Abstract
A gene involved in N-acyl homoserine lactone (N-AHSL) degradation was identified by screening a genomic library of Rhodococcus erythropolis strain W2. This gene, named qsdA (for quorum-sensing signal degradation), encodes an N-AHSL lactonase unrelated to the two previously characterized N-AHSL-degrading enzymes, i.e., the lactonase AiiA and the amidohydrolase AiiD. QsdA is related to phosphotriesterases and constitutes the reference of a novel class of N-AHSL degradation enzymes. It confers the ability to inactivate N-AHSLs with an acyl chain ranging from C6 to C14, with or without substitution at carbon 3. Screening of a collection of 15 Rhodococcus strains and strains closely related to this genus clearly highlighted the relationship between the ability to degrade N-AHSLs and the presence of the qsdA gene in Rhodococcus. Bacteria harboring the qsdA gene interfere very efficiently with quorum-sensing-regulated functions, demonstrating that qsdA is a valuable tool for developing quorum-quenching procedures.
Gram-negative bacteria couple gene expression to population density by a regulatory mechanism named quorum sensing (QS). QS relies upon the production and the perception of one or more signal molecules by the bacterial population. An important class of these signals is the N-acyl homoserine lactone (N-AHSL) class (9). Molecules belonging to this class exhibit a conserved structure, with a backbone composed of a homoserine lactone (HSL) N-linked to an acyl chain via an amide bond. Variation in N-acyl chain length and the oxidation status of N-AHSLs provide for specificity of the signal. Of particular interest is the finding that QS regulates pathogenicity, or pathogenicity-related functions, in bacteria of medical or environmental importance (15, 32, 50).
If QS and N-AHSLs are important components of the strategy of adaptation by bacteria to their biotic environment, especially a plant surface, one might suspect that the eukaryotic hosts and competing bacteria might have developed strategies to interfere with this communication system. Indeed, QS inhibition was reported through the production of antagonists (10) or the production of N-AHSL degradation enzymes by plants (5), animals (2, 34), and a wide range of bacterial genera (14, 16, 18-20, 30, 31, 44, 49). In spite of the large diversity of N-AHSL-degrading bacteria, only two families of N-AHSL-inactivating enzymes (N-AHSLases) have been described to date: the AiiA-like N-AHSL lactonases (6, 19, 49) and the AiiD-like N-AHSL amidohydrolases (14, 20, 30). Whatever their physiological role, N-AHSLases have been used efficiently to interfere with the expression of QS-regulated functions in bacteria (6, 19, 20, 35, 42). This strategy has been termed quorum quenching (QQ). It proved to be a valuable trail toward definition of novel biocontrol agents such as natural isolates degrading N-AHSLs (8, 23, 44). QQ occurs in natural environments as indicated by the coexistence of N-AHSL-producing and -degrading strains in biofilms (14) or in the rhizosphere (4). Among the species harboring an N-AHSLase activity, Rhodococcus erythropolis is remarkable because it is the only bacterium in which three enzymatic activities directed at N-AHSLs have been characterized: an oxidoreductase activity, which reduces 3-oxo-N-AHSLs to their hydroxylated equivalents (43); an amidohydrolase (43); and a lactonase (29). The marked R. erythropolis QQ capabilities suggest that it might be used in biocontrol protocols, especially since it is a natural inhabitant of soils worldwide (44).
In this study we report the isolation of one of the three genes encoding N-AHSLase activities from R. erythropolis strain W2. We show that this gene encodes a phosphotriesterase (PTE)-like broad-spectrum N-AHSL lactonase, which is found only in the Rhodococcus genus and solely in strains capable of degrading N-AHSLs.
MATERIALS AND METHODS
Strains, media, growth conditions, and chemicals.
Bacterial strains and plasmids are listed in Table 1. All strains were grown at 25°C, with the exception of the Chromobacterium violaceum biosensor and Escherichia coli, which were grown at 30°C and 37°C, respectively. Unless otherwise stated, LBm medium was buffered at pH 6.5 to prevent spontaneous lactonolysis of N-AHSLs and used as the rich medium (44). All media were solidified with 16 g/liter of agar. Antibiotics were used at the following final concentrations (in μg/ml; see Table 1 for specific strain requirements): ampicillin, 100; gentamicin, 25; tetracycline, 10; rifampin, 100; and streptomycin, 250. X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) and IPTG (isopropyl-β-d-thiogalactopyranoside) were included in media at 40 μg/ml and 100 μg/ml, respectively. Hexanoyl-HSL (C6-HSL) and its 3-oxo derivative were purchased from Sigma. The other N-AHSLs were generous gifts from S. R. Chhabra and P. Williams (University of Nottingham, United Kingdom).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Characteristics or synonym | Source or reference |
|---|---|---|
| Strains tested for N-AHSL degradation and presence of the qsdA gene | ||
| Actinoplanes utahensis NRRL 12052 | Source of aculeacin A deacylase | 39 |
| Corynebacterium glutamicum ATCC 14752 | Glutamine-producing strain | 38 |
| Escherichia coli Κ-12 | Lab collection | |
| Flavobacterium sp. strain ATCC 27551 | Parathion-degrading strain | 24 |
| Gordonia alkalivorans strain 98 | Petroleum-degrading strain | Lab collection |
| Microbacterium sp. strain POLB142 | Mannopine-degrading rhizospheric strain; soil isolate | 27 |
| Mycobacterium sp. strain Tn20 | Drought-resistant Sahara desert isolate | This study |
| Nocardia asteroides CECT 3051 | ATCC 19247; type strain | CECTa |
| Pseudonocardia autotrophica CECT 3044 | ATCC 13181; type strain | 47 |
| Ralstonia solanacearum GMI 1000 | Type strain | Lab collection |
| Rhodococcus erythropolis | ||
| W2 | N-AHSL-degrading isolate from soil | 44 |
| DCL14 | Limonene-degrading strain | 46 |
| SQ1 | ATCC 4277-1; R. erythropolis type strain | 33 |
| Mic1 | Mycorhizosphere isolate | Lab collection |
| MP50 | Aromatic-nitrile-degrading strain | 41 |
| CECT 3008 | ATCC 11048 | CECT |
| Rhodococcus fascians D188 | Fasciation-causing agent on dicotyledons | 3 |
| Rhodococcus opacus HL PM-1 | 2,4,6-Trinitrophenol-degrading strain | 25 |
| Rhodococcus rhodochrous CECT 3042 | ATCC 6846 | CECT |
| Rhodococcus corynebacteroides CECT 420 | ATCC 14898; soil isolate | CECT |
| Rhodococcus sp. strain RHA1 | Polychlorinated biphenyl-degrading strain | Lab collection |
| N-AHSL biosensors and N-AHSL-producing strains used for QQ assays | ||
| Agrobacterium tumefaciens NTL4(pZLR4) | Hydroxy, oxo, and long-chain N-AHSL sensor strain | 21 |
| Chromobacterium violaceum CV026 | Reduced N-AHSL sensor strain | 22 |
| Pectobacterium carotovorum Pcc797 | Soft-rot-causing agent | Lab collection |
| Pseudomonas fluorescens 1855-344 | Rhizospheric strain | Lab collection |
| Agrobacterium tumefaciens | ||
| 15955trac | Octopine-type strain, constitutive for Ti plasmid conjugal transfer | Lab collection |
| C58C1RS | Ti plasmid-cured C58 derivative resistant to rifampin and streptomycin | Lab collection |
| Escherichia coli strains used for cloning | ||
| DH5α | Lab collection | |
| VCS257 | Stratagene | |
| Plasmids | ||
| pUC19 | Ampr | Lab collection |
| pGEM-T | Ampr | Promega |
| pUC1318::Gm | Ampr Gmr | 40 |
| pME6032 | Tcr | 12 |
| pCP13/B | Tcr; cosmid vector | Lab collection |
| pSU16 | Tcr; cosmid clone conferring N-AHSL degradation capability | This study |
| pSU16-11 | pUC19 with a 3.2-kb EcoRI fragment from pSU16 conferring N-AHSL degradation | This study |
| pSU16-12 | pUC19 with a 3.5-kb BamHI fragment from pSU16 conferring N-AHSL degradation | This study |
| pSU40 | pME6032 harboring the 3.2-kb EcoRI fragment from pSU16-11 | This study |
| pSU8-1 | pGEM-T with a 1.8-kb PCR fragment containing orf1 and qsdA | This study |
| pSU8-1 ΔSalI | pSU8-1 with a SalI deletion in orf1 | This study |
| pSU8-1::Gm | pSU8-1 with insertion of a Gmr cassette at the AgeI site of qsdA | This study |
| pSU8-1 ΔSalI::Gm | pSU8-1 ΔSalI with insertion of a Gmr cassette at the AgeI site of qsdA | This study |
| pSU-qsdAW2 | qsdA ORF amplified and cloned into pET16b | This study |
| pSUreg1 | Upstream regulatory region of qsdA amplified and cloned into pGEM-T | This study |
CECT, Coleccion Española de Cultivos Tipo.
