is dependent on the initial virus count N0 (equation 3).
The reaction order n was determined by minimizing the sum of the mean square errors (MSEs) over a wide range of reaction orders, i.e., averaging the predictive errors in all of the individual kinetics of the complete experimental kinetic data set. Upon fixing the reaction order, the specific inactivation rate constant k′ was obtained regressively (TableCurve 2D statistical package, version 4.0; Systat Software Inc., Richmond, CA) by fitting the kinetic data to equation 2 and assuming that N0 was constant at all experiments performed. Setting k′ independent of N0 was done to obtain comparable values for the inactivation rate, which can be used for secondary modeling. This approximation has only a minor effect on the predictive quality of the model, since variation of n is required only for reaction order identification.
In the present study an empirical polynomial equation (equation 4) was successfully applied to describe the specific rate constant k′ (per second) as a function of pressure and temperature:
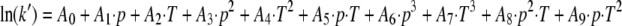 |
(4) |
The parameters A0, A1, A2, A3, A4, A5, A6, A7, A8, and A9 were estimated by regression analysis using a statistical program (TableCurve 3D statistical package, version 3; Systat Software Inc., Richmond, CA). Smeller and Heremans (48) showed that the main form of the elliptical shape of the transition line remains when third-order terms are added to second-order polynomial equations but that the shape of the curve is distorted. Therefore, the significance of the model parameters used in equation 4 was assessed by determining the MSE:
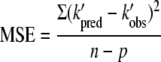 |
(5) |
where n is the number of kinetics used for the model, p is the number of model parameters, k′pred is the predicted inactivation rate constant, and k′obs is the observed inactivation rate constant under certain pressure-temperature combinations. The MSE is the difference between the observed and the predicted values in the model and is usually used in statistics to determine whether the model fits the data or whether the model can be simplified by removing terms. Typically, the smaller the MSE values, the better the fit of the model to the data. Hence, starting from equation 4, third-order model parameters were eliminated until a minimum MSE was found.
The performance of the secondary model (equation 4) was also assessed by calculation of the accuracy factor (Af) (5), which is a simple, multiplicative factor indicating the spread of results around the predicted result:
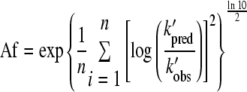 |
(6) |
where the terms are as previously defined. The smaller the Af value, the more accurate are the absolute errors in the model. A value of 1 indicates the perfect agreement of the model to the data fitted.
RESULTS
Thermal inactivation of feline calicivirus.
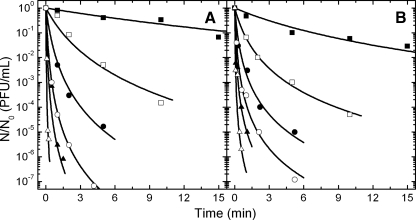
Figure 2A shows the isothermal inactivation kinetics of FCV KS20 in cell culture medium at an ambient pressure between 50 and 75°C. Although the heating phase with an approximate duration of 20 s only minimally reduced the virus titer, it has been taken into account for the calculation of values representing relative reduction in infectivity (N × N0−1). FCV KS20 remained infectious in cell culture medium at up to 45°C (data not shown) and was inactivated by approximately 1 log after 15 min of treatment at 50°C. Higher temperatures accelerated the inactivation reaction. After incubation at 70°C for 90 s, the initial infectivity of approximately 1.5 × 107 PFU FCV KS20 per ml was reduced by 6 log. Figure 2B presents inactivation curves of FCV suspended in mineral water. FCV did not show clear differences between its thermostability in cell culture medium and its thermostability in mineral water (Fig. 2).
FIG. 2.
Thermal inactivation of feline calicivirus FCV KS20 in cell culture medium (A) and still mineral water (B). The symbols denote the applied temperature as follows: ▪, 50°C; □, 55°C; •, 60°C; ○, 65°C; ▴, 70°C; Δ, 75°C.
Combined pressure-temperature inactivation of feline calicivirus.
