Abstract
Lactococcus lactis subsp. lactis biovar diacetylactis CRL264 is a natural strain isolated from cheese (F. Sesma, D. Gardiol, A. P. de Ruiz Holgado, and D. de Mendoza, Appl. Environ. Microbiol. 56:2099-2103, 1990). The effect of citrate on the growth parameters at a very acidic pH value was studied with this strain and with derivatives whose citrate uptake capacity was genetically manipulated. The culture pH was maintained at 4.5 to prevent alkalinization of the medium, a well-known effect of citrate metabolism. In the presence of citrate, the maximum specific growth rate and the specific glucose consumption rate were stimulated. Moreover, a more efficient energy metabolism was revealed by analysis of the biomass yields relative to glucose consumption or ATP production. Thus, it was shown that the beneficial effect of citrate on growth under acid stress conditions is not primarily due to the concomitant alkalinization of the medium but stems from less expenditure of ATP, derived from glucose catabolism, to achieve pH homeostasis. After citrate depletion, a deleterious effect on the final biomass was apparent due to organic acid accumulation, particularly acetic acid. On the other hand, citrate metabolism endowed cells with extra ability to counteract lactic and acetic acid toxicity. In vivo 13C nuclear magnetic resonance provided strong evidence for the operation of a citrate/lactate exchanger. Interestingly, the greater capacity for citrate transport correlated positively with the final biomass and growth rates of the citrate-utilizing strains. We propose that increasing the citrate transport capacity of CRL264 could be a useful strategy to improve further the ability of this strain to cope with strongly acidic conditions.
The industrial relevance of Lactococcus lactis is related to its ability to convert sugars almost exclusively into lactic acid. This trait has been invaluable in food manufacturing because the accompanying acidification prevents spoilage. In recent years, the number of potential applications beyond the food industry increased considerably. In particular, the use of different strains as probiotics or live vehicles for the oral delivery of heterologous proteins of vaccinal or therapeutic interest has been extensively investigated (38).
L. lactis grows optimally at pH values in the range of 6.3 to 6.9, but the lower limit for growth is within the range of 4.0 to 5.0, depending on the strain and the medium composition (12, 14, 15, 31). In most industrial processes, it is the accumulation of lactic acid at low pH that causes growth arrest; hence, the ability to cope with acid stress is very important. Moreover, the efficacy of L. lactis as a live carrier of therapeutics or antigens depends ultimately on the fraction of cells that survive the harsh pH conditions in the upper gastrointestinal tract. Consequently, a deep understanding of the underlying mechanisms of resistance to low pH is of current significance and is essential for the success of strain design.
While it is well known that organic acids inhibit cell growth at low pH, the molecular mechanisms for survival under acid stress conditions are still poorly understood (18, 40). The primary mechanism responsible for pH homeostasis in bacteria relies on the expulsion of protons from the cytoplasm by H+-ATPases (17). The glutamate decarboxylase γ-aminobutyrate antiporter, the arginine deaminase pathway, and the citrate-utilizing pathway are additional processes contributing to pH homeostasis in L. lactis (4, 18, 34, 37).
L. lactis subsp. lactis biovar diacetylactis CRL264 utilizes citrate and is notable for its ability to thrive at pH values that would preclude growth of most L. lactis strains (20, 35). Citrate metabolism in CRL264 has been investigated at the biochemical as well as the genetic level (10, 20, 21, 23). Citrate is transported via a plasmid-encoded carrier (CitP); citrate lyase cleaves citrate into acetate and oxaloacetate, which is subsequently decarboxylated to pyruvate by the action of oxaloacetate decarboxylase. The genes encoding these enzymes are located in a large chromosomal operon; moreover, it has been shown that a low pH, but not citrate, is required for the induction of the catabolic genes as well as the plasmid-borne genes (transporter and regulator).
Given the remarkable ability of strain CRL264 to grow under severe acid stress conditions (20, 22), we deemed it important to obtain further insight into the physiological basis of this trait. Therefore, the growth of CRL264 and isogenic mutant strains with different citrate-uptake capacities was thoroughly characterized under tightly controlled pH conditions. In this way, the cellular response to low pH was assessed independently of the pH changes associated with metabolism. Nuclear magnetic resonance (NMR) was used as a complementary technique to characterize biochemical parameters in living cells.
MATERIALS AND METHODS
Organisms and growth conditions.
L. lactis subsp. lactis biovar diacetylactis strains CRL264, CRL30, CRL30(pCIT), and CHCC2112 were used in this work. Strain CRL264 was obtained from the collection of the Centro de Referencia para Lactobacilos, Tucumán, Argentina (35). It harbors the plasmid pCIT264, which carries the citQRP operon, involved in citrate transport. L. lactis CRL30 was obtained by Magni et al. (21) by treating strain CRL264 with acridine orange to remove pCIT264 and selecting for the inability to utilize citrate in Kempler and McKay medium plates (16). CRL30(pCIT) was constructed for this work by electroporation of CRL30, carried out according to reference 8, with total plasmid DNA from CRL264 prepared as described in reference 1. Positive colonies were selected on commercial M17 agar plates at pH 4.5, supplemented with glucose and citrate (plates in which CRL30 is unable to form colonies), and examined for the presence of the 8.3-kb pCIT264 plasmid. Strain CHCC2112, a natural citrate-utilizing strain, was obtained from the Chr. Hansen Culture Collection (Chr. Hansen A/S, Hørsholm, Denmark). The strains were grown routinely at 30°C without shaking in reconstituted M17 medium prepared by adding the ingredients and quantities specified for M17, except that 5 g/liter yeast extract and 2.5 g/liter beef extract were used. This medium, hereafter designated M17′, was supplemented with glucose (GM17′) or with glucose plus sodium citrate (GCM17′); the concentrations used were 55 mM glucose and 13.2 mM sodium citrate, unless stated otherwise. The pH was adjusted with HCl. Precultures were grown in a medium of exactly the same composition and initial pH as those used for the final cultivations. Fresh medium was inoculated to an initial optical density at 600 nm (OD600) of 0.05.
Fermentation conditions.
