Abstract
Lactobacillus helveticus MIMLh5 was selected for its strong cinnamoyl esterase activity on chlorogenic acid and employed for the preparation of a food product containing a high concentration of free caffeic acid. The novel food product was demonstrated to display high total antioxidant power and potential probiotic properties.
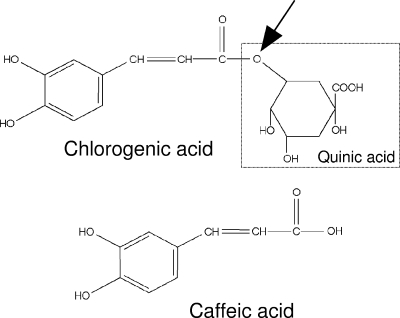
Hydroxycinnamic acids such as caffeic, ferulic, sinapic, and p-coumaric acids are found in almost every plant (11) and represent a major class of phenolic compounds. The most abundant hydroxycinnamic acid is caffeic acid, which is contained in foods mainly as ester with quinic acid, a form called chlorogenic acid (5-caffeoylquinic acid) (Fig. 1).
FIG. 1.
Structures of chlorogenic acid and caffeic acid. The arrow indicates the esterase bond hydrolyzed by cinnamoyl esterase.
Caffeic acid and chlorogenic acid have been demonstrated previously to possess remarkably high levels of antioxidant activity in vitro (8, 12, 13, 18, 25), and they have been associated with several health-promoting effects in vivo (17, 27-30, 33).
The biological properties of the hydroxycinnamic acids depend on their absorption in the gastrointestinal tract. It has been demonstrated previously that the absorption of caffeic acid is drastically reduced when this molecule is ingested as chlorogenic acid (10, 14, 20). The majority of chlorogenic acid, in fact, reaches the large intestine, where it is quickly hydrolyzed by bacteria with cinnamoyl esterase activity and extensively further degraded by the microbiota (10). In contrast, free caffeic acid is well absorbed in the stomach and in the small intestine (20, 23), and its intake is associated with a higher concentration of intact caffeic acid and its tissular metabolites in plasma and a higher level of urinary excretion of these substances than those resulting from the intake of chlorogenic acid (10).
Cinnamoyl esterases have commonly been found in rumen and soil saprophytic microorganisms (6, 7, 16) and also in bacteria from human and animal intestinal microbiota (5, 32). However, very few reports describing cinnamoyl esterases of food-associated bacteria are available (6).
In this study, 100 food and human intestinal bacterial strains (Table 1) were screened for the production of cinnamoyl esterase activity by thin-layer chromatography on silica gel 60 F254 plates (Merck, Milan, Italy). Bacterial strains were grown in 1 ml of liquid medium for 72 h in the presence of a 0.5-mg/ml concentration of the reference substrate, ethyl ferulate or chlorogenic acid (Sigma, St. Louis, MO). Only 12 strains gave undoubtedly positive results on both substrates, and these strains belonged to the species Lactobacillus helveticus, L. acidophilus, and L. fermentum (Table 2).
TABLE 1.
Bacterial strains screened for cinnamoyl esterase activity
| Genus | Species | No. of strainsa | Source |
|---|---|---|---|
| Lactobacillus | L. paracasei | 6 | Human gut |
| L. paracasei | 4 | Fermented milk | |
| L. paracasei | 1 (DSM 5622T) | International culture collection | |
| L. acidophilus | 7 | Fermented milk | |
| L. acidophilus | 2 (ATCC 4356T) | International culture collection | |
| L. helveticus | 9 | Dairy starters | |
| L. casei | 4 | Human gut | |
| L. fermentum | 4 | Human gut | |
| L. rhamnosus | 1 (GG) | Human gut; probiotic commercial strain | |
| Carnobacterium | C. maltaromaticum | 10 | Meat |
| C. maltaromaticum | 2 | Fish | |
| C. maltaromaticum | 1 (DSM 20342T) | Milk | |
| C. divergens | 12 (LMG9199T) | Meat | |
| Enterococcus | E. faecalis | 5 | Cheese |
| E. faecium | 1 | Cheese | |
| Streptococcus | S. thermophilus | 2 (DSM 20617T) | Yogurt |
| Bifidobacterium | B. bifidum | 7 | Human feces |
| B. pseudocatenulatum | 7 | Human feces | |
| B. longum | 7 | Human feces | |
| B. animalis subsp. lactis | 4 (Bb12) | Probiotic commercial product | |
| B. adolescentis | 4 | Human feces |
The names of the most relevant strains included in the study are listed in parentheses.
