Abstract
The actinobacterium Kineococcus radiotolerans is highly resistant to ionizing radiation, desiccation, and oxidative stress, though the underlying biochemical mechanisms are unknown. The purpose of this study was to explore a possible linkage between the uptake of transition metals and extreme resistance to ionizing radiation and oxidative stress. The effects of six different divalent cationic metals on growth were examined in the absence of ionizing radiation. None of the metals tested were stimulatory, though cobalt was inhibitory to growth. In contrast, copper supplementation dramatically increased colony formation during chronic irradiation. K. radiotolerans exhibited specific uptake and intracellular accumulation of copper, compared to only a weak response to both iron and manganese supplementation. Copper accumulation sensitized cells to hydrogen peroxide. Acute-irradiation-induced DNA damage levels were similar in the copper-loaded culture and the age-synchronized no-copper control culture, though low-molecular-weight DNA was more persistent during postirradiation recovery in the Cu-loaded culture. Still, the estimated times for genome restoration differed by only 2 h between treatments. While we cannot discount the possibility that copper fulfills an unexpectedly important biochemical role in a low-radioactivity environment, K. radiotolerans has a high capacity for intracellular copper sequestration and presumably efficiently coordinated oxidative stress defenses and detoxification systems, which confers cross-protection from the damaging effects of ionizing radiation.
Environmental and endogenous sources of reactive oxygen species contribute to the damage of cellular components (20, 23), and a cell's ability to efficiently and effectively repair this damage is an important determinant of survival. All bacteria are equipped with defense mechanisms for coping with DNA damage and oxidative stress; however, species of the genera Deinococcus (1), Arthrobacter (19), Rubrobacter (18), Kineococcus (38), and Chroococcidiopsis (6), among others, are remarkable for their abilities to withstand and survive tremendous cellular insults. Compared to the majority of bacterial species, which generally have relatively low thresholds for stress and tolerances for cellular damage, the extreme-resistant bacteria can survive high doses of ionizing radiation, prolonged desiccation, exposure to strong oxidants, and other DNA-damaging agents. Three primary models have been proposed to explain the extreme resistance phenotype: (i) conventional enzymatic defenses operating at extraordinary efficiency, (ii) the involvement of novel repair functions, and (iii) a highly condensed, multigenomic nucleoid (5, 10, 32, 52). While no single hypothesis explains in full the underlying genetic complexity of the extreme resistance phenotype (e.g., reference 49), the preferential utilization of manganese is thus far the only biochemical strategy shown to be broadly conserved among a diverse, but not comprehensive, collection of extreme-resistant bacteria (13, 14, 22). This finding is important because manganese, unlike iron, does not catalyze hydroxyl radical formation through Fenton/Haber-Weiss chemistry and may also mitigate protein oxidation by scavenging oxygen radicals (14). Elemental ratios of Mn/Fe have been proposed as a potentially useful indicator of a cell's susceptibility to oxidative stress (13, 22). While Mn-accumulating bacteria accrue levels of DNA damage comparable to those for Fe-accumulating bacteria for a given dose of γ-radiation (13), Mn appears to quench secondary chemical reactions that produce reactive oxygen species, thus promoting the effectiveness of enzymatic repair and cell survival.
Kineococcus radiotolerans was isolated within a shielded cell work area containing highly radioactive nuclear waste at the Savannah River Site in Aiken, SC (38). K. radiotolerans is an orange-pigmented, aerobic, nonsporulating actinomycete belonging to the Kineosporiaceae family. While only three species of the genus Kineococcus have been described (30, 38, 50), each containing only a single cultivated representative, Kineococcus-like organisms have been detected on masonry and lime wall paintings (45), in terrestrial soils (39, 48), in a variety of plant samples (28), in marine sediments (30), in the McMurdo Dry Valleys of Antarctica (16), and in hot deserts (21). K. radiotolerans is exceptionally tolerant of environmental stresses, withstanding the damage caused by prolonged desiccation and γ-radiation (38). The physiological determinants and molecular mechanisms that minimize and repair cellular damage in K. radiotolerans have not been studied, and it remains unclear whether this bacterium preferentially incorporates Mn as a means of minimizing the formation of damaging oxygen radicals and speeding recovery and survival following environmental assaults. In this study, we examined K. radiotolerans for preferential utilization of different divalent cationic transition metals for a possible role in radiation resistance and antioxidative defense.
MATERIALS AND METHODS
Culture conditions and chemicals.
Kineococcus radiotolerans (BAA-149) was obtained from the American Type Culture Collection (ATCC; Manassas, VA). Cultures were grown on TGY medium (1.0% tryptone, 0.1% glucose, 0.5% yeast extract) at 28°C and shaken at 150 rpm for liquid cultures. Frozen stocks were prepared using the Microbank bacterial preservation system (Pro-Lab Diagnostics, ON, Canada) and were stored at −80°C. Solutions (50 mM) of various divalent cationic metals were prepared in deionized water and filter sterilized. The metal salts included ferrous ammonium sulfate, manganese chloride, zinc sulfate, cupric sulfate, cobalt sulfate, and sodium molybdate (Sigma-Aldrich, St. Louis, MO).
Gamma irradiation.
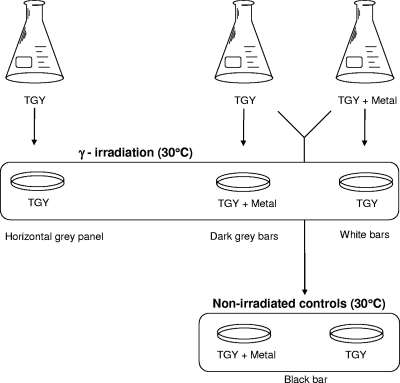
Liquid cultures were grown to mid-exponential phase in TGY medium or TGY medium spiked with 100 μM of Fe2+, Mn2+, Zn2+, Co2+, Cu2+, or Mo2+. Aliquots (25 μl) of each treatment were spread plated on TGY plating medium with or without metal supplementation (100 μM). Plates were exposed to 60 Gy/h (1 Gy = 100 rads) of ionizing radiation for 4 days at a constant temperature of 30°C, and colony outgrowth was counted at 4 days (CFU). Nonirradiated control plates were incubated in the laboratory at 30°C, and CFU were counted after 4 days. A flow diagram illustrating this experimental design is provided in Fig. 1. Our use of CFU is strictly defined by the total number of counted colonies per plate. Because of the inherent clumped growth of K. radiotolerans and the great variation in size and shape among these clumps, CFU cannot be used to reliably approximate cell numbers.
FIG. 1.
Flow diagram illustrating the experiments conducted to determine the effect of transition metals on the growth of K. radiotolerans during gamma irradiation.
