Abstract
Unlike Escherichia coli, the cyanobacterium Synechocystis sp. strain PCC 6803 is insensitive to chill (5°C) in the dark but rapidly losses viability when exposed to chill in the light (100 μmol photons m−2 s−1). Preconditioning at a low temperature (15°C) greatly enhances the chill-light tolerance of Synechocystis sp. strain PCC 6803. This phenomenon is called acquired chill-light tolerance (ACLT). Preconditioned wild-type cells maintained a substantially higher level of α-tocopherol after exposure to chill-light stress. Mutants unable to synthesize α-tocopherol, such as slr1736, slr1737, slr0089, and slr0090 mutants, almost completely lost ACLT. When exposed to chill without light, these mutants showed no or a slight difference from the wild type. When complemented, the slr0089 mutant regained its ACLT. Copper-regulated expression of slr0090 from PpetE controlled the level of α-tocopherol and ACLT. We conclude that α-tocopherol is essential for ACLT of Synechocystis sp. strain PCC 6803. The role of α-tocopherol in ACLT may be based largely on a nonantioxidant activity that is not possessed by other tocopherols or pathway intermediates.
In temperate and subtropical lakes, bloom-forming cyanobacteria, such as Microcystis spp., overwinter in the sediment (25, 37, 40). As the temperature increases in spring, on the surface of sediments where light is sufficient to reinitiate the growth, cyanobacterial cells reinvade the water column (3). Due to the high light availability, early access to increasing temperature, and resuspension by wind-induced mixture or bioturbation in spring, shallow areas of a lake are the main sites for recruitment of benthic cells, providing the inocula for the pelagic population (3, 38, 39). Studies with Synechocystis sp. strain PCC 6803, a widely used mesophilic cyanobacterium research model, showed that light greatly accelerated the loss of cell viability at a chilling temperature (43). Consequently, how to survive the chill-light stress on shallow sediments should be one of the major challenges for overwintering cyanobacteria. In this paper, we report that preconditioning of Synechocystis sp. strain PCC 6803 at 15°C, a typical temperature in autumn or early winter in temperate or subtropical lakes, could greatly enhance its chill-light tolerance.
Either cold or a high level of light leads to photoinhibition, resulting in the production of reactive oxygen species (ROS) in photosynthetic organisms (16); therefore, a combination of chill and light imposes significant photooxidative stress on plants and cyanobacteria. ROS can oxidize biomolecules, forming peroxides and ketones. ROS react especially with the acyl chains of polyunsaturated fatty acids (PUFA) or their membrane lipid residues, triggering the autocatalytic chain reaction of lipid peroxidation (11). Nonenzymatic and enzymatic scavenging mechanisms are involved in ROS detoxification (16). In the higher plant Arabidopsis, tocopherols can limit lipid oxidation during seed storage, germination, and early seedling development (31) and in cooperation with the xanthophyll cycle may protect membrane lipids from photooxidation under chill-light stress (15). However, evidence has also been presented that Arabidopsis tocopherol-deficient mutants exhibit a cold-sensitive phenotype independent of photooxidative damage (22). In Synechocystis sp. strain PCC 6803, tocopherols can protect membranes from lipid peroxidation caused by an exposure to exogenous PUFA (linoleic or linolenic acid) in high-light conditions (21).
Tocopherols are divided into four types, α-, β-, γ-, and δ-tocopherol, which differ from each other in the number and position of methyl substituents on the chromanol ring, and the tocopherol synthesis pathway has been elucidated (7). In Synechocystis sp. strain PCC 6803, the synthesis starts by transformation of p-hydroxyphenylpyruvate into homogentisic acid, which is catalyzed by p-hydroxyphenylpyruvate dioxygenase, encoded by slr0090 (6). Afterwards, homogentisate phytyltransferase, encoded by slr1736, transforms homogentisic acid into 2-methyl-6-phytylbenzoquinone (MPBQ), the first tocopherol intermediate (5, 32, 33). Following the formation of MPBQ, the pathway diverges: (i) catalyzed by tocopherol cyclase encoded by slr1737, MPBQ is cyclized into δ-tocopherol (30), which is further methylated, catalyzed by γ-tocopherol methyltransferase encoded by slr0089, producing β-tocopherol (35), and (ii) catalyzed by MPBQ methyltransferase encoded by sll0418, MPBQ is transformed into 2,3-dimethyl-6-phytylbenzoquinone (DMPBQ) after methylation (1, 4, 34), which is cyclized by the tocopherol cyclase into γ-tocopherol and further methylated by γ-tocopherol methyltransferase, producing α-tocopherol (35). In vitro, each molecule of α-, β-, γ-, and δ-tocopherol is capable of protecting up to 220, 120, 100, and 30 molecules of PUFA, respectively (12). In vivo studies with Arabidopsis seedlings showed that DMPBQ functionally compensated for the absence of tocopherols in protection from lipid peroxidation (31). In Synechocystis sp. strain PCC 6803, DMPBQ partially compensated for the absence of tocopherols in recovery from PUFA treatment in high-light conditions (21). Tocopherols also showed nonantioxidant functions in plants or cyanobacteria (8). In Arabidopsis, tocopherols are required for the development of phloem parenchyma transfer cell walls in proper structure in response to cold (22). In Synechocystis sp. strain PCC 6803, α-tocopherol plays a role in photosynthesis and macronutrient homeostasis that is independent of its antioxidant function (29).
