Abstract
Streptococcus thermophilus is unable to metabolize the galactose moiety of lactose. In this paper, we show that a transformant of S. thermophilus SMQ-301 expressing Streptococcus salivarius galK and galM was able to grow on galactose and expelled at least twofold less galactose into the medium during growth on lactose.
Streptococcus thermophilus is unable to metabolize galactose and thus expels this sugar into the medium during lactose fermentation (6, 7, 10, 17, 18). The genes involved in galactose and lactose metabolism are located on a single locus on the chromosome of S. thermophilus in the following order: galKTEM lacSZ (18, 19). A regulator gene, galR, is located upstream from galK and is transcribed in the opposite direction. The galKTE genes code for the enzymes of the Leloir pathway, and galM codes for a mutarotase involved in the transformation of β-d-galactose into α-d-galactose, while lacS and lacZ code for the lactose transporter LacS and the enzyme β-galactosidase, respectively. The same gene organization is found in the phylogenetically related galactose-positive species Streptococcus salivarius (18). In this paper, we report the growth behavior on lactose and galactose of an S. thermophilus SMQ-301 recombinant strain expressing high levels of S. salivarius galK concurrent with S. salivarius galM.
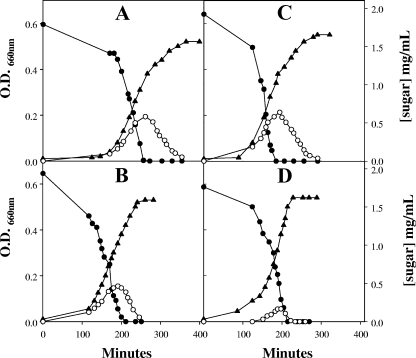
According to the recently proposed classification of S. thermophilus strains based on lactose fermentation (6), strain SMQ-301 belongs to profile C, which means that it is unable to grow on galactose, releases galactose into the medium during growth on lactose, and consumes excreted galactose after the lactose is depleted (18). The inability of strain SMQ-301 to grow on galactose results from a lack of galactokinase, which transforms α-d-galactose into α-d-galactose-1-phosphate (see below) (17, 18). To increase substantially GalK activity in S. thermophilus SMQ-301, we first constructed the vector pH2TK containing S. salivarius ATCC 25975 galK with its GalR binding site and the terminators of the Omegon kanamycin resistance gene from pCIV2 (12). Plasmid pH2TK was constructed from pTRKH2, a high-copy-number plasmid in Lactococcus lactis (13). Plasmid pH2TK was introduced into S. thermophilus SMQ-301 by electroporation (4) to obtain the recombinant strain SMQ-301H2TK. The expression of S. salivarius galK in strain SMQ-301H2TK was verified by measuring galactokinase activity (20) and by testing the ability of the recombinant strain to grow on galactose. The levels of galactokinase activity in the recombinant strain were higher than those reported for S. salivarius (17), a galactose-positive species that does not expel galactose during growth on lactose, and they were approximately 70-fold higher than those determined for strain SMQ-301 (2,508 ± 282 versus 36 ± 12 nmol/mg of protein/min; means ± standard deviations of data obtained from measurements obtained in triplicate with samples from three cultures grown on M17 medium containing 0.5% lactose). The wild-type and recombinant strains grew on 0.2 and 0.5% lactose at the same rate (generation time, 30 ± 3 min). However, while strain SMQ-301 was unable to grow on galactose, strain SMQ-301H2TK was able to grow in M17 broth supplemented with 0.2% galactose with a generation time of 61 ± 3 min (mean ± standard deviation of three separate experiments). Nonetheless, strain SMQ-301H2TK still released an appreciable amount of galactose into the medium during growth on lactose, as did strain SMQ-301 (Fig. 1A and B). Thus, merely increasing galactokinase activity, even to high levels, did not prevent S. thermophilus from releasing galactose during growth on lactose.
FIG. 1.
Growth of S. thermophilus on 0.2% lactose. Cells were grown overnight in the presence of 0.2% lactose. Tubes containing M17 medium supplemented with approximately 0.2% lactose were then inoculated with 300 μl of the overnight cultures and incubated at 42°C. Symbols: ▴, growth; •, concentration of lactose; ○, concentration of galactose in the medium. (A) Growth of strain SMQ-301. (B) Growth of strain SMQ-301H2TK. (C) Growth of strain SMQ-301M1. (D) Growth of strain SMQ-301H2TKM2. The experiments were done in triplicate, and similar results were obtained. O.D. 660 nm, optical density at 660 nm.
