Abstract
The resistance of Listeria monocytogenes to cadmium and arsenic has been used extensively for strain subtyping. However, limited information is available on the prevalence of such resistance among isolates from the environment of food-processing plants. In addition, it is not known whether the resistance of such isolates to heavy metals may correlate with resistance to quaternary ammonium compounds extensively used as disinfectants in the food-processing industry. In this study, we characterized 192 L. monocytogenes isolates (123 putative strains) from the environment of turkey-processing plants in the United States for resistance to cadmium and arsenic and to the quaternary ammonium disinfectant benzalkonium chloride (BC). Resistance to cadmium was significantly more prevalent among strains of serotypes 1/2a (or 3a) and 1/2b (or 3b) (83% and 74%, respectively) than among strains of the serotype 4b complex (19%). Resistance to BC was encountered among 60% and 51% of the serotype 1/2a (or 3a) and 1/2b (or 3b) strains, respectively, and among 7% of the strains of the serotype 4b complex. All BC-resistant strains were also resistant to cadmium, although the reverse was not always the case. In contrast, no correlation was found between BC resistance and resistance to arsenic, which overall was low (6%). Our findings suggest that the processing environment of turkey-processing plants may constitute a reservoir for L. monocytogenes harboring resistance to cadmium and to BC and raise the possibility of common genetic elements or mechanisms mediating resistance to quaternary ammonium disinfectants and to cadmium in L. monocytogenes.
Listeria monocytogenes causes listeriosis, a relatively rare but serious food-borne disease with symptoms such as stillbirths or abortions, septicemia, and meningitis or encephalitis. It has a high mortality rate (ca. 20%) for at-risk populations, which include pregnant women and their fetuses, neonates, and those who are elderly and/or immunocompromised (25). Strains of three serotypes (1/2a, 1/2b, and 4b) are associated with most human listeriosis cases. A substantial fraction of sporadic cases and the majority of food-borne outbreaks are due to serotype 4b strains (12, 25). However, strains of serotype 4b tend to be underrepresented in foods, and the majority of food isolates are of serotype 1/2a or 1/2b (11, 14, 27). The environment of food-processing plants is considered to play a key role in contamination of processed ready-to-eat foods, and substantial evidence points to the ability of certain strains to colonize the processing-plant environment and to persist there, frequently over extended periods of time (for reviews, see references 12 and 13).
Benzalkonium chloride (BC) and other quaternary ammonium compounds (QACs) are extensively used as disinfectants in the food-processing industry (19, 22). Frequent use of BC and other QACs in food-processing plants may constitute a selective pressure for the emergence and establishment of resistance to these compounds among L. monocytogenes isolates that successfully colonize the processing plants and that subsequently become transferred to foods through postprocessing recontamination of the products. It is therefore important to investigate the resistance of L. monocytogenes from the food-processing plant environment to BC and other QACs. Only a few studies, primarily in Europe, have investigated BC resistance in L. monocytogenes from the environment of food (primarily seafood)-processing plants (1, 28). Thus, limited information is currently available on BC resistance in isolates from diverse types of processing plants, especially in the United States.
Studies with L. monocytogenes isolates implicated in the 1998-1999 hot dog-related outbreak in the United States (2) revealed that some of the isolates were resistant to BC (26). Investigations in our laboratory have shown that BC-resistant strains from this outbreak were also resistant to the heavy metal cadmium and that the resistance determinants were harbored on a large (ca. 80-kb) plasmid (7), identified through the genome sequencing of one of the strains, H7858 (23). Resistance to heavy metals, especially cadmium and arsenic, has been extensively used for strain-subtyping purposes in L. monocytogenes (10, 20, 31), and cadmium resistance has been found to be associated with a transposon (Tn5422) commonly harbored on plasmids (16, 18). However, no information has been available on possible associations between BC resistance and resistance to heavy metals in L. monocytogenes from the processing-plant environment. In this study, we investigated the prevalence of resistance to BC, and to the heavy metals cadmium and arsenic, among L. monocytogenes isolates from the environments of several turkey-processing plants in the United States.
MATERIALS AND METHODS
Bacteria and growth conditions.