Strains were grown in 2-liter fermentors at 30°C in GM17′ or GCM17′ medium, with a pH of 4.5 or 6.5, kept constant by the automatic addition of 2 M HCl and 10 M NaOH. Growth without pH controls was performed with an initial pH of 4.5. Typically, the sodium citrate concentration was 13.2 mM, but concentrations of 4.1 and 41.9 mM were used whenever indicated. We verified that Mg2+ was not a limiting factor for growth in the range of citrate concentration examined. Experiments were also performed in GM17′ medium supplemented with 20 mM acetic acid or with a combination of 20 mM acetic acid and 13.2 mM sodium citrate. Anaerobiosis was attained by flushing sterile argon through the medium in the fermentor during 1 h preceding inoculation. Culture samples (3 ml) were taken at regular intervals and centrifuged. The supernatants were filtered through nylon membranes (0.20-μm pore; Millipore Corporation, Bedford, MA) and stored at −20°C until analyzed by high-performance liquid chromatography (HPLC). Growth was quantified by measuring the OD600. The maximum specific growth rate (μmax) was calculated through linear regressions of plots of the ln OD600 versus time during the exponential growth phase. For the yield calculation, unless otherwise stated, two time points were considered: one was immediately after inoculation, and the final point was at the onset of stationary phase, typically after 12 h. ATP production was calculated from the fermentation products, assuming that all ATP was synthesized by substrate-level phosphorylation. TableCurve 2D software (SYSTAT Software Inc., San Jose, CA) was used for the calculation of the average specific consumption rates of glucose and citrate. A factor of 0.3605 was used to convert OD600 into dry weight (mg biomass liter−1), and a factor of 0.59 was used to convert biomass into protein. These values were determined in our laboratory for L. lactis MG1363.
Growth with added lactic acid.
L. lactis CRL264 was grown in 500-ml flasks, using 200 ml of GM17′ or GCM17′, at 30°C without shaking. The medium was supplemented with sodium lactate in a range of concentrations from 20 to 65 mM; medium without lactate addition was used as the control. The initial pH of the medium was adjusted to 4.5. Culture samples were taken at regular intervals and used for OD measurements and HPLC analyses of substrates and end products. Each condition was examined at least twice.
HPLC analysis.
Glucose, citrate, lactate, acetoin, acetate, 2,3-butanediol, ethanol, and formate were quantified in the culture medium by an HPLC (Dionex Corporation, Sunnyvale, CA) equipped with a refractive index detector (Shodex RI-101; Showa Denko K. K., Oita, Japan). The column used was an Aminex HPX-87H anion-exchange column (Bio-Rad Laboratories, Inc., Richmond, CA). H2SO4 (0.005 M) was used as the mobile phase at a flow rate of 0.5 ml min−1. The temperature of the column was 60°C.
NMR experiments.
Cells for NMR experiments were grown under anaerobiosis in a 5-liter fermentor at 30°C in a chemically defined medium as described previously (27). The medium was supplemented with 55 mM glucose plus 13.2 mM sodium citrate. The pH was kept at 5.5. An overnight culture in chemically defined medium (initial pH 5.5) with glucose plus citrate was used to inoculate the fermentor (an initial OD600 of 0.05). Cells were harvested at the logarithmic growth phase, centrifuged, washed twice, and resuspended to a protein concentration of about 18 mg ml−1 in 50 mM KPi or in morpholineethanesulfonic acid/KOH buffer for 13C NMR or 31P NMR experiments, respectively. All NMR spectra of living cells were acquired at 30°C, with a quadruple-nucleus probe head on a Bruker DRX500 spectrometer. In vivo 13C NMR was used to study the metabolism of [2-13C]glucose (40 mM) and [2-13C]glucose plus [2,4-13C]citrate (20 mM) at pH 5.5. The time course of substrate consumption, product formation, and intracellular metabolite pools was monitored (25, 26). To follow the evolution of intracellular and extracellular lactate pools by in vivo 13C NMR, the experiments were run at an external pH of 4.8, which allowed for a good separation of the lactate resonances. [1-13C]glucose (40 mM) was added, and once exhausted, a pulse of citrate (20 mM, final concentration) was supplied. For all the 13C NMR experiments described, sample extracts were prepared and used for the quantification of end products, as described previously (25, 26); intracellular metabolite concentrations were calculated using a value of 2.9 μl mg of protein−1 for the intracellular volume (30). Similar experiments using a 40 mM substrate concentration were performed at pH 5.2 and monitored by 31P NMR to determine intracellular pH. Lower external pH values were examined, but the signal-to-noise ratio in the spectra was too poor to allow for reliable results. Determination of intracellular pH in strain CRL264 was difficult due to (i) the promptness of citrate utilization (20 mM used in less than 2 min) and (ii) the depletion of the inorganic phosphate pool during the period immediately after glucose addition. Each type of NMR experiment was repeated at least twice, and the results were highly reproducible.
Transport assays.
L. lactis cells were grown in 2-liter fermentors at 30°C in GCM17′ medium at a constant pH of 4.5, harvested in the mid-logarithmic growth phase, centrifuged, and washed twice with cold 5 mM KPi buffer (pH 4.5). The pellet was resuspended in 50 mM KPi buffer (pH 4.5) to yield a final OD600 of 2. Samples (3 ml) were incubated for 2 min at 30°C prior to the addition of [1,5-14C]citrate (1.1 μM, corresponding to about 580,000 cpm) plus cold citrate to obtain a final concentration of 50 μM citrate. The concentration of glucose was 50 μM. At consecutive 10-s intervals, 0.5-ml samples were filtered through 0.45-μm nitrocellulose filters (Millipore Corporation, Bedford, MA). Filters were washed once with 10 ml of 50 mM KPi buffer (pH 4.5) and submerged immediately in scintillation fluid. The retained radioactivity was counted in a liquid scintillation counter. Experiments were performed in triplicate.
Analysis of plasmid DNA.
The relative amounts of plasmid pCIT264 in strains CRL264 and CRL30(pCIT) were determined during the cells’ exponential growth phase (OD600 = 0.8). Plasmid DNA was isolated using a commercial kit (Qiagen), and the DNA concentration was measured spectrophotometrically at 260 nm. The 8.3-kb plasmid pCIT264 was linearized by digestion with EcoRI. The plasmid profile was analyzed by electrophoresis in a 1% agarose gel. The relative amounts of pCIT264 were estimated by comparison of the intensities of the corresponding bands, quantified in a Gel-doc apparatus using Quantity One software (Bio-Rad).
Chemicals.
[1-13C]glucose (99% enrichment) and [2-13C]glucose (99% enrichment) were obtained from Campro Scientific and Euriso-top, respectively. [2,4-13C]citrate (99% enrichment) was supplied by CortecNet. [1,5-14C]citrate (107 mCi/mmol) was obtained from Amersham. All other chemicals were reagent grade.
RESULTS
Comparison of growth parameters at a constant pH of 4.5.
Two natural citrate-utilizing strains were analyzed, CRL264 and CHCC2112, as well as two CRL264 derivatives, genetically manipulated in their ability to consume citrate, CRL30(pCIT), where the copy number of the plasmid encoding the citrate transporter gene was approximately halved (see below), and strain CRL30, which was pCIT264 depleted. To examine the effect of low pH, independently of the pH changes concomitant with growth without pH control, this parameter was kept constant at 4.5. Strain CRL264 grew with a μmax of 0.59 h−1, the highest rate among the strains analyzed (Fig. 1A; Table 1). This high μmax value reflects the remarkable ability of strain CRL264 to grow under stressful acidic conditions. This strain also exhibited the highest value of OD600 at the beginning of the stationary period, considering the citrate-utilizing strains only. Even though strain CRL30 grew more slowly than CRL264, with a μmax of 0.49 h−1, it reached a final OD600 which was 13% higher than the value obtained for strain CRL264 (Table 1).
