Abstract
Paecilomyces variotii is a common cosmopolitan species that is able to spoil various food- and feedstuffs and is frequently encountered in heat-treated products. However, isolates from heat-treated products rarely form ascospores. In this study we examined by using molecular techniques and mating tests whether this species can undergo a sexual cycle and form ascospores. The population structure of this species was examined by analyzing the nuclear ribosomal internal transcribed spacer 1 (ITS1) and ITS2 and the 5.8S rRNA gene, as well as partial β-tubulin, actin, and calmodulin gene sequences. Phylogenetic analyses revealed that P. variotii is a highly variable species. Partition homogeneity tests revealed that P. variotii has a recombining population structure. In addition to sequence analyses, mating experiments indicated that P. variotii is able to form ascomata and ascospores in culture in a heterothallic manner. The distribution of MAT1-1 and MAT1-2 genes showed a 1:1 ratio in the progeny of the mating experiments. From the sequence analyses and mating data we conclude that P. variotii is the anamorph of Talaromyces spectabilis and that it has a biallelic heterothallic mating system. Since Paecilomyces sensu stricto anamorphs group within Byssochlamys, a new combination Byssochlamys spectabilis is proposed.
Paecilomyces variotii Bainier (2) is a common cosmopolitan mold found in soils, indoor environments, plants, animals, and foodstuffs (33, 39, 40, 42). It is a fast-growing thermotolerant fungus that is able to grow at low oxygen levels and in the presence of preservatives, able to form the mycotoxin viriditoxin, and able to survive heat treatments (19, 33). The combination of the previously mentioned features makes this species an important organism and one of the most frequently encountered spoilage fungi in various foodstuffs. This species is one of the most heat-resistant fungi known, and its presence in pasteurized beverages causes great economic losses (52). Although P. variotii produces thick-walled hyphae and chlamydospores, ascospores, which are usually considered to be the heat-resistant propagule in other fungi, are rarely found in culture. As a result, the route of P. variotii contamination remains mostly unknown (19). Besides spoiling food, it is also associated with many types of human infections. Endocarditis is the most common infection produced by this species and has a very bad prognosis (5). Industrial applications have also been proposed with P. variotii isolates; examples include tannase production (3, 28) and biofiltration of toluene (7, 10).
Ascomycetes with anamorphs in the genera Penicillium, Aspergillus, and Paecilomyces have been classified in the Trichocomaceae family (2, 29, 30, 34). The connection of Paecilomyces anamorphs with the ascomycete Byssochlamys was first proposed by Stolk and Samson (44). A recent molecular study revealed that P. variotii forms a well-defined clade within the Trichocomaceae, together with other thermophilic Paecilomyces species and Byssochlamys teleomorphs (26). This clade also includes Talaromyces spectabilis Udagawa & S. Suzuki, a species with an anamorph similar to P. variotii. Morphological examination of isolates of this species showed that it has a Byssochlamys instead of a Talaromyces teleomorph (19). Like P. variotii, this species also occurs in heat-processed products due to the formation of heat-resistant ascospores.
The first aim of the present study was to elucidate the genetic diversity among P. variotii isolates and to investigate the population structure of P. variotii using multigene phylogenies. To address this, phylogenetic analyses were conducted by using sequences of the internal transcribed spacer (ITS) region of the nuclear ribosomal DNA gene cluster encompassing ITS1 and ITS2 and the 5.8S rRNA gene, as well as partial β-tubulin, actin, and calmodulin sequences. To reveal the structure of the examined P. variotii population, partition homogeneity tests have also been conducted. A further aim was to determine whether the formation of a sexual state could be elicited in P. variotii. This was done by carrying out mating experiments between selected isolates, which were obtained from heat-treated products. These isolates were chosen because these were most likely to form ascospores since these structures are needed to survive the heat treatment. A final aim was to investigate the presence of the mating-type genes MAT1-1 and MAT1-2 in these isolates. To address this, degenerated primers were designed, and subsequently species-specific primers were made and used for the determination of the presence of these mating genes in individual P. variotii isolates.
MATERIALS AND METHODS
DNA isolation, amplification, and sequencing.
Sixteen P. variotii strains were selected for the phylogenetic analyses, and the type culture of P. brunneolus CBS 370.70, which is a closely related separate species (J. Houbraken et al., unpublished data), was used as an outgroup (Table 1). The majority of the strains came from the United States, and were isolated from independent sources in 2003, 2005, and 2006. The cultures were grown on malt peptone broth using 10% (vol/vol) of malt extract (Oxoid, Basingstoke, United Kingdom) and 0.1% (wt/vol) Difco Bacto peptone (Becton-Dickinson, Le-Pont-de-Claix, France), 2 ml of medium in 15-ml tubes. The cultures were incubated at 25°C for 3 to 4 days. DNA was extracted from the cells by using a Masterpure yeast DNA purification kit (Epicenter Biotechnology, Madison, WI) according to the instructions of the manufacturer. Fragments containing the ITS region were amplified by using primers LS266 and V9G (13). Amplification of part of the β-tubulin gene was performed using the primers Bt2a and Bt2b (14), and amplifications of the partial calmodulin and actin genes were set up as described previously (15). Both strands of the PCR fragments were sequenced with the ABI Prism BigDye Terminator v.3.0 ready reaction cycle sequencing kit (Applied Biosystems, Foster City, CA). Samples were analyzed on an ABI Prism 3700 genetic analyzer (Applied Biosystems, Foster City, CA), and contigs were assembled by using the forward and reverse sequences with the program SeqMan from the LaserGene package (DNAstar, Inc., Madison, WI).
TABLE 1.
