Abstract
We investigated the bacterial communities of nine Bartonella-positive fleas (n = 6 Oropsylla hirsuta fleas and n = 3 Oropsylla montana fleas), using universal primers, clone libraries, and DNA sequencing. DNA sequences were used to classify bacteria detected in a phylogenetic context, to explore community assembly patterns within individual fleas, and to survey diversity patterns in dominant lineages.
Animals harbor microbial communities that play integral roles in organismal function; for example, aphids depend on intracellular endosymbionts for essential nutrients (20); termites harbor protists and endosymbionts to convert cellulose into palatable nutrients (18, 21); marine bivalves harbor endosymbionts in their gill tissue, and these organisms provision energy for the host (10); and photosynthetic microbial eukaryotes inhabiting corals account for the high rate of primary productivity and explain the existence of reefs (9).
Fleas, like other animals, harbor a bacterial community. Previous molecular surveys of flea-related bacteria have been screened for the presence or absence of specific pathogenic lineages (4, 12, 14, 17, 25, 26). However, within an individual flea, pathogens are members of bacterial communities, and they may interact with coexisting bacteria. Bacterial communities in other hematophagous invertebrates including ticks, mosquitoes, lice, and leeches have been targeted by using a clone library approach (1, 13, 24, 28).
Here, we used clone libraries and 16S rRNA gene sequence data to characterize bacterial communities in two flea species, Oropsylla hirsuta, collected from three black-tailed prairie dogs, Cynomys ludovicianus (from the family Sciuridae), and Oropsylla montana, collected from one rock squirrel, Spermophilus variegatus (also from the Sciuridae family). Prairie dogs occur in the plains, and rock squirrels occur in the foothills of the Rocky Mountains; we collected fleas in Boulder County, CO, where these two ecosystems converge. O. hirsuta bacteria were screened for the presence of Bartonella strains before selection for this study at the Centers for Disease Control and Prevention in Fort Collins, CO, by using Bartonella-specific primers to amplify the gltA gene (26). Fleas were kept at −20°C until they were identified based on morphology (3, 5).
We cleaned the fleas to minimize the contribution of ephemeral bacteria on external flea parts. Fleas were washed twice (0.133 M NaCl, 1.11% sodium dodecyl sulfate, 0.0088 M EDTA), treated with a lysozyme (11.6 mg/liter, for 30 min, at 37°C), and washed again (three washes in total). Before DNA was extracted (Qiagen DNeasy tissue kit) from the cleaned fleas, they were crushed and subjected to a lysozyme treatment (2 mg/ml, for 30 min, at 37°C) to lyse spore-forming bacteria.
16S PCR products were generated using the universal primers 27f (5′AGAGTTTGATCCTGGCTCAG) and 1492r (5′GGTTACCTTGTTACGACTT) (11). Purified PCR products were cloned using a pGem-T cloning kit (Promega). Cells were shipped to Functional Biosciences, Inc. (Madison, WI), for two-pass sequencing. Forward and reverse sequencing readings were assembled using Sequencher version 4.6 software (Gene Codes Corp.). We obtained 305 16S rRNA gene sequences (GenBank accession no. EU137334 to EU137638). Sequences were aligned by using the Greengenes NAST aligner (2) imported into ARB (15) and corrected by hand. Aligned sequences were checked for chimeras using Bellerophon software (6). Sequences were classified according to Greengenes phylogeny as implemented in ARB (15). Ten “families” of proteobacteria represent greater than 90% of the total discovered diversity (Table 1).
TABLE 1.