TABLE 2.
Enzymatic activity of the 24 bacterial strains testing positive for the presence of cinnamoyl esterase
| Genus | Species | Strain(s) | Levela of activity as determined by thin-layer chromatography with:
|
% of chlorogenic acid hydrolyzed as determined by HPLC | |
|---|---|---|---|---|---|
| Ethyl ferulate | Chlorogenic acid | ||||
| Lactobacillus | L. helveticus | MIMLh5 | +++ | +++ | 100 |
| L. helveticus | SIM7 | +++ | +++ | 100 | |
| L. helveticus | CBT17 | ++ | +++ | 92.5 | |
| L. helveticus | LH22 | +++ | +++ | 91.5 | |
| L. helveticus | LH28 | ++ | ++ | 70 | |
| L. helveticus | LH23 | ++ | + | <5 | |
| L. helveticus | LH4 | ++ | ± | <5 | |
| L. helveticus | LH164 | ++ | − | NTb | |
| L. acidophilus | ATCC 4356T | +++ | ++ | 81.4 | |
| L. acidophilus | LA47 | ++ | ++ | 70.5 | |
| L. acidophilus | LA48 | +++ | ++ | 59.4 | |
| L. acidophilus | LA51 | +++ | ++ | 56.6 | |
| L. acidophilus | LA53 | ++ | ++ | 54.8 | |
| L. acidophilus | LA46 | +++ | ± | <5 | |
| L. acidophilus | LA50 | ++ | − | NT | |
| L. fermentum | LB12, LB18 | ++ | ± | <5 | |
| L. fermentum | LB19 | ++ | + | 41.6 | |
| L. casei | LB21, LB22 | + | − | NT | |
| L. rhamnosus | GG | + | ± | <5 | |
| Enterococcus | E. faecalis | SMT, TD1, TA15 | + | − | NT |
−, not detectable; ±, very weak or uncertain; +, weak; ++, good; +++, strong.
NT, not tested.
The percentages of chlorogenic acid hydrolyzed by the positive bacterial strains were determined by high-performance liquid chromatography (HPLC) analysis. The HPLC apparatus consisted of an Alliance 2695 pump system (Waters, Milford, MA) equipped with a Waters 996 diode array detector. A Hypersil AA octyldecyl silane column (2.1-mm internal diameter by 250-mm length; film thickness, 5 μm; pore size, 120 Å) from Agilent Technologies (Santa Clara, CA) kept at 30°C was used. Eluting solvents were water containing 4% (vol/vol) formic acid (Sigma) and methanol (Merck, Darmstadt, Germany). The linear elution gradient (200 μl/min) expressed as the methanol proportion was as follows: 0 min, 5%; 0 to 5 min, 5%; 5 to 35 min, 5 to 20%; 35 to 36 min, 20 to 100%; 36 to 38 min, 100%; 38 to 39 min, 5% (run-to-run time, 50 min). UV spectra of chromatographic peaks were acquired by scanning the range from 210 to 400 nm (resolution, 1.2 nm).
Two strains belonging to the L. helveticus species, named MIMLh5 and SIM7, apparently hydrolyzed all the chlorogenic acid used in the experiments (Table 2). In particular, the L. helveticus strain MIMLh5, isolated from an Italian cheese natural whey starter culture, showed good growth in milk and in apple juice, and therefore, it was chosen as a starter for the preparation of the food product.
Development of fermented food product enriched with caffeic acid.
