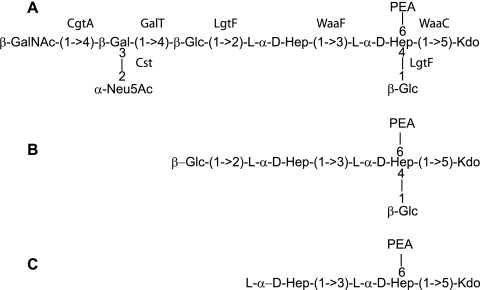Abstract
We report isolation and characterization of Campylobacter jejuni 81-176 lgtF and galT lipooligosaccharide (LOS) core mutants. It has been suggested that the lgtF gene of C. jejuni encodes a two-domain glucosyltransferase that is responsible for the transfer of a β-1,4-glucose residue on heptosyltransferase I (Hep I) and for the transfer of a β-1,2-glucose residue on Hep II. A site-specific mutation in the lgtF gene of C. jejuni 81-176 resulted in expression of a truncated LOS, and complementation of the mutant in trans restored the core mobility to that of the wild type. Mass spectrometry and nuclear magnetic resonance of the truncated LOS confirmed the loss of two glucose residues, a β-1,4-glucose on Hep I and a β-1,2-glucose on Hep II. Mutation of another gene, galT, encoding a glycosyltransferase, which maps outside the region defined as the LOS biosynthetic locus in C. jejuni 81-176, resulted in loss of the β-(1,4)-galactose residue and all distal residues in the core. Both mutants invaded intestinal epithelial cells in vitro at levels comparable to the wild-type levels, in marked contrast to a deeper inner core waaC mutant. These studies have important implications for the role of LOS in the pathogenesis of Campylobacter-mediated infection.
Lipooligosaccharide (LOS) is the major constituent of the outer leaflet of the outer membranes of gram-negative bacteria such as Campylobacter jejuni. LOS consists of two covalently linked domains: (i) the lipid A moiety, a hydrophobic membrane anchor consisting of lipopolysaccharide, and (ii) a nonrepeating core oligosaccharide (OS) consisting of an inner core region and an outer core region. Based on structural studies, the Campylobacter inner core region of LOS has been shown to be highly conserved among C. jejuni serotypes (19, 23). This region consists of a single 3-deoxy-d-manno-octulosonic residue (Kdo) attached to the lipid A and two l-glycero-d-manno-heptose (ld-Hep) residues attached to the Kdo. In addition, the inner core region of certain strains has been shown to contain a glucose residue and a phosphoethanolamine moiety attached to the first heptose residue. The outer core region of LOS of Campylobacter strains consists of various hexoses, N-acetylglucosamine, and N-acetylneuraminic acid (NeuNAc) (sialic acid), which have been shown to mimic human gangliosides (13, 18, 19). Sialylation of the LOS core of C. jejuni is thought to play a role in immune evasion and has been demonstrated to increase resistance to normal human serum (13). However, this molecular mimicry can also result in an autoimmune response that can lead to Guillain-Barre syndrome, a serious postinfection paralysis (23). It is believed that the Campylobacter core OS plays an important role in processes associated with pathogenesis of diarrheal disease, such as colonization and invasion of intestinal epithelial cells (13).
The structure of the LOS core of C. jejuni 81-176 is shown in Fig. 1A. Genomic studies have made predictions of genes involved in LOS biosynthesis, but there have been only limited genetic analyses of LOS biosynthesis in C. jejuni (9). For C. jejuni strain 81-176, inner core mutants (with mutations in waaC and waaF) and outer core mutants (with mutations in cgtA and sialic acid biosynthetic genes) have been described (13, 18, 19). In this study, we identified and characterized the lgtF and galT genes involved in the biosynthesis of the core OS of C. jejuni 81-176.
FIG. 1.
Schematic diagram of the LOS structures of C. jejuni 81-176 and mutants. (A) The structure of the 81-176 LOS has been reported to vary between the structure shown, which mimics GM3 ganglioside, and a structure lacking the terminal GalNAc that mimics GM2 ganglioside (13). The assignment of the roles of the cgtA, waaF, waaC, and cst genes in the biosynthesis of the 81-176 locus has been reported previously (9, 13, 18, 19). (B) Structure of the LOS core of the galT mutant of 81-176. (C) Structure of the LOS core of the lgtF mutant of 81-176.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
C. jejuni wild-type strain 81-176 (Penner serotypes 23 and 36) has been described previously (2-4, 10, 15, 16). C. jejuni strains were grown in Mueller-Hinton (MH) broth under microaerophilic conditions at 37°C. When necessary, the cultures were supplemented with kanamycin (30 μg/ml), ampicillin (100 μg/ml), or chloramphenicol (30 μg/ml). In the invasion experiments, cells were grown in MH biphasic cultures supplemented with antibiotics as appropriate.
DNA cloning and sequence analysis.
Two overlapping plasmids were identified in an ordered library of partially Sau3A-digested 81-176 DNA cloned into λ-ZAPII that included part of the LOS locus (10; L. C. Holder and P. Guerry, unpublished data). DNA sequencing was performed with a Perkin-Elmer Applied Biosystems model 3100 automated DNA sequencer. Custom primers were synthesized with a Perkin-Elmer Applied Biosystems model 292 DNA synthesizer.
Generation of mutants.