Classification of 305 16S rRNA gene sequences from O. hirsuta and O. montana
| Bacteria | No. of gene sequences (no. of fleas harboring the sequence) harbored bya:
|
|
|---|---|---|
| O. hirsuta | O. montana | |
| Acinetobacter | ||
| Propionibacterineae | ||
| Propionibacteriaceae | 2 (2) | 0 |
| Bacteroidetes | ||
| Bacteroidales | ||
| Porphyromonadaceae | 2 (1) | 0 |
| Flavobacteriales | ||
| Flavobacteriaceae | 3 (2) | 0 |
| Firmicutes | ||
| Bacilli | ||
| Lactobacillales | 10 (2) | 0 |
| Staphylococcaceae | 2 (2) | 0 |
| Lachnospiraceae | ||
| Anaerofilum | 1 (1) | 0 |
| Ruminococcus | 1 (1) | 0 |
| Sporobacter | 1 (1) | 0 |
| Unclassified | 1 (1) | 0 |
| Fusobacteria | ||
| Unclassified | 6 (1) | 0 |
| Gemmatimonadetes | ||
| Gemmatimonadales | 1 (1) | 0 |
| Proteobacteria | ||
| Alphaproteobacteria | 172 (6) | 61 (3) |
| Gammaproteobacteria, Betaproteobacteria | 39 (5) | 3 (2) |
| Total | 241 (6) | 64 (3) |
Numbers in parentheses indicate the number of fleas in which a particular lineage was detected. A more detailed presentation of proteobacterial groups is presented in Fig. 1.
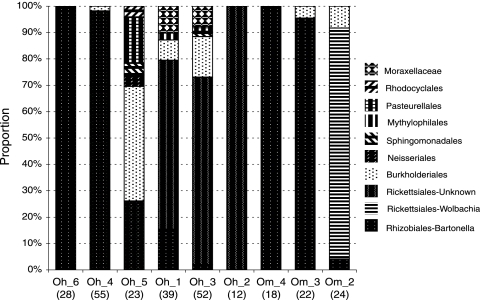
Bacterial diversity is not equally represented among individual fleas; one lineage (e.g., from Bartonella or Rickettsiales) typically dominates community membership (Fig. 1). This pattern has been found in symbiotic communities of other invertebrates. In individual wood-boring bivalves, multiple lineages often coexist, but one lineage typically dominates the symbiotic community (16). Competition among community members, precedence effects related to the timing of colonization, and physiochemical differences among hosts were suggested as possible explanations for these dynamics of dominance. Within fleas, another potential explanation is that bacterial lineages may respond differently to pulses of mammalian blood. Abundance data for lineages within a large number of fleas or laboratory studies are needed to further investigate community assembly patterns.
FIG. 1.
The proportions of 10 proteobacterial “families” within individual fleas are depicted. Samples are arranged based on Bartonella abundance data. Individual fleas tend to be dominated by one bacterial lineage. Numbers in parentheses indicate the total number of proteobacterial DNA sequences obtained from each flea. Fleas were collected from three different prairie dogs (prairie dog 1 harbored O. hirsuta fleas 1 to 3 [Oh_1 to Oh_3]; prairie dog 2, Oh_4; prairie dog 3, Oh_5 to Oh_6).
For dominant lineages (e.g., Bartonella and Rickettsiales), we used ModelGenerator (7) to determine the best model of evolution and MultiPhyl (8) to construct phylogenetic trees (Fig. 2 and 3). Prior to generating the Rickettsiales tree, we removed the most variable nucleotide positions using the LANE mask implemented in ARB.
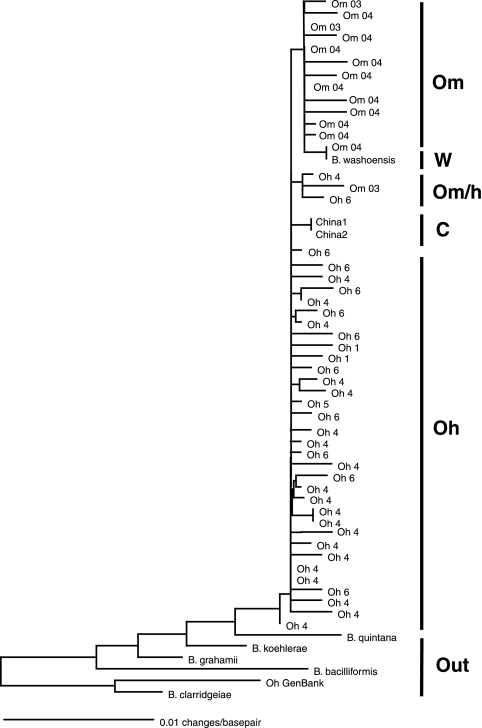
FIG. 2.