Because chill-light stress could be one of the major challenges for overwintering cyanobacteria, it provides an opportunity for investigating the role of tocopherols in natural adaptation of Synechocystis sp. strain PCC 6803. While the wild-type cells synthesize predominantly α-tocopherol, tocopherol synthesis mutants lack tocopherols or accumulate β- or γ-tocopherols (1, 4, 5, 6, 30, 32-35). Using an inducible promoter, the cellular level of α-tocopherol could be controlled (26). Such strains should facilitate studies of the function of a specific tocopherol. In this paper, we report that α-tocopherol is essential for the enhanced chill-light tolerance of Synechocystis sp. strain PCC 6803 after preconditioning at a low temperature.
MATERIALS AND METHODS
Culture conditions and evaluation of chill-light sensitivity.
Synechocystis sp. strain PCC 6803 was from J. Zhao of Peking University and was cultured in BG11 as previously described (43). The antibiotics kanamycin (30 μg ml−1), erythromycin (10 μg ml−1), and spectinomycin (5 μg ml−1) were added to the medium as needed. The ability to reinitiate growth (ARG) or CFU of the wild-type strain was measured as described previously (43). Cells were grown under photoautotrophic conditions at 30°C for 4 days or at 15°C for 6 days or for the indicated period of time. After dilution to an optical density at 730 nm (OD730) of 0.05, cells were exposed to a chill (5°C) with or without light (100 μmol photons m−2 s−1) and allowed to grow mixtrophically (Synechocystis) or photoautotrophically (Microcystis) at 30°C for 4 days. The ARG was calculated as OD730 (treated)/OD730 (control) × 100%, where the OD730 is the turbidity of cells after exposure to chill (treated) or not (control) and growth at 30°C. The relative acquired chill-light tolerance (RACLT) of a mutant was evaluated as
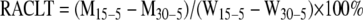 |
(1) |
where M is the turbidity (OD730) of a mutant after exposure to chill-light for 8 days and autotrophic growth at 30°C for 4 days; W is the turbidity of the wild-type after exposure to chill-light and growth at 30°C; 15-5 indicates that cells were grown at 15°C, exposed to chill-light stress, and allowed to grow at 30°C; and 30-5 indicates that cells were grown at 30°C, exposed to chill-light stress, and allowed to grow at 30°C. Because all strains that we tested showed no change in viability after exposure to chill without light in 10 days, the turbidity values used in equation 1 were normalized by dividing by that of the same strain given identical treatments except exposure to chill without light. Data represent the means ± standard deviations of values from three parallel tests. At least two independent experiments were performed to evaluate the RACLT of each mutant, which showed consistent results or as described below. KanC (Table 1), a kanamycin-resistant derivative of Synechocystis sp. strain PCC 6803 showing no phenotypic change under different conditions as described in previous reports (20, 42) and as in our study, was used as the wild-type control for mutants of the same antibiotic resistance marker.
TABLE 1.
Cyanobacterial strains, plasmids, and primers used
| Strain, plasmid, or primer | Derivation and/or relevant characteristicsa | Reference(s) or source |
|---|---|---|
| Synechocystis sp. strainsb | ||
| DRHB451 | Kmr, desD::C.K2 | 43 |
| DRHB816 | Kmr, slr1171 (gpx-1)::C.K2 | This study |
| DRHB819 | Kmr, slr0089::C.K2 | This study |
| DRHB1168 | Kmr, slr0090::C.K2 | This study |
| DRHB1468 | Kmr, slr0091::C.K2 | This study |
| DRHB1471 | Kmr, slr1736::C.K2 | This study |
| DRHB1473 | Kmr, slr1737::C.K2 | This study |
| DRHB819/SRHB2085 | Kmr Cmr Emr, pHB2085 integrated into the genome of mutant slr0089::C.K2 | This study |
| DRHB1168/DRHB2794 | Kmr Smr Spr, omega-PpetE-slr0090 integrated into slr0168 in the genome of mutant slr0090::C.K2 | This study |
| KanC | Kmr, slr0168::C.K2; result of transformation with pKW1188 | 20, 42 |
| PCC 6803 | Wild type | J. Zhao, Peking University |
| Plasmidsc | ||
| pHB794 | Apr; PCR fragment containing slr1171 sequence, amplified with primers slr1171-1 and slr1171-2, cloned into pMD18-T | This study |