FIG. 1.
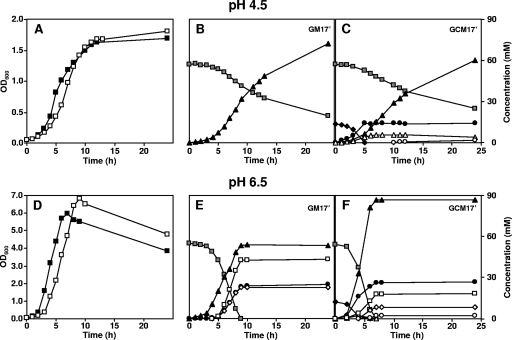
(A) Typical growth curves of L. lactis subsp. lactis biovar diacetylactis CRL264 (▪), CRL30 (○), CRL30(pCIT) (⋄), and CHCC2112 (♦) grown in M17′ medium supplemented with glucose and citrate under controlled pH 4.5 conditions. (B) Fermentation time course of L. lactis CRL264 with (▪) or without (□) citrate under noncontrolled pH initially adjusted to 4.5 and monitored during fermentation. Profile of external pH in the presence (▴) or absence (▵) of citrate in the growth medium.
TABLE 1.
Physiological parameters of strains CRL264, CRL30, CRL30(pCIT), and CHCC2112 grown at a controlled pHa
| Strain | μmax ± SD (h−1) | OD600 ± SDb | GCR* (mmol Glc g biomass−1 h−1) | GCR (mmol Glc g biomass−1 h−1) | CCR (mmol cit g biomass−1 h−1) | YX/Glc (g biomass mol Glc−1) | YX/subst (g biomass mol C−1) | YX/ATP (g biomass mol ATP−1) | V (nmol citrate mg protein−1 min−1) |
|---|---|---|---|---|---|---|---|---|---|
| CRL264 | 0.59 ± 0.02 | 1.62 ± 0.01 | 12.0 | 7.7 | 33.8 | 30.5 | 17.8 | 16.8 | 4.8 |
| CRL30 | 0.49 ± 0.01 | 1.86 ± 0.15 | 11.1 | 0.0 | 23.6 | 23.5 | 12.2 | 0.3 | |
| CRL30(pCIT) | 0.49 ± 0.03 | 1.48 ± 0.05 | 9.4 | 7.1 | 14.9 | 31.6 | 17.2 | 15.4 | 1.9 |
| CHCC2112 | 0.44 ± 0.02 | 1.32 ± 0.10 | 8.9 | 5.5 | 27.8 | 35.1 | 18.0 | 22.5 | 1.3 |
Physiological parameters are shown for strains CRL264, CRL30, CRL30(pCIT), and CHCC2112 grown at a controlled pH of 4.5 in rich medium (M17′) supplemented with 55 mM glucose and 13.2 mM citrate. Mean values correspond to at least two independent determinations. Standard deviations (SD) for the μmax and OD are shown. GCR* is the average specific glucose consumption rate for the period of growth before citrate depletion, and GCR is for the entire growth phase; CCR, average specific citrate consumption rate; YX/Glc, biomass yield relative to glucose consumption; YX/subst, biomass yield relative to glucose plus citrate consumption; YX/ATP, biomass yield relative to ATP production; V, citrate uptake rate of resting cells (at 50 μM citrate).
OD600 values were calculated at the beginning of the stationary phase.
Effect of citrate on the physiology of strain CRL264 grown without pH control.
The growth of strain CRL264 was also analyzed during batch cultivations without pH control to enable a consistent comparison with data at a constant pH. Figure 1B depicts the growth of strain CRL264 with and without the addition of 13.2 mM citrate. Cells grew considerably faster in the presence of citrate, with a μmax of 0.57 h−1, and the final biomass obtained was also higher, reaching an OD600 of 1.3 at stationary phase (Table 2). When citrate was present, the pH of the medium increased transiently to 4.8 during an initial stage and subsequently decreased, reaching 4.4 at the beginning of stationary phase; in contrast, in the absence of citrate, a continuous acidification of the medium occurred, and growth arrest was observed when the pH was 3.9.
TABLE 2.
Growth parameters of strain CRL264a
| Growth conditions |
μmax ± SD (h−1) | OD600 ± SD at stationary phase | CCR (mmol cit g biomass−1 h−1) | GCR* (mmol Glc g biomass−1 h−1) | GCR (mmol Glc g biomass−1 h−1) | YX/Glc* (g biomass mol Glc−1) | YX/Glc** (g biomass mol Glc−1) | YX/Glc (g biomass mol Glc−1) | YX/ATP (g biomass mol ATP−1) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Citrate content | pH controlled | pHin | pHfin | |||||||||
| + | No | 4.5 | 4.4 | 0.57 ± 0.02 | 1.33 ± 0.01 | 32.9 | 12.1 | 8.2 | 54.1 | 22.6 | 32.1 | 18.9 |
| − | No | 4.5 | 3.9 | 0.42 ± 0.002 | 0.81 ± 0.07 | 9.7b | 6.8 | 38.8b | 28.7b | 31.1 | 15.6 | |
| + | Yes | 4.5 | 4.5 | 0.59 ± 0.02 | 1.62 ± 0.01 | 33.8 | 12.0 | 7.7 | 64.8 | 19.9 | 30.5 | 16.8 |
| − | Yes | 4.5 | 4.5 | 0.45 ± 0.01 | 1.67 ± 0.01 | 10.5b | 8.2 | 32.2b | 23.2b | 25.5 | 13.2 | |
| + | Yes | 6.5 | 6.5 | 1.27 ± 0.02 | 5.75 ± 0.27 | 7.8 | 12.7 | 11.2 | 40.5 | 23.9 | 37.7 | 18.2 |
| − | Yes | 6.5 | 6.5 | 0.95 ± 0.01 | 6.90 ± 0.14 | 14.2b | 12.6 | 54.0b | 39.4b | 45.7 | 20.3 | |
Growth parameters are shown for strain CRL264 grown in M17′ medium containing 55 mM glucose with (+) or without (−) 13.2 mM citrate under different pH conditions. pHin, initial pH; pHfin, pH value at the end of growth. The symbols * and ** indicate that the values were calculated during the time of growth up to citrate depletion or after citrate depletion, respectively. CCR, average specific citrate consumption rate; GCR, (without an asterisk) average specific glucose consumption rate for the entire growth phase. YX/Glc*, YX/Glc**, and YX/Glc indicate biomass yields relative to glucose consumption for the periods of growth before and after citrate depletion and for the entire growth phase, respectively. YX/ATP, biomass yield relative to ATP production. The data are mean values of at least two independent determinations. Standard deviations (SD) for μmax and OD are presented.
Value calculated for the time period identical to that used for the corresponding cultivations with citrate.