Mutants were constructed using a Tn5-based in vitro transposition system (Epicenter, Madison, WI) in which the Cmr cassette from pRY109 (30) was cloned into pEZ::TN pMOD as previously described (12). The in vitro reaction was performed according to the manufacturer's instructions using the appropriate cloned DNA as the target. The reaction products were transformed into Escherichia coli DH5α by electroporation. The plasmid DNAs from individual transformants were sequenced using primers that read out from within the Cmr cassette to determine the insertion point and the orientation within each gene. An insertion was selected in which the Cmr cassette had been inserted in the same orientation that the target genes had been transcribed to minimize polarity on downstream genes. Plasmids were used to transform C. jejuni 81-176, with selection on MH agar supplemented with chloramphenicol (15 μg/ml) (28). A successful mutation was verified by performing PCR with primers bracketing the Cmr insertion point to confirm that the DNA had been inserted by a double crossover.
Complementation of lgtF::cat (CJJ1152) and galT::cat (CJJ1165).
The LOS genes of 81-176 are summarized in Table 1. The locus tags are indicated for 81-176 using the TIGR annotation, and the corresponding locus, when present, for NCTC 11168 is also indicated. For simplicity, the 81-176 locus tags were shortened (e.g., CJJ81176_1152 is referred to as CJJ1152). The lgtF gene was PCR amplified from 81-176 using the following primers: LgtFor (5′-CGG GAT CCC GAA GAA CTG ACA CTT TAT CAA GCA C-3′) and LgtRev (5′-GGA ATT CCT TCT ACG TTG TAT ATT GGT ATA ACT ACA CC-3′). In addition, the CJ1165 gene was PCR amplified from 81-176 using the following primers: galTFor (5′-CGG GAT CCC GAT ACG GCT AGA ATT CAA GAA ATG C-3′) and galTRev (5′-GGAATTC CAT CAT AGA AGT AAG TAA ACA ATT GCT ATT TC-3′). The resulting amplicons, which were bracketed with BamHI and EcoRI sites, were cloned behind the σ28 promoter in BamHI-EcoRI-digested pCPE107/28 (21). The resulting kanamycin-resistant shuttle plasmid was transformed into DH5α cells containing RK212.2 and was mobilized into 81-176 lgtF::cat and 81-176 galT::cat with selection on kanamycin and chloramphenicol, respectively.
TABLE 1.
LOS genes of C. jejuni 81-176
| Strain 81-176 locus tag | Strain NCTC 11168 allele | Gene | Annotation | Reference(s) |
|---|---|---|---|---|
| CJJ81176_1150 | Cj1133 | waaC | Heptosyltransferase I | 19 |
| CJJ81176_1151 | Cj1134 | htrB | Lipid A myristoyl acyltransferase | 19 |
| CJJ81176_1152 | Cj1135 | lgtF | Two-domain glucosyltransferase | 19; this study |
| CJJ81176_1153 | Cj1136 | none | Galactosyltransferase | 19 |
| CJJ81176_1155 | Cj1139c | cgtB | β-1,3-Galactosyltransferasea | 19 |
| CJJ81176_1157 | Cj1140 | cst | α-2,3-Sialyltransferase | 13 |
| CJJ81176_1158 | Cj1141 | neuB | Sialic acid synthase | 13 |
| CJJ81176_1159 | Cj1142 | neuC | UDP GlcNAc transferase | 13 |
| CJJ81176_1160 | Cj1143 | cgtA | β-1,4-GalNAc transferase | 13 |
| CJJ81176_1161 | Cj1144 | neuA | CMP-sialic acid synthetase | 13 |
| CJJ81176_1162 | None | none | Acetyltransferase | 9 |
| CJJ81176_1163 | Cj1146c | waaV | Glycosyltransferase | 18 |
| CJJ81176_1164 | Cj1148 | waaF | Heptosyltransferase II | 18 |
| CJJ81176_1165 | None | galT | β-1,4-Galactosyltransferase | 18; this study |
| CJJ81176_1166 | Cj1149c | gmhA1 | Phosphoheptose isomerase | 18 |
cgtB appears to be a pseudogene in 81-176.
Extraction of LOS and preparation of core OS.
LOS samples were prepared from whole-cell lysates that were subjected to complete digestion with proteinase K as described by Hitchcock and Brown (14). LOS extracts were separated on 16% Tricine gels (Invitrogen, Carlsbad, CA) and then silver stained (Bio-Rad, Hercules, CA). Crude LOS for gas-liquid chromatography (GLC)-mass spectrometry (MS) and 1H nuclear magnetic resonance (1H-NMR) was isolated by hot phenol-water extraction (29). The water layer was dialyzed overnight and subjected to ultracentrifugation at 105,000 × g overnight at 4°C. The pellet containing the crude LOS was solubilized in sterile water and lyophilized. The LOS was treated with 1% acetic acid for 1 h at 100°C to cleave the acid-labile ketosidic linkage that connects the core OS to the lipid A. The insoluble lipid A was removed by mild centrifugation (3,000 × g), and the supernatant was applied to a column (1 m by 1 cm) of Bio-Gel P2 for purification. In each case one carbohydrate fraction was obtained after the void volume, as detected by the phenol-sulfuric acid assay (7).
Sugar composition analysis and linkage analysis of core OS.