K. radiotolerans cultures were exposed to acute irradiation followed by pulsed-field gel electrophoresis (PFGE) to determine the effect of copper on DNA damage and the cell's ability to repair the genome. Briefly, acute-irradiation experiments were performed by first harvesting liquid cultures (25 ml) at mid-exponential phase by centrifugation (5,000 × g, 5 min, 4°C). Cell pellets were washed in ice-cold 1× phosphate-buffered saline (PBS; pH 7.3) and suspended in 10 ml 1× PBS to stall further growth and development. Culture suspensions were irradiated at a constant temperature (30°C) to achieve a total dose of 4,000 Gy. Corresponding nonirradiated control cultures were also incubated at 30°C. After exposure, cell concentrates were diluted into fresh TGY medium (n = 3) and allowed to recover at 28°C with shaking (150 rpm) for 6 h.
DNA damage repair.
DNA damage and repair were evaluated by PFGE, using a procedure modified from Kieser et al. (26). Briefly, cell pellets (1 ml) were collected by centrifugation and suspended in 50 μl of TE-25 sucrose buffer. The cell suspensions were combined with equal volumes of molten (55°C) 4% pulsed-field certified agarose and loaded into plug molds (Bio-Rad, Hercules, CA). Solidified plugs were incubated in TE-25 buffer and lysozyme (2 mg/ml) at 37°C for 2 h and then transferred to NDS buffer with proteinase K (1 mg/ml) and incubated at 37°C overnight. Plugs were washed three times in TE (10 mM Tris-HCl [pH 7.4], 1 mM EDTA [pH 8.0]) for 1 h each and stored at 4°C in 0.5 M EDTA prior to electrophoresis. Samples were analyzed in a 1% pulsed-field certified agarose gel and 0.5× Tris-borate-EDTA running buffer by using the Chef-DR III variable-angle system (Bio-Rad) and the following electrophoresis conditions: 6 V/cm, a 120° angle, an initial switch time of 70 s, and a final switch time of 130 s for 24 h at 14°C. Gels were stained for 30 min with ethidium bromide in 0.5× Tris-borate-EDTA and documented under UV illumination by using an AlphaImager 3400 (Alpha Innotech).
Oxidative stress.
Cultures of K. radiotolerans were grown to mid-exponential phase in TGY medium amended with a divalent cationic metal (100 μM) as described above. Cells were harvested (1.0 ml) by centrifugation, washed in an equal volume of ice-cold 1× PBS (pH 7.3), and suspended in an equal volume of 4% H2O2. Cell suspensions were incubated in the dark for 10 min and then inoculated into fresh TGY medium (with no metals) and incubated at 28°C with shaking (150 rpm) for 48 h. Recovery and growth were evaluated by protein quantification using a DC protein assay kit (Bio-Rad, Hercules, CA).
Analytical methods.
Total intracellular metal contents were quantified by inductively coupled plasma-mass spectroscopy (ICP-MS). Briefly, cells were harvested by centrifugation (10,000 × g, 5 min, 15°C) and sequentially washed three times each in 50 mM EDTA-1× PBS (pH 7.5; 1× PBS = 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4) and 25 mM EDTA-1× PBS in order to remove weakly cell surface-adsorbed metal. Cells were then washed three times in PBS, immediately frozen in liquid nitrogen, and stored at −80°C. The effectiveness of the metal chelation wash steps was confirmed by spectrophotometry (7). For intracellular metal analysis, cell pellets were digested at room temperature in 0.1 ml American Chemical Society grade concentrated H2O2 and 0.2 ml concentrated optimum grade HNO3. The samples were then diluted with 18.2 Mohm/cm deionized water and analyzed in standard mode on a Perkin Elmer-Sciex Elan DRC Plus ICP-MS according to EPA method 6020a. External calibration was performed using NIST traceable standards diluted in the same matrix as the samples, and the calibration was verified against a standard with a different lot number.
Electron microscopy.
Intracellular accumulation and subcellular distribution of copper were evaluated by electron microscopy coupled with energy-dispersive X-ray spectroscopy (EDS). Briefly, K. radiotolerans was grown to mid-exponential phase in TGY medium amended with Cu2+ (100 μM), samples (1 ml) were sequentially washed in EDTA and PBS as described above, and cell pellets were fixed overnight in 2.5% cacodylate-buffered (pH 7.2) glutaraldehyde at 4°C.
For scanning electron microscopy (SEM), cell pellets were washed in 0.1 M cacodylate buffer and postfixed in 1% osmium tetroxide for 1 h at 4°C. Cells were washed in 0.1 M cacodylate buffer and dehydrated in a graded series of ethanol (50%, 70%, 80%, 90%, and 100%) for 10 min each and air dried for 2 h. Cells were mounted onto aluminum stubs, and images were collected in a FEI Quanta 200 environmental SEM operated in the environmental SEM mode. The X-ray elemental spectrum of the specimen was obtained using an EDAX EDS system.
For transmission electron microscopy (TEM), cell pellets were processed as described above, except postfixation in osmium tetroxide was omitted. Following ethanol dehydration, pellets were treated with acetone twice at 5 min each and then infiltrated with a 1:1 (vol/vol) mixture of acetone and Spurr's low-viscosity epoxy resin embedding media overnight on a rotator at room temperature. Samples were infiltrated further with a 1:3 (vol/vol) mixture of acetone and Spurr's medium for 6 h on a rotator at room temperature, followed by an overnight incubation in 100% Spurr's medium at room temperature. As a final step, cells were embedded in 100% Spurr's and cured in at 60°C for 24 h.
Ultrathin sections (70 to 80 nm) were collected on Formvar coated gold grids, left unstained, and examined under a JEOL JEM 2100F analytical TEM.
Electron microscopy was performed using a JEOL 2100F 200-kV field emission gun TEM equipped with an Oxford Instruments 30-mm2 solid-state X-ray detector. The images were acquired with a 2,000-by-2,000-pixel charge-coupled-device camera. EDS spectra were collected with the Oxford INCA system at a number of locations, with a live time of 100 seconds. The incoming X-ray count rates were equivalent at all locations due to the ultramicrotomed specimen. An unstained specimen was mounted on Au grids and placed in a Be holder to eliminate spurious copper X rays. In addition, a thick top-hat non-beam-defining aperture was used to reduce stray X rays and high energy electrons from the upper column. No EDS peaks associated with the microscope column were observed.
RESULTS
Effect of metals on growth during chronic irradiation.
The growth response of K. radiotolerans was systematically evaluated according to the exposure of transition metals and chronic irradiation alone and to the combination of transition metals and γ-radiation. Absolute normalization of cell titers between treatments is impractical because K. radiotolerans cells propagate as loosely clumped aggregates and clusters in TGY medium; however, protein determinations and CFU counting have proven to be reliably consistent methods of monitoring growth (R2 ≥ 0.82).