Effect of citrate on the physiology of strain CRL264 at a constant pH of 4.5.
When the pH of the culture was controlled at 4.5, strain CRL264 exhibited biphasic growth: until the depletion of 13.2 mM citrate, the μmax was 0.59 h−1, and thereafter, the specific growth rate decreased to 0.08 h−1 (Fig. 2A and C). When citrate was not added, the growth curve was characterized by a single μmax value of 0.45 h−1 (Fig. 2A and Table 2). Thus, the addition of citrate to the growth medium affected the physiology of strain CRL264 before and after citrate depletion. At this concentration of citrate, the final biomass decreased only slightly. The average specific glucose consumption rate (abbreviated GCR) was calculated for the period of growth before citrate depletion (GCR*; where * is the period of growth before citrate depletion), and for comparison purposes, the same time points were used for calculating the GCR* in cultures where no citrate was added (Table 2). Additionally, the average specific GCR was calculated for the entire growth phase (GCR without an asterisk). The presence of citrate in the medium stimulated the average specific GCR (GCR* increased from 10.5 to 12.0 mmol Glc g of biomass−1 h−1) and provoked a notable enhancement (approximately twofold) of the biomass yield of glucose (YX/Glc*, where Y is the biomass yield, X is the amount of biomass produced per mol of glucose consumed, and * refers to the period of growth before citrate depletion). However, a comparison of the biomass yield values for the period of growth subsequent to citrate depletion (YX/Glc**) shows a reversed relationship, i.e., lower yields from cultures supplied with citrate, indicating a negative effect of citrate metabolism on cell physiology, which became apparent once citrate was exhausted. For the same reason, the average specific GCR decreased from 8.2 (no citrate) to 7.7 (13.2 mM citrate) mmol Glc g biomass−1 h−1 when the entire growth was considered. Interestingly, the beneficial effect of citrate on biomass yield relative to ATP production (YX/ATP) is apparent even when the values for the global fermentation are compared (Table 2).
FIG. 2.
Comparison of the growth of L. lactis CRL264 and the distribution of end products, with or without citrate addition, at a constant pH value of 4.5 or 6.5. Growth profiles in GM17′ (□) or GCM17′ (▪) medium at a controlled pH of 4.5 (A) or pH 6.5 (D). Substrate consumption and end product formation: pH 4.5 in GM17′ medium (B) or GCM17′ medium (C) and pH 6.5 in GM17′ medium (E) or GCM17′ medium (F). , glucose; ♦, citrate; ▴, lactate; •, acetate; ▵, acetoin; ○, 2,3-butanediol; ⋄, ethanol; □, formate.
Effects of the initial citrate concentration on the growth and on the end products of strain CRL264 cultured at a constant pH of 4.5.
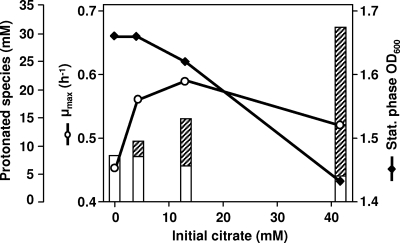
Cells were grown in GM17′ medium containing different concentrations of citrate. A low concentration of citrate (4.1 mM) led to an increase of the μmax compared to that from the experiments without citrate; a further enhancement of the μmax was observed when the citrate concentration was increased to 13.2 mM (Table 3). In this range of citrate concentration (0 to 13.2 mM), the final biomass produced was not greatly affected, although a slightly decreasing trend was apparent (Fig. 3). At 41.9 mM citrate, the μmax and the total biomass production were negatively affected (Fig. 3). At the low working pH used (4.5), growth stopped, even though over 50% of the initial glucose remained in the medium and the amount of glucose consumed decreased with increasing citrate concentration (Table 3). This led us to suspect that acetic acid derived from citrate could be an important factor, since weak organic acids are well-known inhibitors of bacterial growth at low pH. For each citrate concentration examined, the level of the protonated forms of lactic and acetic acids were calculated at the times of growth arrest (Fig. 3). The level of protonated lactic acid decreased with increasing citrate, reflecting the lower amount of glucose consumed; on the other hand, protonated acetic acid increased proportionally with that of citrate, since this substrate was exhausted regardless of the initial concentration supplied.
TABLE 3.
End product accumulation and growth parameters for fermentations of L. lactis CRL264 at controlled pHa
| Concn of citrate added (mM) | μmax ± SD (h−1) | OD600 ± SD at stationary phase | Glucose consumed (mM)b | Amt of lactate (mM) | Amt of acetate (mM) | Amt of acetoin (mM) | Amt of 2,3-butanediol (mM) | YX/Glc* (g biomass mol Glc−1) | YX/Glc (g biomass mol Glc−1) | YX/ATP (g biomass mol ATP−1) | GCR* (mmol Glc g biomass−1 h−1) | GCR (mmol Glc g biomass−1 h−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.0 | 0.45 ± 0.01 | 1.67 ± 0.01 | 23.1 | 43.6 | 0.1 | BD | BD | 25.5 | 13.2 | 8.2 | ||
| 4.1 | 0.56 ± 0.02 | 1.66 ± 0.01 | 22.4 | 42.7 | 4.2 | 1.2 | 0.7 | 41.1 | 23.9 | 12.5 | 11.0 | 7.6 |
| 13.2 | 0.59 ± 0.02 | 1.62 ± 0.01 | 18.1 | 34.4 | 12.8 | 5.6 | 0.6 | 64.8 | 30.5 | 16.8 | 12.0 | 7.7 |
| 41.9 | 0.52 ± 0.03 | 1.43 ± 0.05 | 14.1 | 24.6 | 40.8 | 19.1 | 0.3 | 42.4 | 35.4 | 22.6 | 6.4 | 5.3 |
End product accumulation and growth parameters for fermentation of L. lactis CRL264 grown at controlled pH 4.5 in rich medium containing 55 mM glucose and supplemented with different citrate concentrations. YX/Glc* indicates biomass yield relative to glucose consumption for the period of growth before citrate depletion, and YX/Glc is for the entire growth phase; YX/ATP is biomass yield relative to ATP production. GCR* is the average specific glucose consumption rate for the period of growth before citrate depletion, and GCR (without an asterisk) is for the entire growth phase. CCR, average specific citrate consumption rate; BD, below detection limit. The data correspond to mean values of at least two independent determinations. Standard deviations for μmax and OD600 are presented. Carbon balances were in the range 94 to 96%.
Glucose consumed until growth arrest.
FIG. 3.
Effects of different initial citrate concentrations on the μmax (○) and on the biomass concentration at the onset of stationary phase (⧫) for L. lactis CRL264 grown under constant pH 4.5 conditions. The concentrations of nondissociated organic acids calculated at the time of growth arrest using the Henderson-Hasselbach equation are represented; open bars, lactic acid; hatched bars, acetic acid.