The 192 L. monocytogenes isolates used in this study were from our laboratory's Listeria strain collection and are listed by serotype in Tables S1 to S3 in the supplemental material. Of the 192 isolates, 80 were of serotype 1/2a (or 3a), 46 of 1/2b (or 3b), 13 of 1/2c (or 3c), and 53 of the serotype 4b complex (serotype 4b, 4d, and 4e). The isolates were obtained between 2003 and 2006, as described previously (6), from selective enrichments of environmental samples from six different turkey-processing plants in the United States, with the majority being derived from three plants, A (n = 133), B (n = 28), and C (n = 14) (see Tables S1 to S3 in the supplemental material). The description of the results of this survey regarding the prevalence of L. monocytogenes (including different serotypes and genomic fingerprints) and other Listeria spp. in the plants throughout the survey period will be published elsewhere. Serotype designations were determined by multiplex PCR as described earlier (5), and the genotypic characterization of several isolates of the serotype 4b complex was described previously (6). The serotyping scheme that was employed determined that the isolates were of serotype 1/2a (or 3a), 1/2b (or 3b), or 1/2c (or 3c) or of the serotype 4b complex (4b, 4d, and 4e) (5).
Of these 192 isolates, 87 were derived from multiple (2 to 10) colonies obtained from selective enrichments of 18 different samples (see Tables S1 to S3 in the supplemental material). When isolates representing multiple colonies from the same enrichment shared the same serotype, the same heavy-metal and BC susceptibility profile, and, when known, the same genomic fingerprints based on pulsed-field gel electrophoresis with AscI and ApaI done as described previously (6), only one isolate was included to determine the prevalence of resistance to BC, cadmium, and arsenic. This was done in order to avoid possible bias stemming from inclusion of multiple isolates representing the same strain and sharing the same susceptibility profiles. Thus, a total of 123 strains, including 53 of serotype 1/2a (or 3a), 39 of 1/2b (or 3b), 27 of the 4b complex, and 4 of 1/2c (or 3c), were used for evaluations of the prevalence of resistance to cadmium, BC, and arsenic (see Tables S1 to S3 in the supplemental material). L. monocytogenes H7550, a cadmium- and BC-resistant strain of serotype 4b from the 1998-1999 hot dog-related multistate outbreak (2), and L. monocytogenes J1735, a cadmium- and BC-susceptible strain of serotype 4b from the 2002 turkey delicatessen meat-related multistate outbreak (3), were used as positive and negative controls, respectively, for both cadmium and BC resistance. L. monocytogenes J2213 (an arsenic-resistant serotype 4b sporadic clinical isolate; 2002) was used as a positive control for arsenic resistance. These three strains were provided by the Centers for Disease Control and Prevention (Atlanta, GA) and were chosen based on previous results obtained in our laboratory. The bacteria were routinely grown on blood agar plates containing 5% sheep blood (Remel, Lenexa, KS) at 37°C for 36 h, and long-term storage was at −80°C in brain heart infusion (Becton Dickinson and Co., Sparks, MD) with 20% glycerol (Fisher Scientific, Fairlawn, NJ).
Determination of heavy-metal and quaternary-compound resistance.
A single colony (ca. 1- to 2-mm diameter) from a blood agar plate culture was suspended in 100 μl of tryptic soy broth (Becton Dickinson and Co.). To determine resistance to cadmium, 3 μl of the suspension was spotted in duplicate onto isosensitest agar (ISA) (Oxoid, Basingstoke, England) (control) and ISA containing 70 μg/ml cadmium chloride anhydrous (Sigma, St. Louis, MO). The medium and concentration were based on those employed by McLauchlin et al. (75 μg/ml of cadmium chloride monohydrate) (20). For determination of resistance to arsenic, the cell suspension (3 μl) was spotted in duplicate on ISA containing 500 μg/ml sodium arsenite (Fluka, Buchs, Steinheim, Germany) (20). To determine resistance to BC, the cell suspensions were spotted in duplicate on Mueller-Hinton agar (Mueller Hinton broth with 1.2% Bacto agar (Becton Dickinson and Co.), containing 10 μg/ml benzalkonium chloride (Acros, New Jersey) and 2% defibrinated sheep blood (BBL, Sparks, MD). This concentration of BC (10 μg/ml) was selected after evaluating the MICs for a panel of strains, including those reported to be BC resistant and BC susceptible (26), following the protocol of Soumet et al. (28). Each plate contained the panel of test strains, as well as the designated positive and negative control strains. The plates were incubated at 37°C for 48 h, and the quantity of growth on the test plates was compared with that on the control ISA plates.
Statistical analysis.