Detection of N-AHSLs.
N-AHSLs were separated and visualized on thin-layer chromatography (TLC) plates as described previously (37). Saturated short-chain N-AHSLs were detected by TLC using the Chromobacterium violaceum biosensor CV026 (22). Oxo and hydroxy derivatives and long-chain N-AHSLs were detected using the Agrobacterium biosensor NTL4(pZLR4) (21).
Detection of N-AHSL degradation products.
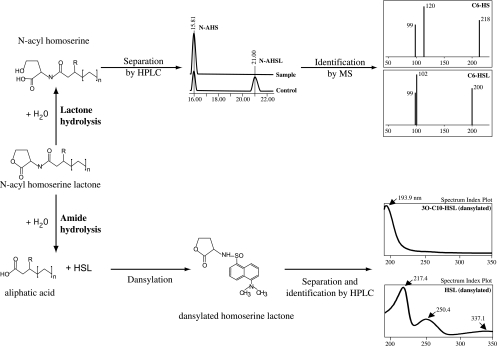
The N-AHSL degradation assays were performed as described earlier (45), using actively growing DH5α cells expressing or not the qsdA gene in pH 6.5 buffered LB medium. The incubation time was set to 24 h, because this allowed work at low concentration and thus reduction of the interference of the medium with the data collected. Under these conditions, a fraction of ca. 25% of the initial amount of C6-HSL is spontaneously converted into C6-HS by chemical lactonolysis in the presence or absence of E. coli cells. The enzymatic degradation of N-AHSLs can proceed through two different routes (Fig. 1), which lead to the formation of either N-AHS (lactonolysis) (Fig. 1, top) or HSL and an alkyl chain (amidolysis) (Fig. 1, bottom). The presence of HSL in the incubation medium was determined after trapping of the free amine with dansyl chloride as described earlier (48). The formation of the ring-opened derivative of N-AHSLs following lactone hydrolysis was investigated using high-pressure liquid chromatography (HPLC) on a Waters chromatograph equipped with a Waters separation module 2659 and an Atlantis reverse-phase column (4.6 by 150 mm; 5 μm) coupled to an electrospray ionization-mass spectrometry detector (Waters Micromass ZQ 200). Retention times and mass spectra were determined for individual molecules in solution as standards. Thirty microliters of the degradation assay sample was injected and eluted with water-0.1% formic acid (solvent A) and acetonitrile-0.1% formic acid (solvent B) with the following elution sequence: 100% solvent A for 5 min, a linear gradient to reach 20% solvent B in 5 min, and 80% solvent A and 20% solvent B for 10 min. Between samples, the column was rinsed by applying 100% B solvent (3 min). The column was then reequilibrated with 100% solvent A for 7 min. The specific fragments expected to appear in the mass spectra of C6-HSL and C6-HS are all present; fragment 200 is characteristic for C6-HSL, while fragment 218 is characteristic for C6-HS. Fragment 102 corresponds to HSL and therefore appears in the C6-HSL spectrum, while fragment 120 is characteristic for HS and appears in the C6-HS spectrum (28).
FIG. 1.
Scheme for identification of N-AHSL-degradation products. N-AHSL inactivation in bacteria proceeds through two known pathways: lactonolysis (top) or amide hydrolysis (bottom). N-AHSL lactonolysis is a reversible reaction which yields the corresponding N-AHS, which can be separated by HPLC and identified by mass spectrometry (MS). Amide hydrolysis is an irreversible reaction which yields HSL and the corresponding acyl side chain. HSL can be detected by HPLC after trapping the free amine with dansyl chloride.
Library construction.
As strain W2 is especially difficult to lyse by conventional methods, genomic DNA preparation was based on the method of Eulberg et al. (7) with the following modifications. Strain W2 was grown overnight in 50 ml of LB medium buffered at pH 8.0 with glycine (3%, wt/vol). Prior to DNA extraction, cells were treated for 2 hours with ampicillin and erythromycin (100 μg/ml, final concentration) to disturb cell wall synthesis and favor the subsequent lysis of the cells. A genomic library of strain W2 was constructed by partially digesting 100 μg of total genomic DNA with Bsp143I (Sau3AI) and ligating the fragments in BamHI-linearized cosmid vector pCP13/B as described by Hayman and Farrand (11). The ligation products were packaged using the Gigapack III Gold packaging kit (Stratagene, La Jolla, CA) as recommended by the manufacturer and recovered by transfection into E. coli VCS257. The W2 genomic library generated in this study contained over 4,000 clones, with an average insert size of 23 kb and fewer than 1% empty clones. This corresponds to a theoretical coverage of 15 times of Rhodococcus genome (1).
Screening of the genomic library for N-AHSL degradation ability.
A total of 2,880 library clones were screened directly in their E. coli strain VCS257 host by use of a modification of the microplate N-AHSL degradation assay described by Uroz et al. (44), using C. violaceum CV026 as an indicator strain. Clones were first grown in presence of antibiotics and then subcultured in medium devoid of antibiotics but supplemented with 25 μM of C6-HSL. Cosmid clones were considered to confer the ability to degrade N-AHSLs only if a total disappearance of the C6-HSL was observed after a 24-h period. The ability of the clones to effectively degrade the N-AHSL was confirmed by separating the degradation products by TLC and detecting the presence of N-AHSL by the appropriate biosensor. This allows the detection of false-positive degradation due to the presence of compounds inhibiting N-AHSL detection or the growth of the biosensor.