Growth experiments were conducted with liquid culture to evaluate the effect of metal supplementation. Protein levels and numbers of CFU were determined for metal-treated (100 μM final concentration) cultures at four distinct phases of growth: early, mid- and late exponential, and stationary (data not shown). Lag times and growth yields from Fe2+-, Cu2+-, Mn2+-, Mo2+-, and Zn2+-treated cultures were the same as those from the no-metal TGY control (R2 = 0.94). Co2+ amendments resulted in an exaggerated lag period, and protein yields and numbers of CFU at stationary phase were approximately half those for the other metal treatments and the no-metal control. Microscopic evaluation of these cultures confirmed that cell clumps were similar in size during all phases of growth and metal treatments.
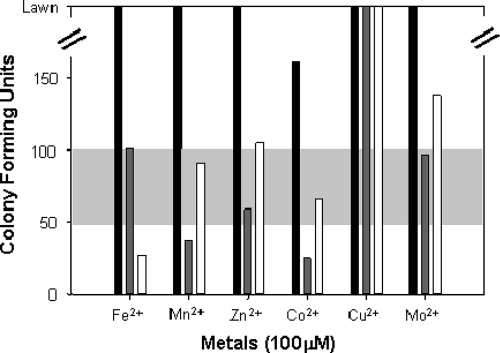
Consistent with the results described above, K. radiotolerans cultures consistently yielded a lawn of growth on TGY plates (with and without metal additions; 100 μM) whether cultures were pregrown in metal-supplemented or no-metal TGY, except Co2+, which generated approximately 150 CFU (Fig. 2) . Because colony formation in response to metal exposure was consistent under ambient laboratory growth conditions, these results were presented simply as a single black bar in Fig. 2. Colony morphology was invariant across all experimental treatments.
FIG. 2.
Effects of different transition metals on the growth of K. radiotolerans during chronic irradiation. Black bars indicate the growth of the no-irradiation control cultures with and without metals. Dark gray bars indicate cultures that were grown to exponential phase in TGY medium and then streak plated onto metal-supplemented TGY plates. White bars indicate cultures that were pregrown to exponential phase in metal-supplemented liquid medium and then streak plated onto non-metal-containing TGY plates. The horizontal baseline (light gray panel) of 75 ± 25 CFU was established from the no-metal, irradiated control cultures. All plates were irradiated for 4 days at 60 Gy/h at a constant temperature of 30°C.
Irradiated no-metal control cultures consistently yielded 75 CFU (Fig. 2). These results were interpreted conservatively by permitting a variance of ±25 CFU around this reference line. These experiments establish the growth responses of K. radiotolerans in the presence of transition metals and chronic radiation (Fig. 2) as individual environmental stressors, thereby permitting evaluation of the effects of the metal additions on growth of K. radiotolerans during chronic irradiation.
The effect of metal supplementation on growth during chronic irradiation (Fig. 2) was neutral, though Mn2+ seemed to have a detrimental effect on growth during chronic irradiation. As expected, cultures plated on Co2+-supplemented medium yielded fewer CFU (∼1/3) than the controls, though the combination of Co2+ and chronic irradiation was not lethal. Conversely, pregrowth in metal-amended medium prior to irradiation on no-metal TGY plates (Fig. 2) yielded different results. In this case, only Fe2+ supplementation was inhibitory to growth. Cobalt appeared to be more toxic to growth than chronic irradiation as growth yields improved when metal stress was relieved. Pregrowth with Mo2+ resulted in higher CFU yields during chronic irradiation, while the effect of both treatments combined was neutral.
The most striking results were obtained for the Cu2+ treatments. Not only did the Cu2+-primed cultures form a lawn during chronic irradiation, but the TGY-grown culture also achieved a lawn of growth during chronic irradiation when Cu2+ was added to the plating medium. Copper-exposed and no-metal control cultures were compared microscopically (phase contrast, scanning confocal laser, and electron microscopy), and no differences were observed in the extents or measured in the sizes of cell clumps and clusters. Furthermore, filtration experiments confirmed that the copper treatments did not result in clump dissociation or the propagation of microcolonies (data not shown).
Growth recovery from oxidative stress.
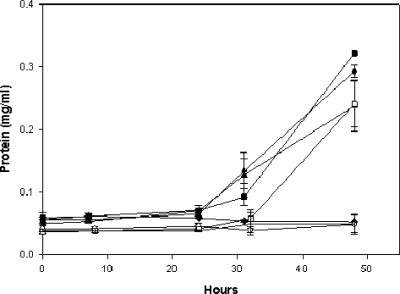
Experiments were conducted to determine whether K. radiotolerans cultures pregrown on TGY in the presence of various divalent cationic metals exhibited differential sensitivity to hydrogen peroxide (Fig. 3). Five percent hydrogen peroxide is lethal to exponentially grown K. radiotolerans cultures in TGY (data not shown), and TGY-grown control cultures resumed cell growth approximately 24 h after exposure to 4% H2O2 (>99% survival; data not shown). The growth recovery levels of Fe2+-, Mn2+-, and Mo2+-pregrown cultures were similar to those of the TGY controls. No increase in protein abundance was measured for the Cu2+-, Zn2+-, and Co2+-grown cultures for up to 48 h following exposure to H2O2.
FIG. 3.
Recovery response of K. radiotolerans following H2O2 exposure. Cultures (n = 3) were grown to exponential phase in TGY or TGY amended with a divalent cationic metal (100 μM), incubated in H2O2 (4%) for 10 min, and then allowed to recover in fresh TGY medium at 28°C and 150 rpm. ▴, TGY (control); ▪, TGY plus Mn2+; ♦, TGY plus Cu2+; •, TGY plus Fe2+; ○, TGY plus Zn2+; □, TGY plus Mo2+; and ▵, TGY plus Co2+.
Intracellular accumulation of metals.
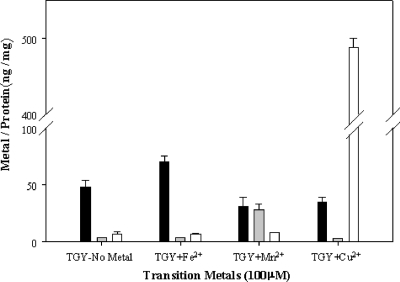
K. radiotolerans cultures were grown to early stationary phase in TGY medium amended with 100 μM Mn2+, Fe2+, or Cu2+, and intracellular metal contents were quantified by ICP-MS (Fig. 4). The biomass yields were not statistically different among each of the metal treatments (P < 0.05; data not shown). The TGY growth medium contained 0.2 μM for both Mn and Cu and 5.8 μM for Fe. K. radiotolerans no-metal control cultures had higher levels of intracellular Fe (∼50 ng/mg protein) than Mn or Cu (<5 ng/mg protein), which capitulated the relative levels in the TGY medium. Metal supplementation of the growth medium significantly increased the intracellular quantities for each of the metals examined relative to the control quantities (P < 0.05). Iron amendment resulted in only a slight increase in intracellular Fe (1.4×), while Mn2+ amendments increased intracellular levels nearly 7×. K. radiotolerans cultures grown in TGY medium with no metal had intracellular Fe contents that were approximately 8× higher than the Cu contents, but cultures actively accumulated Cu when the growth medium was supplemented. Copper amendments increased intracellular Cu 80× over the control levels. It is also interesting to note that Mn2+ and Cu2+ supplementation and enhanced intracellular accumulation of these metals reduced the intracellular quantity of coaccumulated Fe.
