Abstract
The epithelial cell membrane 252-kDa protein (P252) isolated in our laboratory from Bombyx mori midgut was shown to bind strongly with Cry1Aa, Cry1Ab, and Cry1Ac toxins of Bacillus thuringiensis (15). In the current paper, P252 was shown to bind with chlorophyllide (Chlide) to form red fluorescent protein (RFP) complex, termed Bm252RFP, with absorbance and fluorescence emission peaks at 600 nm and 620 nm, respectively. P252 at a concentration of 1 μM is shown to bind with about 50 μM Chlide in a positively cooperative reaction to form Bm252RFP under aerobic conditions and in the presence of light at 37°C. Various parameters influencing this reaction have been optimized for efficient in vitro chemical synthesis of Bm252RFP. Circular dichroism spectra revealed that P252 is composed of a β-structure (39.8% ± 2.2%, based on 5 samples) with negligible contribution of α-helix structure. When bound to Chlide, the β-structure content in the complex is reduced to 21.6% ± 3.1% (n = 5). Since Chlide had no secondary structure, the observed reduction suggests significant conformational changes of P252 during the formation of Bm252RFP complex. Bm252RFP had antimicrobial activity against Escherichia coli, Serratia marcescens, B. thuringiensis, and Saccharomyces cerevisiae with 50% effective concentrations of 2.82, 2.94, 5.88 μM, and 21.6 μM, respectively. This is the first report ever to show clear, concrete binding characteristics of the midgut protein to form an RFP having significant antimicrobial activity.
The actual insecticidal mechanism of Cry toxins of Bacillus thuringiensis and the receptor protein that leads to pore formation are yet to be fully clarified. However, it has generally been accepted that B. thuringiensis Cry1Aa, Cry1Ab, and Cry1Ac insecticidal proteins bind with putative receptor molecules, aminopeptidase N (APN) (21, 33, 40), and/or cadherin-like proteins (17, 28, 39). It is believed that after binding with these receptors, hydrophobic α-helices in domain I of Cry1A toxins penetrate into the brush border membrane (BBM) of midgut epithelial cells. Then, by small-pore formation on the BBM, these cells lose their homeostasis, ultimately leading to the insect's death (7, 22, 45). To understand the complete insecticidal mechanism, we have been interested in elucidating the interaction between epithelial cell membrane proteins and Cry toxins.
During the search for the Cry toxin binding proteins, we found 252-kDa proteins in the membrane of epithelial cells from the Bombyx mori midgut (14-16). The protein (P252) was purified from a Triton X-100 soluble fraction of BBM vesicles (BBMVs) of B. mori, and it was purified to homogeneity by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and gel filtration chromatography. It was shown to have a molecular mass of 252 kDa by SDS-PAGE. But the molecular size of about 985 kDa obtained from gel filtration chromatography suggests that the protein could be a homotetramer, and this P252 was shown to bind strongly with Cry1Aa, Cry1Ab, and Cry1Ac toxins of B. thuringiensis with 30, 180, and 20 nM Kd (dissociation constant) values, respectively (15). Antiserum to the APN with a molecular weight of 120,000 and anti-BtR175 (a cadherin-like protein) antiserum did not recognize P252, and its novelty was suggested; in fact, a homology search using a database for an internal peptide from P252 did not match any sequence.
However, recent database information indicated that three internal peptides derived from P252 have 94 and 100% homology in amino acid sequence with a chlorophyllide (Chlide) binding protein (CBP) (EMBL accession no. AM113746.1). Recently, the same authors reported this CBP to be a novel member of the lipocalin family with 15 lipocalin polypeptide chains; the protein was subsequently named polycalin (26). The investigators characterized this protein by deduced amino acid sequences after they cloned a 302-kDa protein. However, binding between their CBP and Chlide was not experimentally proved, nor were the biochemical characteristics and the physiological role of this red fluorescent pigment protein complex shown.
The silkworm, B. mori, has been examined for the presence of antiviral factors, and activity against B. mori nuclear polyhedrosis virus (BmNPV) factor was reported to be present in the digestive juice of the silkworm (2, 10, 29, 30, 32). Antimicrobial factors were also reported to be present in silkworms (44). Almost 4 decades ago, Aizawa et al. (2) first reported that antiviral activities against BmNPV factors were related to red fluorescence proteins (RFPs). It was reported that RFP could be obtained in vitro from midgut protein and Chlide a, a prosthetic group derived from chlorophyll (Chl) a. Eventually the anti-BmNPV activity was attributed to Chlide a rather than the protein, and it was shown that CBP changed to RFP by binding with Chlide (11).
The molecular size of RFP was first suggested to be large since the protein was eluted at void volume in Sephadex G-200 column chromatography (27). But various RFPs such as 65-kDa RFP (29), 24-kDa serine protease, 30-kDa lipase RFP (30, 32), and recently a 302-kDa lipocalin (26) were reported. Thus, it is reasonable to assume that RFPs with various molecular sizes exist in the digestive juice and/or midgut tracts of insect larvae. Though diversity exists in molecular size, activity, and characteristics, all these reported RFPs were suggested to be derived from the binding between Chlide and insect midgut proteins.
The actual midgut protein(s) which forms RFP and its characteristics is not clear except for the above-cloned 302-kDa lipocalin RFP from midgut searched from the deduced amino acid sequence database. Since the midgut protein P252 shares sequence similarity with the 302-kDa CBP, we carried out binding experiments to explore the possibility of RFP in vitro chemical synthesis by binding with Chlide. This is the first report that presents clear evidence that B. mori midgut membrane protein P252, which has a strong affinity to Cry1A from B. thuringiensis, is also indeed a CBP. Also, this is the first report that successfully shows that antimicrobial activity is exhibited by the B. mori midgut membrane protein P252 that binds to Chlide to form red fluorescent protein, termed Bm252RFP, against Escherichia coli, Serratia marcescens, B. thuringiensis, and even against Saccharomyces cerevisiae.
MATERIALS AND METHODS
Insects.
The silkworm B. mori hybrid Shunrei × Shogetsu was reared on the artificial diet Silkmate (Nosan Kogyo, Yokohama, Japan) and fifth-instar larvae were used.
Culture of microbes and Cry1A toxin production.
E. coli JM109, S. marcescens 2170 (a gift from T. Watanabe, Niigata University), and B. thuringiensis subsp. sotto strain T84A1 (a gift from M. Ohba, Kyushu University) were cultured in Luria-Bertani (LB) medium for the antibacterial activity assay. The yeast S. cerevisiae (a gift from Y. Nakagawa, Niigata University) was cultured with yeast malt broth (YMB) medium (9).