Statistical analysis was done using chi-square tests through SAS (version 9.1.3; SAS Institute, Cary, NC).
RESULTS
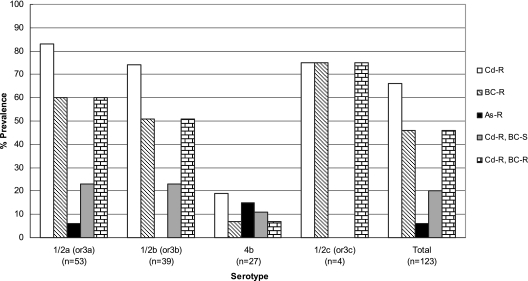
The prevalence of resistance to BC, cadmium, and arsenic was evaluated based on the data for the population of 123 strains obtained following exclusion of isolates from multiple colonies from enrichments of the same sample and harboring the same serotype, susceptibility profile, and (when known) genomic fingerprint. Resistance to cadmium and to BC was frequently encountered in this population; it was detected in 81 (66%) and 57 (46%), respectively, of the 123 strains. In contrast, resistance to arsenic was much less common, being detected in only seven (6%) of the strains (Fig. 1). All 57 BC-resistant strains were also resistant to cadmium, although the reverse was not the case; 30% of the 81 cadmium-resistant strains were susceptible to BC. Furthermore, all seven arsenic-resistant strains were also resistant to cadmium (but only one of the seven was also resistant to BC). Thus, only 1 of the 123 strains was found to be resistant to all three compounds.
FIG. 1.
Prevalence of resistance to cadmium, BC, and arsenic among isolates of L. monocytogenes of different serotypes from the environment of turkey-processing plants. Resistance and susceptibility were determined as described in Materials and Methods. Cd-R, cadmium resistant; BC-R, BC resistant; As-R, arsenic resistant; Cd-R, BC-S, resistant to cadmium but susceptible to BC; Cd-R, BC-R, resistant to both cadmium and BC.
The prevalence of resistance to BC, cadmium, and arsenic varies noticeably among serotypes.
The prevalence of L. monocytogenes with cadmium resistance was much higher in serotypes 1/2a (or 3a) (83%) and 1/2b (or 3b) (74%) than in the serotype 4b complex (19%) (P < 0.0001) (Fig. 1 and Table 1). The prevalence of BC resistance was also significantly higher in strains of serotypes 1/2a (or 3a) and 1/2b (or 3b) (60% and 51%, respectively) than in those of the serotype 4b complex (7%) (P < 0.0002 for 1/2a [or 3a] versus the 4b complex and P < 0.0013 for 1/2b [or 3b] versus the 4b complex). Simultaneous resistance to BC and cadmium was frequent among serotype 1/2a (or 3a) and 1/2b (or 3b) strains (60 and 51%, respectively), with all BC-resistant organisms also being resistant to cadmium, as mentioned above. The prevalences of strains resistant to cadmium but susceptible to BC in serotype 1/2a (or 3a) and 1/2b (or 3b) were similar (ca. 23%) (Fig. 1).
TABLE 1.
Prevalence of resistance to cadmium, BC, and arsenic in isolates of L. monocytogenes of different serotypes and from different turkey-processing plants
| Plant (n) | No./total (%) or % of serotype resistant toa:
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serotype 1/2a, 3a
|
Serotype 1/2b, 3b
|
Serotype 4b
|
Serotype 1/2c, 3c
|
Total
|
|||||||||||
| Cd | BC | As | Cd | BC | As | Cd | BC | As | Cd | BC | As | Cd | BC | As | |
| A (75) | 38/38 | 31/38 | 0/38 | 23/26 | 18/26 | 0/26 | 1/9 | 1/9 | 0/9 | 2/2 | 2/2 | 0/2 | 64/75 (85) | 52/75 (69) | 1/75 (1) |
| B (28) | 1/2 | 0/2 | 0/2 | 3/10 | 0/10 | 0/10 | 4/16 | 1/16 | 4/16 | NA | NA | NA | 8/28 (29) | 1/28 (4) | 4/28 (14) |
| C (14) | 0/8 | 0/8 | 0/8 | 2/2 | 1/2 | 0/2 | 0/2 | 0/2 | 0/2 | 1/2 | 1/2 | 0/2 | 3/14 (21) | 2/14 (14) | 0/14 (0) |
| D-F (6) | 5/6 | 1/6 | 3/6 | 1/6 | 1/6 | 0/6 | NA | NA | NA | NA | NA | NA | 6/6 (100) | 2/6 (33) | 3/6 (50) |
| Total | 44/53 | 32/53 | 3/53 | 29/39 | 20/39 | 0/39 | 5/27 | 2/27 | 4/27 | 3/4 | 3/4 | 0/4 | 81/123 | 57/123 | 7/123 |
| % | 83 | 60 | 6 | 74 | 51 | 0 | 19 | 7 | 15 | 75 | 75 | 0 | 66 | 46 | 6 |
Cd, cadmium; As, arsenic. NA (not applicable), no isolates of the indicated serotype were available from the indicated processing plant.