For analysis of the sugar composition of the core OS, the monosaccharide components were derivatized into the alditol acetate derivatives as described by Sawardeker and Sloneker (27). The core was hydrolyzed in 4 M trifluoroacetic acid at 100°C for 4 h and then reduced in H2O with NaBD4. The alditols were then acetylated in acetic anhydride (100°C for 2 h) using residual sodium acetate as the catalyst. The alditol acetate derivatives were then characterized by GLC (DB-5, 30 m, isothermal at 200°C) and by GLC-MS using the following conditions: for the lgtF sample, DB-5, 30 m, temperature increased from 190 to 240°C at a rate of 2°C/min, and a Hewlett-Packard GC-MS instrument; and for the galT sample, DB-17, 30 m, temperature increased from 150 to 240°C at a rate of 2°C/min, and a ThermoFinnigan PolarisQ GC-MS instrument. For sugar linkage analysis the core was methylated with methyl iodide in dimethyl sulfoxide and NaOH (6). The methylated core was hydrolyzed in 4 M trifluoroacetic acid at 100°C for 4 h, followed by reduction in H2O with NaBD4. The permethylated alditols were then acetylated in acetic anhydride (100°C for 2 h) with residual sodium acetate as the catalyst. The permethylated alditol acetate derivatives were then characterized by GLC (DB-5, 30 m, isothermal at 200°C) and GLC-MS (DB-5, 30 m, 190°C for 60 min).
NMR and MS of core OS.
The 1H- and 31P-NMR experiment was performed using D2O with a Bruker 400-MHz instrument at 21°C with water resonance as the reference (δH 4.828). In the analysis of the galT core OS, 1H-NMR was also performed at 35°C to observe the anomeric resonances coresonating with the HOD peak. The electron spray (ES)-MS experiment with the lgtF core OS was carried out using a Crystal model 310 CE instrument (ATI Unicam, Boston, MA) coupled to a Q-Star quadrupole/time of flight mass spectrometer (Applied Biosystems/Sciex, Concord, Canada) via a micro-ion spray interface (21). The sheath solution (isopropanol-methanol, 2:1) was delivered at a flow rate of 1 ml/min. Separation was obtained using a ca. 90-cm-long bare fused-silica capillary and 10 mM ammonium acetate in deionized water (pH 9.0) containing 5% methanol. A voltage of 15 kV was typically applied at the time of injection. The outlet of the capillary was tapered to obtain an inside diameter of ca. 15 mm using a laser puller (Sutter Instruments, Novato, CA). The ES-MS analysis of the galT core OS was performed with a Waters Micromass Global Q-TOF Ultima mass spectrometer. ES-MS data were acquired in both negative- and positive-ion modes. The voltages applied to the capillary needle and cone were 3.0 kV and 35 V, respectively. The temperature of the source was 80°C. Ammonium acetate (1 to 5 mM) and ammonium hydroxide (0.2 to 2%) were added to the sample prior to analyses.
INT407 cell invasion assays.
Invasion assays were done as previously described (2, 3, 25, 31), except that water was substituted for 0.01% Triton for lysis of the monolayer. Approximately 1.5 × 106 bacteria were added to a monolayer of approximately 4 × 105 INT407 cells grown in minimal essential medium. After centrifugation at 200 × g for 5 min, the assay mixtures were incubated at 37°C for 2 h. Each monolayer was washed twice in Hanks’ balanced salt solution, and fresh, prewarmed minimal essential medium supplemented with 100 μg/ml gentamicin was added to the wells for an additional 2 h of incubation at 37°C to kill the extracellular bacteria. The monolayer was washed four times in Hanks’ balanced salt solution and lysed with water for 30 min at room temperature on an orbital shaker. Released intracellular bacteria were enumerated by plate counting. Invasion was expressed as the percentage of the inoculum surviving the gentamicin treatment. Statistical analyses were done using Graphpad Instat software.
Nucleotide sequence accession numbers.
The DNA sequences of the regions of the 81-176 chromosome containing the lgtF and galT genes were deposited previously in the GenBank database under accession numbers AY862985 and AY423899, respectively.
RESULTS
Isolation and characterization of the C. jejuni 81-176 lgtF mutant.
The region of the 81-176 chromosome containing the lgtF gene (CJJ81176_1152) has been described previously (19) (Table 1). This open reading frame (ORF) encoded a predicted protein consisting of 515 amino acids with a predicted molecular mass of 61 kDa that showed 88% identity and 94% similarity to Cj1135 of C. jejuni NCTC 11168, which was annotated as a putative two-domain glycosyltransferase. In addition, the N-terminal amino acid sequence encoded by this ORF showed significant sequence similarity with the LgtF protein (33% identity and 54% similarity) of Haemophilus ducreyi. The lgtF gene of H. ducreyi has been shown to encode a β-1,4-glucosyltransferase involved in assembly of the inner core OS of LOS (8). The C. jejuni 81-176 lgtF mutant was disrupted by insertional mutagenesis, as described in Materials and Methods. The positions and orientations of the random insertions of the cat gene within the clone were determined by sequence analysis. One insertion that occurred 757 bp from the translational start of lgtF in a nonpolar orientation was selected to generate C. jejuni mutants. Plasmid DNA from this cat insertion was used to electroporate C. jejuni 81-176 with selection for Cmr. Several Cmr transformants were confirmed by PCR in order to verify that the insert had integrated via a double crossover (data not shown).