The concentrations of end products in the culture medium determined at the beginning of stationary phase are summarized in Table 3. The pattern of end products (lactate yield relative to glucose in the range 88 to 95%) was consistent with a homolactic behavior regardless of the initial concentration of citrate examined. In the absence of citrate, the pool of end products comprised lactate, low concentrations of ethanol (0.47 mM), and trace amounts of acetate. When citrate was supplied, the concentration of acetate produced was identical to that of citrate consumed, indicating that acetate originated exclusively from the reaction catalyzed by citrate lyase. Acetoin and 2,3-butanediol, typical products of citrate metabolism, were also produced. The sum of acetoin and 2,3-butanediol accounted well for the stoichiometric consumption of citrate; i.e., pyruvate derived from citrate was essentially directed to the production of these two aromatic compounds. Moreover, the ratio of acetoin/2,3-butanediol increased notably with the initial citrate concentration. Trace amounts of ethanol and formate were also produced.
Effect of citrate on the physiology of strain CRL264 at a constant pH of 6.5.
To verify if the changes in the growth parameters arising from citrate consumption were specific to low pH conditions, cultivations were also performed at a constant pH of 6.5 (the optimal pH for growth). A positive effect of citrate on the μmax, as well as a negative effect on the final biomass produced, was also apparent at this pH value (Fig. 2D and Table 2). The average specific citrate consumption rate was more than fourfold lower at pH 6.5 than at pH 4.5 (Table 2). At the optimum pH, cell growth was strictly carbon limited, as no residual glucose was detected in the medium after growth arrest, in contrast with observations at pH 4.5, where growth stopped despite the high amounts of residual glucose (Fig. 2). At pH 6.5, citrate inhibited the specific GCR* as opposed to the clear stimulatory effect induced at pH 4.5 (Table 2).
A pH change from 4.5 to 6.5 in GM17′ caused a shift of glucose metabolism from typical homolactic to mixed acid fermentation, producing considerable amounts of ethanol, acetate, formate, and lactate in the relative proportions of 1.0:1.0:1.8:2.4 (Fig. 2, compare panels B and E). Lactate represented only about 50% of the glucose consumed (Fig. 2E). Surprisingly, the addition of citrate to the medium at the optimal pH led to a remarkable increase in the lactate yield (from 50% to 79%) and to a very low production of acetoin and 2,3-butanediol. The molar ratios of end products in the presence of citrate were 1.0:3.5:2.5:11.1 for ethanol, acetate, formate, and lactate. The final concentrations of acetate produced were similar when the fermentations with and without citrate were compared (Fig. 2E and 2F); however, after taking into account the equimolar amount of acetate produced in the citrate lyase reaction, it was apparent that the presence of citrate reduced considerably (by approximately 37%) the conversion of pyruvate to acetate.
Effect of added acetic acid on the growth of CRL264 at a constant pH of 4.5.
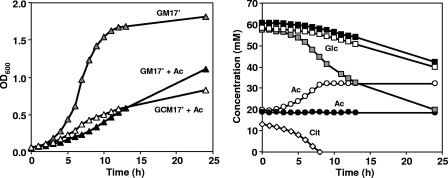
To investigate the relationship between the formation of acetic acid derived from citrate metabolism and the lower amount of final biomass, CRL264 was grown at a controlled pH of 4.5 in GM17′ medium supplemented with 20 mM acetic acid. At this low pH, the addition of the weak acid had a strong negative effect on the μmax as well as on the biomass produced: the μmax decreased from 0.45 to 0.24 h−1, and the OD600 decreased from 1.67 to 0.52 (at 12 h after inoculation). When citrate was present, the μmax increased slightly to 0.26 h−1, but the final biomass decreased even further (Fig. 4A). After citrate depletion, the culture had to cope with a higher concentration of external acetic acid derived from citrate metabolism: up to 32 mM compared to the initial 20 mM (Fig. 4B). It is worth stressing the increase in μmax induced by citrate metabolism, which indicates that the utilization of citrate represented an advantage against the increased toxicity associated with the higher level of acetic acid.
FIG. 4.
(Left panel) Fermentation time courses of L. lactis CRL264 grown at a controlled pH of 4.5 in GM17′ medium (gray symbols), GM17′ medium plus 20 mM acetate (closed symbols), and GCM17′ medium plus 20 mM acetate (open symbols). (Right panel) The time courses for the concentration of selected metabolites are also shown: Glc, glucose; Cit, citrate; Ac, acetate.
Effect of citrate on lactic acid toxicity at low, noncontrolled pH.
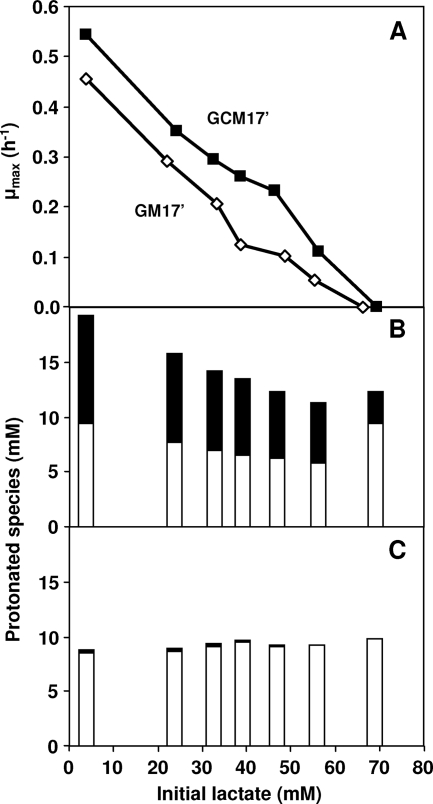
To evaluate the extent to which citrate metabolism counteracts lactic acid toxicity, CRL264 was grown in flasks containing GM17′ medium and different initial concentrations of lactic acid, with or without citrate. At the same initial lactate concentrations, cultures grown with citrate showed greater μmax values (Fig. 5A). For example, at an initial lactic acid concentration of approximately 47.5 mM, the presence of citrate caused an increase of 2.3-fold in the μmax. The critical concentration of initial lactate at which growth was no longer possible was approximately 65 mM. As the pH of the cultures was not controlled, growth arrest occurred at different final pH values, depending on the initial lactate concentration. When citrate was added, the concentration of protonated species at the time of growth arrest was notably higher (Fig. 5B). For the cultures without citrate, the sum of the protonated forms of lactic and acetic acids, calculated at the pH level at which growth arrest occurred, was approximately constant (approximately 9 mM) (Fig. 5C).
FIG. 5.
(A) Effects of different initial concentrations of lactate on the μmax of L. lactis CRL264 grown in flasks containing GM17′ (⋄) medium or GCM17′ (▪) medium. The initial pH was 4.5, and the pH of the cultures was not controlled. The concentrations of nondissociated acid end products at the onset of stationary phase were calculated using the Henderson-Hasselbach equation for growth on glucose plus citrate (B) and for growth on glucose (C). Open bars, lactic acid; closed bars, acetic acid.