DNA sequence analysis.
Sequence analysis was performed with ORF FINDER. Nucleotide and amino acid sequence comparisons were made using the BLAST protocol. Multiple alignments were performed using the Pileup subroutine of the GCG package (version 10; GCG Inc., Madison, WI).
Subcloning of the qsdA region.
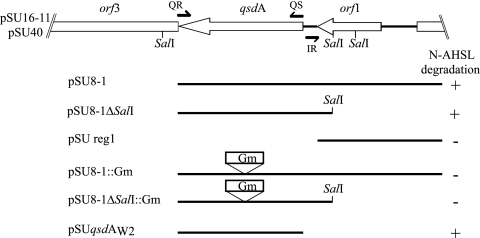
To identify the gene coding for the N-AHSL degradation activity, the 3.2-kb EcoRI fragment was subcloned as shown in Fig. 2 using the primers QS (5′-ATGAGTTCAGTACAAACCGTTCGTG-3′), QR (5′-TCAGCTCTCGAAGTACCGACGTGGG-3′), and IR (5′-TCACCATTTTTCAACGGCCG-3′) and available restriction sites. The qsdA gene was disrupted by insertion of a gentamicin resistance gene from pUC1318::Gm, cloned as an XmaI fragment at the unique AgeI site of the qsdA gene. Southern hybridization was performed at 62°C with a qsdA probe amplified with the QS/QR primer pair and digoxigenin labeled according to the manufacturers' instructions (Roche/Boehringer Mannheim).
FIG. 2.
Identification of the qsdA gene. The genetic organization of the qsdA locus derived from the complete sequence of the 3.2-kb EcoRI fragment conferring C6-HSL degradation upon its host is shown at the top. Broken arrows symbolize the primers used for subcloning and their orientations. Restriction sites also used for subcloning are shown. See Table 1 for a description of the plasmids. Plasmid constructions used to identify the qsdA gene were assayed for their ability (+) or inability (−) to confer N-AHSL degradation upon their host against a set of N-AHSLs, including C6-HSL, O-C6-HSL, C7-HSL, C8-HSL, O-C8-HSL, O-C10-HSL, C12-HSL, O-C12-HSL, and O-C14-HSL. Each construct gave identical degradation results regardless of the N-AHSL present in the medium.
QQ assays.
The ability of the cloned qsdA genes to interfere with the expression of QS-regulated functions was tested in heterologous expression systems. Plasmid pSU40 (qsdAW2 cloned into pME6032) was transferred to E. coli strain DH5α, Pseudomonas fluorescens strain 1855-344, and the octopine-type conjugal transfer constitutive strain Agrobacterium tumefaciens 15955trac to yield DH5α(pSU40), 1855-344(pSU40), and 15955trac (pSU40), respectively. The ability of these strains to interfere with QS-regulated functions was first evaluated using the streak assay described by Molina et al. (23), using C. violaceum CV026 as an indicator strain. The ability to interfere with virulence in Erwinia carotovora was tested in the potato tuber assay as described by Uroz et al. (44). Additionally, the ability of the qsdA locus to interfere intracellularly with QS was tested in Agrobacterium tumefaciens. Ti plasmid conjugation assays were performed essentially as described by Oger et al. (26), using strain 15955trac as a transfer constitutive donor and C58C1RS as a recipient. In all experiments, strains harboring the empty vector pME6032 were used as negative control.
Nucleotide sequence accession numbers.
The qsdA locus sequence has been deposited at GenBank under accession number AY541692, and the qsdA allele sequences have been deposited under accession numbers EF218062, EF218066, EF218065, and EF589962 (R. erythropolis strains SQ1, MP50 CECT 3008, and Mic1, respectively), EF218064 (R. corynebacteroides CECT 420), and EF218063 (R. rhodochrous strain CECT 3042).
RESULTS AND DISCUSSION
Identification of the Rhodococcus erythropolis gene involved in N-AHSL degradation.
Genes involved in QS signal degradation were isolated from a genomic library of R. erythropolis strain W2 on the basis of the ability of specific cosmids to confer C6-HSL degradation capability upon the E. coli host cells. Degradation was clearly evidenced for 20 out of the 2,880 clones screened. Based on their restriction patterns, the 20 cosmids fell into four groups, which all shared single common EcoRI and BamHI fragments of ca. 3.2 and 3.5 kb, respectively. The cosmid pSU16, which harbors the smallest insert, was chosen for further studies. DNA fragments resulting from BamHI or EcoRI restrictions of pSU16 DNA were shotgun subcloned into pUC19. Only two sets of clones, containing either a 3.5-kb BamHI (pSU16-12) (not shown) or a 3.2-kb EcoRI (pSU16-11) (Fig. 2) fragment conferred upon their E. coli host the ability to degrade N-AHSLs with acyl chains ranging from C6- to C12-HSL, independently of the substitution at carbon 3. HPLC analyses of the growth media of these clones confirmed the disappearance of the N-AHSL (data not shown).
The sequence of the EcoRI fragment of pSU16-11 (3,152 bp; GenBank accession number AY541692) contains two incomplete open reading frames (ORFs) and two complete ORFs, which were named orf1 and qsdA (for QS signal degradation) (Fig. 2). The first incomplete ORF (to the right in Fig. 2) shows similarities to alleles of the gntR/fadR family of transcriptional regulators and is most closely related to that of Pseudomonas aeruginosa strain PAO1 (PA1627) (identity, 40%; similarity, 58%). The first complete ORF, orf1, could encode a serine-rich protein of 172 amino acids. It does not show any significant homology with peptide sequences available in the databases, suggesting that it might be a pseudogene. The second complete ORF, qsdA, could encode a protein of 323 amino acids related to members of the PTE superfamily, which is found in a wide range of organisms from bacteria to eukarya. PTEs are zinc metalloenzymes which were initially identified as efficiently catalyzing the hydrolysis of a variety of organophosphorus compounds (13), but a growing number of PTE homologues which also show amidohydrolase or lactonase activities have been characterized (36). Finally, the protein encoded by the incomplete orf3 shows 48% identity with proteins annotated as acyl coenzyme A synthetases (AMP-forming)/AMP-acid ligases II of Ralstonia metallidurans and other enzymes related to the lipid metabolism and transport. Acyl coenzyme A synthetases are involved in both the synthesis and turnover of fatty acids in bacteria.
Because the ORFs qsdA and orf1 were the only uninterrupted ORFs present in pSU16-11, they were likely to determine the C6-HSL degradation ability conferred by that clone. To confirm this hypothesis, various constructions were generated (Fig. 2). As expected, the constructions lacking one or both partial ORFs (e.g., pSU8-1 and pSUqsdAW2) still conferred N-AHSL-degrading capabilities upon E. coli. Conversely, constructions lacking qsdA (pSU reg1) or harboring a disrupted qsdA gene (pSU8-1::Gm) did not confer N-AHSL degradation ability upon their host. From these results, it is clear that qsdA is necessary and sufficient to code for N-AHSL degradation in the original pSU16 cosmid.
qsdA codes for a PTE-like N-AHSL lactonase.