Paecilomyces strains used in this studya
| Culture (CBS no.) | Species | Source, remarks about culture | GenBank no.
|
|||
|---|---|---|---|---|---|---|
| ITS | BenA | Cmd | Act | |||
| 102.74 | Paecilomyces variotii | Unknown source, type of Paecilomyces variotii | EU037055 | EU037073 | EU037038 | EU037021 |
| 101075 | Byssochlamys spectabilis | Heat processed fruit beverage, type of Talaromyces spectabilis, Japan | EU037051 | EU037069 | EU037034 | EU037017 |
| 109072 | Byssochlamys spectabilis | Pectin, isolated by heat shock method, The Netherlands | EU037053 | EU037071 | EU037036 | EU037019 |
| 109073 | Byssochlamys spectabilis | Pectin, isolated by heat shock method, The Netherlands | EU037052 | EU037070 | EU037035 | EU037018 |
| 110431 | Paecilomyces variotii | Rye bread, The Netherlands | EU037054 | EU037072 | EU037037 | EU037020 |
| 121577 | Byssochlamys spectabilis | Spoiled sports drink, United States | EU037066 | EU037084 | EU037049 | EU037032 |
| 121578 | Byssochlamys spectabilis | Spoiled sports drink, United States | EU037065 | EU037083 | EU037048 | EU037031 |
| 121579 | Byssochlamys spectabilis | Sucrose, isolated by heat shock method, United States | EU037064 | EU037082 | EU037047 | EU037030 |
| 121580 | Byssochlamys spectabilis | Spoiled apple juice, United States | EU037063 | EU037081 | EU037046 | EU037029 |
| 121581 | Byssochlamys spectabilis | Spoiled sweetened tea, United States | EU037062 | EU037030 | EU037045 | EU037028 |
| 121582 | Byssochlamys spectabilis | Spoiled sweetened tea, United States | EU037061 | EU037079 | EU037044 | EU037027 |
| 121583 | Byssochlamys spectabilis | Spoiled sports drink, United States | EU037060 | EU037078 | EU037043 | EU037026 |
| 121585 | Byssochlamys spectabilis | Sucrose, isolated by heat shock method, United States | EU037059 | EU037077 | EU037042 | EU037025 |
| 121586 | Byssochlamys spectabilis | Spoiled sweetened tea, United States | EU037058 | EU037076 | EU037041 | EU037024 |
| 121587 | Byssochlamys spectabilis | Spoiled sports drink, United States | EU037057 | EU037075 | EU037040 | EU037023 |
| 121588 | Byssochlamys spectabilis | Sucrose, isolated by heat shock method, United States | EU037056 | EU037074 | EU037039 | EU037022 |
| 370.70 | Paecilomyces brunneolus | Food, type of Paecilomyces variotii var. brunneolus, Japan | EU037050 | EU037068 | EU037033 | EU037016 |
| 121584* | Paecilomyces maximus | Sucrose, isolated by heat shock method, United States | ||||
| 338.51* | Paecilomyves variotii | Fruit juice, Switzerland | ||||
No sequence data are available for the strains marked with an asterisk; these strains are only used in the mating experiments.
Phylogenetic analysis.
Alignment of the sequences was preformed with the CLUSTAL W program in MEGA 3.1 (24, 50), and manual adjustments were made to maximize homology. The phylogenetic analyses of the sequence data were carried out by using PAUP version 4.0b10 (45). Maximum-parsimony analysis was performed for all data sets by using the heuristic search option. The robustness of the most parsimonious trees was evaluated by 1,000 bootstrap replications. Other relevant statistics included tree length, the consistency index (CI), the retention index (RI), and the rescaled consistency index (RC). The partition homogeneity test was used to examine the null hypothesis of recombination. In this test, the observed sites from the four genes for each individual were pooled and resampled without replacement to produce an artificial data set in which sites have been swapped randomly among loci. The obtained ITS, β-tubulin, actin, and calmodulin sequences were deposited at the GenBank nucleotide sequence database (Table 1).
Mating tests.
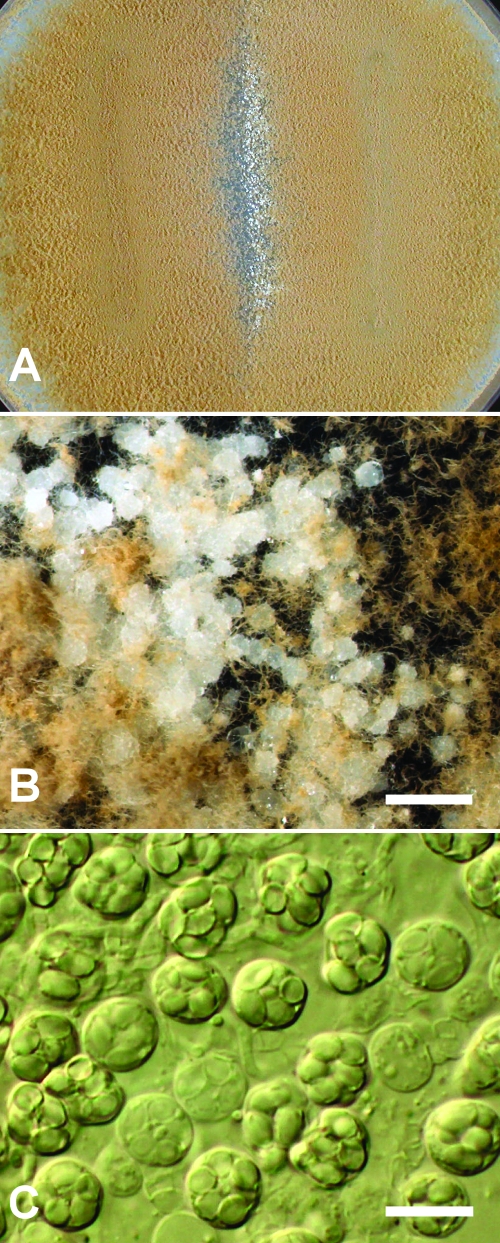
Single conidial isolations of 19 P. variotii isolates (Table 2) were made prior the experiments. Subsequently, the isolates were grown on malt extract agar (Oxoid) for 7 days at 25°C. After incubation the conidia were dislodged from the agar surface by means of a needle, and suspensions were prepared in 30% glycerol and stored at −20°C until use. Two aliquots of 10 μl each, representing two different strains, were pipetted on potato dextrose agar (2% glucose [Merck KGaA, Darmstadt, Germany], 2% agar [Oxoid], 73% distilled water, 23% homemade potato extract) in a streak at a distance of approximately 3 to 4 cm. Isolates were mated in all possible combinations. Controls consisted of inoculations of one isolate to both sides of the plate, and interspecies matings were also tested with P. brunneolus CBS 370.70 and P. maximus CBS 121584. The plates were incubated for 6 to 9 weeks at 30°C in darkness. After incubation, the plates were scored with a dissecting and/or light microscope for the presence of fruiting bodies (Fig. 1).