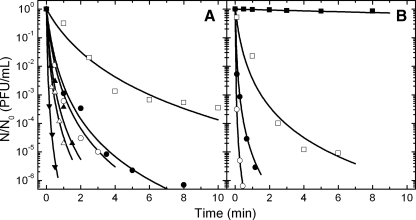
Combined pressure and temperature treatments of FCV KS20 in cell culture medium were conducted between 5 and 65°C and 0.1 and 500 MPa. Inactivation kinetics of FCV at 15°C and 300 to 500 MPa are shown in Fig. 3. The typical compression period, with an approximate duration of 30 s, followed by an immediate rapid decompression already produced a significant reduction in virus numbers of up to 2 log (data not shown), depending on the pressure and temperature applied, which was taken into account for the calculation of relative virus titer values. FCV KS20 was found to be quite pressure sensitive, showing high reductions in titer at 200 MPa and above at 5°C. In mineral water, FCV was stable at 100 MPa and a reduction in titer of approximately 5 log was observed when the virus was treated at 200 MPa and 5°C for 6 min (Fig. 3B). In cell culture medium, FCV appeared to be more resistant to pressure since the same treatment at 200 MPa reduced the titer by 3 log (Fig. 3). Results from treatments performed at higher pressures, for example, 300 MPa for 0.5 min at 10°C, were sufficient to inactivate the virus by 2 and 6 log in cell culture medium and mineral water, respectively (Fig. 3), indicating that higher pressures significantly accelerated the inactivation of FCV KS20.
FIG. 3.
Pressure inactivation of feline calicivirus FCV KS20. Isothermal-isobaric kinetics show the virus inactivation in cell culture medium (A) and in still mineral water (B) at 5°C and 100 (▪), 200 (□), and 250 (•) MPa, at 10°C and 300 MPa (○), and at 20°C and 350 (▴), 400 (Δ), and 450 (▾) MPa.
Modeling the inactivation of feline calicivirus.
Isothermal and isobaric inactivation curves for FCV KS20 typically showed a tailing in the logarithmic plot (Fig. 2 and 3) and thus deviations from log linearity. This is in agreement with the observations of Chen et al. (12) and Kingsley et al. (33), who found good fitting of high-pressure-inactivation kinetics of FCV and a murine norovirus by use of the Weibull and log-logistic models. In our case the Weibull model (43) gave a good description of a single kinetic but showed a poor functional relationship with pressure and temperature in the secondary model approach. In further modeling we therefore used a simple nth-order reaction model to describe the change of virus titer with time under isothermal-isobaric conditions (equation 1). The reaction order n of the virus inactivation was determined by minimizing the cumulative standard error of fit over a range of reaction orders (from 1.0 to 1.65), i.e., by averaging the predictive error for all individual kinetics at all pressure-temperature combinations tested. A minimum value for the error function was found using n = 1.2 and n = 1.25 for FCV KS20 in cell culture medium and in mineral water, respectively. After the reaction order was fixed, the specific inactivation rate constant (k′) was the only parameter remaining and could be estimated in a global approach using linear regression analysis (equations 1 and 2). In Fig. 2 and 3, the lines interpolating the experimental data points of relative virus PFU show the fit of 1.2- and 1.25-order kinetics, respectively. The specific kinetic constants (k′) of all tested pressure-temperature combinations are shown in Table 1 and 2 for FCV KS20 inoculated in cell culture medium and in mineral water, respectively. It is evident from these tables that FCV KS20 inactivation rate constant was reduced when high pressures were applied at temperatures in a range of 20 to 40°C.
The effect of pressure and temperature on the inactivation rate constant k is often expressed by mathematical models combining the equations of Arrhenius and Eyring. However, empirical equations based on Hawley's description of the thermodynamics of the phase boundary of protein unfolding (26) have also been proposed to effectively describe rate constants as a function of pressure and temperature (8, 28, 36). In this study, equation 4 provided a functional relationship that sufficiently fitted the obtained k values found for all pressure-temperature combinations tested. However, it was found that some variables in equation 2 did not significantly contribute to both models of FCV KS20 in cell culture medium and mineral water. Hence, to simplify the model, terms that contributed less than 1% of the total fit standard error for the data set were removed as appropriate. The model parameters in Table 3 were estimated by nonlinear regression fitting of equation 2 to the specific inactivation rate constants k′ found in the pressure-temperature domains investigated.