FIG. 4.
Intracellular accumulation of transition metals in early-stationary-phase K. radiotolerans cultures. Bars indicate normalized metal contents of Fe2+ (solid), Mn2+ (gray), and Cu2+ (white) in no-metal control and metal-supplemented cultures (x axis).
The intracellular metal contents and ratios of transition metals in K. radiotolerans were compared to those of other radiation-resistant and radiation-sensitive bacterial species (Table 1) (13). Grown in TGY medium, K. radiotolerans had lower intracellular Fe levels (0.86 nmol/mg protein) than any of the other radiation-resistant bacterial species (>1.4 nmol/mg protein). Only when the cultures were grown in Fe2+-supplemented TGY medium (1.25 nmol/mg protein) did intracellular iron levels approximate those for Deinococcus spp. (1.45 to 1.7 nmol/mg protein). Likewise, the levels of Mn were also markedly lower (0.075 nmol/mg protein) than the levels previously reported for other radiation-resistant bacterial species (>0.3 nmol/mg protein), though not as low as those for the radiation-sensitive strains (<0.019 nmol/mg protein), but when the cultures were grown in Mn2+-supplemented medium, the levels (0.51 nmol/mg protein) exceeded those for D. radiotolerans (0.36 nmol/mg protein).
TABLE 1.
Intracellular metal contents of extreme-resistant and sensitive bacteriaa
| Species and medium | Radiation resistancec | Ratio (nmol/mg protein) of:
|
||
|---|---|---|---|---|
| Mn/Fe | Mn/Cu | Mn/Fe+Cu | ||
| K. radiotolerans | RT | |||
| TGY | 0.087 | 0.75 | 0.078 | |
| TGY + Fe2+ | 0.0048 | 0.545 | 0.044 | |
| TGY + Mn2+ | 0.91 | 3.778 | 0.734 | |
| TGY + Cu2+ | 0.087 | 0.0071 | 0.0066 | |
| D. radioduransb | RT | 0.24 | ||
| Enterococcus faeciumb | RT | 0.17 | ||
| Pseudomonas putidab | RS | <0.0001 | ||
| E. colib | RS | 0.0072 | ||
| S. oneidensisb | RS | 0.0005 | ||
K. radiotolerans cultures were grown to stationary phase, and cell pellets were washed sequentially in EDTA and PBS prior to metal analysis by ICP-MS.
Comparative data for other bacterial strains were taken directly from Daly et al. (13).
Levels of radiation resistance were categorized by strain as either radiation tolerant (RT) or radiation sensitive (RS).
In general, the Mn/Fe ratios for K. radiotolerans were more closely aligned with the values reported for the radiation-resistant bacteria than the very low ratios for the radiation-sensitive strains, though metal levels varied considerably with growth conditions. Mn/Cu ratios were generally quite high (i.e., a favorable ratio for resistance) except for cultures grown in Cu2+-supplemented medium, and this ratio was more closely aligned with the Mn/Fe ratios for the radiation-sensitive strains. Given the responsiveness of uptake and accumulation mechanisms for Cu2+, Mn/Fe-plus-Cu ratios were also determined. In general, the ratios were more closely aligned with the Mn/Fe ratios of the radiation-tolerant strains than those of the radiation-sensitive strains, except when the cultures were grown in Mn2+-supplemented medium, where the ratio was exceptionally high (i.e., purportedly favorable for resistance), and in Cu2+-supplemented medium, where the ratio was lower than the Mn/Fe ratio for Escherichia coli (i.e., taken to indicate sensitivity).
Subcellular distribution of copper.
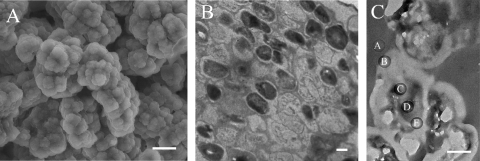
K. radiotolerans was grown to mid-exponential phase in copper-supplemented (100 μM) TGY medium, and cells were prepared for electron microscopy and elemental analysis. A scanning electron micrograph of a typical cell cluster is shown in Fig. 5A. No copper was detected from the extracellular surfaces of intact cells and clusters (data not shown; detection limit, 0.1 wt %).
FIG. 5.
Electron micrographs of K. radiotolerans cell clusters. (A) Scanning electron micrograph. Scale bar = 20 μm. (B) Transmission electron micrograph of a stained thin section. Scale bar = 0.5 μm. (C) Transmission electron micrograph of an unstained thin section. Elemental spectra were collected along a transect (indicated by spots A to E) spanning a single cell within a cluster. Scale bar = 0.5 μm.
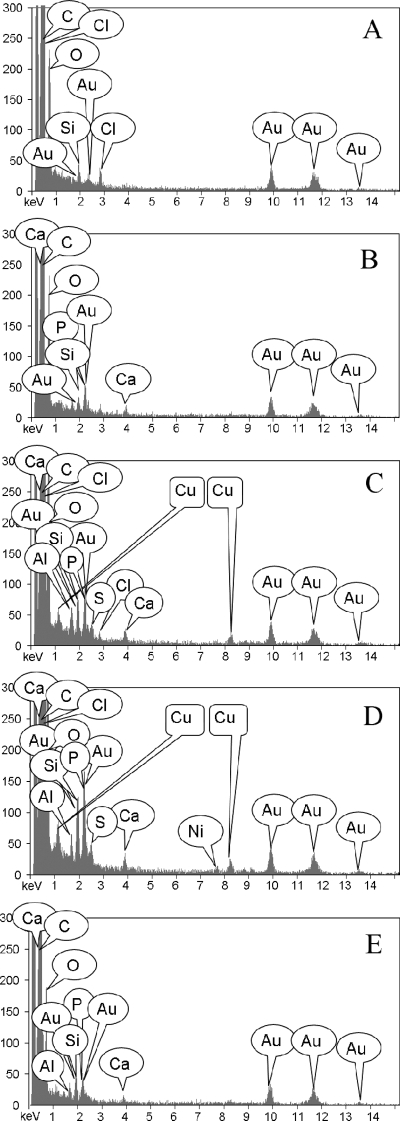
Subcellular distribution of copper was examined from thin cell cluster sections; a stained thin section is shown in Fig. 5B. For elemental analysis, transects were established across three distinct cell clusters and EDS spectra were collected. The trends were consistent across all three transects; the results from one transect are shown in Fig. 5C and 6. The embedding resin was analyzed to establish background composition; no copper was detected (Fig. 5C, spot A, and 6A). No copper was detected within the thick extracellular polymeric substances on the cell surface (Fig. 5C, spot B, and 6B) or collectively from the cell membrane, cell wall, and interstitial space between dividing cells (Fig. 5C, spot E, and 6E). Copper was detected only throughout the cytoplasm (Fig. 5C, spots C and D, and 6C and D).