In contrast to the results with resistance to cadmium and BC, resistance to arsenic was found more frequently in strains of the serotype 4b complex (15%) than in those of serotypes 1/2a (or 3a) (6%) and 1/2b (or 3b) (not detected). The sole strain among the entire collection that was coresistant to all three compounds (strain 1497) indeed belonged to the serotype 4b complex. Resistance to arsenic was encountered in only 3 of the 53 serotype 1/2a (or 3a) strains and was not detected among any of the 39 strains of serotype 1/2b (or 3b) (Fig. 1 and Table 1).
Plant-specific prevalence of resistance to BC, cadmium, and arsenic.
Even though L. monocytogenes isolates were obtained from six plants, the majority were derived from three plants (A, B, and C) (Table 1). Analysis of resistance prevalence data from these three plants revealed significant differences. The prevalence of BC resistance was higher among strains from plant A (69%) than among those from plants B and C (4% and 14%, respectively) (P < 0.0001 for A-B and P = 0.0012 for A-C). Differences were noted in the prevalence of resistance to cadmium (85, 29, and 21% in strains from plants A, B, and C, respectively) (P < 0.0001). Interestingly, arsenic resistance was found only among plant B strains (14%) and among strains from some of the other plants, but not among any of the strains from plant A or C (Table 1).
The prevalence of BC resistance among strains of the same serotype also varied noticeably among plants. BC resistance was more prevalent in serotype 1/2a (or 3a) and 1/2b (or 3b) strains from plant A (82% and 69%, respectively) than in strains of these serotypes obtained from other plants (excluding plants yielding <5 isolates). Similar plant-specific effects were observed in the prevalence of resistance to cadmium in serotype 1/2a (or 3a) and 1/2b (or 3b) strains (Table 1). This was especially pronounced in the case of serotype 1/2a (or 3a) strains from plant C, which were uniformly susceptible to cadmium and constituted the majority (eight of nine) of the cadmium-susceptible strains of this serotype (the other one was a single strain from plant B) (Table 1). Similarly, cadmium resistance was noticeably lower in serotype 1/2b (or 3b) strains from plant B than in those from plant A (P = 0.0018), and plant B isolates contributed the majority (7/10) of the cadmium-susceptible strains of serotype 1/2b (or 3b) (Table 1).
Plant-specific trends in BC resistance were difficult to detect in strains of the serotype 4b complex, due to the low overall prevalence of BC resistance in these strains; only one strain from plant A (1/9) and one from plant B (1/16) were resistant to BC (see Table S3 in the supplemental material). Resistance to cadmium was also low overall among strains of this serotype, regardless of the plant of origin. However, strains from plant B were more likely to be resistant to this heavy metal (25%) than those from plant A (11%) (Table 1).
None of the serotype 1/2a (or 3a) and 1/2b (or 3b) strains from plant A, B, or C were resistant to arsenic. Arsenic-resistant strains of serotype 1/2a (or 3a) were obtained only from plant D (two of three strains) and plant E (one of two strains), but the small total numbers of strains from these plants compromised the ability to identify plant-specific trends. None of the serotype 1/2b (or 3b) isolates were resistant to arsenic, regardless of the plant of origin. A possible plant-specific contribution was identified in strains of the serotype 4b complex, since all four arsenic-resistant strains of this serotype were derived from one facility (plant B) (see Table S3 in the supplemental material).