Both B. thuringiensis subsp. sotto strain T84A1 and B. thuringiensis subsp. kurstaki HD-73 were cultured for production of Cry1Aa and Cry1Ac, respectively, in NYS medium (37). Cry1Ab was obtained from the transformed E. coli JM109 harboring plasmid pYD4.0 encoding cry1Ab (a gift from K. Kanda, Saga University) cultured in LB broth containing 50 μg of ampicillin/ml (19). The insecticidal crystal proteins obtained as above were solubilized and activated with immobilized trypsin as described previously (18) and purified as according to Hossain et al. (15).
Purification of P252 from BBMVs of B. mori.
BBMVs of midgut of B. mori were prepared as described by Wolfersberger et al. (42), and BBMV proteins were solubilized with 1% Triton X-100 according to Shitomi et al. (36). P252 was purified from the soluble BBMV proteins by gel filtration and ion exchange column chromatography and was homogeneous in SDS-PAGE and gel filtration chromatography (15).
Sequence of P252 internal peptides.
The N terminus of P252 was shown to be blocked, and internal peptides were prepared with trypsin and V8 proteases as described by Hossain et al. (15); amino acid sequences of the three peptides were determined according to Shitomi et al. (36).
Preparation of Chlide.
Chlide a was prepared periodically from fresh spinach obtained from a local market, as its stability can be maintained only up to around 20 days at −20°C. The prepared Chlide was compared with the commercial Chlide, purchased from Sigma Aldrich Chemicals (St. Louis, MO). Chl and chlorophyllase were prepared according to the method of Pineau et al. (31) from fresh spinach and Chlide was obtained from the reaction of Chl with chlorophyllase as described by Ardao and Vennesland (3). Chl content was estimated with Arnon's equation (4).
The Chlide generated was separated with thin-layer chromatography (TLC) using precoated TLC plates (Silica Gel 60; Merck, Darmstadt, Germany) with a developing solvent of ethyl acetate-ethanol-methanol (80:20:10, vol/vol/vol) and visualized with UV light (360 nm) according to the method of Aiga and Sasa (1). The band with an Rf value of 0.41 was recovered as Chlide (see Fig. 1B, band 1) and the solvent was evaporated on a rotary evaporator. This Chlide was then suspended again in 50 mM Tris-HCl, pH 8.0, containing 100 mM NaCl and used in various experiments.
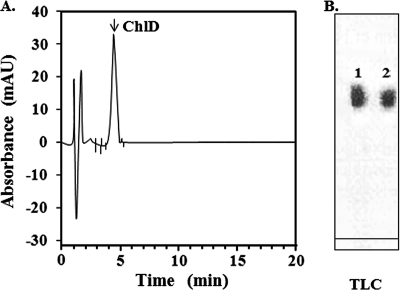
FIG. 1.
HPLC and TLC profiles of purified Chlide. Purity of Chlide prepared from spinach was checked with HPLC with a C18 reverse-phase column and TLC. (A) In HPLC, the sample was eluted with a linear gradient made by solvents A (methanol-28 mM thiobarbituric acid [70:30]) and B (99% methanol). The elution profile was monitored by absorbance at 664 nm. (B) In TLC, the sample was applied onto Silica Gel 60 plates (Merck) and developed with a solvent system (ethyl acetate-ethanol-methanol at 80:20:10, vol/vol/vol). In panel B, commercial Chlide from Sigma Aldrich (1) and indigenously prepared Chlide from commercially available spinach (2) were detected with UV at 360 nm. For experimental details, see “Preparation of Chlide” in Materials and Methods. AU, arbitrary units.
Production of pure Chlide was confirmed using a high-performance liquid chromatography (HPLC) instrument (Class-VP 5032; Shimadzu, Kyoto, Japan) equipped with an Eclipse XDB-C18 column (250 by 4.6 mm; 5-μm particle size; Shimadzu) using a linear gradient made by a mixture of solvents A (methanol-28 mM thiobarbituric acid [70:30]) and B (methanol) (41) with absorbance at 664 nm. The collected fractions containing Chlide were then evaporated to remove organic solvents, and the residue was solubilized in 50 mM Tris-HCl buffer, pH 8.0, where it was found to be stable for at least 20 days at −20°C.
In vitro incubation of P252 and Chlide to form P252-Chlide complex.
Various concentrations of purified P252 (0, 0.4, 0.8, and 1.0 μM) were incubated with 50 μM Chlide under aerobic conditions in the presence of light (aerobic-light) for 60 min at 25°C in a total volume of 1 ml of 50 mM Tris-HCl containing 100 mM NaCl, pH 8.0. The reactants were analyzed with scanning absorbance spectra using a UV-visible light spectrophotometer (UV 160-U; Shimadzu, Kyoto, Japan) with a wavelength scan from 500 nm to 700 nm. The experiment was done in an open-air bench under light at about 520 lx, equivalent to about 6,000 μmol m2 s−1.
The incubation was also done under aerobic-dark, anaerobic-light, and anaerobic-dark conditions to check the effect of air and light on binding reactions. Experiments in the dark were carried out inside a dark room with a safety red lamp, and for further safety, all glass containers were wrapped with aluminum foil. Experiments under anaerobic conditions were performed under streaming N2. P252 and Chlide were also incubated at various temperatures from 20 to 45°C.
HPLC analysis of reactant with fluorescence scanning spectra.
The incubation of P252 and Chlide was done as above, and reactants were lyophilized and redissolved into a small amount of 50 mM Tris-HCl, pH 8.0, containing 100 mM NaCl. The reactants were applied to an HPLC instrument (model HP-1100; Hewlett-Packard, Toronto, Canada) equipped with a C18 column, 250 by 4.6 mm with a 5-μm particle size (model 201TP54; Vydac, CA), and elution profiles were detected by a fluorescence spectrophotometer (RF-5301 PC; Shimadzu, Kyoto, Japan) connected to an 18-μl flow cell. Reactants were eluted with 0.1 M Tris-HCl buffer (pH 7.2) containing 0.1% SDS and 0.1 M NaCl as described previously (25). The fluorescence spectrum was obtained by exciting the samples at 495 nm and scanning between 500 to 700 nm at 25°C.
Hill analysis on the binding of P252 with Chlide.
P252 at a concentration of 1 μM was incubated with different concentrations of Chlide ranging from 0 to 70 μM. The formation of Bm252RFP was expressed based on the absorbance values at 600 nm, and Hill's coefficient was calculated as described previously (13).
Stability of P252-Chlide complex.