TABLE 3.
Estimated model parameter values for feline calicivirus FCV KS20 inactivation based on equation 4 determinations
| Parameter | Value ± SE of regression
|
|
|---|---|---|
| Cell culture medium | Still mineral water | |
| A0 | −8.491 ± 1.118 | −11.430 ± 1.333 |
| A1 | 3.786 × 10−2 ± 0.851 × 10−2 | 4.848 × 10−2 ± 0.711 × 10−2 |
| A2 | −6.503 × 10−2 ± 3.631 × 10−2 | −0.124 ± 0.118 |
| A3 | −3.049 × 10−5 ± 1.721 × 10−5 | 1.085 × 10−5 ± 1.287 × 10−5 |
| A4 | 2.740 × 10−3 ± 0.372 × 10−3 | 7.309 × 10−3 ± 3.182 × 10−3 |
| A5 | −4.655 × 10−4 ± 1.834 × 10−4 | −8.9812 × 10−4 ± 3.083 × 10−4 |
| A6 | ||
| A7 | −4.418 × 10−5 ± 2.362 × 10−5 | |
| A8 | 6.177 × 10−7 ± 4.411 × 10−7 | |
| A9 | 4.850 × 10−6 ± 4.587 × 10−6 | |
| r2 | 0.947 | 0.952 |
| MSE | 0.246 | 0.357 |
| Af | 1.029 | 1.054 |
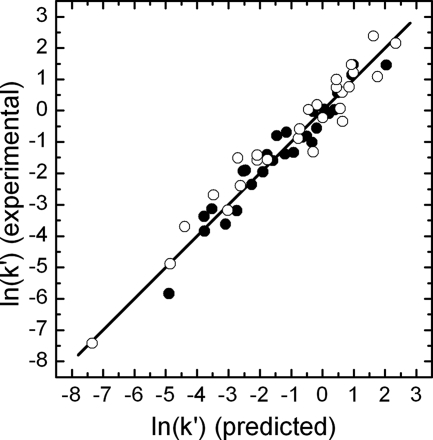
The parity plot of the natural logarithm of the experimental versus the predicted k values (Fig. 4) indicated no heteroscedasticity problems. The deviation from the bisector is considered to be an indicator of the inaccuracy of the models. A good correlation of the predicted and the experimental k values was found, as the coefficient of determination r2 was approximately 0.95 for both models. Further assessment of the models was done by calculation of the accuracy factor (equation 6), yielding values of 1.029 and 1.054 for FCV KS20 in cell culture medium and mineral water, respectively. These values correspond to prediction errors of 2.9% and 5.4%, which shows that the two empirical models are suitable for accurate description of the combined pressure-temperature inactivation of FCV KS20 in cell culture medium and in mineral water, respectively.
FIG. 4.
Correlation between the experimental k′ values of FCV KS20 inactivated [In(k′)] in cell culture medium (•) and in still mineral water (○) determined under isothermal-isobaric conditions and the predicted k′ values determined using equation 4 and the parameter values presented in Table 3.