FIG. 6.
X-ray elemental spectra from a thin K. radiotolerans cell section. Each of the EDS panels corresponds to a specific sampling location from the thin section shown in Fig. 5C. (A) EDS spectrum of the embedding resin. (B) EDS spectrum of the extracellular milieu. (C and D) EDS spectra from two different locations within the cytoplasm. (E) EDS spectrum from the cell membrane and cell wall and the interstitial space between dividing cells within the cluster.
DNA damage repair following acute irradiation.
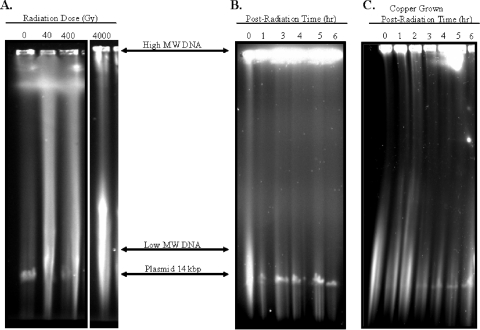
Experiments were conducted to evaluate the effects of copper accumulation on DNA elicited by acute exposure of K. radiotolerans cultures to ionizing radiation. PFGE was used to qualitatively evaluate the extents of radiation-induced DNA damage and repair for copper and no-metal control treatments. K. radiotolerans possesses two plasmids (183 and 14 kbp) (http://www.jgi.doe.gov/), but only the smaller plasmid is reliably resolved on these gels and thus provides a useful visual marker to approximate the completion of genome restitution. Based on protein determinations, cell titers were equivalent for the Cu2+-loaded and control cultures (Fig. 7B and C). In Fig. 7A, the occurrence of DNA double-strand breaks was γ-radiation dose dependent. Relatively low radiation doses (40 Gy) produced a full range of damaged DNA fragments in K. radiotolerans, as indicated by a long smear, and DNA from cells exposed to 4,000 Gy was predominately visualized as low-molecular-weight DNA, which comigrated near the bottom of the gel. In the no-metal TGY-grown control culture, the low-molecular-weight damaged DNA disappeared and the 14-kbp plasmid appeared within 3 h following acute irradiation (Fig. 7B). Comparable levels of radiation-induced DNA damage (i.e., the relative intensity of the smear) were noted for the copper-loaded culture, but the smear itself may have been slightly longer (Fig. 7C). DNA damage repair in the acutely irradiated Cu2+-loaded culture required 4 to 5 h for the low-molecular-weight damaged DNA to fully disappear and for the 14-kbp plasmid to be clearly visible, though the intensity of the plasmid was notably less than that in the control.
FIG. 7.
DNA damage repair in K. radiotolerans cultures following acute irradiation. (A) DNA damage as a function of radiation dose. (B and C) Timing of radiation-induced DNA damage repair for control and Cu-grown cultures. MW, molecular weight.
DISCUSSION
In this study, copper supplementation enhanced the growth and colony formation of Kineococcus radiotolerans during chronic irradiation. Chronic irradiation uniformly reduced the colony yields of K. radiotolerans cultures on TGY plates. While minor variation in absolute numbers of colonies was noted among the various metal treatments tested, the same general response was observed; transition metals had no major effect on the growth responses of irradiated cultures, except for copper. Whether cultures were pregrown in copper-supplemented liquid medium (resulting in intracellular accumulation) or transferred to copper-supplemented plating medium just prior to chronic irradiation, the presence of copper upon irradiation resulted in colony yields that were no different from those of the nonirradiated control cultures. Copper-treated and no-metal control cultures were indistinguishable; absolutely no phenotypic variation was observed or measured for cultures growing in liquid or on solid medium pre- or postirradiation. This result strongly suggests that copper enhances the metabolism and growth of Kineococcus radiotolerans during chronic irradiation, since it is doubtful that a redox active metal could neutralize the direct and indirect chemical reactivities of ionizing radiation.
Copper is an essential cofactor for a variety of enzymes involved in aerobic respiration and energy production; however, excess copper is toxic, and thus, intracellular levels are tightly regulated by the cell (40). Copper toxicity manifests itself through indiscriminant binding to cellular ligands or competitive displacement of other metal cofactors (4, 11) as well in the production of intracellular reactive oxygen species, namely, hydroxyl radicals, via Fenton chemistry (23, 27). While copper-catalyzed reduction of H2O2 to hydroxyl radicals can be demonstrated in vitro (3, 17), the significance of this reaction in mediating DNA damage in vivo remains a controversial issue. This reaction is considered unfavorable on account of the low physiological concentrations of oxygen radicals, the virtual absence of “free” copper inside the cell, and the maintenance of a neutral-pH cytosolic environment (40, 46); however, numerous studies have demonstrated that these reactions do occur (33) and are a significant threat to cell viability and survival (27). Copper homeostasis is critical, as evident by the cell's capacity for copper chelation and complex detoxification systems (15, 29, 37, 40), which are often interconnected through transcriptional regulation of oxidative stress pathways (25, 47). In gram-negative bacteria, periplasmic sequestration of metals is an important resistance mechanism for preventing accumulation of toxic levels of Cu2+ in the cytoplasm (8, 9, 33, 42), but K. radiotolerans is gram positive and accumulates copper intracellularly. Moreover, heightened sensitivity to a strong oxidant and the persistence of low-molecular-weight DNA following acute irradiation of copper-loaded cells are suggestive of copper-catalyzed hydroxyl radical formation.
It is unclear why K. radiotolerans accumulates copper, but it appears that this organism possesses uptake and transport mechanisms that may be Cu2+ specific. Increased intracellular accumulation of Fe and Mn was demonstrated by growing cultures in metal-supplemented medium, but the levels were substantially lower than those of Cu. It would seem unlikely that K. radiotolerans possesses a unique, copper-dependent defense system for oxidative and radiation stress yet more probable that cells are sufficiently equipped to sequester excess copper (or redox active metals) at high efficiency, thus preventing metal toxicity and production of reactive oxygen species. This conclusion would appear to be supported by the observation that Fe-loaded cells were not differentially affected by chronic irradiation or H2O2 relative to the no-metal control. Possible mechanisms for copper sequestration based on preliminary genome sequence examination include the Fe-containing superoxide dismutase, glutathione, and DNA binding protein (Dps). Orthologs of putative low-molecular-weight Cu-induced metallothioneins or metallochaperons have not been identified in K. radiotolerans. Additionally, conventional copper homeostasis pathways (e.g., the cop operon) (46) appear to be absent, though numerous heavy metal transporters and multiple copies of the copC copper resistance gene were identified. Copper toxicity can manifest itself through the displacement of iron, or other metals, for specific ligands and cofactor binding sites, though growth characteristics were unaffected by high cytoplasmic copper levels. Adaptation to copper-supplemented growth medium may induce the expression of additional or redundant defense and detoxification systems that counter metal toxicity as well as excessive production of reactive oxygen species. Intracellular sequestration may be important for copper resistance, but the cupro-chaperons or enzymes involved and the potential coordination with other defense systems have not been experimentally determined.