Stability of the reactants generated by the incubation of P252 and Chlide was monitored. The reactants were lyophilized and redissolved in 50 mM Tris-HCl buffer, pH 8.0, and allowed to stand for 22 h. Every hour, an aliquot of the solution was centrifuged using a 100-kDa filter spin column (YM-100; Microcon, CA), and fluorescent scanning of the filtrates was done with a fluorescence spectrophotometer and also by detecting absorbance values at 600 nm, both as described above.
SDS and native PAGE.
SDS-PAGE was performed as described previously (15). Native PAGE was done according to Kishimoto et al. (20). The proteins were visualized with silver or Coomassie brilliant blue staining. Protein bands having fluorescence were analyzed using an LAS 3000 charged-coupled-device gel analyzer by exciting the gel at 490 nm with a blue epi-illuminator (Fuji Film, Tokyo, Japan), and the emission was observed at 630 nm using a red epi-illuminator.
Protein quantification.
Solubilized or chromatographed BBM proteins were monitored by optical density at 280 nm and quantified by the Bradford method (6). For circular dichroism (CD) analysis, the protein concentration was estimated by the Kjeldahl method (35).
CD spectroscopy.
Far-UV CD experiments were performed with a spectropolarimeter (J-725; Jasco Co. Ltd., Tokyo, Japan) equipped with a 250-W xenon lamp. Spectra were recorded using cells with a 0.1-cm light path at a speed of 50 nm/min over 190 to 260 nm. CD spectra were obtained according to Yang et al. (43) with 20 mM Tris-HCl buffer containing 50 mM NaCl, pH 8.0, and the secondary structure contents were analyzed with the program SSE-338 (Jasco Co., Ltd.). The standard error was calculated from five independent experiments.
Bioassay of antimicrobial activity of Bm252RFP.
S. marcescens 2170, E. coli JM109, B. thuringiensis subsp. sotto TA81, or S. cerevisiae was grown for 12 h in LB broth or YMB medium from a single colony in 24-h precultured agar plates. After growth of bacteria in the LB medium and yeast in YMB medium, each for 12 h, the cell concentration of the cultures was adjusted to a 0.5 McFarland's turbidity standard using a spectrophotometer, as described previously (23). Effectors such as P252, Chlide, and Bm252RFP were dissolved in 50 mM Tris-HCl, pH 8.0, at various concentrations from 1 μM to 22 μM. The culture with adjusted cell number was inoculated with the effectors and incubated for 4 h at 37°C for bacteria and at 30°C for yeast. The aliquots of the cultures treated with effectors were plated in triplicate and incubated overnight at 37°C, and the number of CFU/ml was counted to obtain the 50% effective concentration (showing 50% inhibition in microbial growth [EC50]) calculated as described previously (5).
Bioassay of insecticidal activity.
Insecticidal activity of P252-Chlide was evaluated with a mortality study using 10 individual third-instar larvae of B. mori. P252, Chlide, and P252-Chlide were given to the 10 larvae at 20 μg/g of diet to each larva. Cry1Aa and Cry1Ab were used as the positive controls at 0.6 μg/g of diet and 4 μg/g of diet, respectively.
RESULTS
Amino acid sequence of P252 internal peptides.
Three internal peptides of P252 were sequenced as follows: P252-1, ATYLAGSGGVVTV; P252-2, ATYTAGTGNTVVVQN; and P252-3, ATYTLNSDNTITVFN. Homology searches were then performed. Initially, no homology data were available in the database (15), but recently the peptides were shown to share complete homology with a CBP sequence submitted by B. Mauchamp et al. (EMBL accession no. AM113746.1) except for one amino acid, a serine in CBP which is replaced by leucine in P252-1. Characteristics of the CBP of B. mori strain Nistari filed in the database are summarized as follows: full-length mRNA, 8,310 bp; number of amino acid residues, 2,720; molecular weight, 302,000 (26).
Purity of Chlide.
Chlide was prepared with fresh spinach from a local market, and finally 10 g of spinach yielded 0.08 mg of Chlide by TLC. This Chlide was checked for its purity with HPLC in a C18 reverse-phase column. A single Chlide peak was detected at 4.8 min using absorbance at 664 nm (Fig. 1A), and this retention time corresponded exactly to that of the Chlide from Sigma Aldrich described previously (41). Chlide was shown to have absorbance and fluorescence emission peaks at 664 nm and 670 nm, respectively, similar to a previous report (3).
In TLC, the purified Chlide migrated with an Rf value of 0.41, and the value was the same as that of commercial Chlide from Sigma Aldrich (Fig. 1B). The Chlide prepared from the spinach was estimated to have 93% purity in comparison to the commercial Chlide (Sigma Aldrich), yielding 0.8 mg of purified Chlide.
Chlide was dissolved in 50 mM Tris-HCl buffer, pH 8.0, containing 100 mM NaCl and used for various experiments. The Chlide in this buffer was stable for at least 20 days at −20°C.
Incubation of P252 with Chlide.
RFPs were reported to be synthesized when midgut protein(s) binds with Chlide. Incubation under aerobic-light conditions was reported to be important for RFP formation (12); therefore, we incubated P252 with Chlide to explore the formation of RFP (Fig. 2).
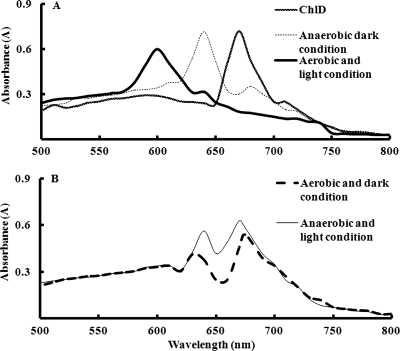
FIG. 2.
Absorption spectra of the mixture of P252 and Chlide incubated under aerobic-light conditions. P252 at various concentrations (line 1, 0; line 2, 0.4; line 3,0.8; and line 4,1 μM) was incubated with 50 μM Chlide for 60 min under aerobic-light conditions at 25°C. All the samples were suspended in a total volume of 1 ml of 50 mM Tris-HCl with 100 mM NaCl, pH 8.0. After the reaction the mixture was subjected to absorbance scanning from 500 nm to 700 nm with a spectrophotometer. The experiment under aerobic conditions was done in an open-air bench in the laboratory under light at about 520 lx, equivalent to about 6,000 μmol m2 s−1.