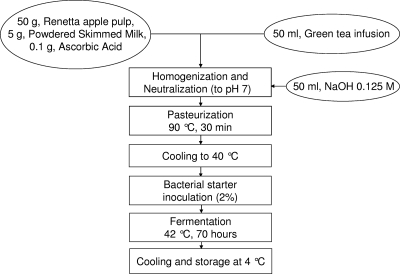
The main intention of this work was the preparation of a food product containing a significant concentration of free caffeic acid, obtained from the chlorogenic acid naturally present in the food through the esterase activity of a food-grade bacterial starter. We selected the ingredients of the food product with the purposes of obtaining an alimentary formulation naturally rich in chlorogenic acid and creating the conditions suitable for the growth of the microbial starter. More specifically (Fig. 2), we chose Renetta cultivar apples because they revealed the highest content of phenolic compounds among the best-known apple cultivars (31), and we included a green tea infusion because of the high concentration of antioxidants, and particularly chlorogenic acid, in green tea (22); ascorbic acid prevented the browning of the apple pulp during production, and the presence of skim milk promoted the growth of the bacterial starter. Nevertheless, we verified good proliferation of the bacterial starter even in the absence of skim milk and in the presence of soy milk.
FIG. 2.
Flow sheet depicting the preparation of the fermented food product.
After fermentation, the food product had the texture of yogurt and a mixed flavor of yogurt and apple. The taste was fresh, and the flavors of apple and green tea were dominant. The homolactic fermentation by the microbial starter reduced the pH from about 7 to 3.6.
At the end of the fermentation process, the final biomass was 8.6 × 108 cells/ml of the food product. Experiments with propidium iodide (1-μg/ml final concentration; Sigma) and counts on MRS agar plates supplemented with 0.05% cysteine hydrochloride showed a log decrease in viable cells not higher than 0.8 after 3 weeks of storage at 4°C.
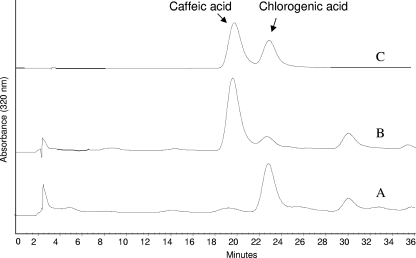
The quantitation of caffeic and chlorogenic acids in unfermented and fermented food products was carried out by HPLC at 320 nm as described above. For the extraction of hydroxycinnamic acids, the pH of the food product was brought to 1.5 with HCl and 1 g of the acidified sample was incubated twice with stirring at 4°C for 1 h in 5 ml of methanol and 0.1% ascorbic acid. Standard solutions of caffeic and chlorogenic acids in methanol at a concentration of 1,000 mg/liter were prepared and gradually diluted. Both spiked and unknown samples were run in duplicate, and three independent determinations were done. The assignment of chromatographic peaks to caffeic and chlorogenic acids was further confirmed by UV spectra and HPLC-electrospray ionization-mass spectrometry experiments. Particularly, the recognition of hydroxycinnamic acids was carried out by different means: (i) selected-ion-monitoring scanning (peak width, 1.0 Da) for specific monoprotonated ions [(M + H+)1+; m/z, 181.2 and 355.3 for caffeic and chlorogenic acids, respectively] and (ii) a full scan at m/z 100 to 400. The mass accuracy was ensured by calibration with a mixture of caffeine and reserpine and the tripeptide PFK (in a 1:1 methanol-water solution with 0.1% acetic acid) infused separately. After 70 h of fermentation, chromatographic peaks with retention times corresponding to those of standard chlorogenic (23.0 min) and caffeic (19.8 min) acids were present in the HPLC pattern of the fermented food product (Fig. 3). The bacterial starter converted most of the chlorogenic acid into caffeic acid, the concentration of which increased from 6.4 ± 3.9 to 149 ± 11 mg/liter during the fermentation process (results are shown as means ± standard deviations; n = 3).
FIG. 3.
HPLC chromatograms of the methanolic extracts of the food product before (A) and after (B) the fermentation with L. helveticus MIMLh5. C, standard solution of caffeic and chlorogenic acids.
The amount of caffeic acid ingested in consuming, for instance, 100 ml of the food product described here may significantly increase the total daily amount of absorbed phenolic compounds (24) and may have a significant biological effect on the consumer (4, 19, 26). Indeed, studies of rats and humans have shown that, different from chlorogenic acid, caffeic acid is efficiently absorbed through the upper part of the gastrointestinal tract and is found in the body either intact or in glucuronidated, sulfated, and O-methylated forms (ferulic and isoferulic acids), increasing the total antioxidant status of plasma (19, 20, 23).
Other potentially functional features.