| pHB795 | Apr; PCR fragment containing slr0089 sequence, amplified with primers slr0089-1 and slr0089-2, cloned into pMD18-T | This study |
| pHB816 | Apr Kmr, slr1171 on pHB794 interrupted by Kmr cassette C.K2 at ClaI site | This study |
| pHB819 | Apr Kmr, slr0089 on pHB795 interrupted by C.K2 at NcoI site | This study |
| pHB977 | Apr; PCR fragment containing slr0089, amplified with primers slr0089-1c and slr0089-2c, cloned into pMD18-T | This study |
| pHB1152 | Apr; PCR fragment containing slr0090 sequence, amplified with primers slr0090-1 and slr0090-2, cloned into pMD18-T | This study |
| pHB1168 | Apr Kmr, slr0090 on pHB1152 interrupted by C.K2 at NcoI site | This study |
| pHB1180 | Apr Tcr; derivative of pKW1188 in which a Kmr cassette was replaced with a Tcr cassette | H. Guo and X. Xu, unpublished data |
| pHB1450 | Apr; PCR fragment containing slr0091 sequence, amplified with primers slr0091-1 and slr0091-2, cloned into pMD18-T | This study |
| pHB1453 | Apr, the PCR fragment containing slr1736 sequence, amplified with primers slr1736-1 and slr1736-2, cloned into pMD18-T | This study |
| pHB1454 | Apr; PCR fragment containing slr1737 sequence, amplified with primers slr1737-1 and slr1737-2, cloned into pMD18-T | This study |
| pHB1468 | Apr Kmr, slr0091 on pHB1450 interrupted by C.K2 at HpaI site | This study |
| pHB1471 | Apr Kmr, slr1736 on pHB1453 interrupted by C.K2 at NcoI site | This study |
| pHB1473 | Apr Kmr, slr1737 on pHB1454 interrupted by C.K2 at NheI site | This study |
| pHB1524 | Apr Smr/Spr; plasmid containing omega-PpetE | 14 |
| pHB2085 | Apr Cmr (Emr); fragment containing slr0089, excised from pHB977, subcloned into pRL1075 | This study |
| pHB2792 | Apr; PCR fragment containing slr0090, amplified with primers slr0090-e1 and slr0090-e2, cloned into pMD18-T | This study |
| pHB2793 | Apr Smr Spr, omega-PpetE excised from pHB1524, cloned into SalI site upstream of slr0090 on pHB2792 | This study |
| pHB2794 | Apr Smr Spr, omega-PpetE-slr0090 excised from pHB2793, cloned into pHB1180, replacing the Tcr cassette | This study |
| pKW1188 | Apr Kmr; plasmid bearing an integrative platform for Synechocystis sp. strain 6803 | 20, 42 |
| pMD18-T | Apr; cloning vector | Takara, Dalian |
| pRL446 | Apr Kmr; plasmid containing the Kmr cassette C.K2 | 9 |
| pRL1075 | Cmr Emr; suicide plasmid with RK2 oriT | 2 |
| Primers | ||
| M13rev | 5′-AGCGGATAACAATTTCACACAGGA-3′ | |
| slr1711-1 | 5′-TGTGGCTCTGTTATTGCTCC-3′ | |
| slr1711-2 | 5′-CTTAATGCGTTGCAGTTCATT-3′ | |
| slr1736-1 | 5′-GCGTCTGGGCTGTGTATCTGT-3′ | |
| slr1736-2 | 5′-ACTGCCAACGACAATGGCGA-3′ | |
| slr1737-1 | 5′-GCCCTGTGTATTCTGACGGT-3′ | |
| slr1737-2 | 5′-TCCCTCAGAATGGCACTGTT-3′ | |
| slr0089-1 | 5′-AGAAATTGCGCTCGGGTCT-3′ | |
| slr0089-2 | 5′-GATGAGCTGACTGATAATCGT-3′ | |
| slr0090-1 | 5′-TGCCGCCCTGTGTTTACGA-3′ | |
| slr0090-2 | 5′-TTTCCTCTTCCTGG GCTTG-3′ | |
| slr0091-1 | 5′-GCTCTCTATGAAGCGGTGGA-3′ | |
| slr0091-2 | 5′-TTTGGTTAGCCCGCCCTGT-3′ | |
| slr0089-1c | 5′-AGAATTGCGCTCGGGTCT-3′ | |
| slr0089-2c | 5′-GATGAGCTGACTGATAATCGTC-3′ | |
| slr0090-e1 | 5′-CTATGAACGGGGATTAGTGCGT-3′ | |
| slr0090-e2 | 5′-CTCTTCCTGGGCTTGAATTTG-3′ |
Ap, ampicillin; Cm, chloramphenicol; Em, erythromycin; Km, kanamycin; Sm, streptomycin; Sp, spectinomycin; Tc, tetracycline.
DRHB(number) refers to a product of double homologous recombination between plasmid pHB(number) and the Synechocystis sp. genome. SRHB(number) refers to a product of single homologous recombination between plasmid pHB(number) and the Synechocystis sp. genome.
Unless stated otherwise, the template for PCRs was Synechocystis sp. genomic DNA.
Plasmid construction.
Plasmids used or constructed and primers used in PCRs are listed in Table 1. Clones of PCR products were confirmed by sequencing.
(i) Plasmid used to inactivate slr1171.
The DNA fragment generated by PCR using primers slr1171-1 and slr1171-2 was cloned into the T-vector pMD18-T (Takara), resulting in pHB794. Plasmid pHB794 was cut with ClaI, blunted with T4 DNA polymerase, and ligated with the kanamycin resistance cassette C.K2 excised from pRL446 (9) with PvuII, resulting in pHB816.