Various concentrations of purified P252 including 0 (Fig. 2, line 1), 0.4 (line 2), 0.8 (line 3), and 1.0 μM (line 4) were incubated with 50 μM Chlide under aerobic-light conditions for 60 min at room temperature, and reactants were subjected to scanning to check absorbance peaks (Fig. 2). When no P252 was incubated, a sharp peak was observed at 664 nm (Fig. 2, line 1). On the other hand, in the incubation of 1.0 μM P252 with Chlide, maximum absorbance was observed at 600 nm while no absorbance at 664 nm was observed (Fig. 2, line 4). Interestingly, when the concentration of P252 incubated with Chlide was reduced to less than 1.0 μM, the peak at 600 nm decreased, and the peak at 664 nm increased. Thus, the substance showing the absorbance at 600 nm was suggested to be a product resulting from the substance showing absorbance at 664 nm. Very interestingly, the absorbance at 600 nm corresponded to the previously reported RFPs (8, 27, 38), and the absorbance at 664 nm was the same as that of Chlide reported previously (3).
Under these conditions when we added 60 μM Chlide, no further increase in the absorbance value at 600 nm was observed, indicating that the production of the compound showing the absorbance was saturated at around 50 μM Chlide with 1 μM P252.
Effect of temperature on formation of P252-Chlide complex.
The reaction for the formation of the compound showing the absorbance peaks at 600 nm and 664 nm was monitored under different temperatures by keeping other parameters constant, as mentioned above (Table 1), and by monitoring the absorbance values at these fixed wavelengths. The maximum absorbance at 600 nm was observed at 37°C; and when carried out at 40°C, the reaction was acutely reduced, and only half the amount of the complex was formed compared to that formed at 37°C (Table 1), indicating that the stability of the complex is jeopardized at 40°C.
TABLE 1.
Influence of temperature on P252-Chlide complex in in vitro chemical synthesisa
| Temp (°C) | A664 | A600 |
|---|---|---|
| 23 | 0.745 | 0.311 |
| 30 | 0.516 | 0.516 |
| 37 | 0.115 | 0.587 |
| 40 | 0.324 | 0.289 |
Purified P252 and Chlide were mixed at various temperatures for 60 min, and the reactions were monitored by 664-nm absorbance, corresponding to specific absorption of Chlide, and by 600-nm absorption, corresponding to absorption of RFP.
Influence of light and air on the formation of complex.
We incubated 1 μM P252 and 50 μM Chlide under anaerobic-dark, anaerobic-light, aerobic-dark, and aerobic-light conditions to check the influence of light and air as these parameters were reported to be important for RFP formation (12). When the reaction was carried out under the aerobic-light conditions as a control, we again obtained a clear single peak at 600 nm without the 664 nm peak shown in Fig. 2 (Fig. 3A). Under anaerobic-dark conditions, however, a clear peak at 640 nm with almost negligible peaks at both 664 nm and 600 nm was observed. Interestingly, the 640-nm absorbance peak was found to be close to the intermediate peak reported by Uchida et al. (38). But in this experiment no peak was observed at 600 nm, which was suggested to be RFP (Fig. 3A, thin dotted line). The peak at 640 nm was also observed under anaerobic-light conditions (Fig. 3B); however, in this case Chlide was not completely utilized, and a peak at 664 nm was also observed (Fig. 3B, thin line). These results suggest that aerobic-light conditions were essential for the efficient in vitro chemical synthesis of the compound with a 600-nm absorbance. The peaks observed at around 640 and 630 nm could correspond to intermediates, as previously suggested (38).
FIG. 3.
Absorption spectra of the mixture of P252 and Chlide (ChlD) incubated under the various air and light conditions. P252 at a concentration of 1 μM was incubated with 50 μM Chlide for 60 min at 25°C under aerobic-light conditions as a control and under anaerobic-dark, aerobic-dark, and anaerobic-light conditions as described in the legend of Fig. 2. After the reactions, the mixture was subjected to absorbance scanning as described in the legend of Fig. 2.
HPLC analysis of product resulted from in vitro incubation of P252 and Chlide.
The ratio of 1 μM P252 with about 50 μM Chlide was suggested to be efficient for the in vitro chemical synthesis of the P252-Chlide complex as described above. The consumption of substrate and production of complex were analyzed using HPLC with a C18 column by scanning the products from 500 to 700 nm by exciting them at 495 nm. A well-defined peak was observed at the retention time of 4.8 min with 670 nm in the mixture before the start of the incubation. On the other hand, after the reaction, the fluorescence emission peak at 4.8 min disappeared almost completely, and a clear new single peak was observed with a 620-nm emission wavelength at 14.1 min. The fluorescent emission at 620 nm also matched that of RFP (8, 27, 38). P252 had no fluorescence within this emission region. This result confirmed that only the P252-Chlide complex could be recovered from the in vitro synthetic reaction.
Native PAGE analysis of the compound synthesized in the incubation of P252 and Chlide.
The reactants obtained in the incubation of P252 with Chlide were subjected to native PAGE to visualize red fluorescence to determine whether the P252-Chlide synthesized also emits red fluorescence as an RFP. The reactants after the incubation were lyophilized, dissolved into a small amount of the Tris-HCl buffer, and applied to a native PAGE gel (Fig. 4). The gel was then excited at 490 nm, and emission was detected at 630 nm (this is the instrument filter wavelength which can also detect fluorescence emission in the red region), as described in Materials and Methods. A clear bright red fluorescence was observed only in the case of the P252-Chlide complex (Fig. 4B). These data evidenced that P252-Chlide certainly emits red fluorescence at the level of an RFP. Thus, the P252-Chlide complex was identified as the B. mori 252-kDa RFP, or Bm252RFP.
FIG. 4.
Native PAGE analysis of P252, Chlide, and their incubated mixture. P252 and Chlide (ChlD) were mixed and incubated as described in the legend of Fig. 2 for 60 min, and after incubation the mixture was applied to a native 5% PAGE gel together with P252 and Chlide as controls. The gels were either stained by Coomassie brilliant blue (A) or left unstained (B). The developed materials in the unstained gel were detected with an excitation wavelength (blue epi-illuminator) at 490 nm and emission wavelength (red epi-illuminator) at 630 nm using an LAS 3000 instrument (Fuji film Co., Kanagawa, Japan) equipped with a charged-coupled device gel analyzer.
Characteristic absorption of Bm252RFP and Chlide.
Various previous reports revealed the absorbance specific to RFP and Chlide as 600 to 605 nm (8, 38) and 663 to 664 (3), respectively. On the other hand, fluorescence emission wavelengths specific to RFP and Chlide are reported as 620 to 625 nm (8, 38) and 670 nm (3), respectively. Our study indicated that Bm252RFP also has absorbance and fluorescence emission peaks at 600 nm and 664 nm, respectively, which closely resembled the values stated above.
Optimal condition of various parameters to produce P252-Chlide complex.
Various experimental conditions were studied as above to find the optimal conditions for efficient Bm252RFP in vitro chemical synthesis through intermediate or direct reactions. Optimum Bm252RFP production was obtained when 1 μM P252 was incubated at 37°C for 60 min under aerobic-light conditions with about 50 μM Chlide. Bm252RFP can also be formed from the intermediate complex obtained under anaerobic-dark conditions, as shown in Fig. 3A, by incubating it under the optimum conditions described above.