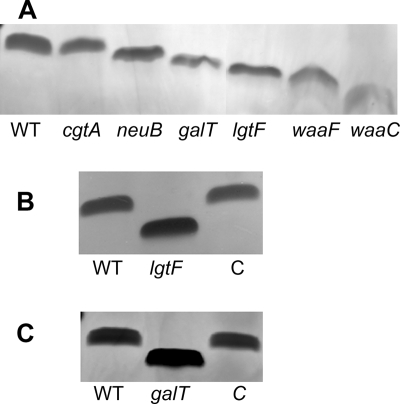
We examined the LOS cores from proteinase K-digested whole cells of wild-type strain 81-176 and the lgtF mutant by electrophoresis on Tricine gels, followed by silver staining. The LOS from the lgtF mutant migrated more quickly than that of parental strain 81-176 (Fig. 2A and B), suggesting a possible defect in the core region of LOS. The 81-176 lgtF mutant also showed no significant difference in growth rate, motility, or capsular expression as determined by immunoblotting compared to the wild type. In addition, the mutant did not show increased sensitivity to antibiotics, such as novobiocin, unlike deeper inner core waaF (18) and waaC (19) mutants. The mobility of the LOS core of the lgtF mutant was restored to that of the wild type following complementation in trans with the expression plasmid pCPE/LGTF (Fig. 2B).
FIG. 2.
Comparison of the mobilities of the LOS cores of 81-176 mutants. Proteinase K-digested whole-cell preparations were electrophoresed on 16% Tricine gels and silver stained. (A) The lanes contained (from left to right) 81-176 (WT), the cgtA mutant (13), the neuB mutant (13), the galT mutant, the waaF mutant (18), and the waaC mutant (19). (B) The lanes contained (from left to right) 81-176, the lgtF mutant, and complement of the lgtF mutant (C). (C) The lanes contained (from left to right) 81-176, the galT mutant, and complement of the galT mutant (C).
Structural analysis of the truncated LOS from the 81-176 lgtF mutant.
Previous studies (1, 13) have described the structure of the C. jejuni 81-176 wild-type core OS (Fig. 1A). The core OS from the lgtF mutant was extracted and analyzed by GLC-MS as described in Materials and Methods.
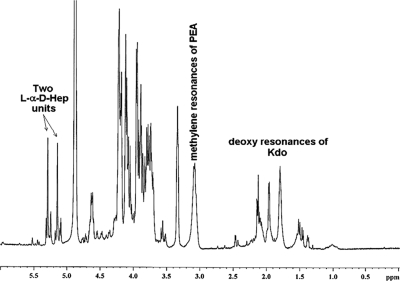
The lgtF core OS mutant was also examined by 1H-NMR and 31P-NMR. The 1H-NMR spectrum (Fig. 3) of the lgtF core OS showed two major equivalent α-anomeric resonances (broad singlets) characteristic of the mannose configuration, in this case ld-Hep, at δH 5.15 and 5.29. The 1H-NMR spectrum also revealed the presence of deoxy resonances characteristic of Kdo between δH 1.4 and 2.15 and a pair of methylene protons pertaining to a 2-amino-ethylphosphate moiety (PEA) (NH2-CH2-CH2-PO4−→) at δH 3.15. Spectroscopic confirmation of the presence of the PEA unit in the lgtF core OS was obtained by 31P-NMR, which revealed a phosphorus resonance at δP 0.75.
FIG. 3.
1H-NMR spectrum of C. jejuni 81-176 lgtF core OS. The spectrum shows two α-anomeric resonances of ld-Hep, the deoxy signals of the Kdo unit, and the methylene resonances of the PEA moiety. No GalNAc N-acetyl resonances were observed.
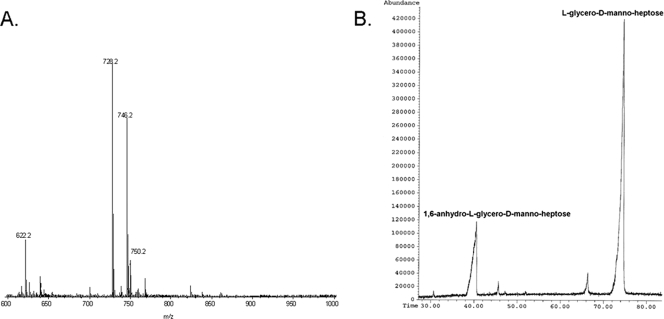
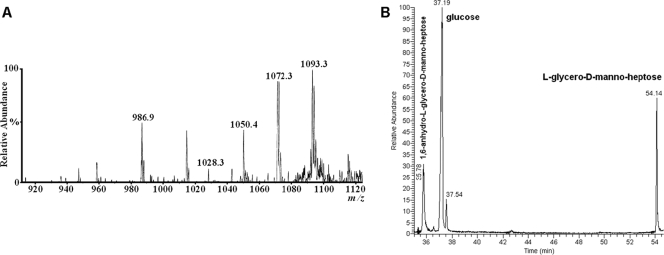
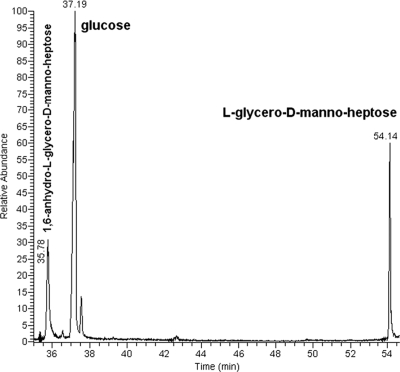
ES-MS of the lgtF core OS (Fig. 4A) yielded a molecular ion at m/z 728.19 and 746.2 (728.2 + H2O) corresponding to a molecule containing two ld-Hep units, one Kdo residue, and one PEA moiety. MS/MS of the m/z 728.19 ion generated secondary ions at m/z 536.2 (728.19-192) for PEA→ld-Hep→Kdo and at m/z 316.15 (728-412) for PEA→ld-Hep. Thus, taking into account the composition and linkage analysis data described above, the primary ion at m/z 728.19 originated from the structure ld-Hep→[PEA→]ld-Hep→Kdo. The sugar composition analysis of the lgtF core OS (Fig. 4B) revealed only the presence of ld-Hep, which also included the alditol acetate derivative of 1,6-anhydro-l-glycero-d-manno-heptose that was generated by the hydrolysis procedure (5). No glucose (Glc), galactose (Gal), and N-acetylgalactosamine (GalNAc) residues found in the parent wild-type core OS (Fig. 1) (1, 13) were detected in the C. jejuni 81-176 lgtF mutant. The sugar linkage analysis indicated that ld-Hep was present as a terminal [ld-Hep-(1→] and as smaller amounts of a 3,6-disubstituted unit [→3/6)-ld-Hep-(1→].