Citrate uptake by whole cells.
Under all the conditions examined, the presence of citrate in the medium resulted in an increased μmax. We wanted to investigate whether acid resistance correlated to the capacity of the citrate transport system in different strains. Citrate uptake was studied in strain CRL264 and in its derivatives, CRL30(pCIT) and CRL30. The natural citrate consumer CHCC2112 was also examined. CRL264 had a very inefficient citrate transport (the substrate uptake rate [V] = 1.0 nmol citrate mg protein−1 min−1) when citrate was the only substrate provided in the transport assays, but the uptake was clearly stimulated when glucose was supplied in addition to citrate (V of 4.8 nmol citrate mg protein−1 min−1). CRL30(pCIT) exhibited an uptake activity 2.5-fold lower than the parental strain under the same conditions. This value is in good agreement with the comparative determination of plasmid copy numbers, which showed that the plasmid content of strain CRL30(pCIT) was approximately half that of strain CRL264 (data not shown). Strain CRL30 presented a very low citrate uptake activity, around 16-fold lower than that of the parental strain (Table 1). The wild-type strain, CHCC2112, had a transport capacity about 3.7-fold lower than that of strain CRL264. The citrate uptake activity in strain CHCC2112 was also very inefficient in the absence of glucose (V = 0.8 nmol citrate mg protein−1 min−1). A positive correlation was found between the citrate uptake capacity and the μmax of the three different citrate-utilizing strains (Table 1).
In vivo NMR evidence for a citrate/lactate exchanger system.
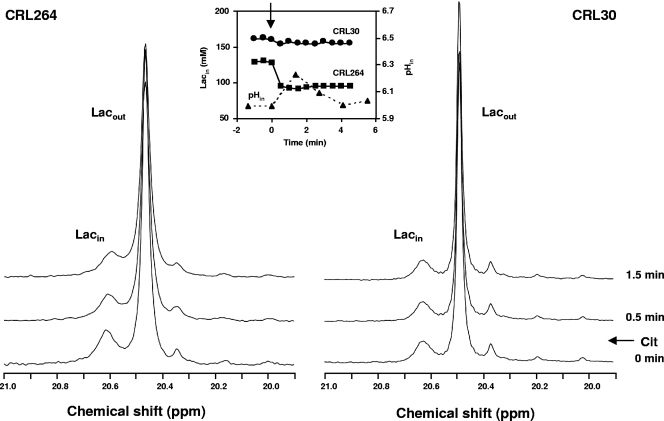
The intracellular and extracellular lactate pools were monitored by 13C NMR during the metabolism of glucose and citrate. Upon addition of 40 mM [1-13C]glucose, resonances due to intracellular and extracellular lactate were clearly observed (Fig. 6). NMR is able to distinguish these two lactate resonances due to the different pH values of the intracellular and extracellular compartments. The effect of citrate on the intracellular lactate pool was evaluated by the subsequent addition of a citrate pulse. Citrate addition resulted in a clear decrease of the intracellular lactate pool in strain CRL264 but not in CRL30, the control strain that had a negligible citrate transport capacity (Table 1). Determination of intracellular pH by 31P NMR in parallel experiments showed that the addition of the citrate pulse caused an increase in the intracellular pH of strain CRL264 (Fig. 6, inset).
FIG. 6.
Selected regions from the 13C NMR spectra of strains CRL264 and CRL30 acquired after the metabolism of 40 mM [1-13C]glucose showing the evolution of intracellular lactate upon the addition of 20 mM citrate (external pH, 4.8). Each spectrum was acquired during 0.5 min. The time courses of the intracellular lactate concentrations are represented in the inset. Plots of the intracellular pH values determined by 31P NMR in a similar experiment with CRL264 at a working pH of 5.2 is also shown. ▴, intracellular pH; ▪, intracellular lactate concentration in strain CRL264; •, intracellular lactate concentration in strain CRL30. The arrow indicates the citrate addition time. Cit, citrate.
DISCUSSION
The beneficial effect of citrate cometabolism on the growth of lactic acid bacteria at an acidic pH is well documented in the literature. Several studies report an increase in the specific growth rate as well as the final biomass under noncontrolled pH conditions, but the underlying molecular mechanisms are poorly understood (3, 11, 13, 33, 37). Alkalinization of the culture medium is observed as a result of citrate metabolism (6, 10, 24, 32), and this led to the assumption that the increase in pH is the main factor responsible for the improved growth. We designed experiments to unequivocally verify whether this simplistic view was correct and used L. lactis subsp. lactis biovar diacetylactis CRL264 as a model organism for lactic acid bacteria with the great ability to grow under severely acidic conditions (10). Surprisingly, the increase in μmax induced by citrate metabolism was the same regardless of whether the fermentation pH of 4.5 was controlled or not (Table 2). This result showed that medium alkalinization was unrelated to the beneficial effect of citrate in respect to the physiological parameter μmax. However, when the pH was not controlled, there was a substantial improvement in the final biomass, while at a constant pH, citrate metabolism had a negative effect on this growth parameter. Acetic acid, which at low pH originates exclusively from the first step in citrate catabolism (citrate lyase reaction), was the main reason for this deleterious effect, as its concentration in the medium at stationary phase correlated with the decrease in the final biomass (Fig. 3). It is well known that the toxicity of an organic acid depends directly on the concentration of uncharged (protonated) species; moreover, it has been suggested that the intracellular pH is the factor that determines growth arrest at low pH (28). At pH 4.5, around 65% of the total acetic acid (pKa, 4.76) is in the neutral form, while only 19% of the lactic acid is protonated (pKa, 3.86). Thus, acetic acid is expected to be a stronger inhibitor of growth at pH 4.5 than lactic acid. The toxic effect of acetic acid is also seen in the fermentations performed without pH control, since growth arrest occurs at a higher pH in the presence of citrate (pH 4.5 with citrate and 3.9 without citrate) (Fig. 1B).
In short, the cometabolism of citrate and glucose has both positive and negative contributions, the latter ones resulting from the production of equimolar amounts of acetic acid that easily cross the cell membrane with the consequent release of protons in the cytoplasm; this implies a higher expenditure of cell energy to achieve pH homeostasis. On the other hand, the beneficial effects of citrate are clearly apparent, since the biomass yields for glucose consumed and ATP produced increased (Table 2). Additionally, citrate metabolism provided an effective way to counteract the stress provoked by acetic and lactic acids (Fig. 4 and 5). As citrate catabolism is not accompanied by substrate-level phosphorylation, the improved energy metabolism provided evidence that less ATP derived from glucose was used for cellular maintenance, including pH homeostasis under acid stress conditions. This view is further corroborated by the observed alkalinization of the cytoplasm induced by citrate utilization (Fig. 6).