Stability of Bm252RFP.
Stability of Bm252RFP is an important issue to assign antimicrobial activity (to be discussed below) only to the complex and not to any metabolites of the Chlide released during the assay. So, stability of the purified Bm252RFP was analyzed by centrifuging the sample periodically in a spin column (YM-100) for 22 h, which is the incubation time period required for the antimicrobial activity assay. But neither Chlide nor any metabolites were observed in the filtrates even up to 20 h, which was indicated by the single characteristic fluorescence emission and absorbance peaks at 620 nm and 600 nm, respectively, as mentioned above (data not shown). Bm252RFP was stable at −20°C at least up to 31 days when stored in 50 mM Tris-HCl buffer, pH 8.0, containing 100 mM NaCl. This Bm252RFP dissolved in the above Tris-HCl buffer was used as the source of Bm252RFP in the following experiments.
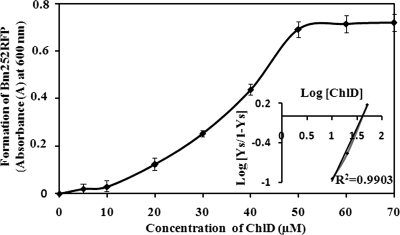
Substrate saturation curve of Bm252RFP formation.
Formation of Bm252RFP by P252 binding with Chlide was calculated and plotted against various concentrations of Chlide to explore the binding saturation. A sigmoidal curve (Fig. 5) was obtained, and Hill's coefficient was calculated as shown previously (13) to be 1.6, which indicates positive cooperation of the binding reaction (Fig. 5, inset). The dissociation constant of this binding was calculated from the Hill plot as 0.4 μM.
FIG. 5.
Chlide saturation curve and Hill's plot. Formation of Bm252RFP was calculated by monitoring the absorbance peak at 600 nm and was plotted against the Chlide (ChlD) concentration. A sigmoidal curve was obtained, and Hill's plot was calculated (inset picture) according to the following equation: log (Ys/[1 − Ys]) = n log [Chlide] − log Kd, where Y is the fractional concentration of Chlide calculated from absorbance values, [Chlide] is Chlide or ligand concentration, Kd is the dissociation constant, and n is the Hill coefficient. Bars represent standard deviations calculated from five different experiments.
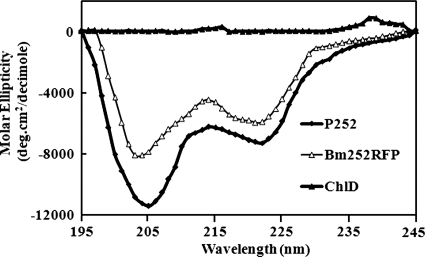
CD spectrum of Bm252RFP.
CD analysis was done to check the effect of a change in the secondary conformation of either P252 or Chlide on the formation of Bm252RFP (Fig. 6). Chlide was shown to have no secondary structure and did not show any specific ellipticity in the 190- to 260-nm region, whereas native P252 was calculated to be composed of 39.8% ± 2.16% β-structure, 58.6% ± 2.27% other conformations, and almost no α-helices. On the other hand, the CD spectrum of Bm252RFP clearly indicated a different pattern from that of P252, and the β-structure substantially decreased to 21.6% ± 3.14%. It also had no α-helices. Since Chlide has no secondary structure, the decrease in the β-content should be due to the change in the P252 conformation.
FIG. 6.
CD spectra of P252, Chlide (ChlD), and Bm252RFP. Far-UV CD experiments were performed with a spectropolarimeter fitted with a 250-W xenon lamp. The samples were solubilized with 20 mM Tris-HCl buffer with 100 mM NaCl, pH 8.0. The standard error was calculated for five experiments. For experimental details, see Materials and Methods. deg, degrees.
Antimicrobial activity of Bm252RFP.
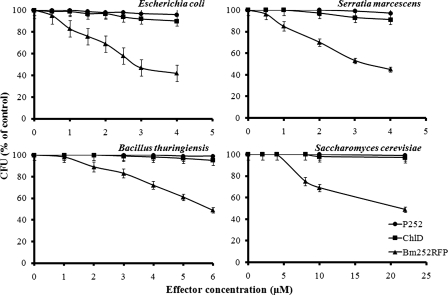
RFPs are known to have antiviral activity (2, 10, 29, 30, 32) which is attributed to the chromophore formed by the Chlide binding reaction. We intended to evaluate if Bm252RFP had antimicrobial activity using microorganisms. Antimicrobial activity of Bm252RFP against E. coli, S. marcescens, B. thuringiensis, and S. cerevisiae was evaluated using various concentrations of effectors and measured as the number of CFU/ml. The effector concentration was increased until EC50 values were obtained, as described in Materials and Methods, and kanamycin was used as the positive control. P252, Chlide, and Tris-HCl buffer were also used alone as controls, but none of them showed any activity against any of the microbes. However, Bm252RFP had antibacterial activity against E. coli, S. marcescens, and B. thuringiensis with EC50s of 2.82, 2.94, and 5.92 μM, respectively. Kanamycin had an EC50 of 1.16 μM. Bm252RFP was also active against S. cerevisiae with an EC50 of 21.6 μM, as seen in Fig. 7.
FIG. 7.
Antimicrobial effect of Bm252RFP against E. coli, S. marcescens, B. thuringiensis, and S. cerevisiae. The microorganisms were grown in LB or YMB medium and inoculated with 50 mM Tris-HCl buffer, pH 8.0, containing effectors of P252, Chlide (ChlD), or Bm252RFP at various concentrations for 4 h at 37 or 30°C. The aliquots of the cultures were plated and incubated overnight at 37°C. The inhibitory effects were expressed based on the number of CFU/ml. Bars represent standard deviations calculated with six different experiments.
Insecticidal activity of Bm252RFP.
Bm252RFP was shown to have antimicrobial activity, and so a bioassay was performed to check whether our in vitro synthesized Bm252RFP can be toxic to B. mori larvae also. Cry1Aa and Cry1Ab were used as the positive controls at 0.6 μg/g of diet and 4 μg/g of diet, respectively, showing 100% mortality of the larvae. However, even 20 μg/g of diet of either P252 or Chlide or Bm252RFP did not kill any B. mori larvae. This indicates that Bm252RFP is toxic only to bacteria and yeast and not to the insect.