FIG. 4.
ES-MS and GLC-MS profiles of the alditol acetate derivatives of C. jejuni 81-176 lgtF core OS. (A) ES-MS analysis showing ions at m/z 728.19 and 746.2 (728.2 + H2O) corresponding to a molecule containing two ld-Hep units, one Kdo residue, and one PEA moiety. MS/MS analysis of m/z 728.19 yielded ions at m/z 536.2 (728.19-192) for PEA→ld-Hep→Kdo and at m/z 316.15 (728-412) for PEA→ld-Hep. (B) GLC-MS analysis of the alditol acetate derivatives showing the presence of only ld-Hep. No Glc, Gal, or GalNAc was detected.
Collectively, the structural data obtained revealed that the lgtF core OS elaborated only the inner core structure of the wild-type core OS (1, 22), a phosphorylated diheptosyl structure terminated at the reducing end by a Kdo residue (Fig. 1C).
Identification of the β-1,4-galactosyltransferase gene.
We also sought to identify the gene encoding the β-1,4-galactosyltransferase (Fig. 1A). A potential candidate, CJJ1165 (Table 1), was located between waaV and gmhA1 (19). This ORF encoded a predicted 252-amino-acid protein with a molecular mass of 61 kDa that was 35% identical to a β-1,4-glucosyltransferase, Lex2B, from H. ducreyi (11). This 81-176 ORF was disrupted by insertional mutagenesis of a clone of this region, as described in Materials and Methods. Several Cmr transformants were confirmed by PCR in order to verify that the insert had integrated via a double crossover (data not shown).
The LOS of the CJJ1165 mutant migrated more quickly on Tricine gels than the LOS of parental strain 81-176 (Fig. 2A and 2C), suggesting a possible defect in the core region of LOS. The CJJ1165 mutant did not differ significantly from the wild type in growth rate, motility, or capsular expression as determined by immunoblotting. The LOS core mobility was restored to that of the wild type (Fig. 2C) when the CJJ1165 mutant strain was complemented in trans. Based on subsequent chemical analysis of this mutant, we annotated CJJ1165 as galT (see below).
Structural analysis of the truncated LOS of the 81-176 galT mutant.
The ES-MS spectra of the galT core OS yielded a molecular ion (M-H) at m/z 1050.4 corresponding to an OS composed of two ld-Hep units, two Glc units, one Kdo residue, and one PEA moiety (Fig. 5A). The sugar composition analysis revealed that the galT core OS was composed of Glc and ld-Hep (Fig. 5B). The sugar linkage analysis indicated that Glc was present as terminal units [Glc-(1→] and that ld-Hep was present as a 2-substituted unit [→2)-ld-Hep-(1→] and as a 3,4-disubstituted ld-Hep unit [→3/4)-ld-Hep-(1→]. Traces of a 3,4,6-trisubstituted ld-Hep [→3/4/6)-ld-Hep-(1→] were also observed.
FIG. 5.
ES-MS and GLC-MS profiles of the alditol acetate derivatives of the core OS of C. jejuni galT. (A) ES-MS analysis of the galT core OS yielded a molecular ion (M-H) at m/z 1050.4 corresponding to an OS composed of two ld-Hep units, two Glc units, one Kdo residue, and one PEA moiety. The higher m/z ions represent Na adducts. (B) GLC-MS analysis showing the presence of Glc and ld-Hep (1,6-anhydro-ld-Hep emanates from ld-Hep during acidic hydrolysis).
The 1H-NMR spectra (Fig. 6) revealed that the galT core OS expressed four anomeric protons, at δH 5.28 (A) and 5.08 (B) corresponding to α-ld-Hep units and at δH 4.85 (C) and 4.62 (D) belonging to β-Glc units. Resonances at δH 1.9 and 2.3 corresponding to the deoxy protons (H-3) of Kdo were also readily observed, as were the methylene resonances of PEA at δH 3.13. The structural data obtained showed that the galT core OS lacked the Gal and GalNAc units, while the remainder of the core was expressed as it was in the wild-type core OS (1, 22) (Fig. 1B).