The experiments performed by Daly et al. (13) have been repeated to evaluate whether certain transition metals might fulfill an important physiological role in the radioresistance phenotype of K. radiotolerans, as they appear to in other extreme-resistant bacterial species. High levels of intracellular Mn relative to Fe levels have been shown to contribute to γ-radiation resistance by mitigating protein oxidation that occurs during and after irradiation (14). Conversely, the high intracellular Fe level, relative to the Mn level, apparently contributes to the sensitivity of bacterial species like E. coli and Shewanella oneidensis to radiation and oxidative stress through the production of reactive oxygen radicals that exacerbate cellular damage. The apparent biochemical preference and utilization of a non-Fenton redox metal shown by the Deinococcus spp. and other extreme-radiation-resistant bacterial species tested by Daly et al. (13), though, does not satisfactorily explain the radiation tolerance of K. radiotolerans, because Cu2+ does participate in Fenton/Haber-Weiss chemistry for the formation of reactive oxygen species (31). These results strongly indicate an important role for Cu2+ in enhancing the metabolic efficiency and/or antioxidative capacity in K. radiotolerans to compensate for the chemical reactivity of this element. It is relatively simple to envision that the copper-induced response could afford cross-protection from other stressors (particularly oxidative stress), but it is not clear how a stress response would dramatically increase energy production and growth only when the compounded stress of ionizing radiation is applied.
Mattimore and Battista (34) postulated that the mechanisms of extreme radioresistance in Deinococcus radiodurans evolved not under direct selection by ionizing radiation but more likely as a consequence of selection for desiccation resistance. This explanation continues to gain credence as more examples arise (e.g., references 2, 16, 21, 41). These observations imply certain overlap among the underlying resistance mechanisms, but there may also be some important stressor-specific distinctions. Here, high intracellular levels of copper prohibited recovery of H2O2-exposed cells, though the expectedly toxic combination of copper and γ-radiation stimulated the growth and colony formation of K. radiotolerans. Exposure of a Cu-loaded culture to 4% hydrogen peroxide may have exceeded the cell's capacity to effectively quench reactive oxidants, resulting in irrecoverable cellular damage, consistent with the expectation that copper catalyzed the production of oxygen radicals. The sensitivity of Cu-loaded cultures to more-closely approximated physiological concentrations of hydrogen peroxide was not determined, though we have measured the heightened sensitivity of Cu-loaded cultures to methyl viologen (0.2 mM), a known producer of superoxide anion (C.E. Bagwell, unpublished data). Thus, the levels of oxidative stress resulting from chronic irradiation of Cu-loaded cells should be less than that imposed by the H2O2 used in these experiments. We presume that exposure of Cu-loaded K. radiotolerans cultures to a strong oxidant is capable of liberating “bound” intracellular Cu, which is then available to react, with lethal consequences to the cell. Consequently, Cu-dependent growth stimulation during chronic irradiation may mean that radiation-induced oxidative stress is below the threshold and that any oxidant produced is readily quenched, so then a beneficial role for copper is conceivable.
The relative amounts of direct DNA damage resulting from acute irradiation were comparable for the Cu-loaded and control cultures, whereas Cu-dependent damage was more pronounced during postirradiation recovery and repair. The Cu-loaded culture may suffer from a higher level of indirect cellular damage due to Cu-dependent production of reactive oxygen species, which would interrupt the efficiency of DNA stabilization and repair. The decreased intensity of the restored plasmid in the Cu-loaded culture implies that some of the low-molecular-weight DNA could not be salvaged for genome reassembly and was either degraded or exported. We presume that the type or extent of DNA damage accrued in the Cu-loaded culture was more severe than that in the control; however, it is interesting to note that DNA stabilization and repair functions were preserved and operated at nearly the same efficiency as those of the control.
Microbial communities do inhabit radioactive environments (19, 24, 38, 43, 51), and bacteria isolated from such habitats are generally much more tolerant to exposure to ionizing radiation and oxidative stress than their counterparts from environments experiencing background levels of radiation (36). Melanin-producing microfungi obtained from the Chernobyl Atomic Energy Station display directional growth of hyphae toward ionizing radiation (51). Dadachova et al. (12) recently demonstrated that melanin enhanced the growth and metabolic activity of certain fungi during chronic exposure to low levels of ionizing radiation relative to what was found for nonmelanized cells. Though the exact mechanism(s) is unknown, melanin may serve to shield these fungi, perhaps scavenging reactive oxygen species (44), but a role for the electron transfer properties of melanin cannot be ignored (12, 35). To the best of our knowledge, this study marks the first documented case whereby bacterial growth is legitimately enhanced during chronic irradiation. Here, growth conditions that were expected to prompt copper-catalyzed production of oxygen radicals actually promoted the growth of K. radiotolerans, and this response could not be duplicated by chronic irradiation or copper supplementation alone.
Acknowledgments
We gratefully acknowledge two anonymous reviewers whose input substantially improved the manuscript. We thank Chris Yeager (Savannah River National Laboratory) and Wayne Outten (University of South Carolina) for helpful discussions and critical review of the manuscript as well as Chris Beam for access to the SRNL Gamma Source Facility and Jason Unrine at the University of Georgia's Savannah River Ecology Laboratory for ICP-MS analysis. We recognize the U.S. Department of Energy's Joint Genome Institute for the complete genome sequencing of K. radiotolerans.
This work was supported by the U.S. Department of Energy, Office of Environmental Management, as administered through the Laboratory Directed Research and Development Program and by the U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research, Environmental Remediation Sciences Program (contract no. KP1302000).
Footnotes
Published ahead of print on 11 January 2008.