(ii) Plasmid used to inactivate slr1736.
The DNA fragment generated by PCR using primers slr1736-1 and slr1736-2 was cloned into pMD18-T, resulting in pHB1453. Plasmid pHB1453 was cut with NcoI, blunted with T4 DNA polymerase, and ligated with C.K2 excised with PvuII, resulting in pHB1471.
(iii) Plasmid used to inactivate slr1737.
The DNA fragment generated by PCR using primers slr1737-1 and slr1737-2 was cloned into pMD18-T, resulting in pHB1454. Plasmid pHB1454 was cut with NheI, blunted with T4 DNA polymerase, and ligated with C.K2 excised with PvuII, resulting in pHB1473.
(iv) Plasmid used to inactivate slr0089.
The PCR product generated using primers slr0089-1 and slr0089-2 was cloned into pMD18-T, resulting in pHB795. Plasmid pHB795 was cut with NcoI, blunted with T4 DNA polymerase, and ligated with C.K2 excised with PvuII, resulting in pHB819.
(v) Plasmid used to inactivate slr0090.
The PCR product generated using primers slr0090-1 and slr0090-2 was cloned into pMD18-T, resulting in pHB1152. Plasmid pHB1152 was cut with NcoI, blunted with T4 DNA polymerase, and ligated with C.K2 excised with PvuII, resulting in pHB1168.
(vi) Plasmid used to inactivate slr0091.
The PCR product generated using primers slr0091-1 and slr0091-2 was cloned into pMD18-T, resulting in pHB1450. Plasmid pHB1450 was cut with HpaI and ligated with C.K2 excised with PvuII, resulting in pHB1468.
(vii) Plasmid used to complement the slr0089::C.K2 mutant.
The DNA fragment containing slr0089 generated by PCR using primers slr0089-1c and slr0089-2c was cloned into pMD18-T and confirmed by sequencing, resulting in pHB977. Plasmid pHB977 was cut with BamHI, blunted with T4 DNA polymerase, and ligated with EcoRV-cut pRL1075 (2), resulting in pHB2085.
(viii) Plasmid used to regulate slr0090 with PpetE.
The DNA fragment containing slr0090 generated with primers slr0090-e1 and slr0090-e2 was cloned into pMD18-T and confirmed by sequencing, resulting in pHB2792. omega-PpetE excised from pHB1524 (14) with BamHI and SalI, blunted with T4 DNA polymerase, was cloned into pHB2792 cut with SalI and blunted with T4 DNA polymerase, resulting in pHB2793. The fragment containing omega-PpetE::slr0090 was excised from pHB2793 with HindIII and EcoRI, blunted with T4 DNA polymerase, and cloned into pHB1180 (Table 1) (modified from pKW1188 [42]) cut with EcoRI and blunted with T4 DNA polymerase, resulting in pHB2794. The omega cassette contains a streptomycin/spectinomycin resistance gene bracketed by two stem-loop structures that may terminate transcription (24, 41).
Mutant construction and complementation.
Transformation of Synechocystis sp. strain PCC 6803 was performed as described by Williams (42). For targeted insertion of a gene, Synechocystis sp. strain PCC 6803 was transformed with plasmids, and the resulted transformants were streaked on plates and cultured in liquid medium under selective pressure of antibiotics until complete segregation was confirmed by PCR using primers listed in Table 1.
The mutant slr0089::C.K2 was complemented by pHB2085, which was introduced into the cyanobacterium by conjugation (10) and integrated into the genome via single-crossover recombination. The homologous integration was confirmed with PCR using primers M13rev/slr0089-2.
Tocopherol measurements.
Ten milliliters of cells grown at 30°C (OD730 of around 1.0) was collected by centrifugation at 5,000 × g for 10 min and washed twice with 25 mM HEPES buffer (pH 7.0). Tocopherols were extracted in 0.5 ml of methanol with 0.1% (wt/vol) butylated hydroxytoluene (BHT) at 4°C. After centrifugation and filtration, 10 μl was subjected to high-pressure liquid chromatography (Shimadzu CBM-10A, Japan) on a reverse-phase C18 column (Shimadzu Shim-Pack CLC-ODS; 5 μm, 4.6150 mm) using 100% methanol at a flow rate of 1 ml min−1. Tocopherols were detected with the RF-10AXL fluorescence detector (excitation, 290 nm; emission, 325 nm) (Shimadzu) and quantified against standard curves generated with commercially available tocopherols (Sigma).
Detection of lipid peroxides.
The level of lipid peroxide in intact cyanobacterial cells was measured using the ferrous oxidation-xylenol orange method as previously described (18, 27). One and a half milliliters of cells grown at 30°C or 15°C (OD730 of 0.8 to 1.2) or 40 ml of cells exposed to chill-light (OD730 of 0.1) was collected by centrifugation, and the pellets were resuspended in 0.8 ml of methanol containing 0.01% (wt/vol) BHT. After addition of 0.1 ml of reagent A (2.5 mM ammonium ferrous sulfate, 0.25 M sulfuric acid) and 0.1 ml of reagent B (40 mM BHT, 1.25 mM xylenol orange in methanol), samples were quickly centrifuged to remove cell debris. The OD560 of the supernatant was measured immediately. The amount of lipid peroxide was calculated from an apparent extinction coefficient (E560, 43,000 M−1 cm−1).