FIG. 6.
1H-NMR spectrum of C. jejuni 81-176 galT core OS. The spectrum shows two α-anomeric resonances of ld-Hep units (A and B) and two β-anomeric resonances of Glc units (C and D); the deoxy signals of the Kdo unit and the methylene resonances of the PEA moiety are also evident. The inset shows the anomeric region of the 1H-NMR spectrum obtained at 35°C to reveal anomeric resonance C, which coresonates with the HOD peak at 21°C. No GalNAc N-acetyl resonances were detected.
Effect of LOS mutations on invasion of INT407 cells.
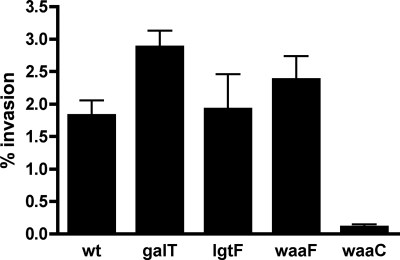
Invasion of intestinal epithelial cells is thought to be a critical step in the pathogenesis of C. jejuni (2, 3, 32). We previously found that a waaF mutant of 81-176 was defective in invasion of INT407 cells using an assay in which the monolayer was lysed with 0.01% Triton (18). However, additional characterization of the waaF mutant indicated that it showed enhanced sensitivity to detergent compared to the wild type (data not shown). Using a modified invasion assay in which the monolayer was lysed with water rather than detergent, there was no statistically significant difference in the invasion levels of wild-type strain 81-176 (1.82% ± 0.7% of the input bacteria) and waaF (2.37% ± 0.97%), lgtF (1.92% ± 1.43%), and galT (2.88% ± 0.56%) mutants, as shown in Fig. 7. There was a statistically significant difference (P < 0.05) between wild-type strain 81-176 and the waaC mutant (0.1% ± 0.085%), however, as originally reported (19).
FIG. 7.
Invasion of INT407 cells by strain 81-176 and isogenic LOS mutants. Invasion assays were performed as described in Materials and Methods using water to lyse the monolayers. The data are the means and standard deviations of four to nine independent experiments done in duplicate. The only mutant that showed a statistically significant difference from wild-type strain 81-176 (wt) was the waaC mutant (P < 0.05).
DISCUSSION
Although isolation and characterization of lgtF mutants of a number of other organisms, such as Neisseria meningitidis and H. ducreyi, have been reported (8, 17), this is the first report of such a mutant of C. jejuni. Based on sequence alignment with other characterized glycosyltransferases, the homologs of lgtF in various strains of C. jejuni have been suggested to have two domains that are responsible for the transfer of a β-1,4-glucose residue on heptosyltransferase I (Hep I) and a β-1,2-glucose residue on Hep II (9). The N-terminal domain (amino acid residues 1 to 250) shares significant sequence homology to β-1,4-glucosyltransferases of N. meningitidis and H. ducreyi (8, 17), whereas the second domain, consisting of residues 250 to 515, was suggested to be responsible for the transfer of a β-1,2-glucose residue on Hep II. The structural data presented here confirmed that the LOS core of the 81-176 lgtF mutant showed loss of both the β-1,4 glucose residue on Hep I and the β-1,2-glucose residue on Hep II (Fig. 3 and 4). The mass spectrum of the truncated LOS species in the lgtF mutant may be interpreted as the mass spectrum of an LOS species that contains two ld-Hep residues, phosphoethanolamine, and a Kdo residue (Fig. 1C and 4). However, unequivocal evidence of the dual function of the Campylobacter LgtF protein as a β-1,4- and β-1,2-glucosyltransferase requires additional biochemical characterization. The previously published structure of a waaF mutant of 81-176, which contained a core composed of a single heptose, a β-1,4-linked glucose, phosphoethanolamine, and a Kdo residue (18), suggests that LgtF is capable of transferring the β-1,4-glucose independent of the β-1,2 linkage. It may be that addition of the β-1,4-glucose is a prerequisite for addition of the β-1,2-glucose.
The core OS domain of C. jejuni 81-176 contains only one β-1,4-galactose residue attached to the β-1,2 glucose on Hep II. Although the gene adjacent to lgtF, CJJ1153, which corresponds to Cj1136, was annotated as a galactosyl transferase gene (9), the data presented here indicate that CJJ1165, which encoded a protein that was 35% identical to Lex2B, a β-1,4-glucosyltransferase from H. ducreyi, encodes the β-1,4-galactosyltransferase, and this gene has been annotated as galT. This is the first LOS gene that has been identified that maps outside the region defined as the LOS locus between waaC and waaF as defined by Parker et al. (9). Thus far, a limited number of the sequenced strains of Campylobacter possess this galT homolog. We propose that these strains have a β-1,4-galactose moiety in their LOS cores in place of the more common β-1,3-galactose moiety that is seen in Campylobacter strains such as NCTC 11168 (23). However, additional biochemical evidence is required to confirm that GalT is a galactosyltransferase. This study represents the completion of the identification of the genes encoding enzymes that transfer sugar residues involved in biosynthesis of the 81-176 LOS, as shown in Fig. 1A and 2A. There are two putative glycosyltransferases in this region whose function remains to be determined, CJJ1153 and CJJ1163.