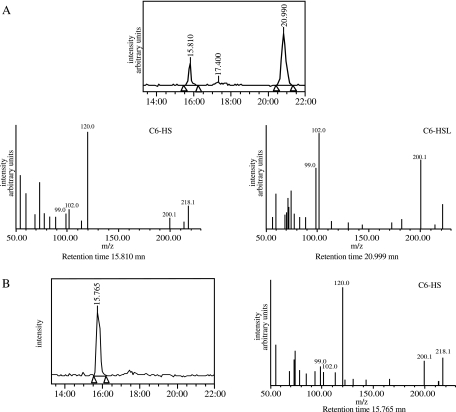
The identification of the degradation products of N-AHSLs was performed by a combination of HPLC and mass spectrometry analyses to detect the presence of HSL or N-AHS generated by amidolysis or lactonolysis, respectively (Fig. 1). The spontaneous degradation was evaluated in control experiments that used uninoculated LB medium or medium inoculated with E. coli DH5α or DH5α with the empty cloning vector. Figure 3A presents results obtained for strain DH5α(pME6032). In each experiment, two peaks were visible on the HPLC spectra after a 24-h incubation. Their position at 15.8 and 21 min as well as mass spectra correspond to C6-HS and C6-HSL, respectively. The presence of C6-HS in the control experiments results from the spontaneous lactonolysis of C6-HSL in aqueous medium. Each control condition yielded the same amount of spontaneous lactonolysis (ca. 25%; data not shown), showing that DH5α does not itself facilitate the degradation of N-AHSLs. At the end of experiments performed with E. coli DH5α(pSU40) expressing qsdA, no N-AHSL could be detected (Fig. 3B), indicating that a complete conversion of the initial C6-HSL occurred. Attempts to detect the presence of HSL, which would indicate a cleavage of the N-AHSL molecules by an amidohydrolase, failed (data not shown). At the same time, the presence of C6-HS was clearly visible (Fig. 3). Thus, qsdA must code for an N-AHSL lactonase activity, the presence of which in Rhodococcus has been recently identified by Park and colleagues (29).
FIG. 3.
QsdA lactonase activity. E. coli strains DH5α(pME6032) and DH5α(pSU40) were incubated in pH 6.5 buffered LBm medium with 50 μM C6-HSL for 24 h. The medium was analyzed at 0 and 24 h by HPLC-mass spectrometry. Under the experimental conditions used, C6-HS (molecular weight, 217) and C6-HSL (molecular weight, 199) had retention times of 15.8 and 21 min, respectively, and mass spectra were composed of the following main fragments: m/z = 218, 200, and 120 for C6-HS, and m/z = 200, 102, and 99 for C6-HSL. (A) Spontaneous degradation of C6-HSL in aqueous medium. (B) DH5α(pSU40) after 24 h of incubation. A single peak at a retention time of 15.8 min is visible on the HPLC spectrum, which is identified as C6-HS. C6-HSL has completely disappeared from the medium. The formation of C6-HS, correlated with the absence of HSL in the medium, is indicative of a lactonase activity.
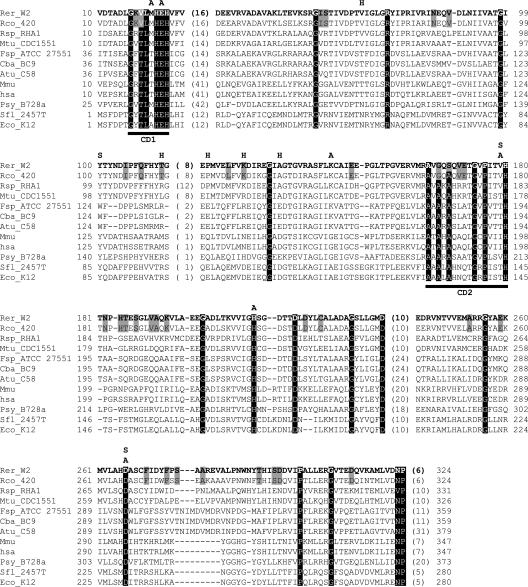
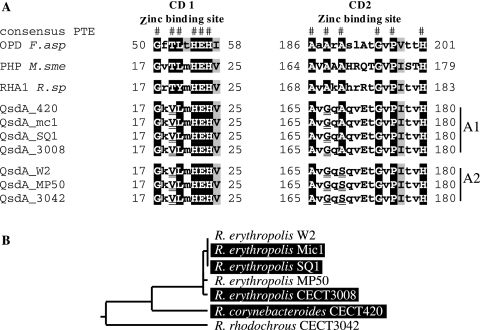
QsdA clearly belongs to the PTE family of zinc-dependent metalloproteins (Fig. 4). It is totally unrelated to the known bacterial N-AHSL lactonases, which belong to the Zn-dependent glyoxylase family, or to the known N-AHSL amidohydrolases, which belong to the cluster of β-lactam acylases. Thus, the present study extends the number of families of N-AHSL-degrading enzymes of bacterial origin to three and to include the PTE family. PTEs were first described for their ability to cleave the phosphotriester bond in phosphotriesters but were later shown to be promiscuous enzymes, harboring lactonase or amidohydrolase activities (36). QsdA, however, does not confer the ability to degrade the prototypic phosphotriester methyl parathion, nor does the prototype PTE gene, opd, code for N-AHSL degradation (data not shown). Although QsdA exhibits the canonical structure of PTEs, the sequence of its metal binding site, which is central to the enzyme activity by fixing two molecules of zinc and forming the catalytic pocket in which the substrate inserts, differs from the consensus sequence of PTEs at 3 of 12 positions (CD1 and CD2 domains in Fig. 4). In contrast, the sole PTE/QsdA homologue known from Rhodococcus, i.e., the TIG:13193 gene from strain RHA1 (Rsp_RHA1 in Fig. 4), as well as the closest homologues of QsdA, i.e., the PTE alleles of several Mycobacterium strains and other actinobacteria (Mtu_CDC1551 in Fig. 4), all present the canonical signature of the PTEs, including the 12 conserved amino acids from the zinc binding domain. Interestingly, strain RHA1 and Mycobacterium sp. strain Tn20 are both unable to degrade N-AHSLs. In addition, when cloned and expressed in E. coli, the TIG:13193 ORF from RHA1 does not confer N-AHSL degradation capabilities to its host. Thus, the lack of N-AHSL degradation in strain RHA1 is due not to a lack of expression of the protein from its native promoter but to the lack of N-AHSL lactonase activity of the TIG:13193 protein, which will consequently be referred to not as a QsdA but as a classical PTE homologue. QsdA is therefore the first prokaryotic member of the PTE family with demonstrated N-AHSL lactonase activity.
FIG. 4.