REFERENCES
- 1.Anderson, A. W., H. C. Nordon, R. F. Cain, G. Parrish, and D. Duggan. 1956. Studies on a radio-resistant micrococcus. I. Isolation, morphology, cultural characteristics, and resistance to gamma radiation. Food Technol. 10:575-578. [Google Scholar]
- 2.Arrage, A. A., T. J. Phelps, R. E. Benoit, and D. C. White. 1993. Survival of subsurface microorganisms exposed to UV radiation and hydrogen peroxide. Appl. Environ. Microbiol. 59:3545-3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aruoma, O. I., B. Halliwell, E. Gajewski, and M. Dizdaroglu. 1991. Copper-ion-dependent damage to the bases in DNA in the presence of hydrogen peroxide. Biochem. J. 273:601-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asmuss, M., L. H. F. Mullenders, A. Eker, and A. Hartwig. 2000. Differential effects of toxic metal compounds on the activities of Fpg and XPA, two zinc finger proteins involved in DNA repair. Carcinogenesis 21:2097-2104. [DOI] [PubMed] [Google Scholar]
- 5.Battista, J. R. 1997. Against all odds: the survival strategies of Deinococcus radiodurans. Annu. Rev. Microbiol. 51:203-224. [DOI] [PubMed] [Google Scholar]
- 6.Billi, D., E. I. Friedmann, K. G. Hofer, M. G. Caiola, and R. Ocampo-Friedmann. 2000. Ionizing-radiation resistance in the desiccation-tolerant cyanobacterium Chroococcidiopsis. Appl. Environ. Microbiol. 66:1489-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenner, A. J., and E. D. Harris. 1995. A quantitative test for copper using bicinchoninic acid. Anal. Biochem. 226:80-84. [DOI] [PubMed] [Google Scholar]
- 8.Cha, J., and D. A. Cooksey. 1991. Copper resistance in Pseuodomonas syringae mediated by periplasmic and outer membrane proteins. Proc. Natl. Acad. Sci. USA 88:8915-8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooksey, D. A., and H. R. Azad. 1992. Accumulation of copper and other metals by copper-resistant plant-pathogenic and saprophytic pseudomonads. Appl. Environ. Microbiol. 58:274-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cox, M. M., and J. R. Battista. 2005. Deinococcus radiodurans—the consummate survivor. Nat. Rev. Microbiol. 3:882-892. [DOI] [PubMed] [Google Scholar]
- 11.Culotta, V. C., H. D. Joh, S. J. Lin, K. H. Slekar, and J. Strain. 1995. A physiological role for Saccharomyces cerevisiae copper/zinc superoxide dismutase in copper buffering. J. Biol. Chem. 270:29991-29997. [DOI] [PubMed] [Google Scholar]
- 12.Dadachova, E., R. A. Bryan, X. Huang, T. Moadel, A. D. Schweitzer, P. Alsen, J. D. Nosanchuk, and A. Casadevall. 2007. Ionizing radiation changes the electronic properties of melanin and enhances the growth of melanized fungi. PLoS ONE 5:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daly, M. J., E. K. Gaidamakova, V. Y. Matrosova, A. Vasilenko, M. Zhai, A. Venkateswaran, M. Hess, M. V. Omelchenko, H. M. Kostandarithes, K. S. Makarova, L. P. Wackett, J. K. Fredrickson, and D. Ghosal. 2004. Accumulation of Mn(II) in Deinococcus radiodurans facilitates gamma-radiation resistance. Science 306:1025-1028. [DOI] [PubMed] [Google Scholar]
- 14.Daly, M. J., E. K. Gaidamakova, V. Y. Matrosova, A. Vasilenko, M. Zhai, R. D. Leapman, B. Lai, B. Ravel, S-M. W. Li, K. M. Kemner, and J. K. Fredrickson. 2007. Protein oxidation implicated as the primary determinant of bacterial radioresistance. PLoS Biol. 5:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dameron, C. T., and M. D. Harrison. 1998. Mechanisms for protection against copper toxicity. Am. J. Clin. Nutr. 67:1091S-1097S. [DOI] [PubMed] [Google Scholar]
- 16.de la Torre, J. R., B. M. Goebel, E. I. Friedmann, and N. R. Pace. 2003. Microbial diversity of cryptoendolithic communities from the McMurdo dry valleys, Antarctica. Appl. Environ. Microbiol. 69:3858-3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dizdaroglu, M., G. Rao, B. Halliwell, and E. Gajweski. 1991. Damage to the DNA bases in mammalian chromatin by hydrogen peroxide in the presence of ferric and cupric ions. Arch. Biochem. Biophys. 285:317-324. [DOI] [PubMed] [Google Scholar]
- 18.Ferreira A. C., M. F. Nobre, E. Moore, F. A. Rainey, J. R. Battista, and M. S. da Costa. 1999. Characterization and radiation resistance of new isolates of Rubrobacter radiotolerans and Rubrobacter xylanophilus. Extremophiles 3:235-238. [DOI] [PubMed] [Google Scholar]
- 19.Fredrickson, J. K., J. M. Zachara, D. L. Balkwill, D. Kennedy, S. W. Li, H. M. Kostandarithes, M. J. Daly, M. F. Romine, and F. J. Brockman. 2004. Geomicrobiology off high-level nuclear wate-contaminated vadose sediments at the Hanford Site, Washington State. Appl. Environ. Microbiol. 70:4230-4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fridovich, I. 1978. The biology of oxygen radicals. Science 201:875-880. [DOI] [PubMed] [Google Scholar]
- 21.Garrity, G. M., B. K. Heimbuch, and M. Gagliardi. 1996. Isolation of zoosporogenous actinomycetes from desert soils. Ind. Microbiol. Biotechnol. 17:268-280. [Google Scholar]
- 22.Ghosal, D., M. V. Omelchenko, E. K. Gaidamakova, V. Y. Matrosova, A. Vasilenko, A. Venkateswaran, M. Zhai, H. M. Kostandarithes, H. Brim, K. S. Makarova, L. P. Wackett, J. K. Fredrickson, and M. J. Daly. 2005. How radiation kills cells: survival of Deinococcus radiodurans and Shewanella oneidensis under oxidative stress. FEMS Microbiol. Rev. 29:361-375. [DOI] [PubMed] [Google Scholar]
- 23.Halliwell, B., and J. M. C. Gutteridge. 1984. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 219:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ivanova, A. E., K. B. Aslanidi, Iu. V. Karpenko, and T. A. Belozerskaia. 2005. The effect of hydrogen peroxide on the growth of microscopic mycelial fungi isolated from habitats with different levels of radioactive contamination. Mikrobiologiia 74:756-765. [PubMed] [Google Scholar]
- 25.Jamers, A., K. Van der Ven, L. Moens, J. Robbens, G. Potters, Y. Guisez, R. Blust, and W. De Coen. 2006. Effect of copper exposure on gene expression profiles in Chlamydomonas reinhardtii based on microarray analysis. Aquat. Toxicol. 80:249-260. [DOI] [PubMed] [Google Scholar]
- 26.Kieser, H. M., T. Kieser, and D. A. Hopwood. 1992. A combined genetic and physical map of the Streptomyces coelicolor A3(2) chromosome. J. Bacteriol. 174:5496-5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimura, T., and H. Nishioka. 1997. Intracellular generation of superoxide by copper sulphate in Escherichia coli. Mutat. Res. 389:237-242. [DOI] [PubMed] [Google Scholar]