TABLE 2.
Schematic representation of the results of the mating study using P. variotii isolates
| Strain (CBS no.) | Detection of ascomataa
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 121577 | 121578 | 121579 | 121580 | 121581 | 121582 | 121583 | 121585 | 121586 | 121587 | 121588 | 109072 | 109073 | 101075 | 102.74 | 110431 | 338.51 | 121584 | 370.70 | |
| 121577 | − | − | − | + | + | − | + | + | + | + | − | − | − | − | − | − | − | − | − |
| 121578 | − | − | + | + | − | + | + | + | + | − | − | − | − | − | − | − | − | − | |
| 121579 | − | + | + | − | + | + | + | + | − | − | − | − | − | − | − | − | − | ||
| 121580 | − | − | + | − | − | − | − | + | + | + | + | − | − | − | − | − | |||
| 121581 | − | + | − | − | − | − | + | + | + | + | − | − | − | − | − | ||||
| 121582 | − | + | + | + | + | − | − | − | − | − | − | − | − | − | |||||
| 121583 | − | − | − | − | + | + | + | + | − | − | − | − | − | ||||||
| 121585 | − | − | − | + | + | + | + | − | − | − | − | − | |||||||
| 121586 | − | − | + | + | + | + | − | − | − | − | − | ||||||||
| 121587 | − | + | + | + | + | − | − | − | − | − | |||||||||
| 121588 | − | − | − | − | − | − | − | − | − | ||||||||||
| 109072 | − | − | − | − | − | − | − | − | |||||||||||
| 109073 | − | − | − | − | − | − | − | ||||||||||||
| 101075 | − | − | − | − | − | − | |||||||||||||
| 102.74 | − | − | − | − | − | ||||||||||||||
| 110431 | − | − | − | − | |||||||||||||||
| 338.51 | − | − | − | ||||||||||||||||
| 121584 | − | − | |||||||||||||||||
| 370.70 | − | ||||||||||||||||||
The results are scored with “−” when no ascomata are formed and with “+” when ascomata were detected.
FIG. 1.
Mating experiments between P. variotii strains. (A) Detail image of a potato dextrose agar plate where two P. variotii strains of different mating types were inoculated on opposite sides; ascomata are formed in the middle. (B) Higher-magnification view of the ascomata. Scale bar, 1 mm. (C) Micrograph of the ascomata, showing asci with ascospores. Scale bar, 10 μm.
For screening the fertility of the progeny (F1), 15 single ascospore isolates from four different parent strains (CBS 121582 × CBS 121583, CBS 121581 × CBS 121582, CBS 121581 × CBS 121578, and CBS 121581 × CBS 121577) were obtained, resulting in 60 single ascospore isolates. The single ascospore isolations were made by isolating the ascomata of a crossing and subsequently making ascospore suspensions by using glass beads. The conidia were killed by heat treatment for 10 min at 85°C, and a decimal dilution was made from each suspension. After plating, single colonies were picked after 2 to 3 days of incubation and transferred to a malt extract agar plate. Crossings with these isolates were made as described above. The only difference was that mating-type tester strains CBS 121581 and CBS 121582 were used to determine the mating type. Negative controls with one isolate were used; the mating-type tester strains were used as a positive control.
Designing MAT1-1- and MAT1-2-specific primers for P. variotii isolates.
Degenerate primers for the amplification of the MAT1-1 and MAT1-2 genes were developed. These primers were designed based on the full genome data of Aspergillus species (MAT1-1 [GenBank accession numbers XM655267, XM001263835, and AY898661] and MAT1-2 [GenBank accession numbers AY898661 and XM749896]) and the mating-type gene sequences of Penicillium marneffei (GenBank accession numbers DQ340761 and DQ340762). The mating-type genes were amplified in two PCR steps. For amplification of the MAT1-1 gene, the MAT1-1 degenerated primers MAT1-F2new/MAT1-R1old were used in the first PCR run (MAT1-F2new [5′-WCCYCTYSARCGWGCYTTYAA-3′] and MAT1-R1old [5′-TYRTCNCKKATKATNSWRTANGC-3′]). The PCRs were performed in 12.5-μl mixtures, containing 0.5 μl of genomic DNA, 1.5 μl of MgCl2 (25 mM), 1.25 μl of PCR buffer, 0.93 μl of deoxynucleoside triphosphate (1 mM), 0.05 μl of Taq polymerase (5 U/μl; Genaxxon Bioscience GmbH, Biberach, Germany), 5.77 μl of demineralized water, and 2.5 μl of both primers (10 μM). PCRs were carried out on a Thermolyne Amplitron II thermal cycler (Barnstead International, Dubuque, IA) according to the following program: predwelling at 94°C for 4 min, followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 60°C for 50 s, and extension at 72°C for 1 min, with a final postdwelling at 72°C for 5 min. In the subsequent seminested PCR, the primers MAT1-F2new and MAT1-R1new were used (MAT1-R1new [5′-GDAYRATKGARTASGCYTTYGC-3′]). The PCR mixture and protocol were similar to that described above; the only modification was the annealing temperature, which was set to 63°C. For amplification of the MAT1-2 gene, the degenerated primers HMG-F1 (5′-CCNAAYGCNTTYATHYTNTAYMG-3′) and HMG-R1(5′-YNKYTCNSWNGGYTTNCKNGG-3′) were used. The PCR mixture and protocol were similar to that described above, with the exception of the annealing temperature, which was set to 48°C. The following seminested PCR was performed with the primer pair HMG-F1 and HMG-R2 (5′-KNGGNGYRTANTGRTARTYNGG-3′). The PCR conditions were similar to the first run, except for the annealing temperature, which was 56°C. After we performed the seminested PCR, we sequenced the amplicons as described above. The contigs were assembled by using the forward and reverse sequences with the program SeqMan from the LaserGene package (DNAstar, Inc., Madison, WI). The obtained sequences were used to develop P. variotii specific mating-type primers.