DISCUSSION
Resistance to heavy metals (especially cadmium and arsenic) has been extensively employed as a strain-subtyping tool for L. monocytogenes (10, 20, 31), but the possible role of such resistance in the ecology of the bacterium in habitats of relevance to food contamination, e.g., the food-processing environment and foods themselves, has remained elusive. Substantial attention has been directed to attributes that may contribute to the establishment and persistence of the pathogen in the environment of food-processing plants, including (but not limited to) the abilities to form biofilms, to colonize surfaces, and to resist disinfectants (13). However, possible correlations between heavy-metal resistance and disinfectant resistance in L. monocytogenes have not been recognized. The findings from this study provide for the first time, to our knowledge, strong evidence that in L. monocytogenes from the processing-plant environment resistance to the quaternary ammonium disinfectant BC is strongly correlated with resistance to the heavy metal cadmium. Without exception, all isolates found to be BC resistant were also resistant to cadmium. However, ca. 30% of cadmium-resistant strains lacked resistance to BC, suggesting that cadmium and BC resistance were not always linked.
The molecular mechanisms responsible for BC resistance always being accompanied by resistance to cadmium remain unknown at this time. Genetic studies have shown that efflux systems mediating resistance to cadmium are associated with a transposable element (Tn5422) frequently harbored on plasmids (16-18). Genome-sequencing studies have revealed additional cadmium efflux systems on plasmids pLM80 of L. monocytogenes H7858 (23) and pLI100 of Listeria innocua CLIP 11262 (9) and, in the case of L. monocytogenes strain EGDe, on the chromosome (9). Preliminary studies in our laboratory suggested that BC resistance is also mediated by a plasmid-borne efflux system (7), even though chromosomal genes involved in multidrug resistance have also been implicated in acquired resistance to this disinfectant (21, 26). One may speculate that the strains characterized in the current study acquired BC resistance determinants in addition to preexisting cadmium resistance plasmids. Such plasmids have been identified before in L. monocytogenes, especially in serogroup 1/2 (16). Since selective pressure for BC resistance may be relatively recent, this may account for such resistance always being accompanied by the earlier acquired trait of resistance to cadmium.
In this study, resistance of L. monocytogenes to BC (51 to 60%) was significantly more prevalent than observed in previous studies (10% or lower) (1, 21). The high prevalence of BC resistance and cadmium resistance (74 to 83%) observed in the current study in strains of serotype 1/2a (or 3a) and 1/2b (or 3b) is significant, considering that strains of these serotypes make a major contribution to the environmental L. monocytogenes burden in processing plants. Overall, serotype 1/2a (or 3a) and 1/2b (or 3b) were recovered from 48% and 39%, respectively, of the L. monocytogenes-positive samples from the turkey-processing plants from which the strains investigated here were derived (R. M. Siletzky and S. Kathariou, unpublished findings). Similar predominance of strains with these serotypes from processing-plant environmental samples has been reported by others, as well (15, 24, 28, 29). Earlier studies also reported that resistance to cadmium in food and clinical strains of serotypes 1/2a and 1/2b was significantly higher than in serotype 4b strains (20).
One may speculate that the lower prevalence of BC and cadmium resistance among the isolates of the serotype 4b complex that were investigated here may reflect the relatively low propensity of serotype 4b strains to harbor plasmids. Earlier studies indicated that plasmids were more common in serogroup l strains (35%) than in strains of serogroup 4 (15%) (16). However, it must be kept in mind that at least two multistate outbreaks have involved serotype 4b strains with plasmid-borne cadmium resistance and, in the case of the 1998-1999 strains, BC resistance as well (7, 23). Thus, resistance to BC and cadmium, even though relatively rare overall in serotype 4b, may still represent an important adaptation for strains implicated in food contamination and human illness.
Interestingly, our findings indicate that resistance to arsenic was more prevalent in strains of the serotype 4b complex than in other serotypes. Similar findings were reported with food and clinical strains in earlier studies (20). Such findings suggest that serotype 4b strains may have a fitness advantage in habitats with arsenic contamination. It is noteworthy that, in addition to natural and industrial sites with heavy-metal contamination, the arsenic burden is also likely to be elevated in conventional, intensive poultry production systems due to the frequent administration of organoarsenates (e.g., roxarsone) as coccidiostatic agents (4). This may represent a previously unrecognized selective pressure for serotype 4b strains in such environments and is worthy of further investigation.
Arsenic resistance was not encountered among any of the serotype 1/2b (or 3b) strains that we investigated, and in other studies, it was also found to be rather rare in food and clinical strains of serotype 1/2b (20). This is intriguing, considering the fact that 1/2b and 4b strains are members of the same evolutionary branch within L. monocytogenes (12, 32). Further studies are needed to identify possible barriers to acquisition of arsenic resistance determinants by strains of serotype 1/2b and to characterize dissemination mechanisms for arsenic resistance in L. monocytogenes.