Invasion of intestinal epithelial cells is thought to be a critical step in the pathogenesis of C. jejuni 81-176 (20), and we used a genetic approach to study the role of LOS in this process. Two outer core 81-176 LOS mutants, with mutations in the neuC1 and cgtA genes, have also been assayed to determine their abilities to invade human INT407 cells (13). The neuC1 mutant, which lacked NeuNAc in the LOS core, invaded INT407 cells at the same levels as the wild-type strain but showed increased sensitivity to human serum. The cgtA mutant, encoding an N-acetylgalactosaminyl transferase involved in transfer of the terminal GalNAc (13), showed a modest, but statistically significant, increase in invasion of INT407 cells (13). In this study, two additional mutants, lgtF and galT mutants, were compared to the wild type and the previously described waaF and waaC mutants using a modified invasion assay that released intracellular bacteria with water lysis in place of detergent. Most of these mutants invaded INT407 cells at levels comparable to the wild-type levels; the only exception was the deepest rough mutant, the waaC mutant, whose level of invasion was significantly reduced. The lgtF and galT mutants of 81-176 described here did not show any changes in novobiocin resistance, unlike the more deeply truncated waaC and waaF mutants (18, 19). These data collectively suggest that the waaC mutant is more defective for intracellular survival, likely because of associated membrane changes that often occur in deep rough mutants (24, 26). However, the waaC mutant has also been shown to lack the 3-O methyl group on the heptose of the capsular polysaccharide, indicating possible coordinate regulation of capsular and LOS biosynthesis (19). Thus, the current data cannot exclude the possibility that the capsular change is also involved in the invasion defect. The availability of a complete set of 81-176 mutants with increasing truncations of the core, as shown in Fig. 2A, should facilitate additional studies of the role of LOS in the virulence of C. jejuni.
Acknowledgments
M.I.K. gives special thanks to the members of the Guerry laboratory for technical assistance.
This work was supported by NMRC work unit 6000.RAD1.DA3.A0308 to P.G. from the Military Infectious Diseases Program. M.I.K. was a recipient of an ASEE-NAVY summer faculty internship. M.A.M. was supported in part from a grant from the National Sciences and Engineering Research Council of Canada.
The views expressed in this paper are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, the Department of Defense, or the U.S. Government.
Footnotes
Published ahead of print on 21 December 2007.
REFERENCES
- 1.Aspinall, G., A. McDonald, T. Raju, H. Pang, A. Moran, and J. Penner. 1993. Chemical structures of the core regions of Campylobacter jejuni serotypes O:1, O:4, O:23, and O:36 lipopolysaccharides. Eur. J. Biochem. 2131017-1027. [DOI] [PubMed] [Google Scholar]
- 2.Bacon, D. J., R. A. Alm, D. H. Burr, L. Hu, D. J. Kopecko, C. P. Ewing, T. J. Trust, and P. Guerry. 2000. Involvement of a plasmid in virulence of Campylobacter jejuni 81-176. Infect. Immun. 684384-4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bacon, D. J., C. M. Szymanski, D. H. Burr, R. P. Silver, R. A. Alm, and P. Guerry. 2001. A phase-variable capsule is involved in virulence of Campylobacter jejuni 81-176. Mol. Microbiol. 40769-777. [DOI] [PubMed] [Google Scholar]
- 4.Black, R. E., M. M. Levine, M. L. Clements, T. P. Hughes, and M. J. Blaser. 1988. Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 157472-479. [DOI] [PubMed] [Google Scholar]
- 5.Chaby, R., and P. Szabo. 1976. Formation of 1,6-anhydro-l-glycero-d-manno-heptopyranose during acidic hydrolysis and methanolysis of a polysaccharide from Bordetella pertussis. Carbohydr. Res. 49489-493. [DOI] [PubMed] [Google Scholar]
- 6.Ciucanu, I., and F. Kerek. 1984. A simple and rapid method for the permethylation of carbohydrates. Carbohydr. Res. 131209-217. [Google Scholar]
- 7.Dubois, M., K. Giles, J. Hamilton, P. Rebers, and F. Smith. 1956. Colormetric method for the determination of sugars and related substances. Anal. Chem. 28350-356. [Google Scholar]
- 8.Filiatrault, M. J., B. W. Gibson, B. Schilling, S. Sun, R. S. Munson, Jr., and A. A. Campagnari. 2000. Construction and characterization of Haemophilus ducreyi lipooligosaccharide (LOS) mutants defective in expression of heptosyltransferase III and β-1,4-glucosyltransferase: identification of LOS glycoforms containing lactosamine repeats. Infect. Immun. 683352-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert, M., M. F. Karwaski, S. Bernatchez, N. M. Young, E. Taboada, J. Michniewicz, A. M. Cunningham, and W. W. Wakarchuk. 2002. The genetic bases for the variation in the lipo-oligosaccharide of the mucosal pathogen, Campylobacter jejuni. Biosynthesis of sialylated ganglioside mimics in the core oligosaccharide. J. Biol. Chem. 277327-337. [DOI] [PubMed] [Google Scholar]
- 10.Goon, S., J. F. Kelly, S. M. Logan, C. P. Ewing, and P. Guerry. 2003. Pseudaminic acid, the major modification on Campylobacter flagellin, is synthesized via the Cj1293 gene. Mol. Microbiol. 50659-671. [DOI] [PubMed] [Google Scholar]
- 11.Griffin, R., A. D. Cox, K. Makepeace, J. C. Richards, E. R. Moxon, and D. W. Hood. 2003. The role of lex2 in lipopolysaccharide biosynthesis in Haemophilus influenzae strains RM7004 and RM153. Microbiology 1493165-3175. [DOI] [PubMed] [Google Scholar]
- 12.Guerry, P., C. P. Ewing, T. E. Hickey, M. M. Prendergast, and A. P. Moran. 2000. Sialylation of lipooligosaccharide cores affects immunogenicity and serum resistance of Campylobacter jejuni. Infect. Immun. 686656-6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guerry, P., C. M. Szymanski, M. M. Prendergast, T. E. Hickey, C. P. Ewing, D. L. Pattarini, and A. P. Moran. 2002. Phase variation of Campylobacter jejuni 81-176 lipooligosaccharide affects ganglioside mimicry and invasiveness in vitro. Infect. Immun. 70787-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hitchcock, P., and T. Brown. 1983. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 154269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu, L., and D. Kopecko. 1999. Campylobacter jejuni 81-176 associates with microtubules and dynein during invasion of human intestinal cells. Infect. Immun. 674171-4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu, L., and D. Kopecko. 2000. Interactions of Campylobacter with eukaryotic cells: gut luminal colonization and mucosal invasion mechanisms, p. 191-217. In I. Nachamkin and M. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, DC.