RESULTS
ACLT in a unicellular cyanobacterium.
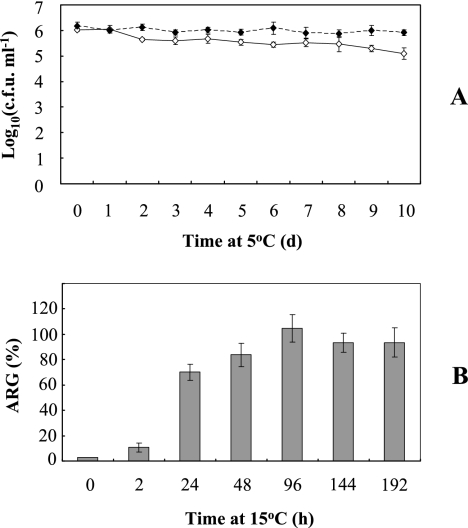
Synechocystis sp. strain PCC 6803 is a mesophilic cyanobacterium, showing no growth at 5°C. Previously, we found that when exposed to 5°C and light at 100 μmol photons m−2 s−1, cells grown at 30°C lost viability within 10 days (43). In temperate and subtropical regions, cyanobacteria experience a decrease of temperature in autumn before exposure to the more severe cold and light stress in winter. We tested the effect of pretreatment at a low temperature (15°C) on chill-light tolerance in Synechocystis sp. strain PCC 6803. Cells grown at 15°C for 6 days showed little decrease in viability under chill-light stress within 10 days, as seen by CFU (Fig. 1A) or the ARG (data not shown), and the effect of light on the chill stress became negligible. Within a month, cells exposed to 5°C in the dark showed no loss of viability when pretreated at 15°C (ARG, 115.7% ± 4.9%) or a partial loss of viability when not pretreated at low temperature (ARG, 64.1% ± 11.6%). Synechocystis sp. strain PCC 6803 pretreated at 15°C was sampled at different time points and examined for chill-light tolerance. Preconditioning at 15°C for 2 h gave rise to a very slight increase in chill-light tolerance as seen by ARG; after 2 days, the chill-light tolerance reached the maximal level (Fig. 1B). A similar phenomenon was found for Microcystis sp. strain PCC 7806, a bloom-forming species (see Fig. S1 in the supplemental material). The phenomenon that the chill-light tolerance of a cyanobacterium is greatly enhanced by preconditioning at a low temperature is called acquired chill-light tolerance (ACLT).
FIG. 1.
ACLT of Synechocystis sp. strain PCC 6803 as seen with CFU or ARG. (A) Cells pretreated at 15°C at a photosynthetic photon flux density of 30 μmol photons m−2 s−1 for 6 days were exposed to a chill with (diamonds) or without (squares) light stress (100 μmol photons m−2 s−1) for different periods of time and transferred to 30°C on plates to determine log10(CFU·ml−1). (B) Cells pretreated at 15°C for different period of time were exposed to chill-light stress for 8 days and transferred to 30°C for mixotrophic growth in liquid medium to determine the ARG. Error bars indicate standard deviations.
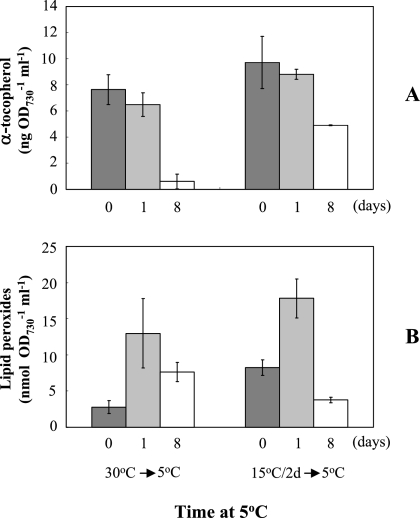
Because chill-light stress may exert oxidative stress on cyanobacterial cells, we tested the effects of preconditioning on levels of tocopherols and lipid peroxides. Under our conditions, only α-tocopherol was detectable in the wild type. Levels of α-tocopherol and lipid peroxides were measured in cells grown at 30°C, pretreated at 15°C, and exposed to chill and light for 1 day and 8 days. Figure 2A shows that α-tocopherol was reduced in cells exposed to chill-light stress, and the reduction was much greater if cells were not pretreated at 15°C. Figure 2B shows that the level of lipid peroxides increased on the first day of exposure to chill-light stress and decreased afterwards, but preconditioning promoted recovery from lipid peroxidation. The effect of preconditioning on the level of α-tocopherol appeared to be much greater than that on lipid peroxidation.
FIG. 2.