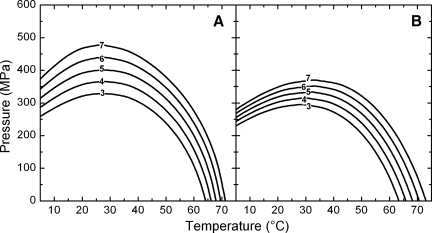
The pressure-temperature diagram with isorate lines is the most concise way of presenting the combined effects of pressure and temperature on a system. Replacing k′ of equation 1 by the functional association of equation 4, pressure-temperature combinations corresponding to a specified reduction of virus titer within an explicit exposure time can be calculated. Using the pressure as the independent variable and setting the inactivation ratio (N × N0−1) and treatment time t constant, the equation can be solved for the temperature to obtain, for example, a 5-log reduction in virus titer after 30 to 480 s (Fig. 5) and a 3- to 7-log titer reduction of FCV KS20 within 1 min of isothermal-isobaric treatment (Fig. 6). It is evident that pressure and temperature have synergistic effects on virus reduction, since in both pressure-temperature diagrams (Fig. 5 and 6) the predicted isorate curves all bend to the left in the high-temperature region. However, the elliptic shape of the curves shows that the pressure stability of FCV KS20 is highest in the range of 25 to 35°C. At lower temperatures the required pressure to achieve the same inactivation rate is clearly decreased. No evidence was found for a pressure-induced increase in thermostability as is often described for bacterial phages (21, 38) and proteins (4, 27). While the thermostability of FCV KS20 in mineral water is similar to that of FCV KS20 in cell culture medium, it is evident from Fig. 5 and 6 that the maximal pressure stability is reduced in mineral water. Whereas in mineral water FCV KS20 is inactivated by more than 6 log after 1 min of treatment at 30°C, the same treatment conditions applied to the virus in cell culture medium reduced the titer by only 4 log. High-pressure-induced virus inactivation occurred in a narrower pressure range in mineral water than in cell culture medium. Therefore, the pressure and temperature requirements for a 5-log reduction were very similar for 480 s of treatment time (Fig. 5) and the pressure and temperature required for a 3-log inactivation for 60 s of treatment time in cell culture medium were very similar to those required for mineral water (Fig. 6).
FIG. 5.
Pressure-temperature isorate diagram for 5-log inactivation of feline calicivirus FCV KS20 in cell culture medium (A) and still mineral water (B) after 30 to 480 s of isothermal-isobaric treatment.
FIG. 6.
Pressure-temperature isorate diagram for 3- to 7-log inactivation of feline calicivirus FCV KS20 in cell culture medium (A) and still mineral water (B) after 60 s of isothermal-isobaric treatment.
DISCUSSION
A low infectious dose was estimated for norovirus (10). But little is known about the norovirus content of water and foods. Stool specimens from patients may contain up to 109 copies of norovirus genome per gram (23). Vomiting may release an estimated 30 million virus particles from the infected person (10). Shellfish are filter feeders and concentrate virus from surrounding sewage-contaminated water. Data from Beuret et al. (6, 7) suggest sporadic contamination of mineral water by norovirus and the occurrence of low concentrations of norovirus sequences in mineral water. Because of the limited knowledge about levels of contamination in food, we used high titers of virus to study the possible worst-case scenarios.
Cooking inactivates human norovirus (45). However, heating of this virus at 60°C for 30 min was insufficient to inactivate the virus completely (14). Because of its nonculturability, detailed inactivation experiments had to be performed using surrogates such as feline calicivirus. Feline calicivirus in cockle meat was completely inactivated within 1 min by heating to an internal temperature of 78°C (47). A 6.5-log reduction of this virus was achieved at 70°C in 3 min (15). Duizer et al. (16) reported that heating to 71.3°C resulted in 3-log inactivation in 1 min for a canine calicivirus and a feline calicivirus. The titers of feline calicivirus were reduced by 4 log PFU ml−1 at 59.3°C in 7 min (12). In this study, FCV KS20 showed only minor temperature stability differences in mineral water and in cell culture medium. We found high stability of the virus at temperatures below 50°C. A 5-log reduction was determined after 5 min at 60°C and after 1 min of isothermal heating at 70°C. This is in good agreement with FCV results reported by other authors (15, 47). Determination of the log-linearity behavior of the inactivation curve is a prerequisite for determination of the D value. However, due to the strong tailing of the inactivation kinetics of feline calicivirus, it is not suitable for calculation of D values.
To our knowledge, pressure inactivation of human norovirus has never been reported. Pressurization of a murine norovirus at 350 MPa resulted in 1.15-log inactivation at 30°C and 5.56 log inactivation at 5°C after 5 min (33). The two reports of high hydrostatic pressure treatment of the related feline calicivirus both found a temperature-dependent reduction below 300 MPa (12, 32). A 7-log reduction was achieved at 275 MPa and the ambient temperature in 5 min (32). At −10°C, the titers were reduced by 5 log after 4 min at 200 MPa (12). The inactivation of murine norovirus and feline calicivirus as a function of treatment time resulted in pronounced tailing and therefore in nonlinear models fitted better to inactivation curves (12, 33).