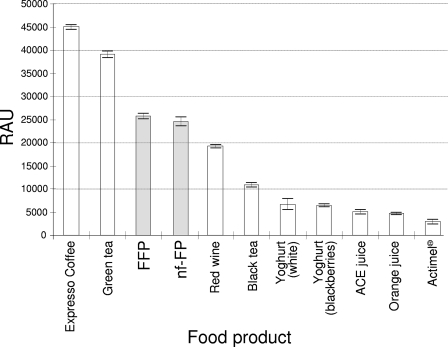
We verified the total antioxidant capacity by evaluating the ability of the food product to reduce Fe3+ ions into Fe2+ ions under acidic conditions as demonstrated by assaying the ferric reducing ability of plasma (see the supplemental material for details on the experimental protocol), a method already successfully applied to a variety of food products (3, 21). The total antioxidant capacity of our fermented product proved to be much higher than those of the other fermented dairy products tested and also higher than those of beverages with high concentrations of antioxidant compounds, such as red wine and black tea (Fig. 4), suggesting that the fermented food described herein can be considered an antioxidant alimentary product. The antioxidant power of the fermented product was not significantly different from that of the unfermented food. This result indicates that the activity of the bacterial starter did not alter the reducing potential of the food or eventually counteracted its loss.
FIG. 4.
Antioxidant activities of 10 different food products as determined by assaying the ferric reducing ability of plasma. RAU, relative antioxidant units; FFP, functional food product developed in this work; nf-FP, nonfermented FFP; ACE juice, commercial orange and carrot juice enriched with vitamins A, C, and E; Actimel, probiotic fermented milk from Danone. Vertical bars represent standard deviations. Unpaired Student's t test revealed a statistically significant difference between FFP and nf-FP and either red wine or green tea (P < 0.05).
L. helveticus MIMLh5 belongs to a species that is generally recognized as safe and has been often associated with health-promoting effects (1, 15). We studied its ability to resist gastrointestinal transit and to bind to intestinal epithelial cells in vitro (data not shown; see the supplemental material for details on the experimental protocols). Experiments with simulated gastric juices at pH 2 and pH 3 showed that L. helveticus MIMLh5 can survive for 3 h, very similar to the control strain L. rhamnosus GG, which is the most studied probiotic and is known to survive gastrointestinal transit (9). We also observed that MIMLh5 can survive very well in the presence of 0.3% oxgall and can grow in MRS liquid medium supplemented with 0.05% cysteine hydrochloride and 0.2% oxgall. Finally, we found that L. helveticus MIMLh5 can adhere to cells of the Caco-2 human epithelial cell line, very similar to the control strain L. rhamnosus GG, which is known to be able to transiently colonize the human intestinal tract (2) (data not shown).
In conclusion, the alimentary product prepared and characterized in this work is a novel functional food exhibiting high antioxidant power and presenting probiotic potential. Furthermore, the novelty of the present work resides in the use of a bacterial probiotic starter, selected for its high level of cinnamoyl esterase activity, for the production of a significant concentration of free caffeic acid during the fermentation of a food matrix rich in antioxidants (S. Guglielmetti and D. Mora, 27 April 2007, European patent application EP 07008593.1). The promising potential health-promoting effects of this food product need to be proven by upcoming studies of human nutrition and physiology.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Ministry of the University and Technological and Scientific Research (First 2005).
We are grateful to Daniele Ramponi for his invaluable technical assistance.