In this study, surveys of different plants suggested heterogeneity in the prevalence of resistance to the compounds that were tested. For instance, serotype 1/2a (or 3a) strains from plant C were uniformly susceptible to BC and cadmium and in fact were the major contributors to the average estimates for susceptibility to cadmium and BC among strains of this serotype. Accurate determinations of plant-specific impacts on prevalence were compromised by the fact that the numbers of strains from the different plants were variable, and in several cases they were low (due to small numbers of L. monocytogenes-positive samples from some of the plants). Nonetheless, the current data suggest the importance of including strains from more than one plant, both for overall evaluations of resistance prevalence and for evaluations of resistance among strains of a specific serotype. Previously, a genotypic comparison of strains of the serotype 4b complex from two of the plants (plants A and B) revealed plant-specific strains (6), and similar preliminary data have been obtained with serotype 1/2a (or 3a) and 1/2b (or 3b) strains (S. Mullapudi, R. M. Siletzky, and S. Kathariou, unpublished results). The presence of plant-specific strain types has also been reported in other studies focusing on different types of processing plants (8, 30). We are currently investigating the possible associations between resistance to BC, cadmium, and arsenic and the apparent dissemination and persistence of the organism in the processing plant. In one earlier study, strains repeatedly isolated from foods were found to be more likely to be resistant to cadmium than sporadic strains (10).
In conclusion, investigation of strains from turkey-processing plants has revealed a previously unidentified correlation between resistance to the quaternary ammonium disinfectant BC and to the heavy metal cadmium. Concurrent resistance to these agents was common in serogroup 1/2 strains from some plants, whereas resistance to arsenic was most commonly encountered in strains of the serotype 4b complex. Further studies are needed to characterize the underlying resistance mechanisms and to evaluate the possible impact of such resistance attributes on the ecology and adaptations of L. monocytogenes isolates that contaminate the environment of these and other food-processing plants.
Supplementary Material
Acknowledgments
This project was partially funded by USDA grant 2006-35201-17377. Isolation and serotyping of the L. monocytogenes isolates from the processing plants was done in association with a project funded by the USDA National Alliance for Food Safety and Security (NAFSS) as a cooperative agreement with USDA-ARS.
The NAFSS project involved collaborations among L.-A. Jaykus (North Carolina State University), J. Eifert (Virginia Tech), E. Ryser (Michigan State University), R. Meinersmann (USDA-ARS, Athens, GA), and M. Berrang (USDA-ARS, Athens, GA). We thank all members of our laboratory for discussions, encouragement, and support in the course of the project.
Footnotes
Published ahead of print on 11 January 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Aase, B., G. Sundheim, S. Langsrud, and L. M. Rorvik. 2000. Occurrence of and a possible mechanism for resistance to a quaternary ammonium compound in Listeria monocytogenes. Int. J. Food Microbiol. 62:57-63. [DOI] [PubMed] [Google Scholar]
- 2.CDC. 1998. Multistate outbreak of listeriosis—United States, 1998. MMWR Morb. Mortal. Wkly. Rep. 47:1085-1086. [PubMed] [Google Scholar]
- 3.CDC. 2002. Public health dispatch: outbreak of listeriosis-north eastern United States, 2002. MMWR Morb. Mortal. Wkly. Rep. 51:950-951. [PubMed] [Google Scholar]
- 4.Chapman, H. D., and Z. B. Johnson. 2002. Use of antibiotics and roxarsone in broiler chickens in the USA: analysis for the years 1995 to 2000. Poult. Sci. 81:356-364. [DOI] [PubMed] [Google Scholar]
- 5.Doumith, M., C. Buchrieser, P. Glaser, C. Jacquet, and P. Martin. 2004. Differentiation of the major Listeria monocytogenes serovars by multiplex PCR. J. Clin. Microbiol. 42:3819-3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eifert, J. D., P. A. Curtis, M. C. Bazaco, R. J. Meinersmann, M. E. Berrang, S. Kernodle, C. Stam, L. A. Jaykus, and S. Kathariou. 2005. Molecular characterization of Listeria monocytogenes of the serotype 4b complex (4b, 4d, 4e) from two turkey processing plants. Foodborne Pathog. Dis. 2:192-200. [DOI] [PubMed] [Google Scholar]
- 7.Elhanafi, D., and S. Kathariou. 2007. Genetic characterization of benzalkonium chloride resistance mechanism in the food-borne pathogen Listeria monocytogenes, abstr. P73. International Symposium on Problems of Listeriosis XVI, Savannah, GA.