The pressure stability of FCV KS20 examined in cell culture medium and in mineral water appeared to be maximal at 25 to 35°C. Figure 5A indicates that only 250 MPa is required to obtain a 5-log reduction of FCV number in cell culture medium after 4 min at 5°C, while more than 300 MPa is needed to reach the same rate of inactivation at 20°C. This agrees with the findings of Chen et al. (12) and Kingsley et al. (33) that high-pressure inactivation of caliciviruses is enhanced when applied at refrigeration temperatures. Antagonistic effects of pressure and temperature in the low-temperature region have been reported for picornavirus stability (41) and are a common phenomenon in protein denaturation (20, 27). Thermodynamically cold denaturation of proteins under pressure can be explained by a synergistic destabilization of locally hydrogen bonds and hydration of hydrophobic groups within the peptide (44). As a consequence, tertiary and quaternary protein structures are disrupted and the protein partly unfolds.
Unlike bacteria, viruses such as those of the Caliciviridae family encode no repair mechanisms. Thus, an adaptation to short-term pressure stress, making the surviving virus particles more resistant, could not explain the nonlinear inactivation curves of feline calicivirus. Possible explanations may be its heterogeneous sensitivities to pressure and/or its self-association.
Most of the studied viruses were markedly inactivated at pressures ≤ 500 MPa, as summarized by Grove et al. (22). This pressure sensitivity does not depend on whether a lipid-protein envelope or a protein capsid surrounds these viruses. Our studies of the enveloped avian influenza A virus (28) and nonenveloped feline calicivirus (this report) confirmed this observation. However, some single-stranded, positive-sense RNA viruses, such as tobacco mosaic virus, poliovirus 1, coxsackievirus strain B5, and Aichi virus, showed a remarkable barotolerance at 920 MPa and 600 MPa (19, 35, 50). Their RNA genomes are small, ranging from 6.4 kb to 8.3 kb, and represent a small target of high-pressure treatment. A few structural proteins protect these small RNA genomes. Members of the Picornaviridae family, comprising poliovirus 1, coxsackievirus strain B5, and Aichi virus, are distantly related to the family of Caliciviridae. The two families share a spherical capsid assembled by a few structural proteins. Nevertheless, viruses of the genus Enterovirus within the family of Picornaviridae, such as coxsackievirus strains and poliovirus 1, differed widely in their sensitivity to high pressure (35). Studies published to date have shown that barotolerance is restricted to some virus strains that have a small single-stranded RNA genome protected by structural proteins.
This study showed efficient inactivation of feline calicivirus by more than 7 log10 PFU per ml in cell culture medium or mineral water at 75°C for 2 min and at 450 MPa and 15°C for 1 min. Models of nth orders successfully predicted the nonlinear heat and pressure inactivation kinetics of the norovirus surrogate. It is proposed that this nonthermal high-pressure treatment, when applied under the appropriate pressure, temperature, and process time conditions, is suitable to reduce the risk of norovirus infections by food products.
Acknowledgments
Our work was supported by the Bundesministerium für Bildung und Forschung Verbundsprojekt Effizienzanalyse von Prozess- und Anlagenkonzepten zur schonenden Haltbarmachung von Lebensmitteln mittels neuartiger Hochdruckverfahren.
Footnotes
Published ahead of print on 21 December 2007.
REFERENCES
- 1.Adler, J. L., and R. Zickl. 1969. Winter vomiting disease. J. Infect. Dis. 119:668-673. [DOI] [PubMed] [Google Scholar]
- 2.Appleton, H., and M. S. Pereira. 1977. A possible virus aetiology in outbreaks of food-poisoning from cockles. Lancet i:780-781. [DOI] [PubMed] [Google Scholar]
- 3.Arabas, J., J. Szczepek, L. Dmowki, V. Heinz, and M. Fonberg-Broczek. 1999. New technique for kinetic studies of pressure-temperature induced changes of biological materials, p. 537-540. In H. Ludwig (ed.), Advances in high pressure bioscience and biotechnology. Springer, Berlin, Germany.