Alignment of selected PTEs from bacterial and eukaryotic origins. In addition to QsdA from R. erythropolis strain W2 (Rer_W2), PTE homologues used in this alignment include a putative QsdA homologue from Rhodococcus corynebacterioides CECT 420 (Rco_420, this study); putative PTEs from Rhodococcus sp. strain RHA1 (Rsp_RHA1, accession no. TIG:13193), Agrobacterium tumefaciens strain C58 (Atu_C58, accession no. AAL43882), Pseudomonas syringae pv. Syringae strain B728a (Psy_B728a, accession no. YP_233869), Shigella flexneri strain 2457T (Sfl_2457T, accession no. AAP19319), Mycobacterium tuberculosis strain CDC1551 (Mtu_CDC1551, accession no. AAK44461), and Escherichia coli K-12 (Eco_K12, accession no. AAC76404); PTEs from Chryseobacterium balustinum strain BC9 (Cba_BC9, accession no. CAD19996); OPD from Flavobacterium sp. strain ATCC 27551 (Fsp_ATCC27551, accession no. CAD13181); and PTER from Homo sapiens sapiens (Hsa, accession no. Q96BW5) and from Mus musculus (Mmu, accession no. Q60866). The positions of the essential amino acids residues of the catalytic site (A), the substrate binding site (S), and the dimerization domains (H) are shown above the alignment. CD1 and CD2, zinc binding conserved domains 1 and 2. The 27 residues conserved among known PTE sequences are shown in reverse font. QsdA-specific amino acid substitutions which are not observed in other PTEs (including alleles not shown in the figure) are highlighted by a gray background.
QsdA is a Rhodococcus-specific PTE-like N-AHSL lactonase.
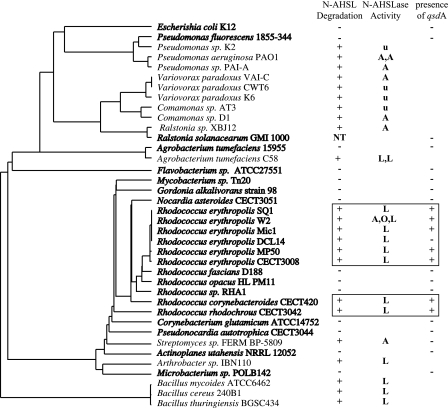
The wide occurrence of PTE homologues in species closely related to R. erythropolis and the specific signature and activity of QsdA prompted us to determine the phylogenetic distribution of this gene in the actinobacterial cluster. We screened a collection of related strains belonging to the Rhodococcus genus, i.e., R. corynebacteroides (one strain), R. erythropolis (five strains), R. fascians (one strain), R. rhodochrous (one strain), and R. opacus (one strain), for their ability to inactivate N-AHSLs and the presence of the qsdA gene. The ability to degrade N-AHSLs appeared ubiquitous among strains of R. erythropolis, since all assayed isolates (5/5) degraded a range of N-AHSLs identical to that previously reported for strain W2 (acyl chain length from C6 to C14, with or without substitution on C3 [see Table S1 in the supplemental material]). N-AHSL degradation was observed in three of the five Rhodococcus species tested, i.e., R. erythropolis, R. rhodochrous, and R. corynebacteroides, but not in R. fascians and R. opacus, although these species are more closely related to R. erythropolis than are R. rhodochrous and R. corynebacteroides (Fig. 5), and not in the polychlorinated biphenyl-degrading Rhodococcus sp. strain RHA1. The degradation ability was also tested in members of genera closely related to Rhodococcus, such as Pseudonocardia autotrophica (strain CECT 3044), Corynebacterium glutamicum (strain ATCC14752), Gordonia alkalivorans (strain 98), Microbacterium sp. (strain POLB142), Mycobacterium sp. (strain Tn20), Nocardia asteroides (strain CECT 3051), Actinoplanes utahensis (strain NRRL 12052), and Flavobacterium sp. (strain ATCC 27551). None of these assayed strains degraded N-AHSLs.
FIG. 5.
Phylogenetic distribution of N-AHSL degradation in Rhodococcus. The ability to degrade N-AHSLs among bacteria is highlighted on a phylogenetic tree based on the 16S rRNA gene. Strains shown in bold were tested in the present study. N-AHSL degradation data from the literature are reported for all other strains. Wherever possible the nature of the enzymatic activity is noted: A, N-AHSL amidohydrolase; L, N-AHSL lactonase; O, N-AHSL oxidoreductase; u, undetermined. The number of identified activities is also reported (e.g., A, A indicates two amidohydrolases). The presence (+) or absence (−) of a qsdA homologue was determined by hybridization with a qsdA probe. The N-AHSL-degrading strains belonging to the Rhodococcus genus are boxed. Agrobacterium tumefaciens strain C58 harbors two N-AHSL lactonases (AttM and AiiB), and R. erythropolis strain W2 harbors at least three N-AHSL modification/degradation enzymes: an amidohydrolase (A), an oxidoreductase (O), and a lactonase (L). NT, not tested.
The qsdA gene was detected by Southern hybridization, under low-stringency conditions using the cloned qsdA gene from strain W2 as a probe, in all but one of the strains able to degrade N-AHSLs, including R. corynebacteroides, R. erythropolis, and R. rhodochrous. Southern hybridization performed on genomic DNA restricted with EcoRI, BamHI, and HindIII confirmed that the gene is present as a single copy in the genomes of these strains. The only R. erythropolis strain that did not hybridize with the qsdA probe was strain DCL14 (46). This strain is indistinguishable from strain W2 in terms of 16S rRNA gene sequence and ability to inactivate QS molecules. This result is, however, consistent with the observation by Uroz et al. (43), who demonstrated the occurrence of N-AHSLase activities other than lactonase in R. erythropolis strains W2 and DCL14. The qsdA gene could not be detected in any of the Rhodococcus strains unable to degrade N-AHSLs or in the strains from the related genera.
A total of six qsdA alleles (in addition to qsdA from strain W2) were cloned into pGEM-T Easy following PCR amplification from the genomic DNAs of the four R. erythropolis clones, as well as the R. rhodochrous and R. corynebacteroides strains showing a positive hybridization signal in Southern analysis. All alleles conferred the ability to degrade N-AHSLs upon E. coli DH5α. The deduced QsdA peptides fell into two homology groups, which were termed A1 and A2 (Fig. 6A). Sequences (DNA and protein) were almost identical within each group and were ca. 88% identical and 93% similar at the protein level between groups A1 and A2. The conserved zinc binding domains CD1 and CD2 of the QsdA alleles of clusters A1 and A2 diverge at one position (residue 167) (Fig. 6A). Meanwhile, domains CD1 and CD2 of allele clusters A1 and A2 also diverge from the consensus PTE domains at two or three positions (QsdA residues 19, 167, 169), suggesting that the six alleles might derive from the same PTE ancestor. However, the qsdA allele phylogeny is not congruent with the Rhodococcus 16S rRNA gene phylogeny (Fig. 6B). Furthermore, the Rhodococcus species harboring these alleles do not appear to form a clade within the Rhodococcus genus (Fig. 5). This suggests that qsdA is a Rhodococcus-specific N-AHSL lactonase that evolved in this genus and was transferred horizontally at several points during the speciation of Rhodococcus.
FIG. 6.