- 8.Giovannacci, I., C. Ragimbeau, S. Queguiner, G. Salvat, J. L. Vendeuvre, V. Carlier, and G. Ermel. 1999. Listeria monocytogenes in pork slaughtering and cutting plants: use of RAPD, PFGE and PCR-REA for tracing and molecular epidemiology. Int. J. Food Microbiol. 53:127-140. [DOI] [PubMed] [Google Scholar]
- 9.Glaser, P., L. Frangeul, C. Buchrieser, C. Rusniok, A. Amend, F. Baquero, P. Berche, H. Bloecker, P. Brandt, T. Chakraborty, A. Charbit, F. Chetouani, E. Couve, A. de Daruvar, P. Dehoux, E. Domann, G. Dominguez-Bernal, E. Duchaud, L. Durant, O. Dussurget, K. D. Entian, H. Fsihi, F. Garcia-del Portillo, P. Garrido, L. Gautier, W. Goebel, N. Gomez-Lopez, T. Hain, J. Hauf, D. Jackson, L. M. Jones, U. Kaerst, J. Kreft, M. Kuhn, F. Kunst, G. Kurapkat, E. Madueno, A. Maitournam, J. M. Vicente, E. Ng, H. Nedjari, G. Nordsiek, S. Novella, B. de Pablos, J. C. Perez-Diaz, R. Purcell, B. Remmel, M. Rose, T. Schlueter, N. Simoes, A. Tierrez, J. A. Vazquez-Boland, H. Voss, J. Wehland, and P. Cossart. 2001. Comparative genomics of Listeria species. Science 294:849-852. [DOI] [PubMed] [Google Scholar]
- 10.Harvey, J., and A. Gilmour. 2001. Characterization of recurrent and sporadic Listeria monocytogenes isolates from raw milk and nondairy foods by pulsed-field gel electrophoresis, monocin typing, plasmid profiling, and cadmium and antibiotic resistance determination. Appl. Environ. Microbiol. 67:840-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang, B., S. Eglezos, B. A. Heron, H. Smith, T. Graham, J. Bates, and J. Savill. 2007. Comparison of multiplex PCR with conventional biochemical methods for the identification of Listeria spp. isolates from food and clinical samples in Queensland, Australia. J. Food Prot. 70:1874-1880. [DOI] [PubMed] [Google Scholar]
- 12.Kathariou, S. 2002. Listeria monocytogenes virulence and pathogenicity, a food safety perspective. J. Food Prot. 65:1811-1829. [DOI] [PubMed] [Google Scholar]
- 13.Kornacki, J. L., and J. B. Gurtler. 2007. Incidence and control of Listeria in food processing facilities, p. 681-766. In E. T. Ryser and E. H. Marth (ed.), Listeria, listeriosis and food safety, 3rd ed. CRC Press, Boca Raton, FL.
- 14.Latorre, L., A. Parisi, R. Fraccalvieri, G. Normanno, M. C. Nardella La Porta, E. Goffredo, L. Palazzo, G. Ciccarese, N. Addante, and G. Santagada. 2007. Low prevalence of Listeria monocytogenes in foods from Italy. J. Food Prot. 70:1507-1512. [DOI] [PubMed] [Google Scholar]
- 15.Lawrence, L. M., and A. Gilmour. 1995. Characterization of Listeria monocytogenes isolated from poultry products and from the poultry-processing environment by random amplification of polymorphic DNA and multilocus enzyme electrophoresis. Appl. Environ. Microbiol. 61:2139-2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lebrun, M., J. Loulergue, E. Chaslus-Dancla, and A. Audurier. 1992. Plasmids in Listeria monocytogenes in relation to cadmium resistance. Appl. Environ. Microbiol. 58:3183-3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lebrun, M., A. Audurier, and P. Cossart. 1994. Plasmid-borne cadmium resistance genes in Listeria monocytogenes are similar to cadA and cadC of Staphylococcus aureus and are induced by cadmium. J. Bacteriol. 176:3040-3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lebrun, M., A. Audurier, and P. Cossart. 1994. Plasmid-borne cadmium resistance genes in Listeria monocytogenes are present on Tn5422, a novel transposon closely related to Tn917. J. Bacteriol. 176:3049-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonnell, G., and A. D. Russell. 1999. Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 12:147-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLauchlin, J., M. D. Hampton, S. Shah, E. J. Threlfall, A. A. Wieneke, and G. D. W. Curtis. 1997. Subtyping of Listeria monocytogenes on the basis of plasmid profiles and arsenic and cadmium susceptibility. J. Appl. Microbiol. 83:381-388. [DOI] [PubMed] [Google Scholar]
- 21.Mereghetti, L., R. Quentin, N. Marquet-van der Mee, and A. Audurier. 2000. Low sensitivity of Listeria monocytogenes to quaternary ammonium compounds. Appl. Environ. Microbiol. 66:5083-5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merianos, J. J. 1991. Quaternary ammonium antimicrobial compounds, p. 225-255. In S. S. Block (ed.), Disinfection, sterilization and preservation, 4th ed. Lea & Feigner, Malvern, PA.