- 4.Balny, C., and P. Masson. 1993. Effects of high pressure on proteins. Food Rev. Int. 9:611-628. [Google Scholar]
- 5.Baranyi, J., C. Pin, and T. Ross. 1999. Validating and comparing predictive models. Int. J. Food Microbiol. 48:159-166. [DOI] [PubMed] [Google Scholar]
- 6.Beuret, C., D. Kohler, A. Baumgartner, and T. M. Lüthi. 2002. Norwalk-like virus sequences in mineral water: one-year monitoring of three brands. Appl. Environ. Microbiol. 68:1925-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beuret, C., D. Kohler, and T. M. Lüthi. 2000. Norwalk-like virus sequences detected by reverse transcription-polymerase chain reaction in mineral water imported into or bottled in Switzerland. J. Food Prot. 63:1576-1582. [DOI] [PubMed] [Google Scholar]
- 8.Buckow, R., V. Heinz, and D. Knorr. 2005. Two fractional model for evaluating the activity of glucoamylase from Aspergillus niger under combined pressure and temperature conditions. Food Bioprod. Process 83:220-228. [Google Scholar]
- 9.Cannon, R. O., J. R. Poliner, R. B. Hirschborn, D. C. Rodeheaver, P. R. Silverman, E. A. Brown, G. H. Talbot, S. E. Stine, S. S. Monroe, D. T. Dennis, and R. I. Glass. 1991. A multistate outbreak of Norwalk virus gastroenteritis associated with consumption of commercial ice. J. Infect. Dis. 164:860-863. [DOI] [PubMed] [Google Scholar]
- 10.Caul, E. O. 1994. Small round structured viruses: airborne transmission and hospital control. Lancet 343:1240-1242. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. 2002. Outbreaks of gastroenteritis associated with noroviruses on cruise ships—United States. Morb. Mort. Wkly. Rep. 51:1112-1114. [PubMed] [Google Scholar]
- 12.Chen, H., D. G. Hoover, and D. H. Kingsley. 2005. Temperature and treatment time influence high hydrostatic pressure inactivation of feline calicivirus, a norovirus surrogate. J. Food Prot. 68:2389-2394. [DOI] [PubMed] [Google Scholar]
- 13.Crandell, R. A., C. G. Fabricant, and W. A. Nelson-Rees. 1973. Development, characterization and viral susceptibility of a feline (Felis catus) renal cell line (CFRK). In Vitro 9:176-185. [DOI] [PubMed] [Google Scholar]
- 14.Dolin, R., N. R. Blacklow, H. DuPont, S. Formal, R. F. Buscho, J. A. Kasel, R. P. Chames, R. Hornick, and R. M. Channock. 1972. Biological properties of Norwalk agent of acute infectious nonbacterial gastroenteritis. Proc. Soc. Exp. Biol. Med. 140:578-583. [DOI] [PubMed] [Google Scholar]
- 15.Doultree, J. C., J. D. Druce, C. J. Birch, D. S. Bowden, and J. A. Marshall. 1999. Inactivation of feline calicivirus, a Norwalk virus surrogate. J. Hosp. Infect. 41:51-57. [DOI] [PubMed] [Google Scholar]
- 16.Duizer, E., P. Bijerk, B. Rockx, A. de Groot, F. Twisk, and M. Koopmans. 2004. Inactivation of calicivirus. Appl. Environ. Microbiol. 70:4538-4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eyles, M. J., G. R. Davey, and E. J. Huntley. 1981. Demonstration of viral contamination of oysters responsible for an outbreak of viral gastroenteritis. J. Food Prot. 44:294-296. [DOI] [PubMed] [Google Scholar]
- 18.Gerba, C. P., and D. Kayed. 2003. Caliciviruses: a major cause of foodborne illness. J. Food Prot. 68:1136-1142. [Google Scholar]
- 19.Giddings, N. J., H. A., Allard, and B. H. Hite. 1929. Inactivation of the tobacco mosaic virus by high pressure. Phytopathology 19:749-750. [Google Scholar]
- 20.Gross, M., and R. Jaenicke. 1994. Proteins under pressure: the influence of high hydrostatic pressure on structure, function and assembly of proteins and protein complexes. Eur. J. Biochem. 221:617-630. [DOI] [PubMed] [Google Scholar]
- 21.Gross, P., and H. Ludwig. 1992. Pressure-temperature-phase diagram for the stability of bacteriophage T4, p. 57-59. In C. Balny, R. Hayashi, K. Heremans, and P. Masson (ed.), High pressure and biotechnology. John Libbey Eurotext, Montrouge, France.