- 28.Kudo, T., K. Matsushima, T. Itoh, J. Sasaki, and K. Suzuki. 1998. Description of four new species of the genus Kineosporia: Kineosporia succinea sp. nov., Kineosporia rhizophila sp. nov., Kineosporia mikuniensis sp. nov. and Kineosporia rhamnosa sp. nov., isolated from plant samples, and amended description of the genus Kineosporia. Int. J. Syst. Bacteriol. 48:1245-1255. [DOI] [PubMed] [Google Scholar]
- 29.Ledin, M. 2000. Accumulation of metals by microorganisms—processes and importance for soil systems. Earth Sci. Rev. 51:1-31. [Google Scholar]
- 30.Lee, S. D. 2006. Kineococcus marinus sp. nov., isolated from marine sediments of the coast of Jeju, Korea. Int. J. Syst. Evol. Microbiol. 56:1279-1283. [DOI] [PubMed] [Google Scholar]
- 31.Letelier, M. E., A. M. Lepe, M. Faundez, J. Salazar, R. Marin, P. Aracena, and H. Speisky. 2005. Possible mechanisms underlying copper-induced damage in biological membranes leading to cellular toxicity. Chem. Biol. Interact. 151:71-82. [DOI] [PubMed] [Google Scholar]
- 32.Levin-Zaidman, S., J. Englander, E. Shimoni, A. K. Sharma, K. W. Minton, and A. Minsky. 2003. Ringlike structures of the Deinococcus radiodurans genome: a key to radioresistance? Science 299:254-256. [DOI] [PubMed] [Google Scholar]
- 33.Macomber, L., C. Rensing, and J. A. Imlay. 2007. Intracellular copper does not catalyze the formation of oxidative DNA damage in Escherichia coli. J. Bacteriol. 189:1616-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mattimore, V., and J. R. Battista. 1996. Radioresistance of Deinococcus radiodurans: functions necessary to survive ionizing radiation are also necessary to survive prolonged desiccation. J. Bacteriol. 178:633-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menter, J. M., and I. Willis. 1997. Electron transfer and photoprotective properties of melanins in solution. Pigment Cell Res. 10:214-217. [DOI] [PubMed] [Google Scholar]
- 36.Mironenko, N. V., I. A. Alekhina, N. N. Zhdanova, and S. A. Bulat. 2000. Intraspecific variation in gamma-radiation resistance and genome structure in the filamentous fungus Alternaria alternate: a case study of strains inhabiting Chernobyl Reactor No. 4. Ecotoxicol. Environ. Saf. 45:177-187. [DOI] [PubMed] [Google Scholar]
- 37.Nair, S., and S. E. Finkel. 2004. Dps protects cells against multiple stresses during stationary phase. J. Bacteriol. 186:4192-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phillips, R. W., J. Wiegel, C. J. Berry, C. Fliermans, A. D. Peacock, D. C. White, and L. J. Shimkets. 2002. Kineococcus radiotolerans sp. nov., a radiation-resistant, Gram-positive bacterium. Int. J. Syst. Evol. Microbiol. 52:933-938. [DOI] [PubMed] [Google Scholar]
- 39.Radajewski, S., and T. Duxbury. 2001. Motility responses and desiccation survival of zoospores from the actinomycete Kineosporia sp. strain SR11. Microb. Ecol. 41:233-244. [DOI] [PubMed] [Google Scholar]
- 40.Rae, T. D., P. J. Schmidt, R. A. Pufahl, V. C. Culotta, and T. V. O'Halloran. 1999. Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science 284:805-808. [DOI] [PubMed] [Google Scholar]
- 41.Rainey, F. A., K. Ray, M. Ferreira, B. Z. Gatz, M. F. Nobre, D. Bagaley, B. A. Rash, M.-J. Park, A. M. Earl, N. C. Shank, A. M. Small, M. C. Henk, J. R. Battista, P. Kämpfer, and M. S. da Costa. 2005. Extensive diversity of ionizing-radiation resistant bacteria recovered from Sonoran desert soils and description of nine new species of the genus Deinococcus obtained from a single soil sample. Appl. Environ. Microbiol. 71:5225-5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rensing, C., and G. Grass. 2003. Escherichia coli mechanisms of copper homeostasis in a changing environment. FEMS Microbiol. Rev. 27:197-213. [DOI] [PubMed] [Google Scholar]
- 43.Romanovskaia V. A., S. M. Stoliar, I. Malashenko, and E. S. Shatokhina. 1996. The effect of long-acting radiation on the diversity of heterotrophic bacteria in the soils of a 10-kilometer area around the Chernobyl Atomic Electric Power Station. Mikrobiol. Z. 58(5):3-12. (In Russian.) [PubMed] [Google Scholar]
- 44.Różanowska, M., T. Sarna, E. J. Land, and T. G. Truscott. 1999. Free radical scavenging properties of melanin interaction of eu- and pheo-melanin models with reducing and oxidizing radicals. Free Radic. Biol. Med. 26:518-525. [DOI] [PubMed] [Google Scholar]
- 45.Schabereiter-Gurtner, C., G. Piñar, D. Vybiral, W. Lubitz, and S. Rolleke. 2001. Rubrobacter-related bacteria associated with rosy discolouration of masonry and lime wall paintings. Arch. Microbiol. 176:347-354. [DOI] [PubMed] [Google Scholar]
- 46.Solioz, M., and J. V. Stoyanov. 2003. Copper homeostasis in Enterococcus hirae. FEMS Microbiol. Rev. 27:183-195. [DOI] [PubMed] [Google Scholar]
- 47.Teitzel, G. M., A. Geddie, S. K. De Long, M. J. Kirisits, M. Whiteley, and M. R. Parsek. 2006. Survival and growth in the presence of elevated copper: transcriptional profiling of copper-stressed Pseudomonas aeruginosa. J. Bacteriol. 188:7242-7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tringe, S. G., C. von Mering, A. Kobayashi, A. A. Salamov, K. Chen, H. W. Chang, M. Podar, J. M. Short, E. J. Mathur, J. C. Detter, P. Bork, P. Hugenholtz, and E. M. Rubin. 2005. Comparative metagenomics of microbial communities. Science 308:554-557. [DOI] [PubMed] [Google Scholar]
- 49.Udupa, K. S., P. A. O'Cain, V. Mattimore, and J. R. Battista. 1994. Novel ionizing radiation-sensitive mutants of Deinococcus radiodurans. J. Bacteriol. 176:7439-7446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yokota, A., T. Tamura, T. Nishii, and T. Hasegawa. 1993. Kineococcus aurantiacus gen. nov., sp. nov., a new aerobic, gram-positive, motile coccus with meso-diaminopimelic acid and arabinogalactan in the cell wall. Int. J. Syst. Bacteriol. 43:52-57. [Google Scholar]
- 51.Zhdanova, N. N., T. Tugay, J. Dighton, V. Zheltonozhsky, and P. McDermott. 2004. Ionizing radiation attracts soil fungi. Mycol. Res. 108:1089-1096. [DOI] [PubMed] [Google Scholar]
- 52.Zimmerman, J. M., and J. R. Battista. 2005. A ring-like nucleoid is not necessary for radioresistance in the Deinococcaceae. BMC Microbiol. 5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]