Maximum likelihood (GTR plus G correction model) tree of Bartonella from O. hirsuta (Oh) and O. montana (Om), with a number of sequences from GenBank. The clade with both O. hirsuta and O. montana is indicated by Om/h. Bartonella strains obtained from GenBank in the tree include B. washoensis (w; accession no. AF070463), two Bartonella sequences from Spermophilus sp. in China (C; accession no. DQ641913 and DQ641912), B. quintana (accession no. M73228), B. koehlerae (accession no. AF076237), B. grahamii (accession no. Z31349), B. bacilliformis (accession no. M65249), B. clarridgeiae (accession no. X97822), and a Bartonella sequence from another O. hirsuta (accession no. DQ473482). The tree was constructed using all of the Bartonella sequences detected, but some were removed to minimize the figure's size.
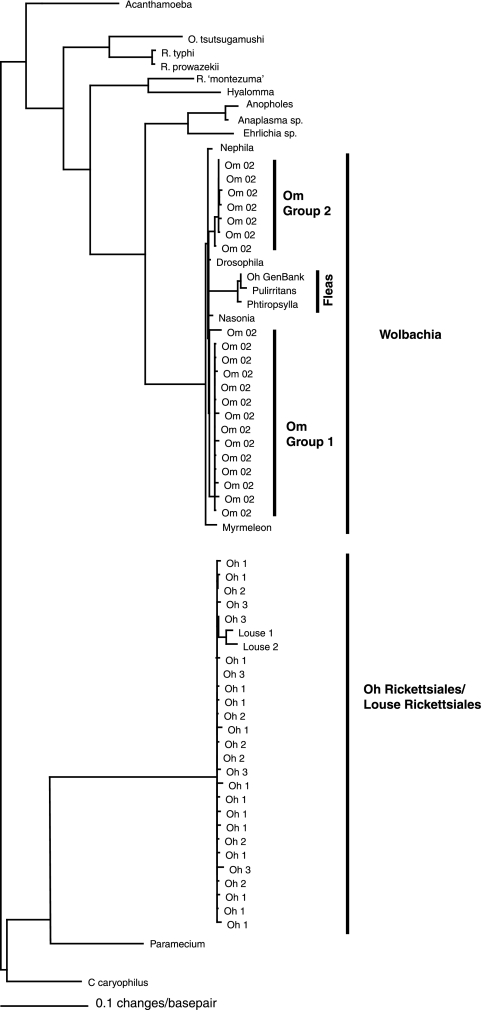
FIG. 3.
Maximum likelihood (GTR plus G correction model) tree of Rickettsiales detected in O. hirsuta (Oh) and O. montana (Om). Except for R. typhi, R. prowazekii, Rickettsiales bacterium “Montezuma” (R. ‘montezuma’), Orientalis tsutsugamushi, Anaplasma sp., Ehrlichia sp., and C. caryophilus, the taxon names indicate the genus of the host in which the bacteria were found (e.g., Wolbachia bacteria were found in a Nasonia parasitoid wasp host). Sequences from GenBank in the tree include Caedibacter caryophilus (accession no. AY753195), O. tsutsugamushi (accession no. U17258), Rickettsia typhi (accession no. AE017197), R. prowazekii (accession no. M21789), Rickettsiales bacterium “Montezuma” (accession no. AF493952), Anaplasma sp. (accession no. AY527214), Ehrlichia sp. (accession no. DQ324367), Hyalomma (accession no. AM181356), Acanthamoeba (accession no. AF132137), Anopheles (accession no. AY837738), Oh_GenBank (accession no. AY335925), Phtiropsylla (accession no. AY335932), Pulirritans (accession no. AY335926), Nephila (accession no. AF232234), Drosophila (accession no. AY833061), Nasonia (acces sion no. M84688), Myrmeleon (accession no. DQ068803), Paramecium (accession no. X58198), Louse 1 (accession no. AF467369), and Louse 2 (accession no. AF467370). The tree was constructed using all of the Rickettsiales sequences detected, but some were removed to minimize the figure's size.