Effects of preconditioning on the contents of α-tocopherol (A) and lipid peroxides (B) in Synechocystis sp. strain PCC 6803. Dark gray bars, 30°C or 15°C as indicated; light gray bars, exposed to chill (5°C)-light (100 μmol photons m−2 s−1) for 1 day; empty bars, exposed to chill-light for 8 days. Error bars indicate standard deviations.
Lack of ACLT in a γ-tocopherol methyltransferase gene mutant.
The effect of a single gene on the RACLT could be evaluated by comparing a mutant and the wild type according to equation 1, whose principle is schematically shown in Fig. S2 in the supplemental material. The RACLT of a mutant is the percent enhanced ARG after preconditioning at 15°C relative to the wild type. To minimize the deviation caused by the difference in growth of individual samples at 30°C or 15°C, we set controls exposed to chill without light in parallel to samples exposed to chill-light stress. Before use in calculation, values were normalized according to the controls. We chose slr0089, encoding γ-tocopherol methyltransferase (35), and slr1171 (gpx-1), encoding NADPH-dependent lipid peroxidase (13), to test. Like tocopherols, a lipid peroxidase protects membranes from lipid peroxidation in plants and cyanobacteria (13). The two genes were inactivated in Synechocystis sp. strain PCC 6803, and the resulting mutants both remained unchanged in autotrophic growth at 15°C (data not shown). The RACLT for the slr0089 mutant was 1.9% ± 0.6%, and that for the slr1171 mutant was 81.4% ± 5.2%. These results indicated that ACLT was virtually absent in the slr0089 mutant and slightly reduced in the gpx-1 mutant. The minor effect of loss of gpx-1 function on ACLT could be due to the presence of slr1992 (gpx-2), another gene with the same function, in the genome of Synechocystis sp. strain PCC 6803 (13).
α-Tocopherol is essential for ACLT of Synechocystis sp. strain PCC 6803.
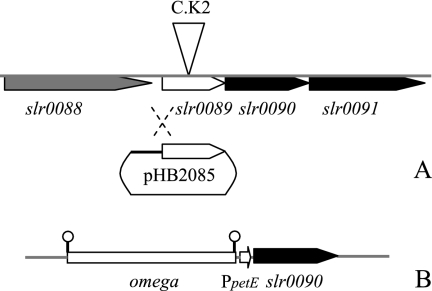
In addition to slr0089::C.K2, we constructed mutants of Synechocystis sp. strain PCC 6803 in which different steps of the synthesis pathway were blocked, including slr0090::C.K2, slr1736::C.K2, and slr1737::C.K2. The tocopherol contents in the wild type and different mutants (Table 2) were basically consistent with previous reports except for reference 33. The γ-tocopherol content in the slr0089 mutant was higher than that reported by Sakuragi et al. (29), which could be due to differences in culture conditions (1). We complemented the slr0089 mutant with pHB2085 carrying the wild-type slr0089 through homologous single crossover with the genome (Fig. 3A). In the complemented strain, pHB2085 was integrated upstream of the kanamycin resistance marker in slr0089 as detected by PCR (data not shown), and the synthesis of α-tocopherol was restored to the wild-type level (Table 2). All the mutants grew at 15°C without apparent differences from the wild type, and the reduction of the growth rate, if any, was less than 10%.
TABLE 2.
Correlations between ACLT and tocopherols in Synechocystis sp. strain 6803a
| Strain | Tocopherol (μg·g−1 [wet wt])
|
RACLT (%) | |||
|---|---|---|---|---|---|
| α | β | γ | δ | ||
| Wild type | 5.1 ± 0.6 | ND | ND | ND | 100 |
| slr0089::C.K2 | ND | ND | 1.4 ± 0.02 | ND | 1.9 ± 0.6 |
| slr0089::C.K2, complemented | 7.1 ± 0.2 | ND | ND | ND | 94 ± 6.0 |
| slr0090::C.K2 | ND | ND | ND | ND | −2.0 ± 2.7 |
| PpetE-slr0090 | |||||
| With Cu | 9.3 ± 1.8 | ND | ND | ND | 96 ± 2.4 |
| Without Cu | 0.9 ±0.1 | ND | ND | ND | 9.5 ± 0.6 |
| slr0091::C.K2 | 6.1 ± 2.0 | ND | ND | ND | 96 ± 4.4 |
| slr1736::C.K2 | ND | ND | ND | ND | 3.3 ± 0.6 |
| slr1737::C.K2 | ND | ND | ND | ND | −0.3 ± 2.9 |
Cells were grown at 30°C with 30 μmol photons m−2 s−1. Values are means and standard deviations. ND, not detectable.
FIG. 3.
Genetic modifications of the slr0089 and slr0090 genes. (A) Insertion of a kanamycin resistance cassette in slr0089 and complementation of the slr0089 mutant with the suicide plasmid pHB2085 (Table 1) carrying the wild type slr0089. (B) omega-PpetE-slr0090 integrated in a neutral integrative platform (20, 42) in mutant slr0090::C.K2. The stem-loop structures bracketing the omega cassette are transcriptional terminators.