- 22.Grove, S. F., A. Lee, T. Lewis, C. M. Stewart, H. Chen, and D. G. Hoover. 2006. Inactivation of foodborne viruses of significance by high pressure and other processes. J. Food Prot. 69:957-968. [DOI] [PubMed] [Google Scholar]
- 23.Häfliger, D., M. Gilgen, J. Lüthy, and P. Hübner. 1997. Seminested RT-PCR systems for small round structured viruses and detection of enteric viruses in seafood. Int. J. Food Microbiol. 37:27-36. [DOI] [PubMed] [Google Scholar]
- 24.Hansman, G. S., H. Saito, C. Shibata, S. Ishizuka, M. Oseto, T. Oka, and N. Takeda. 2007. Outbreak of gastroenteritis due to sapovirus. J. Clin. Microbiol. 45:1347-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardy, M. E. 1999. Norwalk and “Norwalk-like viruses” in epidemic gastroenteritis. Clin. Lab. Med. 19:675-690. [PubMed] [Google Scholar]
- 26.Hawley, S. A. 1971. Reversible pressure-temperature denaturation of chymotrypsinogen. Biochemistry 10:2436-2442. [DOI] [PubMed] [Google Scholar]
- 27.Heremans, R., and L. Smeller. 1998. Protein structure and dynamics at high pressure. Biochim. Biophys. Acta 1386:353-370. [DOI] [PubMed] [Google Scholar]
- 28.Isbarn, S., R. Buckow, A. Himmelreich, A. Lehmacher, and V. Heinz. 2007. Inactivation of avian influenza virus by heat and high hydrostatic pressure. J. Food. Prot. 70:667-673. [DOI] [PubMed] [Google Scholar]
- 29.Jiang, X., M. Wang, K. Wang, and M. K. Estes. 1993. Sequence and genomic organization of Norwalk virus. Virology 195:51-61. [DOI] [PubMed] [Google Scholar]
- 30.Karst, S. M., C. E. Wobus, M. Lay, J. Davidson, and H. W. Virgin. 2003. STAT1-dependent innate immunity to a Norwalk-like virus. Science 299:1575-1578. [DOI] [PubMed] [Google Scholar]
- 31.Keswick, B. H., T. K. Satterwhite, P. C. Johnson, H. L. DuPont, S. L. Secor, J. A. Bitsura, G. W. Gary, and J. C. Hoff. 1985. Inactivation of Norwalk virus in drinking water by chlorine. Appl. Environ. Microbiol. 50:261-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kingsley, D. H., D. G. Hoover, E. Papafragkou, and G. P. Richards. 2002. Inactivation of hepatitis A virus and a calicivirus by high hydrostatic pressure. J. Food Prot. 65:1605-1609. [DOI] [PubMed] [Google Scholar]
- 33.Kingsley, D. H., D. R. Holliman, K. R. Calci, H. Chen, and G. J. Flick. 2007. Inactivation of a norovirus by high-pressure processing. Appl. Environ. Microbiol. 73:581-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kingsley, D. H., G. K. Meade, and G. P. Richards. 2002. Detection of both hepatitis A virus and Norwalk-like virus in imported clams associated with food-borne illness. Appl. Environ. Microbiol. 68:3914-3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kingsley, D. H., H. Chen, and D. G. Hoover. 2004. Hydrostatic pressure application to selected picornavirus. Virus Res. 102:221-224. [DOI] [PubMed] [Google Scholar]
- 36.Ludikhuyze, L., A. Van Loey, I. Indrawati, C. Smout, and M. Hendrickx. 2003. Effects of combined pressure and temperature on enzymes related to quality of fruits and vegetables: from kinetic information to process engineering aspects. Crit. Rev. Food Sci. Nutr. 43:527-586. [DOI] [PubMed] [Google Scholar]