In the Bartonella phylogenetic tree, the Bartonella strain from an O. hirsuta flea is in a clade with a strain isolated from a Spermophilus squirrel in China, and the Bartonella strain from O. montana is in a clade with B. washoensis (with the exception of one sequence that clustered with the O. hirsuta strains) (Fig. 2). Interestingly, the Bartonella strain from an O. hirsuta flea in GenBank (accession no. DQ473482) clusters with Bartonella clarridgeiae; this suggests that specific strains of Bartonella have not coevolved with specific flea species. Our finding that the Bartonella strain in Boulder, CO, is closely related to a strain detected in China suggests that Bartonella dispersed widely and frequently.
The Rickettsiales phylogenetic tree shows that the Rickettsiales strains detected in this study are unique relative to those of the known diversity (Fig. 3). The Rickettsiales sequences detected in O. hirsuta are most closely related to a sequence detected in the bacteria of a pocket gopher louse (95% sequence similarity) but are not closely related to any other known diversity. Both of the Rickettsiales lineages detected in O. montana are related to those of Wolbachia, but they are more closely related to the Wolbachia lineage detected in flies than to that of Wolbachia from fleas.
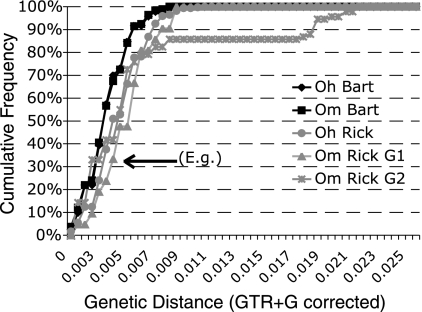
Strains of Rickettsiales and Bartonella have different transmission strategies that may affect the accumulation of genetic diversity within a lineage. Bartonella bacteria are horizontally transmitted, and fleas acquire Bartonella lineages through the environment, from host blood, flea feces, etc. Rickettsial lineages, on the other hand, have either a strictly vertical transmission strategy or a mixture of vertical and horizontal transmission strategies (23). We investigated diversity patterns using PAUP version 4.0b10-Altivec (Sinauer Associates) to generate pairwise distances (general time reversible [GTR] plus gamma correction) between all members of each Bartonella and Rickettsiales lineage. We used full sequences for these comparisons (i.e., no LANE mask). A cumulative frequency distribution of pairwise distances depicts patterns of genetic diversity within a lineage (Fig. 4). The average genetic similarity is greater within the Bartonella lineages than within the Rickettsiales lineages. Vertically transmitted bacterial lineages have been found to have a higher rate of evolution (19, 22, 27), and this may explain the higher diversity found in Rickettsiales.
FIG. 4.
Cumulative frequency distributions of pairwise genetic distances (GTR plus G corrected) for Bartonella and Rickettsiales strains from each flea species show that Rickettsiales lineages harbor more genetic diversity than Bartonella lineages. As genetic diversity within a lineage increases, the frequency distribution shifts to the right. Distributions of Bartonella genetic distances are presented as black lines, and Rickettsiales distances are presented as gray lines. Distributions for the two Bartonella lineages almost completely overlap and fall to the left of those for the Rickettsiales lineages. As an example of how to interpret this figure, the data point marked by the arrow indicates that 33.33% of the O. montana (Om) Rickettsiales (Rick) group 1 (G1) shares 99.65% or more sequence similarity.
Acknowledgments
We thank the following funding sources: Department of Ecology and Evolutionary Biology of the University of Colorado—Boulder, the William H. Burt Fund of the University of Colorado Museum, the Beverly Sears Student Award of the University of Colorado, the Colorado Mountain Club, and NSF.