Most living cells possess a galactose mutarotase (GalM), which rapidly catalyzes the conversion of β-d-galactose into α-d-galactose (1). The activity of this enzyme is vital for the metabolism of lactose since the hydrolysis of lactose by β-galactosidase generates β-d-galactose, while the substrate of galactokinase is α-d-galactose. Because water-dependent spontaneous mutarotation is a rather slow process (9), the presence of GalM is essential for the efficient metabolism of lactose (2). We previously reported that galM is weakly transcribed in S. thermophilus (18). To determine whether an increase in GalM activity could improve the metabolism of galactose in S. thermophilus, we introduced plasmid pNT1M containing S. salivarius galM under the control of a constitutive promoter into S. thermophilus SMQ-301 and SMQ-301H2TK, generating strains SMQ-301M1 and SMQ-301H2TKM2. Plasmid pNT1M was constructed as follows (Fig. 2). The cloning vector pNT1 was constructed by cloning the cat gene of pC194 into the AatII site of S. thermophilus plasmid pSMQ172 (5, 16). The resulting plasmid was then digested with ScaI and ligated, thereby removing nucleotides between positions 2 and 3,812 of pSMQ172. This resulted in inactivation of orf4, which codes for a protein with an unknown function. orf3 (mob gene) of pSMQ172 was also inactivated by adding the multiple cloning site of pBlueScript KS (Stratagene) between the BstEII and HaeII sites. A 1,158-bp DNA fragment containing galM was PCR amplified from S. salivarius chromosomal DNA with primers galM-Kpn2 (5′-AAAGGGTACCATGATCGAGGATAATT-3′), which contained an engineered KpnI site (underlined), and galM-R-SacI (5′-TTAGAGCTCTTTTTACTAAACTTTTAC-3′), which contained an engineered SacI site (underlined), and was cloned into pCR2.1 (Invitrogen) to obtain plasmid pCR2.1M2. The sequence-confirmed DNA fragment cloned into pCR2.1M2 contained the stop codon of galE, which is located upstream from galM, the ribosome binding site of galM, the entire galM gene, and the intergenic region downstream from galM. This DNA fragment was excised from pCR2.1M2 and cloned into pNT1 with the same enzymes to obtain plasmid pNT1M. In this plasmid, galM was located within mob with the same transcriptional orientation and thus was under the control of the constitutive mob promoter. However, the presence of the galE stop codon at the 5′ end of the DNA fragment containing galM prevented the synthesis of a Mob-GalM fusion protein.
FIG. 2.
Scheme for construction of pNT1M, the plasmid bearing S. salivarius galM.
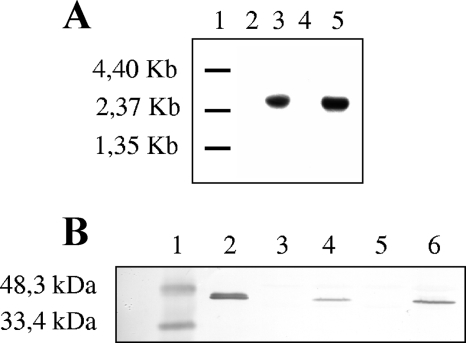
The expression of GalM in strains SMQ-301M1 and SMQ-301H2TKM2 was demonstrated by Northern and Western blot experiments (Fig. 3). The Northern blot experiments were conducted using a galM-specific probe, while the Western blot experiments were conducted with cellular extracts prepared by grinding cells with alumina (18) using rabbit polyclonal antibodies against S. salivarius His6-GalM, which shows over 94% identity with the S. thermophilus orthologue (18). His6-GalM was obtained from Escherichia coli BL21(DE3)(pMc1His3), which contains S. salivarius galM cloned into pET-29a(+) (Novagen).
FIG. 3.
Northern (A) and Western (B) blot analyses. (A) The lanes contained total RNA isolated from S. thermophilus cells grown on M17 medium containing 0.5% lactose and harvested in the mid-exponential phase of growth. The experiment was performed with an S. salivarius galM-specific probe. Lane 1, positions of RNA markers obtained from Invitrogen; lane 2, S. thermophilus SMQ-301; lane 3, S. thermophilus SMQ-301M1; lane 4, S. thermophilus SMQ-301H2TK; lane 5, S. thermophilus SMQ-301H2TKM2. (B) Cells were grown on 0.5% lactose. The proteins (15 μg in lanes 3 to 7) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to a nitrocellulose sheet. Samples were probed with anti-GalM rabbit polyclonal antibodies and revealed with anti-rabbit alkaline phosphatase conjugate (Sigma-Aldrich). Lane 1, prestained molecular size standards obtained from Bio-Rad; lane 2, purified recombinant His6-GalM (0.11 μg); lane 3, S. thermophilus SMQ-301; lane 4, S. thermophilus SMQ-301M1; lane 5, S. thermophilus SMQ-301H2TK; lane 6, S. thermophilus SMQ-301H2TKM2.