Screening of P. variotii mating type-specific primers.
DNA was extracted as described above and used as a template for amplification of the MAT1-1 and MAT1-2 genes. The primers used for the amplification of a part of the MAT1-1 and MAT1-2 were designed from sequences obtained via the previously described seminested PCRs and were the aligned with sequences of P. marneffei (GenBank accession numbers DQ340761 for MAT1-1 and DQ340762 for MAT1-2). For the amplification of the MAT1-1 gene, the primers MAT1-F1-VarSp (5′-TATGCCTCCTGGTGAGCTGG-3′) and MAT1-R2-VarMar 5′-GATCCCRGAYTTSGYCTTCTG-3′) were used. The primers MAT2-F1Paec (5′-AYCAYCAYCCKATYGTCAAAGC-3′) and MAT2-R1Paec (5′-GYTTGCGYTTTATCTSCTCYGC-3′) were used for the amplification of a part of MAT1-2 gene. Multiplex PCR was used to screen for the presence of the MAT1-1 or MAT1-2 gene of P. variotii in one reaction. The reaction mixtures were performed in 25-μl mixtures containing 1.0 μl of genomic DNA; 3.0 μl of MgCl2 (25 mM); 2.5 μl of PCR buffer; 1.85 μl of deoxynucleoside triphosphate (1 mM); 0.10 μl of Taq polymerase (5 U/μl; Genaxxon Bioscience GmbH, Biberach, Germany); 14.65 μl of demineralized water; 0.50 μl of the primers MAT1-F1-VarSp, MAT2-F1Paec, and MAT2-R1Paec; and 2.50 μl of the primer MAT1-R2-VarMar (all primers were at a concentration of 10 mM). PCR products were obtained by using the following PCR program: predwelling at 94°C for 4 min, followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 58°C for 1 min, and extension at 72°C for 1 min, followed in turn by postdwelling at 72°C for 5 min.
RESULTS
Phylogenetic analyses.
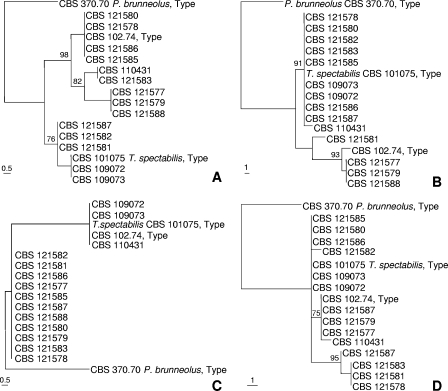
For examining the genetic diversity and population structure of P. variotii isolates, 16 strains were selected, together with the type strain of T. spectabilis. Most of these strains originated from heat-treated products or were isolated by a heat shock method. To address the question of reproductive mode, partial sequences of three protein coding genes (β-tubulin, actin, and calmodulin) and the ITS region were analyzed (Fig. 2). Phylogenetic analyses of the ITS, β-tubulin, actin and calmodulin data sets revealed the existence of two, five, six, and five genotypes, respectively. The MP trees based on ITS, β-tubulin, and actin sequences all had consistency index of 1.000, indicating that there was no homoplasy within any of these data sets, with no evidence for intragenic recombination. The partition homogeneity test was used to examine the null hypothesis of recombination. Under clonality, the sums of the lengths of the gene trees for the observed and resampled data should be similar, while under recombination the sums of the tree lengths should be longer than that of the actual data (12). The average summed tree length of the combined artificial data set was 15 steps longer than the actual summed tree length, which was 37 steps. The analysis showed that the four data sets could not be combined (P = 0.001), which indicates that recombination occurred between these loci.
FIG. 2.
One most parsimonious tree from each of the four analyzed gene regions sequenced. (A) β-Tubulin (9/475 parsimony informative characters; tree length, 16; CI, 1.0000; RI, 1.0000; RC, 1.000). (B) Calmodulin (14/546 parsimony informative characters; tree length, 44; CI, 0.909; RI, 0.922; RC, 0.838). (C) ITS (13/575 parsimony informative characters; tree length, 13; CI, 1.0000; RI, 1.0000; RC, 1.000). (D) Actin (5/313 parsimony informative characters; tree length, 20; CI, 1.000; RI, 1.000; RC, 1.000). Bootstrap values above 70% are indicated above the nodes. P. brunneolus was used as an outgroup.
Mating experiments.
Seventeen P. variotii strains were crossed in all possible pairings to identify the capacity to undergo teleomorph stage development. Fruitbodies were detected in 14 isolates (Table 2). Strikingly, all of these strains were isolated from heat-treated products or isolated by a heat shock method. Mating was also observed between P. variotii isolates and the type strain of T. spectabilis. Ascoma development was not observed in any mating tests which involved isolate CBS 102.74, the type culture of P. variotii, CBS 338.51 originating from fruit juice and CBS 110431, a strain isolated from rye bread. No interspecies mating was observed with P. maximus CBS 121584, which was isolated from heat-shocked sucrose and with the closely related species P. brunneolus CBS 370.70. Of the analyzed fertile strains, six belonged to one mating type, and eight belonged to the other. This indicates that heat resistance is not connected to the mating type.