We evaluated the RACLTs of these tocopherol-deficient mutants. As in mutant slr0089::C.K2, ACLT was completely abolished in mutants slr0090::C.K2, slr1736::C.K2, and slr1737::C.K2 (Table 2). slr1736 and slr1737 are next to each other, and downstream of slr1737 is a gene in the opposite orientation. Genes slr0089, slr0090, and slr0091 are clustered in the same orientation. Inactivation of slr0091 showed no effect on either tocopherol synthesis or ACLT (Table 2). The mutation of slr1737 or slr0090 did not exert any polar effect on the downstream genes. If exposed to chill without light, all the mutants showed no or slight differences from the wild-type strain in final turbidity after reinitiating growth at 30°C. For mutants slr0089::C.K2, slr0090::C.K2, slr1736::C.K2, and slr1737::C.K2, the ratios of mutant to wild type (OD730) were 81% ± 1.2%, 111% ± 0.6%, 76% ± 3.2%, and 105% ± 8.7%, respectively. In parallel to the synthesis of α-tocopherol, complementation of the slr0089 mutant with the wild-type gene restored the ACLT (Table 2).
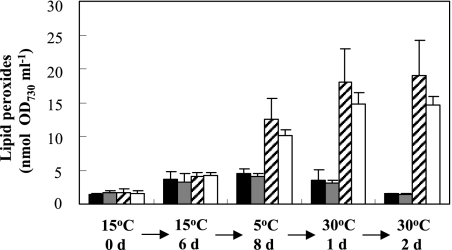
We detected lipid peroxides in cells after preconditioning at 15°C, exposure to 5°C in the light, or transfer back to 30°C. As shown in Fig. 4, the wild-type strain and the slr1737 and slr0089 mutants showed no difference in lipid peroxidation at 30°C or 15°C. However, in mutants exposed to chill-light stress for 8 days, lipid peroxides increased to about twofold or more of the levels in the wild-type strain and the complemented slr0089 mutant. After exposure to the chill-light stress, cells were allowed to reinitiate growth at 30°C; lipid peroxides were gradually lowered to the original level in the wild-type strain and the complemented slr0089 mutant but remained at a relatively high level in the α-tocopherol-deficient strains. Lipid peroxides were also detected in the wild-type and slr1737 mutant cells exposed to chill-light stress for 1 day. At this time point, the mutant contained less lipid peroxide (10.1% ± 2.4 ng OD730−1 ml−1) than in the wild type (20.2% ± 2.8 ng OD730−1 ml−1).
FIG. 4.
Lipid peroxides produced in the wild type (dark bars), the complemented slr0089 mutant (gray bars), the slr0089 mutant (hatched bars), and the slr1737 mutant (empty bars) before pretreatment at 15°C (day 0) and after pretreatment at 15°C for 6 days, exposure to 5°C and light for 8 days, and transfer to 30°C for 1 day and 2 days during the ACLT test. Photon flux densities at 15°C, 5°C, and 30°C were 30, 100, and 30 μmol photons m−2 s−1, respectively. Error bars indicate standard deviations.
We employed a copper-regulated promoter, PpetE (44), to control the expression of slr0090 and the amount of α-tocopherol in cells. A fragment containing omega-PpetE-slr0090 was integrated into a neutral platform in the genome of the slr0090::C.K2 mutant (Fig. 3B). The copper concentration in the medium regulated the synthesis of α-tocopherol and, concomitantly, the ACLT of Synechocystis sp. strain PCC 6803 (Table 2).
DISCUSSION
In Escherichia coli, cells grown at 37°C lost viability rapidly at 4°C, while pretreatment at 16°C before exposure to 4°C induced the accumulation of trehalose in cells and greatly increased the viability (19). Unlike E. coli, Synechocystis sp. strain PCC 6803 grown at 30°C remains viable at 5°C in the dark for 2 months; the presence of light at 100 μmol photons m−2 s−1, however, remarkably promoted cell death at 5°C (43). In this study, we found great enhancement of the chill-light tolerance of Synechocystis sp. strain PCC 6803 after preconditioning at 15°C. One can conjecture that in temperate or subtropical lakes, exposure of cyanobacteria to suboptimal growth temperatures in late autumn and early winter may greatly enhance their ability to survive the chill-light stress in shallow areas in winter.
We tested the ACLTs in different mutants of Synechocystis sp. strain PCC 6803 defective in tocopherol synthesis. Because α-tocopherol may be required for maintaining cellular homeostasis of nutrients in the cyanobacterium and mutants lacking α-tocopherol were unable to grow under mixotrophic conditions (29), we avoided the use of glucose in the tests. All four mutants lacking α-tocopherol showed virtually no ACLT. Among the tested genes, slr0089 and slr0090 are positioned in the same cluster and both were involved in the synthesis of tocopherol, while the downstream gene slr0091 was required for neither tocopherol synthesis nor ACLT. The phenotype of slr0089 and slr0090 mutants should not be due to a polar effect on slr0091. The slr0089 mutant synthesized γ-tocopherol, indicating that slr0090, as the gene responsible for the first step toward tocopherol synthesis, was active in the mutant. In addition, the complementing plasmid pHB2085 integrated upstream of the C.K2 cassette fully restored the synthesis of α-tocopherol and ACLT in the slr0089 mutant. The phenotype of the slr0089 mutant, including the lack of ACLT, should not be due to a polar effect on slr0090. In the PpetE-slr0090 strain, the level of tocopherol and ACLT were both regulated by cupric ions. These lines of evidence together support the idea that α-tocopherol is essential for ACLT and that its function in ACLT could not be compensated for by other pathway intermediates. Because of its essentiality in ACLT, α-tocopherol may play an important role in the overwintering mechanism of certain groups of cyanobacteria.