The presence of S. salivarius galM alone did not allow strain SMQ-301 to grow on galactose and did not prevent or reduce the expulsion of galactose during growth on lactose (Fig. 1C). The galK-galM recombinant strain SMQ-301H2TKM2 and the wild-type strain had the same generation times on 0.2 and 0.5% lactose (∼30 min), but unlike the wild-type strain, strain SMQ-301H2TKM2 was able to grow on galactose (76 ± 2 min). The generation time of strain SMQ-301H2TKM2 on galactose was slightly greater than that of strain SMQ-301H2TK. The increase in the generation time may be explained by the fact that strain SMQ-301H2TKM2 contained an additional plasmid, the replication of which required extra energy. The slower growth of SMQ-301H2TKM2 on galactose could also have resulted from the fact that expression of galM from pNT1M was not coordinately controlled with the other genes involved in galactose metabolism, resulting in futile energy expenditure. Unlike strain SMQ-301H2TK, strain SMQ-301H2TKM2 released three- to fourfold less galactose into the medium than the wild-type strain during growth on 0.2% lactose (Fig. 1D). Moreover, the small amount of galactose released into the medium was rapidly metabolized. To determine whether the amount of lactose in the medium influenced the ability of SMQ-301H2TKM2 to metabolize the galactose moiety of lactose, we measured the amount of galactose expelled during growth in the presence of 0.5% lactose. We found that even at a higher concentration of lactose, the amounts of galactose released into the medium by strain SMQ-301H2TKM2 were two- to threefold lower than the amounts expelled by SMQ-301 (not shown). Lastly, using a Dionex-500 high-performance liquid chromatography system (14), we determined the amounts of lactic acid in the culture medium produced by strains SMQ-301 and SMQ-301H2TKM2 after complete depletion of lactose and galactose. The amounts of lactic acid produced by strains SMQ-301 and SMQ-301H2TKM2 grown on 0.2 and 0.5% lactose corresponded to the amounts expected following the complete transformation of lactose into lactic acid. For instance, the amounts of lactic acid produced by strains SMQ-301 and SMQ-301H2TKM2 grown with 0.5% ± 0.03% lactose were 5.4 ± 0.1 and 4.7 ± 0.01 mg/ml (means ± standard deviations of two experiments), respectively. Thus, the two sugar moieties of lactose were converted into lactic acid by both strains.
Galactose-positive strains of S. thermophilus may be valuable for the dairy industry to prevent problems caused by the accumulation of galactose in fermented milk products (11, 15). Such strains may also produce larger amounts of exopolysaccharides (8), which play a major role in the organoleptic properties of yogurts (3, 21). The results presented here point to an interesting avenue for restoring galactose metabolism in S. thermophilus and reducing galactose expulsion during growth on lactose. Our results show that the expulsion of galactose during growth on lactose was significantly decreased when both GalM and GalK activities were increased. These results suggest that the expulsion of galactose by S. thermophilus LacS results, in part, from the inability of S. thermophilus to rapidly convert β-d-galactose into α-d-galactose-1-phosphate.
Acknowledgments
We thank Israël Casabon for valuable discussions and Gene Bourgeau for editorial assistance.
We thank FQRNT-NOVALAIT-MAPAQ funds in collaboration with the Canadian Institutes of Health Research (grant MOP 36338 to C.V. and M.F.), Agriculture and AgriFood Canada, and the Natural Sciences and Engineering Research Council of Canada (discovery grant to S.M.) for financial support.
Footnotes
Published ahead of print on 7 December 2007.