- 37.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Müller-Merbach, M., T. Rauscher, and J. Hinrichs. 2005. Inactivation of bacteriophages by thermal and high-pressure treatment. Int. Dairy J. 15:777-784. [Google Scholar]
- 39.Murphy, A. M., G. S. Grobmann, P. J. Christopher, W. A. Lopez, G. R. Davey, and R. H. Millsom. 1979. An Australia-wide outbreak of gastroenteritis from oysters caused by Norwalk virus. Med. J. Aust. 2:329-333. [DOI] [PubMed] [Google Scholar]
- 40.Noel, J. S., B. J. Liu, C. D. Humphrey, E. M. Rodriguez, P. R. Lambden, I. N. Clarke, D. M. Dwyer, T. Ando, R. I. Glass, and S. S. Monroe. 1997. Parkville virus: a novel genetic variant of human calicivirus in the Sapporo virus clade, associated with an outbreak of gastroenteritis in adults. J. Med. Virol. 52:173-178. [PubMed] [Google Scholar]
- 41.Oliveira, A. C., D. Ishimaru, R. B. Goncalves, T. J. Smith, P. Mason, D. Sa-Carvalho, and J. L. Silva. 1999. Low temperature and pressure stability of picornaviruses: implications for virus uncoating. Biophys. J. 76:1270-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parshionikar, S. U., S. Willian-True, G. S. Fout, D. E. Robbins, S. A. Seys, J. D. Cassady, and R. Harris. 2003. Waterborne outbreak of gastroenteritis associated with a norovirus. Appl. Environ. Microbiol. 69:5263-5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peleg, M., and M. B. Cole. 1998. Reinterpretation of microbial survival curves. Crit. Rev. Food Sci. Nutr. 38:353-380. [DOI] [PubMed] [Google Scholar]
- 44.Scharnagl, C., M. Reif, and J. Friedrich. 2005. Stability of proteins: temperature, pressure and the role of the solvent. Biochim. Biophys. Acta 1749:187-213. [DOI] [PubMed] [Google Scholar]
- 45.Scientific Committee on Veterinary Measures Relating to Public Health. 2002. Norwalk-like viruses. 8-3-2002 report. Health and Consumer Protection Directorate—General European Commission, Brussels, Belgium.
- 46.Seymour, I. J., and H. Appleton. 2001. Foodborne viruses and fresh products. J. Appl. Microbiol. 91:759-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Slomka, M. J., and H. Appleton. 1998. Feline calicivirus as a model system for heat inactivation studies of small round structured viruses in shellfish. Epidemiol. Infect. 121:401-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smeller, L., and K. Heremans. 1997. Some thermodynamic and kinetic consequences of the phase diagram of protein denaturation, p. 55-58. In K. Heremans (ed.), High pressure research in bioscience and biotechnology. Leuven University Press, Leuven, Belgium.
- 49.Wheeler, J. G., D. Sethi, J. M. Cowden, P. G. Wall, L. C. Rodrigues, D. S. Tompkins, M. J. Hudson, and P. J. Roderick. 1999. Study of infectious intestinal disease in England: rates in the community, presenting to general practice, and reported to national surveillance. BMJ 318:1046-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilkinson, N., A. S. Kurdzeil, S. Langton, E. Needs, and N. Cook. 2001. Resistance of poliovirus to inactivation by high hydrostatic pressures. Innov. Food Sci. Emerg. Technol. 2:95-98. [Google Scholar]