Alignment of the conserved zinc biding domains of QsdA homologues. (A) OPD F.asp, canonical PTE sequence from Flavobacterium sp. strain ATCC 27551; RHA1 R.sp, deduced peptide sequence from the PTE homologue from Rhodococcus sp. strain RHA1 encoded by gene TIG:13193; PHP M.sme, PTE homologue from Mycobacterium smegmatis; consensus PTE, the 12 conserved amino acids from the PTE zinc binding domains CD1 and CD2 are indicated (#). Nonconserved positions are indicated in lowercase letters. qsdA-deduced protein sequences used in this alignment include those from Rhodococcus corynebacteroides strain CECT 420 (QsdA_420), Rhodococcus rhodochrous strain CECT 3042 (QsdA_3042), and Rhodococcus erythropolis strains SQ1 (QsdA_SQ1), CECT 3008 (QsdA_3008), W2 (QsdA_W2), Mic1 (QsdA_Mic1), and MP50 (QsdA_MP50). Divergences from the consensus PTE zinc domain sequence are underlined in the QsdA sequences. Two divergent alleles of QsdA (A1 and A2) are found in Rhodococcus. (B) Taxonomic relationship between strains harboring allele A1 (black background) and strains harboring allele A2 (white background) based on a 16S rRNA gene phylogeny.
qsdA confers QS quenching abilities in heterologous systems.
We tested the ability of all the cloned qsdA alleles, including qsdAW2 as well as qsdA420, qsdASQ1, qsdA3008, qsdAMP50, qsdAMic1 and qsdA3042, to interfere with the expression of QS-regulated functions, using three different quenching assays in which qsdA is expressed in heterologous systems.
All alleles conferred upon their host the ability to inactivate the same range of N-AHSLs as the wild-type strain W2 (i.e., N-AHSLs with or without substitution on carbon 3 and with an acyl chain ranging from 6 to 14 carbons [data not shown]), regardless of the plasmid vector used to express the gene. They were able to quench the synthesis of violacein by C. violaceum CV026 grown in the presence of C6-HSL with the same efficiency as the wild-type R. erythropolis strain W2 (data not shown).
When expressed in P. fluorescens strain 1855-344, qsdAW2 conferred quenching capabilities closely matching that of the wild-type R. erythropolis strain W2 (Table 2). Strains 1855-344(pSU40) and 1855-344(pME6032) were inoculated independently at various ratios with the potato soft rot pathogen P. carotovorum strain PCC797 on potato tubers. In the absence of the quencher, the maceration zones averaged 2.8 cm on the potato tubers. Conversely, in the presence of the quenching strain expressing qsdAW2, a clear reduction of symptom severity was visible, since no maceration occurred at quencher/pathogen ratios of 10:1 and 1:1 whatever the concentration of pathogen used for the assay (105 or 106 cells ml−1).
TABLE 2.
QQ capabilities of P. fluorescens strain 1855-344 harboring qsdA
| P. carotovorum PCC 797 concn (cells ml−1)a | Pathogen/quencher ratio | Avg extent of maceration zone (cm) ± SE with P. fluorescens 1855-344 carryingb:
|
|
|---|---|---|---|
| pME6032 | pSU40 | ||
| 105 | 1:10 | 2.6 ± 0.5 | NM |
| 1:1 | 2.8 ± 0.5 | NM | |
| 10:1 | 2.5 ± 0.6 | 2.2 ± 0.8 | |
| 106 | 1:10 | 2.8 ± 0.4 | NM |
| 1:1 | 2.8 ± 0.5 | NM | |
| 10:1 | 2.8 ± 0.5 | 2.8 ± 0.8 | |
Concentration of the P. carotovorum cell suspension used for inoculation of potato tubers.
Taken from three repeats. NM, no maceration observed.
When expressed in the Agrobacterium tumefaciens conjugal transfer constitutive strain 15955trac, qsdAW2 prevented the accumulation in the medium of the N-AHSLs normally produced by this strain (data not shown). In addition, the qsdAW2 gene conferred upon this strain the ability to degrade N-AHSLs provided exogenously (data not shown). Furthermore, the expression of the qsdAW2 gene totally abolished Ti plasmid conjugal transfer by this strain. Indeed, 15955trac(pME6032) transferred its Ti plasmid to the recipient strain C58C1RS at high frequency (1.7 10−2 transconjugants per donor cells), while 15955trac(pSU40) no longer conjugated its Ti plasmid to detectable levels; i.e., transfer frequencies were below the detection level of 5 × 10−8 transconjugants per donor cell. Therefore, the qsdA-based N-AHSL-turnover was sufficient to prevent the accumulation of the typical A. tumefaciens QS signal molecules in the growth medium and the QS regulation of Ti plasmid conjugal transfer.
Due to the worldwide distribution of this genus in the soil and to the marked quenching abilities of the wild-type strains and cloned qsdA genes, Rhodococcus isolates and derivatives harboring this gene could become interesting natural biocontrol agents directed toward QS-regulated traits in plant pathogens.
Role of qsdA in Rhodococcus.
The above observations raise questions regarding the role of qsdA in Rhodococcus. The analysis of the neighboring sequences (encoding an acyl coenzyme A synthetase and a FadR peptide analogous to a fatty acid biosynthesis regulatory protein) suggests a possible involvement in fatty acid metabolism. The observation that even closely related clones, such as R. erythropolis strain DCL14, or species, such as R. opacus and R. fascians, do not possess the qsdA gene or a N-AHSLase activity supports the view that the function encoded by qsdA may be either nonessential or redundant. This view is also supported by the observation that a W2 qsdA mutant (harboring a disrupted qsdA gene) has growth properties and N-AHSL degradation ability similar to those of the wild-type parent (data not shown). In such a light, the hypothesis suggested by Kaufmann et al. (17) that 3-oxo-dodecanoyl-HSL and spontaneous reorganization products could play a role as antibiotics targeting specifically gram-positive bacteria is a very tempting alternative explanation accounting for the presence of qsdA within the Rhodococcus genus. Strain W2 and related N-AHSL degraders therefore appear very well equipped to resist N-AHSLs produced by gram-negative soil competitors, with a complete degradative arsenal composed of at least two N-AHSL degradation activities, including a lactonase and an amidohydrolase, and an additional N-AHSL modification activity (29, 43). The putative toxicity of N-AHSLs on Rhodococcus remains to be demonstrated, since it has not been observed in preliminary experiments performed with the wild-type strains used in the present work.
Supplementary Material
Acknowledgments
This work was made possible by grants from the European Union 5th Framework Program EcoSafe (QLK3-2000-01759) to Y.D., from the French Ministère de la Recherche et de la Technologie GEOMEX program to P.M.O., and from the French Ministère de la Recherche et de la Technologie to S.U., all of which are gratefully acknowledged.
We thank Claudine Elmerich and Carmela Giglionne (CNRS, Gif-sur-Yvette), Annie Chaboud (IBCP-Lyon), Mohammed Bendahmane (ENS-Lyon), Robert van der Geize (University of Groningen), and Paul Williams (University of Nottingham) for helpful discussions. We especially acknowledge all the colleagues who provided the strains tested for N-AHSL degradation in this study.