We also thank Boulder City OSMP and Boulder County Open Space for access to land and animals. We also thank Amelia Markeson, Jory Brinkerhoff, and Adam Mitchell for help with collecting and identifying fleas.
Footnotes
Published ahead of print on 18 January 2008.
REFERENCES
- 1.Benson, M. J., J. D. Gawronski, D. E. Eveleigh, and D. R. Benson. 2004. Intracellular symbionts and other bacteria associated with deer ticks (Ixodes scapularis) from Nantucket and Wellfleet, Cape Cod, Massachusetts. Appl. Environ. Microbiol. 70:616-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeSantis, T. Z., P. Hugenholtz, K. Keller, E. L. Brodie, N. Larsen, Y. M. Piceno, R. Phan, and G. L. Andersen. 2006. NAST: a multiple sequence alignment server for comparative analysis of 16S rRNA genes. Nucleic Acids Res. 34:W394-W399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furman, D. P. a. E. P. C. 1982. Order Siphonoptera, p. 207. In Manual of medical entomology. Cambridge University Press, Cambridge, United Kingdom.
- 4.Gorham, C. H., Q. Q. Fang, and L. A. Durden. 2003. Wolbachia endosymbionts in fleas (Siphonaptera). J. Parasitol. 89:283-289. [DOI] [PubMed] [Google Scholar]
- 5.Hubbard, C. A. 1947. Fleas of western North America. Hafner Publishing Company, New York, NY.
- 6.Huber, T., G. Faulkner, and P. Hugenholtz. 2004. Bellerophon: a program to detect chimeric sequences in multiple sequence alignments. Bioinformatics 20:2317-2319. [DOI] [PubMed] [Google Scholar]
- 7.Keane, T. M., C. J. Creevey, M. M. Pentony, T. J. Naughton, and J. O. McLnerney. 2006. Assessment of methods for amino acid matrix selection and their use on empirical data shows that ad hoc assumptions for choice of matrix are not justified. BMC Evol. Biol. 6:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keane, T. M., Naughton, T. J., and J. O. McInerney. 2007. MultiPhyl: a high-throughput phylogenomics webserver using distributed computing. Nucleic Acids Res. 35:W33-W37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knowlton, N., and F. Rohwer. 2003. Multispecies microbial mutualisms on coral reefs: the host as a habitat. Am. Nat. 162:S51-S62. [DOI] [PubMed] [Google Scholar]
- 10.Krueger, D. M., and C. M. Cavanaugh. 1997. Phylogenetic diversity of bacterial symbionts of Solemya hosts based on comparative sequence analysis of 16S rRNA genes. Appl. Environ. Microbiol. 63:91-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lane, D. J. 1991. 16S/23S rRNA sequencing, p. 115-175. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley and Sons, West Sussex, England.
- 12.Lappin, M. R., B. Griffin, J. Brunt, A. Riley, D. Burney, J. Hawley, M. M. Brewer, and W. A. Jensen. 2006. Prevalence of Bartonella species, haemoplasma species, Ehrlichia species, Anaplasma phagocytophilum, and Neorickettsia risticii DNA in the blood of cats and their fleas in the United States. J. Feline Med. Surg. 8:85-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindh, J. M., O. Terenius, and I. Faye. 2005. 16S rRNA gene-based identification of midgut bacteria from field-caught Anopheles gambiae sensu lato and A. funestus mosquitoes reveals new species related to known insect symbionts. Appl. Environ. Microbiol. 71:7217-7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loftis, A. D., W. K. Reeves, D. E. Szumlas, M. M. Abbassy, I. M. Helmy, J. R. Mortarity, and G. A. Dasch. 2006. Surveillance of Egyptian fleas for agents of public health significance: Anaplasma, Bartonella, Coxiella, Ehrlichia, Rickettsia, and Yersinia pestis. Am J. Trop. Med. Hyg. 75:41-48. [PubMed] [Google Scholar]