- 23.Nelson, K. E., D. E. Fouts, E. F. Mongodin, J. Ravel, R. T. DeBoy, J. F. Kolonay, D. A. Rasko, S. V. Angiuoli, S. R. Gill, I. T. Paulsen, J. Peterson, O. White, W. C. Nelson, W. Nierman, M. J. Beanan, L. M. Brinkac, S. C. Daugherty, R. J. Dodson, A. S. Durkin, R. Madupu, D. H. Haft, J. Selengut, S. Van Aken, H. Khouri, N. Fedorova, H. Forberger, B. Tran, S. Kathariou, L. D. Wonderling, G. A. Uhlich, and D. O. Bayles. 2004. Whole genome comparisons of serotype 4b and 1/2a strains of the food-borne pathogen Listeria monocytogenes reveal new insights into the core genome components of this species. Nucleic Acids Res. 32:2386-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ojeniyi, B., H. C. Wegener, N. E. Jensen, and M. Bisgaard. 1996. Listeria monocytogenes in poultry and poultry products: epidemiological investigations in seven Danish abattoirs. J. Appl. Bacteriol. 80:395-401. [DOI] [PubMed] [Google Scholar]
- 25.Painter, J., and L. Slutsker. 2007. Listeriosis in humans, p. 85-109. In E. T. Ryser and E. H. Marth (ed.), Listeria, listeriosis and food safety, 3rd ed. CRC Press, Boca Raton, FL.
- 26.Romanova, N., S. Favrin, and M. W. Griffiths. 2002. Sensitivity of Listeria monocytogenes to sanitizers used in the meat processing industry. Appl. Environ. Microbiol. 68:6405-6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen, Y., Y. Liu, Y. Zhang, J. Cripe, W. Conway, J. Meng, G. Hall, and A. A. Bhagwat. 2006. Isolation and characterization of Listeria monocytogenes isolates from ready-to-eat foods in Florida. Appl. Environ. Microbiol. 72:5073-5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soumet, C., C. Ragimbeau, and P. Maris. 2005. Screening of benzalkonium chloride resistance in Listeria monocytogenes strains isolated during cold smoked fish production. Lett. Appl. Microbiol. 41:291-296. [DOI] [PubMed] [Google Scholar]
- 29.Thevenot, D., M. L. Delignette-Muller, S. Christieans, and C. Vernozy-Rozand. 2005. Prevalence of Listeria monocytogenes in 13 dried sausage processing plants and their products. Int. J. Food Microbiol. 102:85-94. [DOI] [PubMed] [Google Scholar]
- 30.Unnerstad, H., E. Bannerman, J. Bille, M. L. Danielsson-Tham, E. Waak, and W. Tham. 1996. Prolonged contamination of a dairy with Listeria monocytogenes. Neth. Milk Dairy J. 50:493-499. [Google Scholar]
- 31.Vaz-Velho, M., G. Duarte, J. McLauchlin, and P. Gibbs. 2001. Characterization of Listeria monocytogenes isolated from production lines of fresh and cold-smoked fish. J. Appl. Microbiol. 91:556-562. [DOI] [PubMed] [Google Scholar]
- 32.Wiedmann, M., J. L. Bruce, C. Keating, A. E. Johnson, P. L. McDonough, and C. A. Batt. 1997. Ribotypes and virulence gene polymorphisms suggest three distinct Listeria monocytogenes lineages with differences in pathogenic potential. Infect. Immun. 65:2707-2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.