A shift from a homolactic to a mixed acid fermentation and the consequently higher ATP production are findings that have been evoked to explain the positive effect of citrate on the growth of other lactic acid bacteria, such as Oenococcus oeni (13, 24, 39). However, the strains analyzed in this study did not display such metabolic behavior (Table 3). The same conclusion was obtained from in vivo 13C NMR data with resting cells: all the acetate formed was derived from citrate, and glucose was converted almost exclusively to lactate.
This raises the question of what the mechanism was for ATP conservation in this L. lactis strain. The possibility that citrate transport contributes to the formation of a proton gradient, a favorable membrane potential, or both has been extensively investigated in lactic acid bacteria (2, 7, 19, 22, 24, 32). Bandell et al. provided strong evidence supporting the involvement of a secondary metabolic energy generating pathway in the metabolism of citrate by Leuconostoc mesenteroides: the membrane potential is generated in the transport step (citrate2−/lactate− exchange), and the proton gradient results from the consumption of one proton in the oxaloacetate decarboxylation step (2). These authors proposed a similar mechanism for L. lactis strain NCDO176 and more recently also for L. lactis strain CRL264 (22). In the present work, in vivo 13C NMR data provided direct evidence for the operation in CRL264 of the citrate/lactate exchanger (Fig. 6), corroborating earlier hypotheses (22). In fact, a citrate/lactate exchanger could provide the cells with an advantage against toxicity due to lactate, which accumulated inside the cells to high concentrations (approximately 160 mM) (Fig. 6). However, the fermentation profiles show that at low, controlled pH levels, when citrate depletion occurred, the lactate concentration in the medium was around 60% (Fig. 2C) of that expected for a citrate/lactate exchanger, with a 1:1 stoichiometry. Two modes of operation have been proposed for the citrate transporter in Leuconostoc mesenteroides: (i) as a citrate/lactate exchanger when lactate is available or (ii) as a citrate/proton symporter in the absence of lactate. Taking into account the high sequence similarity between the citrate transporters of Leuconostoc mesenteroides and that of L. lactis (36), we speculate that both modes are also operating in L. lactis CRL264.
At the optimal pH for growth, 6.5, citrate addition still increased the μmax and decreased the final biomass, but contrary to the situation at low pH, citrate had a negative effect on biomass yields relative to glucose consumption and ATP production (Table 2). This result further substantiates the beneficial effect of citrate metabolism under acid stress conditions. The shift to a more homolactic fermentation observed at a neutral pH upon the addition of citrate can justify the decreased biomass yields and final biomass obtained, since the pathway for acetate formation is accompanied by ATP production. The reason why glucose metabolism is homolactic at low pH and becomes mixed acid at optimal pH is not known but has been observed previously for another citrate consuming strain, CNRZ125 (5), and for carbon-limited chemostat cultures of L. lactis subsp. cremoris (9, 29). It is noteworthy that the metabolic deviation to lactic acid, which has a lower pKa value than acetic acid, will ultimately result in a milder growth inhibition at a low pH.
This study provided the first quantitative analysis of the contribution of citrate metabolism to the growth and energetic parameters of L. lactis. Given the fact that alkalinization was not the main cause for the beneficial effect of citrate during cometabolism at a low pH, we hypothesized that the capacity of the citrate transport system could be a determining factor. Interestingly, the efficiency of the transport of the citrate-utilizing strains examined correlated positively with the better growth performance at low pH (Table 1). We propose that increasing the citrate transport capacity could be a useful strategy to improve growth under severely acidic conditions, a trait of great industrial importance. While the current constraints on the use of genetically modified organisms are not relaxed, the practical option is to select for natural strains with the highest citrate transport capacity.
Acknowledgments
This work was supported by funds from the Commission of the European Communities (contract QLK1-CT-2002-02388) and from Fundação para a Ciência e a Tecnologia, Portugal, and FEDER (POCTI-BIO/48333/2002). M.M.D.S. received a fellowship from FCT, Portugal (SFRH/BPD/14562/2003).
We thank Carla Jorge for assistance contributed during the radioactivity assays.
Footnotes
Published ahead of print on 21 December 2007.
REFERENCES
- 1.Anderson, D. G., and L. L. McKay. 1983. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl. Environ. Microbiol. 46:549-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bandell, M., M. E. Lhotte, C. Marty-Teysset, A. Veyrat, H. Prévots, V. Dartois, C. Diviès, W. N. Konings, and J. S. Lolkema. 1998. Mechanism of the citrate transporters in carbohydrate and citrate cometabolism in Lactococcus and Leuconostoc species. Appl. Environ. Microbiol. 64:1594-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boquien, C. Y., G. Corrieu, and M. J. Desmazeaud. 1988. Effect of fermentation conditions on growth of Streptococcus cremoris AM2 and Leuconostoc lactis CNRZ 1091 in pure and mixed cultures. Appl. Environ. Microbiol. 54:2527-2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Budin-Verneuil, A., E. Maguin, Y. Auffray, S. D. Ehrlich, and V. Pichereau. 2004. An essential role for arginine catabolism in the acid tolerance of Lactococcus lactis MG1363. Lait 84:61-68. [Google Scholar]
- 5.Cachon, R., and C. Diviès. 1994. Generalized model of the effect of pH on lactate fermentation and citrate bioconversion in Lactococcus lactis subsp. lactis biovar diacetylactis. Appl. Microbiol. Biotechnol. 41:694-699. [Google Scholar]
- 6.Cogan, T. M., M. O′Dowd, and D. Mellerick. 1981. Effects of pH and sugar on acetoin production from citrate by Leuconostoc lactis. Appl. Environ. Microbiol. 41:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.David, S., M. E. van der Rest, A. J. M. Driessen, G. Simons, and W. M. de Vos. 1990. Nucleotide sequence and expression in Escherichia coli of the Lactococcus lactis citrate permease gene. J. Bacteriol. 172:5789-5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dornan, S., and M. A. Collins. 1990. High efficiency electroporation of Lactococcus lactis subsp. lactis LM0230 with plasmid pGB301. Lett. Appl. Microbiol. 11:62-64. [DOI] [PubMed] [Google Scholar]