- 15.Ludwig, W., O. Strunk, R. Westram, L. Richter, H. Meier, Yadhukumar, A. Buchner, T. Lai, S. Steppi, G. Jobb, W. Forster, I. Brettske, S. Gerber, A. W. Ginhart, O. Gross, S. Grumann, S. Hermann, R. Jost, A. Konig, T. Liss, R. Lussmann, M. May, B. Nonhoff, B. Reichel, R. Strehlow, A. Stamatakis, N. Stuckmann, A. Vilbig, M. Lenke, T. Ludwig, A. Bode, and K. H. Schleifer. 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32:1363-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luyten, Y. A., J. R. Thompson, W. Morrill, M. F. Polz, and D. L. Distel. 2006. Extensive variation in intracellular symbiont community composition among members of a single population of the wood-boring bivalve Lyrodus pedicellatus (Bivalvia: Teredinidae). Appl. Environ. Microbiol. 72:412-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marie, J. L., P. E. Fournier, J. M. Rolain, S. Briolant, B. Davoust, and D. Raoult. 2006. Molecular detection of Bartonella quintana, B. elizabethae, B. koehlerae, B. doshiae, B. taylorii, and Rickettsia felis in rodent fleas collected in Kabul, Afghanistan. Am. J. Trop. Med. Hyg. 74:436-439. [PubMed] [Google Scholar]
- 18.Martin, M. M. 1991. The evolution of cellulose digestion in insects. Philos. Trans. R. Soc. Lond. B 333:281-288. [Google Scholar]
- 19.Moran, N. A. 1996. Accelerated evolution and Muller's rachet in endosymbiotic bacteria. Proc. Natl. Acad. Sci. USA 93:2873-2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moran, N. A., G. R. Plague, J. P. Sandstrom, and J. L. Wilcox. 2003. A genomic perspective on nutrient provisioning by bacterial symbionts of insects. Proc. Natl. Acad. Sci. USA 100:14543-14548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohkuma, M. 2003. Termite symbiotic systems: efficient bio-recycling of lignocellulose. Appl. Microbiol. Biotechnol. 61:1-9. [DOI] [PubMed] [Google Scholar]
- 22.Peek, A. S., R. C. Vrijenhoek, and B. S. Gaut. 1998. Accelerated evolutionary rate in sulfur-oxidizing endosymbiotic bacteria associated with the mode of symbiont transmission. Mol. Biol. Evol. 15:1514-1523. [DOI] [PubMed] [Google Scholar]
- 23.Perlman, S. J., M. S. Hunter, and E. Zchori-Fein. 2006. The emerging diversity of Rickettsia. Proc. R. Soc. Lond. B 273:2097-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reed, D. L., and M. S. Hafner. 2002. Phylogenetic analysis of bacterial communities associated with ectoparasitic chewing lice of pocket gophers: a culture-independent approach. Microbiol. Ecol. 44:78-93. [DOI] [PubMed] [Google Scholar]
- 25.Reeves, W. K., M. P. Nelder, and J. A. Korecki. 2005. Bartonella and Rickettsia in fleas and lice from mammals in South Carolina, USA. J. Vector Ecol. 30:310-315. [PubMed] [Google Scholar]
- 26.Stevenson, H. L., Y. Bai, M. Y. Kosoy, J. A. Montenieri, J. L. Lowell, M. C. Chu, and K. L. Gage. 2003. Detection of novel Bartonella strains and Yersinia pestis in prairie dogs and their fleas (Siphonaptera: ceratophyllidae and pulicidae) using multiplex polymerase chain reaction. J. Med. Entomol. 40:329-337. [DOI] [PubMed] [Google Scholar]
- 27.Woolfit, M., and L. Bromham. 2003. Increased rates of sequence evolution in endosymbiotic bacteria and fungi with small effective population sizes. Mol. Biol. Evol. 20:1545-1555. [DOI] [PubMed] [Google Scholar]
- 28.Worthen, P. L., C. J. Gode, and J. Graf. 2006. Culture-independent characterization of the digestive-tract microbiota of the medicinal leech reveals a tripartite symbiosis. Appl. Environ. Microbiol. 72:4775-4781. [DOI] [PMC free article] [PubMed] [Google Scholar]