Footnotes
Published ahead of print on 28 December 2007.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Aihara, K., O. Kajimoto, H. Hirata, R. Takahashi, and Y. Nakamura. 2005. Effect of powdered fermented milk with Lactobacillus helveticus on subjects with high-normal blood pressure or mild hypertension. J. Am. Coll. Nutr. 24:257-265. [DOI] [PubMed] [Google Scholar]
- 2.Alander, M., R. Satokari, R. Korpela, M. Saxelin, T. Vilpponen-Salmela, T. Mattila-Sandholm, and A. Von Wright. 1999. Persistance and colonization of human colonic mucosa by a probiotic strain, Lactobacillus rhamnosus GG, after oral consumption. Appl. Environ. Microbiol. 65:351-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benzie, I. F., and J. J. Strain. 1996. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 239:70-76. [DOI] [PubMed] [Google Scholar]
- 4.Chiang, L. C., L. T. Ng, W. Chiang, M. Y. Chang, and C. C. Lin. 2003. Immunomodulatory activities of flavonoids, monoterpenoids, triterpenoids, iridoid glycosides and phenolic compounds of Plantago species. Planta Med. 69:600-604. [DOI] [PubMed] [Google Scholar]
- 5.Couteau, D., A. L. McCartney, G. R. Gibson, G. Williamson, and C. B. Faulds. 2001. Isolation and characterization of human colonic bacteria able to hydrolyse chlorogenic acid. J. Appl. Microbiol. 90:873-881. [DOI] [PubMed] [Google Scholar]
- 6.Donaghy, J., P. F. Kelly, and A. M. McKay. 1998. Detection of ferulic acid esterase production by Bacillus spp. and lactobacilli. Appl. Microbiol. Biotechnol. 50:257-260. [DOI] [PubMed] [Google Scholar]
- 7.Faulds, C. B., and G. Williamson. 1991. The purification and characterization of 4-hydroxy-3-methoxycinnamic (ferulic) acid esterase from Streptomyces olivochromogenes. J. Gen. Microbiol. 137:2339-2345. [DOI] [PubMed] [Google Scholar]
- 8.Foley, S., S. Navaratnam, D. J. McGarvey, E. J. Land, T. G. Truscott, and C. A. Rice-Evans. 1999. Singlet oxygen quenching and the redox properties of hydroxycinnamic acids. Free Radic. Biol. Med. 26:1202-1208. [DOI] [PubMed] [Google Scholar]
- 9.Goldin, B. R., S. L. Gorbach, M. Saxelin, S. Barakat, L. Gualtieri, and S. Salminen. 1992. Survival of Lactobacillus species (strain GG) in human gastrointestinal tract. Dig. Dis. Sci. 37:121-128. [DOI] [PubMed] [Google Scholar]
- 10.Gonthier, M., M. Verny, C. Besson, C. Rémésy, and A. Scalbert. 2003. Chlorogenic acid bioavailability largely depends on its metabolism by the gut microflora in rats. J. Nutr. 133:1853-1859. [DOI] [PubMed] [Google Scholar]
- 11.Herrmann, K. 1976. Flavonols and flavones in food plants: a review. J. Food Technol. 11:433-438. [Google Scholar]
- 12.Kasai, H., S. Fukada, Z. Yamaizumi, S. Sugie, and H. Mori. 2000. Action of chlorogenic acid in vegetables and fruits as an inhibitor of 8-hydroxydeoxyguanosine formation in vitro and in a rat carcinogenesis model. Food Chem. Toxicol. 38:467-471. [DOI] [PubMed] [Google Scholar]
- 13.Kono, Y., H. Shibata, Y. Kodama, and Y. Sawa. 1995. The suppression of the N-nitrosating reaction by chlorogenic acid. Biochem. J. 312:947-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lafay, S., A. Gil-Izquierdo, C. Manach, C. Morand, C. Besson, and A. Scalbert. 2006. Chlorogenic acid is absorbed in its intact form in the stomach of rats. J. Nutr. 136:1192-1197. [DOI] [PubMed] [Google Scholar]
- 15.Leblanc, J., I. Fliss, and C. Matar. 2004. Induction of a humoral immune response following an Escherichia coli O157:H7 infection with an immunomodulatory peptic fraction derived from Lactobacillus helveticus-fermented milk. Clin. Diagn. Lab. Immunol. 11:1171-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McSweeney, C. S., A. Dulieu, and R. Bunch. 1998. Butyrivibrio spp. and other xylanolytic microorganisms from the rumen have cinnamoyl esterase activity. Anaerobe 4:57-65. [DOI] [PubMed] [Google Scholar]