DISCUSSION
In the search for Cry1A binding proteins, we purified and characterized a 252-kDa protein, P252, from the B. mori midgut epithelial cell membrane that had been shown to bind strongly with B. thuringiensis Cry1Aa, Cry1Ab, and Cry1Ac with 30, 180 and 20 nM Kd values, respectively (15). Immunohistochemical analysis of P252 using the antiserum showed that, although the majority of P252 is localized on the surface of the epithelial cells of the midgut, a significant amount is also localized in the basolateral or even the basement region of the midgut cells (14, 16). These findings together with the Kd values suggested that at least the P252 located on the apical membrane can compete for binding to the Cry1A toxins in the midgut tract with APN and/or a cadherin-like protein having a Kd in the nanomolar range. These characteristics resembling that of a receptor led us to investigate the role of P252 in the Cry toxin-mediated insecticidal mechanisms.
A homology search using the three internal peptides of P252 indicated a very high sequence similarity with the CBP present in the B. mori midgut. This search result led us to explore the ability of P252 to bind with Chlide. As we have characterized sequentially above, we have clearly shown that P252 binds with Chlide and that in this pigment-protein binding reaction, a red fluorescent complex, termed Bm252RFP, was formed. Various characteristics, such as absorbance at about 600 to 605 nm and a fluorescence emission peak at about 620 to 625 nm, of Bm252RFP matched with the previously reported data corresponding to historically reported RFPs (8, 26, 27, 30, 38). These RFPs reported were found in not only the midgut membrane but also the digestive juice and feces of silkworm larvae (2, 10, 29, 32). In addition, very interestingly, Bm252RFP was also shown to have strong antibacterial activity.
Initial in vitro synthesis experiments with BBM proteins suggested that RFP formation occurs through the binding of midgut protein with Chlide (10). Also, the formation of Chlide-midgut protein complex was shown to first form an intermediate complex having a 650-nm absorbance peak and then was shown to eventually form RFP with an absorbance peak at about 601.5 nm under aerobic-light conditions (38). Analogous with the bile pigment metabolism (34), Chlide is suggested to be converted to α-hydroxy-Mg/CBP complex in the presence of oxygen. The porphyrin ring is then cleaved to form a pigment-protein complex (RFP) containing three conjugated pyrrole groups, and the prosthetic group is accepted to be closely related to biladiene-like compounds as described in Kusuda et al. (24).
Our P252-Chlide reaction mixture also showed an absorbance peak at 640 nm under anaerobic-dark conditions (Fig. 3A). This is suggested to be an intermediate with a partially cloven porphyrin ring of Chlide, as described previously (24, 38). However, under aerobic-light conditions, such a cleavage might have been directly completed, and a clear 600-nm peak was observed as previously reported (38); and in our optimum reaction, 10 μg of P252 protein was required to form RFP in 60 min. RFP in vitro chemical synthesis experiments were done previously (10, 38) with midgut protein fractions whose characteristics are unknown, and in these studies about 4 mg of midgut protein fractions was employed, which might be due to the low purity of the actual protein.
Recently Mauchamp et al. cloned the cDNA of CBP, and the molecular size was deduced to be 302 kDa. The structure was also deduced to have 15 lipocalin repeats and was termed polycalin, a novel member of the lipocalin family (26). Since P252 internal peptides were homologous to these nucleotide sequences and since P252 was also evidenced as CBP, it was suggested to be similar, if not the same, as lipocalin CBP even though three internal small peptides had no lipocalin repeat. Lipocalin is a cytosolic fatty acid binding protein having a characteristic β-barrel structure. Interestingly, the secondary structure of P252 was also rich in β-structures but not in α-structures (Fig. 6). Based on these data, it is highly possible that P252, whose native molecular mass is about 980 kDa, also has a β-barrel structure. Although evidence of a β-barrel in P252 and the binding site of Chlide has not yet been ascertained, it is fascinating to envision that Chlide binds with this barrel. A Hill's coefficient of 1.6 indicated a positive cooperative reaction such that binding of one molecule of Chlide with P252 increases the affinity of other Chlide molecules as an allosteric enzyme.
If the binding of Chlide with P252 takes place via the β-barrel structure de facto, the conformation of the structure must drastically change upon binding with Chlide, since secondary structure analysis by CD suggested that contents of the β-structure in native P252 was reduced by 50% on the formation of Bm252RFP (Fig. 6). We also suggest that the binding between P252 and Chlide was strong due to a low dissociation constant, 0.4 μM. Furthermore, the binding was observed to be very stable in storage at least up to 31 days at −20°C. Bm252RFP was allowed to stand for various time intervals, and the supernatants were filtered through a 100,000-molecular-weight-cutoff membrane, but neither Chlide nor Chlide derivatives were observed even up to 22 h. Bm252RFP showed almost the same intensity in red fluorescence and also held its typical spectral characteristics during the incubation time period necessary to carry out the assay of antimicrobial activity. Bm252RFP was stable at pH 9.5 for a month (data not shown), which is the approximate pH of the lepidopteran midgut juice; this suggests that Bm252RFP may be stable in the B. mori midgut juice until almost the last stadium larvae even at room temperature. If this is the case, antibacterial activity of Bm252RFP must be important for the defense of B. mori against harmful microbes.
Bm252RFP had antibacterial activity against E. coli, S. marcescens, and B. thuringiensis with EC50s of 2.4, 2.9, and 5.9 μM, respectively. These values were comparable to that of kanamycin, estimated as 1.16 μM in our experiments. It also had comparatively less activity against S. cerevisiae, with an EC50 of 21.6 μM. P252 did not show any inhibitory effect against any of the above microbes. Therefore, only Bm252RFP can work as an antibacterial factor. An excess amount of Chlide showed only a weak inhibitory activity, which might be due to the photo-oxidation of a small amount of Chlide during our experiment (Fig. 7). If this is true, the reaction of Chlide to form a chromophore that can have antimicrobial activity may not easily occur spontaneously, and catalysts such as P252 (CBP) might be necessary. This activity may be due to the formation of a stable radical generated by the binding of Chlide; however, at this moment we have no evidence to advance the discussion further.
We checked the insecticidal activity of Bm252RFP using the third instar of B. mori larvae, but no activity was observed even at 20 μg/g of diet. Neither Chlide nor P252 had any insecticidal effect on the larvae at the same concentration. Thus, Bm252RFP has adverse effects only on the microbes and not on the insects. So, as we discussed above, it can possibly play an immune role against pathogenic microbes in the insect larvae.
It will be interesting to explore the role of Bm252RFP-Cry1A in the insecticidal mechanism. This study will help us to understand the physiological function(s) of Bm252RFP.
Acknowledgments
This work was supported, in part, by research grants from the Ministry of Education, Culture, Sports, Science and Technology (grants 13306006 and 12558069 to H.H.). A grant for the Promotion of the Niigata University Research Project (2004; to H.H.) also partially supported this work.