Footnotes
Published ahead of print on 11 January 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Bigey, F., G. Janbon, A. Arnaud, and P. Galzy. 1995. Sizing of the Rhodococcus sp. R312 genome by pulsed-field gel electrophoresis. Localization of genes involved in nitrile degradation. Antonie Leeuwenhoek 68:173-179. [DOI] [PubMed] [Google Scholar]
- 2.Chun, K. C., E. A. Ozer, M. J. Welsh, J. Zabner, and E. P. Greenberg. 2004. Inactivation of a Pseudomonas aeruginosa quorum-sensing signal by human airway epithelia. Proc. Natl. Acad. Sci. USA 101:3587-3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crespi, M., D. Vereecke, W. Temmerman, M. Van Montagu, and J. Desomer. 1994. The fas operon of Rhodococcus fascians encodes new genes required for efficient fasciation of host plants. J. Bacteriol. 176:2492-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Angelo-Picard, C., D. Faure, I. Penot, and Y. Dessaux. 2005. Diversity of N-acyl homoserine lactone-producing and -degrading bacteria in soil and tobacco rhizosphere. Environ. Microbiol. 7:1796-1808. [DOI] [PubMed] [Google Scholar]
- 5.Delalande, L., D. Faure, A. Raffoux, S. Uroz, C. D'Angelo-Picard, M. Elasri, A. Carlier, R. Berruyer, A. Petit, P. Williams, and Y. Dessaux. 2005. Plant-, temperature- and pH-dependent stability of hexanoyl-homoserine lactone, a mediator of quorum-sensing regulation. FEMS Microbiol. Ecol. 52:13-20. [DOI] [PubMed] [Google Scholar]
- 6.Dong, Y. H., J. L. Xu, X. Z. Li, and L. H. Zhang. 2000. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc. Natl. Acad. Sci. USA 97:3526-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eulberg, D., L. A. Golovleva, and M. Schlomann. 1997. Characterization of catechol catabolic genes from Rhodococcus erythropolis 1CP. J. Bacteriol. 179:370-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fray, R. G. 2002. Altering plant-microbe interaction through artificially manipulating bacterial quorum sensing. Ann. Bot. (London) 89:245-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuqua, C., M. R. Parsek, and E. P. Greenberg. 2001. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 35:439-468. [DOI] [PubMed] [Google Scholar]
- 10.Givskov, M., R. de Nys, M. Manefield, L. Gram, R. Maximilien, L. Eberl, S. Molin, P. D. Steinberg, and S. Kjelleberg. 1996. Eukaryotic interference with homoserine lactone-mediated prokaryotic signalling. J. Bacteriol. 178:6618-6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayman, G. T., and S. K. Farrand. 1988. Characterization and mapping of the agrocinopine-agrocin 84 locus on the nopaline Ti plasmid pTiC58. J. Bacteriol. 170:1759-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heeb, S., Y. Itoh, T. Nishijyo, U. Schnider, C. Keel, J. Wade, U. Walsh, F. O'Gara, and D. Haas. 2000. Small, stable shuttle vectors based on the minimal pVS1 replicon for use in gram-negative, plant-associated bacteria. Mol. Plant-Microbe Interact. 13:232-237. [DOI] [PubMed] [Google Scholar]
- 13.Hong, S. B., and F. M. Raushel. 1996. Metal-substrate interactions facilitate the catalytic activity of the bacterial phosphotriesterase. Biochemistry 35:10904-10912. [DOI] [PubMed] [Google Scholar]
- 14.Huang, J. J., J. Han, L. Zhang, and J. R. Leadbetter. 2003. Utilization of acyl-homoserine lactone quorum signals for growth by a soil pseudomonad and Pseudomonas aeruginosa PAO1. Appl. Environ. Microbiol. 69:5941-5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones, S., B. Yu, N. J. Bainton, M. Birdsall, B. W. Bycroft, S. R. Chhabra, A. J. Cox, P. Golby, P. J. Reeves, S. Stephens, et al. 1993. The lux autoinducer regulates the production of exoenzyme virulence determinants in Erwinia carotovora and Pseudomonas aeruginosa. EMBO J. 12:2477-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang, B. R., J. H. Lee, S. J. Ko, Y. H. Lee, J. S. Cha, B. H. Cho, and Y. C. Kim. 2004. Degradation of acyl-homoserine lactone molecules by Acinetobacter sp. strain C1010. Can. J. Microbiol. 50:935-941. [DOI] [PubMed] [Google Scholar]
- 17.Kaufmann, G. F., R. Sartorio, S.-H. Lee, C. J. Rogers, M. M. Meijler, J. A. Moss, B. Clapham, A. P. Brogan, T. J. Dickerson, and K. D. Janda. 2005. Revisiting quorum sensing: discovery of additional chemical and biological functions for 3-oxo-N-acylhomoserine lactones. Proc. Natl. Acad. Sci. USA 102:309-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leadbetter, J. R., and E. P. Greenberg. 2000. Metabolism of acyl-homoserine lactone quorum-sensing signals by Variovorax paradoxus. J. Bacteriol. 182:6921-6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, S. J., S. Y. Park, J. J. Lee, D. Y. Yum, B. T. Koo, and J. K. Lee. 2002. Genes encoding the N-acyl homoserine lactone-degrading enzyme are widespread in many subspecies of Bacillus thuringiensis. Appl. Environ. Microbiol. 68:3919-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin, Y. H., J. L. Xu, J. Hu, L. H. Wang, S. L. Ong, J. R. Leadbetter, and L. H. Zhang. 2003. Acyl-homoserine lactone acylase from Ralstonia strain XJ12B represents a novel and potent class of quorum-quenching enzymes. Mol. Microbiol. 47:849-860. [DOI] [PubMed] [Google Scholar]
- 21.Luo, Z. Q., S. Su, and S. K. Farrand. 2003. In situ activation of the quorum-sensing transcription factor TraR by cognate and noncognate acyl-homoserine lactone ligands: kinetics and consequences. J. Bacteriol. 185:5665-5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McClean, K. H., M. K. Winson, L. Fish, A. Taylor, S. R. Chhabra, M. Camara, M. Daykin, J. H. Lamb, S. Swift, B. W. Bycroft, G. S. Stewart, and P. Williams. 1997. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143:3703-3711. [DOI] [PubMed] [Google Scholar]
- 23.Molina, L., F. Constantinescu, L. Michel, C. Reimmann, B. Duly, and G. Défago. 2003. Degradation of pathogen quorum-sensing molecules by soil bacteria: a preventive and curative biological control mechanism. FEMS Microbiol. Ecol. 45:71-81. [DOI] [PubMed] [Google Scholar]
- 24.Mulbry, W. W., and J. S. Karns. 1989. Parathion hydrolase specified by the Flavobacterium opd gene: relationship between the gene and protein. J. Bacteriol. 171:6740-6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nga, D. P., J. Altenbuchner, and G. S. Heiss. 2004. NpdR, a repressor involved in 2,4,6-trinitrophenol degradation in Rhodococcus opacus HL PM-1. J. Bacteriol. 186:98-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oger, P., K. S. Kim, R. L. Sackett, K. R. Piper, and S. K. Farrand. 1998. Octopine-type Ti plasmids code for a mannopine-inducible dominant-negative allele of traR, the quorum-sensing activator that regulates Ti plasmid conjugal transfer. Mol. Microbiol. 27:277-288. [DOI] [PubMed] [Google Scholar]