- 17.Kahler, C. M., R. W. Carlson, M. M. Rahman, L. E. Martin, and D. S. Stephens. 1996. Two glycosyltransferase genes, lgtF and rfaK, constitute the lipooligosaccharide ice (inner core extension) biosynthesis operon of Neisseria meningitidis. J. Bacteriol. 1786677-6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanipes, M. I., L. C. Holder, A. T. Corcoran, A. P. Moran, and P. Guerry. 2004. A deep-rough mutant of Campylobacter jejuni 81-176 is noninvasive for intestinal epithelial cells. Infect. Immun. 722452-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanipes, M. I., E. Papp-Szabo, P. Guerry, and M. A. Monteiro. 2006. Mutation of waaC, encoding heptosyltransferase I in Campylobacter jejuni 81-176, affects the structure of both lipooligosaccharide and capsular carbohydrate. J. Bacteriol. 1883273-3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kopecko, D. J., L. Hu, and K. J. Zaal. 2001. Campylobacter jejuni—microtubule-dependent invasion. Trends Microbiol. 9389-396. [DOI] [PubMed] [Google Scholar]
- 21.Larsen, J. C., C. Szymanski, and P. Guerry. 2004. N-linked protein glycosylation is required for full competence in Campylobacter jejuni 81-176. J. Bacteriol. 1866508-6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, J., A. D. Cox, D. W. Hood, E. R. Moxon, and J. C. Richards. 2004. Application of capillary electrophoresis-electrospray-mass spectrometry to the separation and characterization of isomeric lipopolysaccharides of Neisseria meningitidis. Electrophoresis 252017-2025. [DOI] [PubMed] [Google Scholar]
- 23.Moran, A., J. Penner, and G. Aspinall. 2000. Campylobacter lipopolysaccharides, p. 241-257. In I. Nachamkin and M. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, DC.
- 24.Nikaido, H. 1976. Outer membrane of Salmonella typhimurium. Transmembrane diffusion of some hydrophobic substances. Biochim. Biophys. Acta 433118-132. [DOI] [PubMed] [Google Scholar]
- 25.Oelschlaeger, T. A., P. Guerry, and D. J. Kopecko. 1993. Unusual microtubule-dependent endocytosis mechanisms triggered by Campylobacter jejuni and Citrobacter freundii. Proc. Natl. Acad. Sci. USA 906884-6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roantree, R. J., T. T. Kuo, and D. G. MacPhee. 1977. The effect of defined lipopolysaccharide core defects upon antibiotic resistances of Salmonella typhimurium. J. Gen. Microbiol. 103223-234. [DOI] [PubMed] [Google Scholar]
- 27.Sawardeker, J., and J. Sloneker. 1967. Quantitative determination of monosaccharides as their alditol acetates by gas liquid chromatography. Anal. Chem. 371602-1604. [Google Scholar]
- 28.Wang, Y., and D. Taylor. 1990. Natural transformation in Campylobacter species. J. Bacteriol. 172949-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westphal, O. K., and K. Jann. 1965. Bacterial lipopolysaccharide. Extraction with phenol-water and further applications of the procedure. Methods Carbohydr. Chem. 583-91. [Google Scholar]
- 30.Yao, R., R. A. Alm, T. J. Trust, and P. Guerry. 1993. Construction of new Campylobacter cloning vectors and a new mutational cat cassette. Gene 130127-130. [DOI] [PubMed] [Google Scholar]
- 31.Yao, R., D. H. Burr, P. Doig, T. J. Trust, H. Niu, and P. Guerry. 1994. Isolation of motile and non-motile insertional mutants of Campylobacter jejuni: the role of motility in adherence and invasion of eukaryotic cells. Mol. Microbiol. 14883-893. [DOI] [PubMed] [Google Scholar]
- 32.Yao, R., D. H. Burr, and P. Guerry. 1997. CheY-mediated modulation of Campylobacter jejuni virulence. Mol. Microbiol. 231021-1031. [DOI] [PubMed] [Google Scholar]