Footnotes
Published ahead of print on 11 January 2008.
REFERENCES
- 1.Aiga, I., and T. Sasa. 1970. Studies on chlorophyllase of Chlorella protothecoides II. Formation of atypical chlorophyllide a. Plant Cell Physiol. 11:161-165. [Google Scholar]
- 2.Aizawa, K. 1962. Antiviral substance in the gut-juice of the silkworm, Bombyx mori (Linnaeus). J. Insect Pathol. 4:72-76. (In Japanese.) [Google Scholar]
- 3.Ardao, C., and B. Vennesland. 1960. Chlorophyllase activity of spinach chloroplastin. Plant Physiol. 35:368-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnon, D. I. 1949. Copper enzyme in isolated chloroplast. Polyphenol oxidase in Beta vulgaris. Plant Physiol. 24:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergman, P., L. Johansson, H. Wan, A. Jones, R. L. Gallo, G. H. Gudmundsson, T. Hökfelt, A. B. Jonsson, and B. Agerberth. 2006. Induction of the antimicrobial peptide CRAMP in the blood-brain barrier and meninges after meningococcal infection. Infect. Immun. 74:6982-6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 7.Bravo, A., I. Gomez, J. Conde, C. Munoz-Garay, J. Sanchez, R. Miranda, M. Zhuang, S. S. Gill, and M. Soberon. 2004. Oligomerization triggers binding of a Bacillus thuringiensis Cry1Ab pore-forming toxin to aminopeptidase N receptor leading to insertion into membrane microdomains, Biochim. Biophys. Acta 1667:38-46. [DOI] [PubMed] [Google Scholar]
- 8.Doi, M., Y. Shioi, and T. Sasa. 1986. Purification and characterization of a blue-colored red-fluorescent protein from the silkworm (Bombyx mori L.) larvae. Comp. Biochem. Physiol. 83B:569-573. [Google Scholar]
- 9.Haley, L. D. 1971. Identification of yeasts in clinical microbiology laboratories. Am. J. Med. Technol. 37:125-131. [PubMed] [Google Scholar]
- 10.Hayashiya, K. 1978. Red fluorescent protein in the digestive juice of the silkworm larvae fed on host-plant mulberry leaves. Entomol. Exp. Appl. 24:228-236. [Google Scholar]
- 11.Hayashiya, K., J. Nishida, and U. Uchida. 1976. The mechanism of formation of the red fluorescent protein in the digestive juice of silkworm larvae—the formation of chlorophyllide-a. J. Appl. Entomol. Zool. 20:37-43. (In Japanese.) [Google Scholar]
- 12.Hayashiya, K., U. Uchida, and J. Nishida. 1976. Comparison of anti-viral activities of the silkworm larvae reared in light and in darkness in relation to the formation of red fluorescent protein (RFP). J. Appl. Entomol. Zool. 20:139-143. (In Japanese.) [Google Scholar]
- 13.Hill, A. V. 1910. The possible effects of the aggregation of the molecules of hemoglobin on its dissociation curves. J. Physiol. 40:4-7. [Google Scholar]
- 14.Hossain, D. M., T. Hayakawa, Y. Shitomi, K. Itoh, T. Mitsui, S. Sato, and H. Hori. 2007. Histochemical analysis of Bacillus thuringiensis Cry1A toxin binding to midgut epithelial cells of Bombyx mori. Pestic. Biochem. Physiol. 87:30-38. [Google Scholar]
- 15.Hossain, D. M., Y. Shitomi, T. Hayakawa, M. Higuchi, T. Mitsui, R. Sato, and H. Hori. 2004. Characterization of a novel plasma membrane protein, expressed in the midgut epithelia of Bombyx mori that binds to Cry1A toxins. Appl. Environ. Microbiol. 70:4604-4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hossain, D. M., Y. Shitomi, Y. Nanjo, D. Takano, T. Nishiumi, T. Hayakawa, T. Mitsui, R. Sato, and H. Hori. 2005. Localization of a novel 252-kDa plasma membrane protein that binds Cry1A toxins in the midgut epithelia of Bombyx mori. Appl. Entomol. Zool. 40:125-135. [Google Scholar]
- 17.Ihara, H., T. Uemura, M. Masuhara, S. Ikawa, S. Sugimoto, A. Wadano, and M. Himeno. 1998. Purification and partial amino acid sequences of the binding protein from Bombyx mori for CryIAa δ-endotoxin of Bacillus thuringiensis. Comp. Biochem. Physiol. B 120:197-204. [DOI] [PubMed] [Google Scholar]
- 18.Indrasith, L. S., K. Ogiwara, M. Minami, T. Iwasa, T. Maruyama, N. Suzuki, S. Asano, K. Sakanaka, and H. Hori. 1991. Processing of delta endotoxin from Bacillus thuringiensis subsp. Kurstaki HD-1 and HD-73 by immobilized trypsin and chymotrypsin. Appl. Entomol. Zool. 26:485-492. [Google Scholar]
- 19.Kim, Y. S., K. Kanda, F. Kato, and A. Murata. 1998. Effect of the carboxyl-terminal portion of Cry1Ab in Bacillus thuringiensis on toxicity against the silkworm, Bombyx mori. Appl. Entomol. Zool. 33:473-477. [Google Scholar]
- 20.Kishimoto, T., H. Hori, D. Takano, Y. Nakano, M. Watanabe, and T. Mitsui. 2001. Rice α-mannosidase digesting the high mannose glycopeptide of glutelin. Physiol. Plant 112:15-24. [DOI] [PubMed] [Google Scholar]
- 21.Knight, P. J., N. Crickmore, and D. J. Ellar. 1994. The receptor for Bacillus thuringiensis Cry1A(c) δ-endotoxin in the brush border membrane of the lepidopteran Manduca sexta is aminopeptidase N. Mol. Microbiol. 11:429-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knowles, B. H., and D. J. Ellar. 1987. Colloid-osmotic lysis is a general feature of the mechanism of action of Bacillus thuringiensis δ-endotoxins with different insect specificity. Biochim. Biophys. Acta 924:509-518. [Google Scholar]
- 23.Koch, A. L. 1970. Turbidity measurements of bacterial cultures in some available commercial instruments. Anal. Biochem. 38:252-259. [DOI] [PubMed] [Google Scholar]
- 24.Kusuda, J., and J. Mukai. 1971. A biliprotein from the digestive juice of Bombyx mori L., its purification and partial structural study of the chromophore. Comp. Biochem. Physiol. 39B:317-323. [Google Scholar]