To test the fertility of the progeny, 15 single-ascospore isolates were obtained from four different pairings (CBS 121582 × CBS 121583, CBS 121581 × CBS 121582, CBS 121581 × CBS 121578, and CBS 121581 × CBS 121577), resulting in 60 single ascospore isolates. These isolates were crossed against mating-type tester strains, and 29 of them were found to represent each mating type, indicating that fertile mating occurs in culture. Two isolates did not mate with the tester strains and were scored as infertile. The 1:1 ratio is in agreement with the Mendelian segregation of mating type and suggests the presence of a biallelic mating-type system in P. variotii.
Detection of MAT1-1 and MAT1-2 idiomorphs.
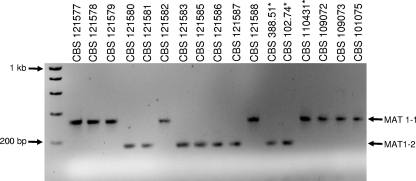
Amplification reactions, using the designed P. variotii specific mating-type primers, resulted in a 334-bp fragment for MAT1-1 and a 188-bp fragment for MAT1-2 (Fig. 3). Sequences of the amplified fragments were deposited in GenBank (accession numbers EU037085 for MAT1-1 and EU037067 for MAT1-2) and were found to be homologous to mating-type genes of other fungi. The distribution of MAT-specific PCR amplicons correlated perfectly with the mating-type phenotype obtained from the conventional crossings. The type strain of T. spectabilis was found to carry MAT1-1. The results of the conventional mating-type study showed that three P. variotii strains (CBS 338.51, CBS 102.74, and CBS 110431) were not able to mate with any of the tested strains; nevertheless, their MAT idiomorphs could be detected. The strain isolated from rye bread had a MAT1-1 idiomorph, whereas the type culture of P. variotii and CBS 338.51, an isolate from fruit juice, had a MAT1-2 motif.
FIG. 3.
Multiplex PCR amplicons obtained from P. variotii isolates showing the MAT1-1 (334 bp) and MAT1-2 (188 bp) genes.
DISCUSSION
The genetic variability of P. variotii observed in this study was remarkably high compared to that of other species in the Trichocomaceae. For example, regarding the variability of the ITS region, six parsimony informative sites were present, while some Aspergillus species cannot even be distinguished using ITS sequence data alone (41, 55). The two clades identified in P. variotii based on ITS sequence data are less related to each other than, e.g., A. niger to A. tubingensis (41) or A. ibericus to A. carbonarius (43). However, mating experiments revealed that these clades represent a single biological species. Similarly high variability was observed in the protein coding regions (Fig. 2). Further studies are needed to clarify the significance of this large intraspecific diversity.
Two basic population structures are known in fungi, the clonal and the recombining one. In the case of clonal reproduction, each progeny has only one parent, and its genome is an exact mitotic copy of its parent, while recombining populations develop through meiotic recombination after mating or through a parasexual cycle (mitotic recombination [49, 54]). Several methods have been described for distinguishing recombination from clonality in fungal populations (1, 49). All of these methods are based on the assumption that under clonality different regions of the genome are inherited as a unit, while in the case of recombination they may have different evolutionary histories. We applied one of these methods, the partition homogeneity test, to reveal the structure of the examined P. variotii population. This test has previously been used to examine the population structures of several fungi, including A. flavus (12), Coccidioides immitis (22), Letharia species (23), and Metarrhizium anisopliae (4). The partition homogeneity tests revealed that the four data sets are incongruent, indicating that P. variotii has a recombining population structure. The observed recombining population structure can be the result of either parasexual processes, past meiotic exchanges, or the presence of an undetected, “cryptic” sexual stage. To examine whether recombination was caused by the presence of a “cryptic” sexual cycle in P. variotii, mating experiments have been carried out. These experiments revealed that P. variotii isolates are able to reproduce sexually if isolates carrying opposite mating types are crossed, i.e., this species has a heterothallic lifestyle. The majority of species in the Trichocomaceae family are homothallic, with eight confirmed exceptions. Six of the exceptions belong to the genus Neosartorya (N. fennelliae, N.spathulata, N. nishimurae, N. udagawae, N. indohii, and N. tsurutae [16, 17, 25, 46, 48]), one belongs to the genus Emericella (E. heterothallica [36]), and Talaromyces derxii belongs to Penicillium subgenus Biverticillium (47).
Sexual reproduction in filamentous ascomycetes is controlled by idiomorphic mating-type alleles, MAT1-1 and MAT1-2, which contain one to three genes. Of these genes, MAT1-1-1 and MAT1-2-1 encode putative transcription factors and are thus considered to be the major regulators of sexual communication and mating (21). Homothallic species contain both mating types, while for heterothallic fungi, the two mating types carry either of the two idiomorphs. Examination of the frequency and occurrence of mating-type genes in a population is a method to test the possibility of sexual reproduction and was used to reveal that fungi including, e.g., P. marneffei (56) and A. fumigatus (31, 35), could have a heterothallic mode of reproduction. Distribution of the MAT idiomorphs in the progeny of the mating tests was found to be 1:1, indicating the presence of a biallelic heterothallic mating system in P. variotii. Previous phylogenetic studies revealed that P. variotii is closely related to the homothallic species Byssochlamys nivea and B. fulva, raising questions about the evolution of sexual reproduction in this genus (26). In the Aspergillus genus, homothallism was suggested to be an ancient character based on phylogenetic analysis of β-tubulin and hydrophobin sequences (11) and on genomic data (9). However, more recently, heterothallism has been proposed to be ancient in aspergilli (38) and in several other fungal groups, including the genus Cochliobolus (57), yeasts (6, 8), and Fusarium species (20, 51). We presume that heterothallism is the ancient character in this genus as well; however, further studies are needed to clarify the evolution of sex in the Byssochlamys genus.