REFERENCES
- 1.Beebe, J. A., and P. A. Frey. 1998. Galactose mutarotase: purification, characterization, and investigations of two important histidine residues. Biochemistry 37:14989-14997. [DOI] [PubMed] [Google Scholar]
- 2.Bouffard, G. G., K. E. Rudd, and S. L. Adhya. 1994. Dependence of lactose metabolism upon mutarotase encoded in the gal operon in Escherichia coli. J. Mol. Biol. 244:269-278. [DOI] [PubMed] [Google Scholar]
- 3.Broadbent, J. R., D. J. McMahon, D. L. Welker, C. J. Oberg, and S. Moineau. 2003. Biochemistry, genetics, and applications of exopolysaccharide production in Streptococcus thermophilus: a review. J. Dairy Sci. 86:407-423. [DOI] [PubMed] [Google Scholar]
- 4.Buckley, N. D., C. Vadeboncoeur, D. J. Leblanc, L. N. Lee, and M. Frenette. 1999. An effective strategy, applicable to Streptococcus salivarius and related bacteria, to enhance or confer electroporation competence. Appl. Environ. Microbiol. 65:3800-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corneau, N., É. Émond, and G. LaPointe. 2004. Molecular characterization of three plasmids from Bifidobacterium longum. Plasmid 51:87-100. [DOI] [PubMed] [Google Scholar]
- 6.de Vin, F., P. Rådström, L. Herman, and L. De Vuyst. 2005. Molecular and biochemical analysis of the galactose phenotype of dairy Streptococcus thermophilus strains reveals four different fermentation profiles. Appl. Environ. Microbiol. 71:3659-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hutkins, R. W., and H. A. Morris. 1987. Carbohydrate metabolism by Streptococcus thermophilus: a review. J. Food Prot. 50:876-884. [DOI] [PubMed] [Google Scholar]
- 8.Levander, F., M. Svensson, and P. Rådström. 2002. Enhanced exopolysaccharide production by metabolic engineering of Streptococcus thermophilus. Appl. Environ. Microbiol. 68:784-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy, G. B., and E. S. Cook. 1954. A rotographic study of mutarotase. Biochem. J. 57:50-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mora, D., M. G. Fortina, C. Parini, G. Ricci, G. Giraffa, and P. L. Manachini. 2002. Genetic diversity and technological properties of Streptococcus thermophilus strains isolated from dairy products. J. Appl. Microbiol. 93:278-287. [DOI] [PubMed] [Google Scholar]
- 11.Mukherjee, K. K., and R. W. Hutkins. 1994. Isolation of galactose-fermenting thermophilic cultures and their use in the manufacture of low browning Mozzarella cheese. J. Dairy Sci. 77:2839-2849. [Google Scholar]
- 12.Okada, N., R. T. Geist, and M. G. Caparon. 1993. Positive transcriptional control of mry regulates virulence in the group A streptococcus. Mol. Microbiol. 7:893-903. [DOI] [PubMed] [Google Scholar]
- 13.O'Sullivan, D. J., and T. R. Klaenhammer. 1993. High- and low-copy-number Lactococcus shuttle cloning vectors with features for clone screening. Gene 137:227-231. [DOI] [PubMed] [Google Scholar]
- 14.Robitaille, G., S. Moineau, D. St. Gelais, C. Vadeboncoeur, and M. Britten. 2007. Galactose metabolism and capsule formation in a recombinant strain of Streptococcus thermophilus with a galactose-fermenting phenotype. J. Dairy Sci. 90:4051-4057. [DOI] [PubMed] [Google Scholar]
- 15.Tinson, W., M. F. Ratcliff, A. J. Hillier, and G. R. Jago. 1982. Metabolism of Streptococcus thermophilus. 3. Influence on the level of bacterial metabolites in Cheddar cheese. Aust. J. Dairy Technol. 37:17-21. [Google Scholar]
- 16.Turgeon, N., and S. Moineau. 2001. Isolation and characterization of a Streptococcus thermophilus plasmid closely related to the pMV158 family. Plasmid 45:171-183. [DOI] [PubMed] [Google Scholar]
- 17.Vaillancourt, K., J.-D. LeMay, M. Lamoureux, M. Frenette, S. Moineau, and C. Vadeboncoeur. 2004. Characterization of a galactokinase-positive recombinant strain of Streptococcus thermophilus. Appl. Environ. Microbiol. 70:4596-4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaillancourt, K., S. Moineau, M. Frenette, C. Lessard, and C. Vadeboncoeur. 2002. Galactose and lactose genes from the galactose-positive bacterium Streptococcus salivarius and the phylogenetically related galactose-negative bacterium Streptococcus thermophilus: organization, sequence, transcription, and activity of the gal gene products. J. Bacteriol. 184:785-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaughan, E. E., P. T. C. van den Bogaard, P. Catzeddu, O. P. Kuipers, and W. M. de Vos. 2001. Activation of silent gal genes in the lac-gal regulon of Streptococcus thermophilus. J. Bacteriol. 183:1184-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verhees, C. H., D. G. M. Koot, T. J. G. Ettema, C. Dijkema, W. M. de Vos, and J. van der Oost. 2002. Biochemical adaptations of two sugar kinases from the hyperthermophylic archaeon Pyrococcus furiosus. Biochem. J. 366:121-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welman, A. D., and I. S. Maddox. 2003. Exopolysaccharides from lactic acid bacteria: perspectives and challenges. Trends Biotechnol. 21:269-274. [DOI] [PubMed] [Google Scholar]