- 27.Oger, P. M., H. Mansouri, X. Nesme, and Y. Dessaux. 2004. Engineering root exudation of Lotus toward the production of two novel carbon compounds leads to the selection of distinct microbial populations in the rhizosphere. Microb. Ecol. 47:96-103. [DOI] [PubMed] [Google Scholar]
- 28.Ortori, C. A., S. Atkinson, S. R. Chhabra, M. Camara, P. Williams, and D. A. Barrett. 2007. Comprehensive profiling of N-acylhomoserine lactones produced by Yersinia pseudotuberculosis using liquid chromatography coupled to hybrid quadrupole-linear ion trap mass spectrometry. Anal. Bioanal. Chem. 387:497-511. [DOI] [PubMed] [Google Scholar]
- 29.Park, S. Y., B. J. Hwang, M. H. Shin, J. A. Kim, H. K. Kim, and J. K. Lee. 2006. N-acyl homoserine lactone producing Rhodococcus spp. with different AHL-degrading activities. FEMS Microbiol. Letters 261:102-108. [DOI] [PubMed] [Google Scholar]
- 30.Park, S. Y., H. O. Kang, H. S. Jang, J. K. Lee, B. T. Koo, and D. Y. Yum. 2005. Identification of extracellular N-acylhomoserine lactone acylase from a Streptomyces sp. and its application to quorum quenching. Appl. Environ. Microbiol. 71:2632-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park, S. Y., S. J. Lee, T. K. Oh, J. W. Oh, B. T. Koo, D. Y. Yum, and J. K. Lee. 2003. AhlD, an N-acylhomoserine lactonase in Arthrobacter sp., and predicted homologues in other bacteria. Microbiology 149:1541-1550. [DOI] [PubMed] [Google Scholar]
- 32.Piper, K. R., S. Beck von Bodman, and S. K. Farrand. 1993. Conjugation factor of Agrobacterium tumefaciens regulates Ti plasmid transfer by autoinduction. Nature 362:448-450. [DOI] [PubMed] [Google Scholar]
- 33.Powell, J. A., and J. A. Archer. 1998. Molecular characterisation of a Rhodococcus ohp operon. Antonie Leeuwenhoek 74:175-188. [DOI] [PubMed] [Google Scholar]
- 34.Rasmussen, T. B., and M. Givskov. 2006. Quorum sensing inhibitors: a bargain of effects. Microbiology 152:895-904. [DOI] [PubMed] [Google Scholar]
- 35.Reimmann, C., N. Ginet, L. Michel, C. Keel, P. Michaux, V. Krishnapillai, M. Zala, K. Heurlier, K. Triandafillu, H. Harms, G. Defago, and D. Haas. 2002. Genetically programmed autoinducer destruction reduces virulence gene expression and swarming motility in Pseudomonas aeruginosa PAO1. Microbiology 148:923-932. [DOI] [PubMed] [Google Scholar]
- 36.Roodveldt, C., and D. S. Tawfik. 2005. Shared promiscuous activities and evolutionary features in various members of the amidohydrolase superfamily. Biochemistry 44:12728-12736. [DOI] [PubMed] [Google Scholar]
- 37.Shaw, P. D., G. Ping, S. L. Daly, C. Cha, J. E. Cronan, Jr., K. L. Rinehart, and S. K. Farrand. 1997. Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc. Natl. Acad. Sci. USA 94:6036-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simic, P., H. Sahm, and L. Eggeling. 2001. l-Threonine export: use of peptides to identify a new translocator from Corynebacterium glutamicum. J. Bacteriol. 183:5317-5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takeshima, H., J. Inokoshi, Y. Takada, H. Tanaka, and S. Omura. 1989. A deacylation enzyme for aculeacin A, a neutral lipopeptide antibiotic, from Actinoplanes utahensis: purification and characterization. J. Biochem. (Tokyo) 105:606-610. [DOI] [PubMed] [Google Scholar]
- 40.Tichi, M. A., and F. R. Tabita. 2002. Metabolic signals that lead to control of cbb gene expression in Rhodobacter capsulatus. J. Bacteriol. 184:1905-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trott, S., S. Burger, C. Calaminus, and A. Stolz. 2002. Cloning and heterologous expression of an enantioselective amidase from Rhodococcus erythropolis strain MP50. Appl. Environ. Microbiol. 68:3279-3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ulrich, R. L. 2004. Quorum quenching: enzymatic disruption of N-acylhomoserine lactone-mediated bacterial communication in Burkholderia thailandensis. Appl. Environ. Microbiol. 70:6173-6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uroz, S., S. R. Chhabra, M. Càmara, P. Wiliams, P. M. Oger, and Y. Dessaux. 2005. N-Acylhomoserine lactone quorum-sensing molecules are modified and degraded by Rhodococcus erythropolis W2 by both amidolytic and novel oxidoreductase activities. Microbiology 151:3313-3322. [DOI] [PubMed] [Google Scholar]
- 44.Uroz, S., C. D'Angelo-Picard, A. Carlier, M. Elasri, C. Sicot, A. Petit, P. Oger, D. Faure, and Y. Dessaux. 2003. Novel bacteria degrading N-acylhomoserine lactones and their use as quenchers of quorum-sensing-regulated functions of plant-pathogenic bacteria. Microbiology 149:1981-1989. [DOI] [PubMed] [Google Scholar]
- 45.Uroz, S., P. Oger, S. R. Chhabra, M. Camara, P. Williams, and Y. Dessaux. 2007. N-acyl homoserine lactones are degraded via an amidolytic activity in Comamonas sp strain D1. Arch. Microbiol. 187:249-256. [DOI] [PubMed] [Google Scholar]
- 46.Van der Werf, M. J., H. J. Swarts, and J. A. de Bont. 1999. Rhodococcus erythropolis DCL14 contains a novel degradation pathway for limonene. Appl. Environ. Microbiol. 65:2092-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Warwick, S., T. Bowen, H. McVeigh, and T. M. Embley. 1994. A phylogenetic analysis of the family Pseudonocardiaceae and the genera Actinokineospora and Saccharothrix with 16S rRNA sequences and a proposal to combine the genera Amycolata and Pseudonocardia in an emended genus Pseudonocardia. Int. J. Syst. Bacteriol. 44:293-299. [DOI] [PubMed] [Google Scholar]
- 48.Yates, E. A., B. Philipp, C. Buckley, S. Atkinson, S. R. Chhabra, R. E. Sockett, M. Goldner, Y. Dessaux, M. Camara, H. Smith, and P. Williams. 2002. N-Acylhomoserine lactones undergo lactonolysis in a pH-, temperature-, and acyl chain length-dependent manner during growth of Yersinia pseudotuberculosis and Pseudomonas aeruginosa. Infect. Immun. 70:5635-5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang, H. B., L. H. Wang, and L. H. Zhang. 2002. Genetic control of quorum-sensing signal turnover in Agrobacterium tumefaciens. Proc. Natl. Acad. Sci. USA 99:4638-4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang, L., P. J. Murphy, A. Kerr, and M. E. Tate. 1993. Agrobacterium conjugation and gene regulation by N-acyl-L-homoserine lactones. Nature 362:446-448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.