P. variotii is a heat-resistant spoilage fungus that is frequently encountered from pasteurized products. We have detected P. variotii as a heat-resistant spoilage organism in various food products and ingredients, including pectin and beverages (Emilia Rico, unpublished data). In these isolates ascospores were rarely observed and, although the D-value was determined (19), the heat resistance was often questioned. Our observation that P. variotii represents a heterothallic fungus, which possibly produces its heat-resistant ascospores in raw materials or in habitats where mating can take place, gives more insight in the presence of this species in pasteurized foods and beverages. This is the first report on a heterothallic species causing food spoilage. Ascospore production in nature may contribute to its heat resistance, thus posing a selection pressure on maintaining their mating abilities. The type strain of P. variotii and two other isolates did not mate with any of the tested strains, indicating that they lost the ability to reproduce sexually. This could have been caused by the long-term maintenance and subculturing in the culture collection. Gradual degeneration of various traits due to long-term subculturing, including virulence, spore-forming abilities (37), metabolite production (27), the formation of mycotoxins and sclerotia (18), and mating abilities (J. Varga, unpublished data) have previously been observed in several fungi. However, another possibility is that some of these isolates lost their mating abilities due to the lack of selection pressure from the environment. For example, strain CBS 110431 was isolated from rye bread, where the production of heat-resistant ascospores is not a prerequisite for spoilage. This isolate might have lost its mating ability via repeated clonal reproduction in the production plant environment.
During PCR amplification of MAT genes, complete correlation was observed between the results of the mating experiments and the presence of either MAT1-1 or MAT1-2 in the isolates. We could not detect any correlation between the presence of either MAT genes in the isolates, and their position on the trees, as expected for a recombining population. The results of the phylogenetic analyses and the mating experiments confirmed that P. variotii represents the anamorph of T. spectabilis. However, placement of this species in the genus Talaromyces is not correct and should be transferred to Byssochlamys (19). The following new combination is proposed: Byssochlamys spectabilis (Udagawa et Suzuki) Houbraken & Samson comb. nov. Basionym: Talaromyces spectabilis Udagawa et S. Suzuki (Mycotaxon 50:82, 1994). Anamorph: Paecilomyces variotii Bainier (Bull. Trimest. Soc. Mycol. Fr. 23:26, 1907).
Twenty-four single spore isolations have been made from the type culture of B. spectabilis CBS 101075. These isolates were tested for the presence of mating-type idiomorphs, using the multiplex PCR as described above. The results showed that all isolates had a MAT1-1 idiomorph. Since the teleomorph was detected in this strain by Udagawa and Suzuki (53), it is very likely that the original material was a mixed culture containing both mating types of this heterothallic species.
Examination of the population structure by phylogenetic methods revealed the presence of recombination in several species in the Trichocomaceae family that were considered previously to reproduce only asexually (clonally), including A. flavus, A. fumigatus, A. nomius, A. lentulus, and P. marneffei (11, 31, 32, 56; S. A. Balajee and D. Nickle, unpublished data). However, the sexual stage of none of these anamorphs could be recovered thus far. In addition, the present study is one of the few examples in the order Eurotiales in which a teleomorph-anamorph relationship could be established based on both the phylogenetic and the biological species concept.
Acknowledgments
We thank Eddy van Breemen (CBS Fungal Biodiversity Centre) for excellent technical assistance and Martin Meijer (CBS Fungal Biodiversity Centre) for help in the development of the degenerate primers. We appreciate the help of Ferry Hagen (CBS Fungal Biodiversity Centre) with the phylogenetic analysis sequences.
Footnotes
Published ahead of print on 11 January 2008.
REFERENCES
- 1.Anderson, J. B., and L. M. Kohn. 1998. Genotyping, gene genealogies and genomics bring fungal population genetics above ground. Trends Ecol. Evol. 13:444-449. [DOI] [PubMed] [Google Scholar]
- 2.Bainier, G. 1907. Mycothèque de l'école de Pharmacie. XI. Paecilomyces, genre nouveau de Mucédinées. Bull. Trimest. Soc. Mycol. Fr. 23:26-27. [Google Scholar]
- 3.Battestin, V., and G. A. Macedo. 2007. Tannase production by Paecilomyces variotii. Bioresour. Technol. 98:1832-1837. [DOI] [PubMed] [Google Scholar]
- 4.Bidochka, M. J., C. L. N. Small, and M. Spironello. 2005. Recombination within sympatric cryptic species of the insect pathogenic fungus Metarhizium anisopliae. Environ. Microbiol. 7:1361-1368. [DOI] [PubMed] [Google Scholar]
- 5.Castro, L. G. M., A. Salebian, and M. N. Sotto. 1990. Hyalophyphomycosis by Paecilomyces lilacinus in a renal transplant recipient and a review of human Paecilomyces species infections. J. Med. Vet. Mycol. 28:15-26. [PubMed] [Google Scholar]
- 6.Coppin, E., R. Debuchy, S. Arnaise, and M. Picard. 1997. Mating types and sexual development in filamentous ascomycetes. Microbiol. Mol. Biol. Rev. 61:411-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Estevez, E., M. C. Veiga, and C. Kennes. 2005. Biodegradation of toluene by the new fungal isolates Paecilomyces variotii and Exophiala oligosperma. J. Ind. Microbiol. Biotechnol. 32:33-37. [DOI] [PubMed] [Google Scholar]
- 8.Fabre, E., H. Muller, P. Therizols, I. Lafontaine, B. Dujon, and C. Fairhead. 2005. Comparative genomics in hemiascomycete yeasts: evolution of sex, silencing, and subtelomeres. Mol. Biol. Evol. 22:856-873. [DOI] [PubMed] [Google Scholar]