- 17.Mori, H., T. Tanaka, H. Shima, T. Kuniyasu, and M. Takahashi. 1986. Inhibitory effect of chlorogenic acid on methilazoxymethanol acetate-induced carcinogenesis in large intestine and liver of hamsters. Cancer Lett. 30:49-54. [DOI] [PubMed] [Google Scholar]
- 18.Nardini, M., M. D'Aquino, G. Tomassi, V. Gentili, M. Di Felice, and C. Scaccini. 1995. Inhibition of human low-density lipoprotein oxidation by caffeic acid and other hydroxycinnamic acid derivatives. Free Radic. Biol. Med. 19:541-552. [DOI] [PubMed] [Google Scholar]
- 19.Nardini, M., E. Cirillo, F. Natella, and C. Scaccini. 2002. Absorption of phenolic acids in humans after coffee consumption. J. Agric. Food Chem. 50:5735-5741. [DOI] [PubMed] [Google Scholar]
- 20.Olthof, M. R., P. C. Hollman, and M. B. Katan. 2001. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 131:66-71. [DOI] [PubMed] [Google Scholar]
- 21.Pellegrini, N., M. Serafini, B. Colombi, D. Del Rio, S. Salvatore, M. Bianchi, and F. Brighenti. 2003. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nutr. 133:2812-2819. [DOI] [PubMed] [Google Scholar]
- 22.Rababah, T. M., N. S. Hettiarachchy, and R. Horax. 2004. Total phenolics and antioxidant activities of fenugreek, green tea, black tea, grape seed, ginger, rosemary, gotu kola, and ginkgo extracts, vitamin E, and tert-butylhydroquinone. J. Agric. Food Chem. 52:5183-5186. [DOI] [PubMed] [Google Scholar]
- 23.Rechner, A. R., J. P. E. Spencer, G. Kuhnle, U. Hahn, and C. A. Rice-Evans. 2001. Novel biomarkers of the metabolism of caffeic acid derivates in vivo. Free Radic. Biol. Med. 30:1213-1222. [DOI] [PubMed] [Google Scholar]
- 24.Scalbert, A., and G. Williamson. 2000. Dietary intake and bioavailability of polyphenols. J. Nutr. 130:2073S-2085S. [DOI] [PubMed] [Google Scholar]
- 25.Shibata, H., Y. Sakamoto, M. Oka, and Y. Kono. 1999. Natural antioxidant, chlorogenic acid, protects against DNA breakage caused by monochloramine. Biosci. Biotechnol. Biochem. 63:1295-1297. [DOI] [PubMed] [Google Scholar]
- 26.Simonetti, P., C. Gardana, and P. Pietta. 2001. Plasma levels of caffeic acid and antioxidant status after red wine consumption. J. Agric. Food Chem. 49:5964-5968. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki, A., D. Kagawa, R. Ochiai, I. Tokimitsu, and I. Saito. 2002. Green coffee bean extract and its metabolites have a hypotensive effect in spontaneously hypertensive rats. Hypertens. Res. 25:99-107. [DOI] [PubMed] [Google Scholar]
- 28.Takeda, H., M. Tsuji, M. Inazu, T. Egashira, and T. Matsumiya. 2002. Rosmarinic acid and caffeic acid produce antidepressive-like effect in the forced swimming test in mice. Eur. J. Pharmacol. 449:261-267. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka, T., T. Kojima, T. Kawamori, A. Wang, M. Suzui, K. Okamoto, and H. Mori. 1993. Inhibition of 4-nitroquinoline-1-oxide-induced rat tongue carcinogenesis by the naturally occurring plant phenolics caffeic, ellagic, chlorogenic, and ferulic acid. Carcinogenesis 14:1321-1325. [DOI] [PubMed] [Google Scholar]
- 30.Tsuchiya, T., O. Suzuki, and K. Igarashi. 1996. Protective effects of chlorogenic acid on paraquat-induced oxidative stress in rats. Biosci. Biotechnol. Biochem. 60:765-768. [DOI] [PubMed] [Google Scholar]
- 31.Vrhovsek, U., A. Rigo, D. Tonon, and F. Mattivi. 2004. Quantitation of polyphenols in different apple varieties. J. Agric. Food Chem. 52:6532-6538. [DOI] [PubMed] [Google Scholar]
- 32.Wang, X., X. Geng, Y. Egashira, and H. Sanada. 2004. Purification and characterization of a feruloyl esterase from the intestinal bacterium Lactobacillus acidophilus. Appl. Environ. Microbiol. 70:2367-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou, J., F. Ashoori, S. Suzuki, I. Nischicaghi, and K. Yagi. 1993. Protective effect of chlorogenic acid on lipid-peroxidation induced in the liver of rats by carbon-tetrachloride or Co-60-irradiation. J. Clin. Biochem. Nutr. 15:119-125. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.