- 25.Mantoura, R. F. C., and C. A. Llewellyn. 1983. The rapid determination of algal chlorophyll and carotenoid pigments and their breakdown products in natural waters by reverse-phase high-performance liquid chromatography. Anal. Chim. Acta 151:297-314. [Google Scholar]
- 26.Mauchamp, B., C. Royer, A. Garel, A. Jalabert, M. D. Rocha, A.-M. Grenier, V. Labas, J. Vinh, K. Mita, K. Kadono, and G. Chavancy. 2006. Polycalin (chlorophyllide a binding protein): a novel, very large fluorescent lipocalin from the midgut of the domestic silkworm Bombyx mori L. Insect Biochem. Mol. Biol. 36:623-633. [DOI] [PubMed] [Google Scholar]
- 27.Mukai, J., J. Inouye, and S. Akune. 1969. A red, fluorescent protein from silkworm. Agric. Biol. Chem. 33:125-127. [Google Scholar]
- 28.Nagamatsu, Y., S. Toda, T. Koike, Y. Miyoshi, S. Shigematsu, and M. Kogure. 1998. Cloning, sequencing, and expression of the Bombyx mori receptor for Bacillus thuringiensis insecticidal CryIA(a) toxin. Biosci. Biotechnol. Biochem. 62:727-734. [DOI] [PubMed] [Google Scholar]
- 29.Nagaraja-Sethuraman, B., N. Nagaraju, and R. K. Datta. 1993. Purification and partial characterization of antiviral protein in silkworm, Bombyx mori. Indian J. Sericult. 32:63-67. [Google Scholar]
- 30.Nakazawa, H., E. Tsuneishi, K. M. Ponnuvel, S. Furukawa, A. Asaoka, H. Tanaka, J. Ishibashi, and M. Yamakawa. 2004. Antiviral activity of a serine protease from the digestive juice of Bombyx mori larvae against nucleopolyhedrovirus. Virology 321:154-162. [DOI] [PubMed] [Google Scholar]
- 31.Pineau, B., G. Dubertret, J. Joyard, and R. Douce. 1986. Fluorescence properties of the envelope membranes from spinach chloroplasts. Detection of protochlorophyllide. J. Biol. Chem. 261:9210-9215. [PubMed] [Google Scholar]
- 32.Ponnuvel, K. M., H. Nakazawa, S. Furukawa, A. Asaoka, J. Ishibashi, H. Tanaka, and M. Yamakawa. 2003. A lipase isolated from the silkworm Bombyx mori shows antiviral activity against nucleopolyhedrovirus. J. Virol. 77:10725-10729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sangadala, S., F. S. Walters, L. H. English, and M. J. Adang. 1994. A mixture of Manduca sexta aminopeptidase and phosphatase enhances Bacillus thuringiensis insecticidal CryIA(c) toxin binding and 86Rb+-K+ efflux in vitro. J. Biol. Chem. 269:10088-10092. [PubMed] [Google Scholar]
- 34.Schmidt, R., and A. F. McDonagh. 1979. Formation and metabolism of bile pigments in vivo, p. 257-292. In D. Dolphin (ed.), The porphyrins, vol. VI. Academic Press, New York, NY. [Google Scholar]
- 35.Shaw, J., and L. C. Beadle. 1949. A simplified ultra-micro Kjeldahl method for the estimation of protein and total nitrogen in fluid samples of less than 1.0 μl. J. Exp. Biol. 26:15-23. [DOI] [PubMed] [Google Scholar]
- 36.Shitomi, Y., T. Hayakawa, D. M. Hossain, M. Higuchi, K. Miyamoto, K. Nakanishi, R. Sato, and H. Hori. 2006. A novel 96-kDa aminopeptidase localized on epithelial cell membranes of Bombyx mori midgut, which binds to Cry1Ac toxin of Bacillus thuringiensis. J. Biochem. (Tokyo) 139:223-233. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki, M., H. Hori, K. Ogiwara, S. Asano, R. Sato, M. Ohba, and H. Iwahana. 1992. Insecticidal spectrum of a novel isolate of Bacillus thuringiensis serovar japonensis. Biol. Control 2:138-142. [Google Scholar]
- 38.Uchida, Y., and K. Hayashiya. 1981. Biosynthesis of a red fluorescent protein (RFP) in the digestive juice of the silkworm larvae, Bombyx mori L. (Lepidoptera: Bombycidae). Formation of a chlorophyllide-a midgut protein complex. J. Appl. Entomol. Zool. 25:94-100. (In Japanese.) [Google Scholar]
- 39.Vadlamudi, R. K., E. Weber, I. Ji, T. H. Ji, and L. A. Bulla, Jr. 1995. Cloning and expression of a receptor for an insecticidal toxin of Bacillus thuringiensis. J. Biol. Chem. 270:5490-5494. [DOI] [PubMed] [Google Scholar]
- 40.Valaitis, A. P., M. K. Lee, F. Rajamohan, and D. H. Dean. 1995. Brush border membrane aminopeptidase-N in the midgut of the gypsy moth serves as the receptor for the CryIA(c) δ-endotoxin of Bacillus thuringiensis. Insect Biochem. Mol. Biol. 25:1143-1151. [DOI] [PubMed] [Google Scholar]
- 41.Van Heukelem, L., and C. S. Thomas. 2001. Computer-assisted high-performance liquid chromatography method development with applications to the isolation and analysis of phytoplankton pigments. J. Chromatogr. A 910:31-49. [DOI] [PubMed] [Google Scholar]
- 42.Wolfersberger, M., P. Luethy, A. Maurer, P. Parenti, F. V. Sacchi, B. Giordana, and G. M. Hanozet. 1987. Preparation and partial characterization of amino acid transporting brush border membrane vesicles from the larval midgut of the cabbage butterfly (Pieris brassicae). Comp. Biochem. Physiol. 86A:301-308. [Google Scholar]
- 43.Yang, J. T., C. S. Wu, and H. M. Martinez. 1986. Calculation of protein conformation from circular dichroism. Methods Enzymol. 130:208-269. [DOI] [PubMed] [Google Scholar]
- 44.Yao, H.-P., X.-F. Wu, and K. Gokulamma. 2006. Antiviral activity in the mulberry silkworm, Bombyx mori L. J. Zhejiang Univ. Sci. A 7:350-356. [Google Scholar]
- 45.Zhang, X., M. Candas, N. B. Griko, R. Tausigg, and L. A. Bulla, Jr. 2006. A mechanism of cell death involving an adenylyl cyclase/PKA signaling pathway is induced by the Cry1Ab toxin of Bacillus thuringiensis. Proc. Natl. Acad. Sci. USA 103:9897-9902. [DOI] [PMC free article] [PubMed] [Google Scholar]