- 9.Galagan, J. E., S. E. Calvo, C. Cuomo, L. J. Ma, J. R. Wortman, S. Batzoglou, S. I. Lee, M. Basturkmen, C. C. Spevak, J. Clutterbuck, V. Kapitonov, J. Jurka, C. Scazzocchio, M. Farman, J. Butler, S. Purcell, S. Harris, G. H. Braus, O. Draht, S. Busch, C. D'Enfert, C. Bouchier, G. H. Goldman, D. Bell-Pedersen, S. Griffiths-Jones, J. H. Doonan, J. Yu, K. Vienken, A. Pain, M. Freitag, E. U. Selker, D. B. Archer, M. A. Penalva, B. R. Oakley, M. Momany, T. Tanaka, T. Kumagai, K. Asai, M. Machida, W. C. Nierman, D. W. Denning, M. Caddick, M. Hynes, M. Paoletti, R. Fischer, B. Miller, P. Dyer, M. S. Sachs, S. A. Osmani, and B. W. Birren. 2005. Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature 438:1105-1115. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Pena, I., S. Hernandez, R. Auria, and S. Revah. 2005. Correlation of biological activity and reactor performance in biofiltration of toluene with the fungus Paecilomyces variotii CBS 115145. Appl. Environ. Microbiol. 71:4280-4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geiser, D. M., J. C. Frisvad, and J. W. Taylor. 1998. Evolutionary relationships in Aspergillus section Fumigati inferred from partial β-tubulin and hydrophobin DNA sequences. Mycologia 90:831-845. [Google Scholar]
- 12.Geiser, D. M., J. I. Pitt, and J. W. Taylor. 1998. Cryptic speciation and recombination in the aflatoxin-producing fungus Aspergillus flavus. Proc. Natl. Acad. Sci. USA 95:388-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerrits van den Ende, A. H. G., and G. S. De Hoog. 1999. Variability and molecular diagnostics of the neurotropic species Cladophialophora bantiana. Stud. Mycol. 43:152-162. [Google Scholar]
- 14.Glass, N. L., and G. C. Donaldson. 1995. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 61:1323-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong, S. B., S. J. Ho, H. D. Shin, J. C. Frisvad, and R. A. Samson. 2005. Polyphasic taxonomy of Aspergillus fumigatus and related species. Mycologia 97:1316-1329. [DOI] [PubMed] [Google Scholar]
- 16.Horie, Y., M. Miyaji, K. Nishimura, M. F. Franco, and K. I. R. Coelho. 1995. New and interesting species of Neosartorya from Brazilian soil. Mycoscience 36:199-204. [Google Scholar]
- 17.Horie, Y., P. Abliz, K. Fukushima, K. Okada, and G. M. Campos-Takagi. 2003. Two new species of Neosartorya from Amazonian soil, Brazil. Mycoscience 44:397-402. [Google Scholar]
- 18.Horn, B. W., and J. W. Dorner. 2002. Effect of competition and adverse culture conditions on aflatoxin production by Aspergillus flavus through successive generations. Mycologia 94:741-751. [PubMed] [Google Scholar]
- 19.Houbraken, J., R. A. Samson, and J. C. Frisvad. 2006. Byssochlamys: significance of heat resistance and mycotoxin production, p. 211-224. In A. D. Hocking, J. I. Pitt, R. A. Samson, and U. Thrane (ed.), Advances in food mycology: advances in experimental medicine and biology, vol. 571. Springer Science-Business Media, Inc., New York, NY. [DOI] [PubMed] [Google Scholar]
- 20.Kerényi, Z., A. Moretti, C. Waalwijk, B. Oláh, and L. Hornok. 2004. Mating type sequences in asexually reproducing Fusarium species. Appl. Environ. Microbiol. 70:4419-4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keszthelyi, A., A. Jeney, Z. Kerényi, O. Mendes, C. Waalwijk, and L. Hornok. 2007. Tagging target genes of the MAT1-2-1 transcription factor in Fusarium verticillioides (Gibberella fujikuroi MP-A). Antonie van Leeuwenhoek 91:373-391. [DOI] [PubMed] [Google Scholar]
- 22.Koufopanou, V., A. Burt, and J. W. Taylor. 1997. Concordance of gene genealogies reveals reproductive isolation in the pathogenic fungus Coccidioides immitis. Proc. Natl. Acad. Sci. USA 94:5478-5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kroken, S., and J. W. Taylor. 2001. Outcrossing and recombination in the lichenized fungus Letharia. Fungal Genet. Biol. 34:83-92. [DOI] [PubMed] [Google Scholar]
- 24.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for molecular evolutionary genetic analysis and sequence alignment. Brief. Bioinform. 5:150-163. [DOI] [PubMed] [Google Scholar]
- 25.Kwon-Chung, K. J., and S. J. Kim. 1974. A second heterothallic Aspergillus. Mycologia 66:628-638. [PubMed] [Google Scholar]
- 26.Luangsa-ard, J. J., N. L. Hywel-Jones, and R. A. Samson. 2004. The polyphyletic nature of Paecilomyces sensu lato based on 18S-generated rDNA phylogeny. Mycologia 96:773-780. [DOI] [PubMed] [Google Scholar]
- 27.MacDonald, K. D. 1968. Degeneration of penicillin titre in cultures of Penicillium chrysogenum. Nature 218:371-372. [DOI] [PubMed] [Google Scholar]
- 28.Mahendran, B., N. Raman, and D. J. Kim. 2006. Purification and characterization of tannase from Paecilomyces variotii: hydrolysis of tannic acid using immobilized tannase. Appl. Microbiol. Biotechnol. 70:444-450. [DOI] [PubMed] [Google Scholar]
- 29.Malloch, D., and R. F. Cain. 1972. The Trichocomaceae: ascomycetes with Aspergillus, Paecilomyces, and Penicillium imperfect states. Can. J. Bot. 50:2613-2628. [Google Scholar]
- 30.Malloch, D., and R. F. Cain. 1973. The Trichocomaceae (ascomycetes): synonyms in recent publications. Can. J. Bot. 51:1647-1648. [Google Scholar]
- 31.Paoletti, M., C. Rydholm, E. Schwier, M. Anderson, G. Szakács, F. Lutzoni, J. Debeaupuis, J. Latgé, D. Denning, and P. Dyer. 2005. Evidence for sexuality in the opportunistic fungal pathogen Aspergillus fumigatus. Curr. Biol. 15:1242-1248. [DOI] [PubMed] [Google Scholar]
- 32.Peterson, S. W., Y. Ito, B. W. Horn, and T. Goto. 2001. Aspergillus bombycis, a new aflatoxigenic species and genetic variation in its sibling species, A. nomius. Mycologia 93:689-703. [Google Scholar]
- 33.Pitt, J. I., and A. D. Hocking. 1997. Fungi and food spoilage, 2nd ed. University Press, Cambridge, United Kingdom.