- 9.Even, S., N. D. Lindley, and M. Cocaign-Bousquet. 2003. Transcriptional, translational and metabolic regulation of glycolysis in Lactococcus lactis subsp. cremoris MG 1363 grown in continuous acidic cultures. Microbiology 149:1935-1944. [DOI] [PubMed] [Google Scholar]
- 10.García-Quintáns, N., C. Magni, D. de Mendoza, and P. López. 1998. The citrate transport system of Lactococcus lactis subsp. lactis biovar diacetylactis is induced by acid stress. Appl. Environ. Microbiol. 64:850-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harvey, R. J., and E. B. Collins. 1963. Roles of citrate and acetoin in the metabolism of Streptococcus diacetilactis. J. Bacteriol. 86:1301-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harvey, R. J. 1965. Damage to Streptococcus lactis resulting from growth at low pH. J. Bacteriol. 90:1330-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hugenholtz, J. 1993. Citrate metabolism in lactic acid bacteria. FEMS Microbiol. Rev. 12:165-178. [Google Scholar]
- 14.Hutkins, R. W., and N. L. Nannen. 1993. pH homeostasis in lactic acid bacteria. J. Dairy Sci. 76:2354-2365. [Google Scholar]
- 15.Kashket, E. R. 1987. Bioenergetics of lactic acid bacteria: cytoplasmic pH and osmotolerance. FEMS Microbiol. Rev. 46:233-244. [Google Scholar]
- 16.Kempler, G. M., and L. L. McKay. 1980. Improved medium for detection of citrate-fermenting Streptococcus lactis subsp. diacetylactis. Appl. Environ. Microbiol. 39:926-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi, H. 1985. A proton-translocating ATPase regulates pH of the bacterial cytoplasm. J. Biol. Chem. 260:72-76. [PubMed] [Google Scholar]
- 18.Konings, W. N. 2002. The cell membrane and the struggle for life of lactic acid bacteria. Antonie van Leeuwenhoek 82:3-27. [PubMed] [Google Scholar]
- 19.Lolkema, J. S., B. Poolman, and W. N. Konings. 1995. Role of scalar protons in metabolic energy generation in lactic acid bacteria. J. Bioenerg. Biomembr. 27:467-473. [DOI] [PubMed] [Google Scholar]
- 20.López de Felipe, F. L., C. Magni, D. de Mendoza, and P. López. 1995. Citrate utilization gene cluster of the Lactococcus lactis biovar diacetylactis: organization and regulation of expression. Mol. Gen. Genet. 246:590-599. [DOI] [PubMed] [Google Scholar]
- 21.Magni, C., F. L. López de Felipe, F. Sesma, P. López, and D. de Mendoza. 1994. Citrate transport in Lactococcus lactis subsp. lactis biovar diacetylactis. Expression of the citrate permease P. FEMS Microbiol. Lett. 118:75-82. [Google Scholar]
- 22.Magni, C., D. de Mendoza, W. N. Konings, and J. S. Lolkema. 1999. Mechanism of citrate metabolism in Lactococcus lactis: resistance against lactate toxicity at low pH. J. Bacteriol. 181:1451-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martín, M. G., P. D. Sender, S. Peirú, D. de Mendoza, and C. Magni. 2004. Acid-inducible transcription of the operon encoding the citrate lyase complex of Lactococcus lactis biovar diacetylactis CRL264. J. Bacteriol. 186:5649-5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marty-Teysset, C., C. Posthuma, J. S. Lolkema, P. Schmitt, C. Divies, and W. N. Konings. 1996. Proton motive force generation by citrolactic fermentation in Leuconostoc mesenteroides. J. Bacteriol. 178:2178-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neves, A. R., A. Ramos, M. C. Nunes, M. Kleerebezem, J. Hugenholtz, W. M. de Vos, J. Almeida, and H. Santos. 1999. In vivo nuclear magnetic resonance studies of glycolytic kinetics in Lactococcus lactis. Biotechnol. Bioeng. 64:200-212. [DOI] [PubMed] [Google Scholar]
- 26.Neves, A. R., A. Ramos, C. Shearman, M. J. Gasson, J. S. Almeida, and H. Santos. 2000. Metabolic characterization of Lactococcus lactis deficient in lactate dehydrogenase using in vivo 13C-NMR. Eur. J. Biochem. 267:3859-3868. [DOI] [PubMed] [Google Scholar]
- 27.Neves, A. R., A. Ramos, C. Shearman, M. J. Gasson, and H. Santos. 2002. Catabolism of mannitol in Lactococcus lactis MG1363 and a mutant defective in lactate dehydrogenase. Microbiology 148:3467-3476. [DOI] [PubMed] [Google Scholar]
- 28.O'Sullivan, E., and S. Condon. 1997. Intracellular pH is a major factor in the induction of tolerance to acid and other stresses in Lactococcus lactis. Appl. Environ. Microbiol. 63:4210-4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Sullivan, E., and S. Condon. 1999. Relationship between acid tolerance, cytoplasmic pH, and ATP and H+-ATPase levels in chemostat cultures of Lactococcus lactis. Appl. Environ. Microbiol. 65:2287-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poolman, B., E. J. Smid, H. Veldkamp, and W. N. Konings. 1987. Bioenergetic consequences of lactose starvation for continuously cultured Streptococcus cremoris. J. Bacteriol. 169:1460-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rallu, F., A. Gruss, and E. Maguin. 1996. Lactococcus lactis and stress. Antonie van Leeuwenhoek 70:243-251. [DOI] [PubMed] [Google Scholar]
- 32.Ramos, A., B. Poolman, H. Santos, J. S. Lolkema, and W. N. Konings. 1994. Uniport of anionic citrate and proton consumption in citrate metabolism generates a proton motive force in Leuconostoc oenos. J. Bacteriol. 176:4899-4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salou, P., P. Loubiere, and A. Pareilleux. 1994. Growth and energetics of Leuconostoc oenos during cometabolism of glucose with citrate or fructose. Appl. Environ. Microbiol. 60:1459-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanders, J. W., K. Leenhouts, J. Burghoorn, J. R. Brands, G. Venema, and J. Kok. 1998. A chloride-inducible acid resistance mechanism in Lactococcus lactis and its regulation. Mol. Microbiol. 27:299-310. [DOI] [PubMed] [Google Scholar]
- 35.Sesma, F., D. Gardiol, A. P. de Ruiz Holgado, and D. de Mendoza. 1990. Cloning and expression of the citrate permease gene of Lactococcus lactis subsp. lactis biovar diacetylactis in Escherichia coli. Appl. Environ. Microbiol. 56:2099-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sobczak, I., and J. S. Lolkema. 2005. The 2-hydroxycarboxylate transporter family: physiology, structure, and mechanism. Microbiol. Mol. Biol. Rev. 69:665-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Starrenburg, M., and J. Hugenholtz. 1991. Citrate fermentation by Lactococcus and Leuconostoc spp. Appl. Environ. Microbiol. 57:3535-3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steidler, L., and P. Rottiers. 2006. Therapeutic drug delivery by genetically modified Lactococcus lactis. Ann. N. Y. Acad. Sci. 1072:176-186. [DOI] [PubMed] [Google Scholar]
- 39.Torino, M. I., M. P. Taranto, and G. Font de Valdez. 2005. Citrate catabolism and production of acetate and succinate by Lactobacillus helveticus ATCC 15807. Appl. Microbiol. Biotechnol. 69:79-85. [DOI] [PubMed] [Google Scholar]
- 40.van de Guchte, M., P. Serror, C. Chervaux, T. Smokvina, S. D. Ehrlich, and E. Maguin. 2002. Stress response in lactic acid bacteria. Antonie van Leeuwenhoek 82:187-216. [PubMed] [Google Scholar]