- 34.Pitt, J. I., R. A. Samson, and J. C. Frisvad. 2000. List of accepted species and their synonyms in the family Trichocomaceae, p. 9-49. In R. A. Samson and J. I. Pitt (ed.), Integration of modern taxonomic methods for Penicillium and Aspergillus classification. Plenum Press, Inc., New York, NY.
- 35.Poggeler, S. 2002. Genomic evidence for mating abilities in the asexual pathogen Aspergillus fumigatus. Curr. Genet. 42:153-160. [DOI] [PubMed] [Google Scholar]
- 36.Raper, K. B., and D. I. Fennell. 1965. The genus Aspergillus. The Williams & Wilkins Co., Baltimore, MD.
- 37.Ryan, M. J., P. D. Bridge, D. Smith, and P. Jeffries. 2002. Phenotypic degeneration occurs during sector formation in Metarhizium anisopliae. J. Appl. Microbiol. 93:163-168. [DOI] [PubMed] [Google Scholar]
- 38.Rydholm, C., P. S. Dyer, and F. Lutzoni. 2007. DNA sequence characterization and molecular evolution of MAT1 and MAT2 mating-type loci of the self-compatible ascomycete mold Neosartorya fischeri. Eukaryot. Cell 6:868-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samson, R. A. 1974. Paecilomyces and some allied hyphomycetes. Stud. Mycol. 6:1-119. [Google Scholar]
- 40.Samson, R. A., E. S. Hoekstra, and J. C. Frisvad (ed.). 2004. Introduction to food- and airborne fungi, 7th ed. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
- 41.Samson, R. A., J. A. M. P. Houbraken, A. F. A. Kuijpers, J. M. Frank, and J. C. Frisvad. 2004. New ochratoxin or sclerotium producing species in Aspergillus section Nigri. Stud. Mycol. 50:45-61. [Google Scholar]
- 42.Samson, R. A., J. Houbraken, R. C. Summerbell, B. Flannigan, and J. D. Miller. 2001. Common and important species of fungi and actinomycetes in indoor environment, p. 287-474. In B. Flannigan, R. A. Samson, and J. Miller (ed.), Microorganisms in home and indoor work environments. CRC Press LLC, Boca Raton, FL.
- 43.Serra, R., F. J. Cabañes, G. Perrone, G. Castella, A. Venancio, G. Mule, and Z. Kozakiewicz. 2006. Aspergillus ibericus: a new species of section Nigri isolated from grapes. Mycologia 98:295-306. [DOI] [PubMed] [Google Scholar]
- 44.Stolk, A. C., and R. A. Samson. 1972. Studies on Talaromyces and related genera II: the genus Talaromyces. Stud. Mycol. 2:1-65. [Google Scholar]
- 45.Swofford, D. L. 2000. PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4.0b4a. Sinauer Associates, Sunderland, MA.
- 46.Takada, M., and S.-I. Udagawa. 1985. A new species of heterothallic Neosartorya. Mycotaxon 24:395-402. [Google Scholar]
- 47.Takada, M., and S. Udagawa. 1988. A new species of heterothallic Talaromyces. Mycotaxon 31:417-425. [Google Scholar]
- 48.Takada, M., Y. Horie, and P. Abliz. 2001. Two new heterothallic Neosartorya from African soil. Mycoscience 42:361-367. [Google Scholar]
- 49.Taylor, J. W., D. M. Geiser, A. Burt, and V. Koufopanou. 1999. The evolutionary biology and population genetics underlying fungal strain typing. Clin. Microbiol. Rev. 12:126-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tóth, B., Á. Mesterházy, P. Nicholson, J. Téren, and J. Varga. 2004. Mycotoxin production and molecular variability of European and American Fusarium culmorum isolates. Eur. J. Plant Pathol. 110:587-599. [Google Scholar]
- 52.Tournas, V. 1994. Heat-resistant fungi of importance to the food and beverage industry. Crit. Rev. Microbiol. 20:243-263. [DOI] [PubMed] [Google Scholar]
- 53.Udagawa, S., and S. Suzuki. 1994. Talaromyces spectabilis, a new species of food-borne ascomycetes. Mycotaxon 50:81-88. [Google Scholar]
- 54.Varga, J., and B. Tóth. 2003. Genetic variability and reproductive mode of Aspergillus fumigatus: a review. Infect. Genet. Evol. 3:3-17. [DOI] [PubMed] [Google Scholar]
- 55.Varga, J., S. Kocsubé, B. Tóth, J. C. Frisvad, G. Perrone, A. Susca, M. Meijer, and R. A. Samson. 2007. Aspergillus brasiliensis sp. nov., a biseriate black Aspergillus species with worldwide distribution. Int. J. Syst. Evol. Microbiol. 57:1925-1932. [DOI] [PubMed] [Google Scholar]
- 56.Woo, P. C. Y., K. T. K. Chong, H. Tse, J. J. Cai, C. C. Y. Lau, A. C. Zhou, S. K. P. Lau, and K. Yuen. 2006. Genomic and experimental evidence for a potential sexual cycle in the pathogenic thermal dimorphic fungus Penicillium marneffei. FEBS Lett. 580:3409-3416. [DOI] [PubMed] [Google Scholar]
- 57.Yun, S. H., M. L. Berbee, O. C. Yoder, and B. G. Turgeon. 1999. Evolution of the fungal self-fertile reproductive lifestyle from self-sterile ancestors. Proc. Natl. Acad. Sci. USA 96:5592-5597. [DOI] [PMC free article] [PubMed] [Google Scholar]