At 15°C, the chill-light tolerance of Synechocystis sp. strain PCC 6803 increased rapidly in the first 24 h and reached a stationary phase after 48 h (Fig. 1B), but the level of α-tocopherol remained almost unchanged in 48 h (Fig. 2A). Therefore, the ACLT could not be attributed to an increase of α-tocopherol before exposure to chill-light stress. The rapid increase in the ACLT may depend or partially depend on the up-regulation of early responsive genes, such as desA, desB, and desD, encoding fatty acid desaturases (23, 28); rbp1, encoding an RNA-binding protein; crhL, encoding an RNA helicase; and many others found by DNA microarray analyses (17, 36). Compared to cells directly transferred from 30°C, those preconditioned at 15°C maintained a much higher level of α-tocopherol after 8 days of exposure to chill-light stress (Fig. 2A). Preconditioning at 15°C reduced the consumption or enhanced the recycling/synthesis of α-tocopherol, or both, in cells exposed to chill-light stress.
In Arabidopsis, leaf discs rather than whole plants of single mutants deficient in tocopherol synthesis showed photooxidative damage under chill-light stress (15). A subsequent report, however, showed that the same tocopherol-deficient mutants were actually sensitive to nonfreezing cold temperatures independent of the light level (22). In Synechocystis sp. strain PCC 6803, tocopherol deficiency led to loss of ACLT rather than tolerance to chill without light or growth at low temperature. Under favorable or suboptimal growth conditions, the wild-type and mutant strains lacking α-tocopherol or tocopherols showed no difference in lipid peroxidation. On the first day of exposure to chill-light stress, Synechocystis sp. strain PCC 6803 produced lipid peroxides rapidly, and the level of lipid peroxides in an α-tocopherol-deficient mutant was not more than that in the wild type. After exposure for 8 days, cells showed reduced lipid peroxidation relative to that after exposure for 1 day. In the slr0089 and slr1737 mutants, lipid peroxides remained at a remarkably higher level than in the wild type. It appears that the recovery from photooxidative damage was slowed in a mutant lacking α-tocopherol compared to the wild type. In vivo, lack of protection from lipid peroxidation by tocopherols can be compensated for fully or partially by DMPBQ, which is accumulated in an slr1737 mutant (21, 31). A phenotype specifically dependent on α-tocopherol can be independent of its antioxidant activity (29). More likely, the requirement of α-tocopherol in the ACLT of Synechocystis sp. strain PCC 6803 was based on a nonantioxidant activity that is not possessed by other tocopherols or pathway intermediates, while the slowed recovery from lipid peroxidation of tocopherol mutants is a result of their sensitivity to chill-light stress.
Supplementary Material
Acknowledgments
This study was supported by the National Natural Science Foundation of China (30330030) and the Key Project (KSCX2-SW-332) of Knowledge Innovation Program of the Chinese Academy of Sciences. Y.Y. was partially supported by a scholarship from China Postdoctoral Science Foundation.
Footnotes
Published ahead of print on 28 December 2007.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Backasch, N., R. Schulz-Friedrich, and J. Appel. 2005. Influences on tocopherol biosynthesis in the cyanobacterium Synechocystis sp. PCC 6803. J. Plant Physiol. 162758-766. [DOI] [PubMed] [Google Scholar]
- 2.Black, T. A., and C. P. Wolk. 1993. Spatial expression and autoregulation of hetR, a gene involved in the control of heterocyst development in Anabaena. Mol. Microbiol. 977-84. [DOI] [PubMed] [Google Scholar]
- 3.Brunberg A.-K., and P. Blomqvist. 2003. Recruitment of Microcystis (Cyanophyceae) from lake sediments: the importance of littoral inocula. J. Phycol. 3959-63. [Google Scholar]
- 4.Cheng, Z. G., S. Sattler, H. Maeda, Y. Sakuragi, D. A. Bryant, and D. DellaPenna. 2003. Highly divergent methyltransferases catalyze a conserved reaction in tocopherol and plastoquinone synthesis in cyanobacteria and photosynthetic eukaryotes. Plant Cell 152343-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collakova, E., and D. DellaPenna. 2001. Isolation and functional analysis of homogentisate phytyltransferase from Synechocystis sp. PCC 6803 and Arabidopsis. Plant Physiol. 1271113-1124. [PMC free article] [PubMed] [Google Scholar]
- 6.Dahnhardt, D., J. Falk, J. Appel, T. A. van der Kooij, R. Schulz-Friedrich, and K. Krupinska. 2002. The hydroxyphenylpyruvate dioxygenase from Synechocystis sp. PCC 6803 is not required for plastoquinone biosynthesis. FEBS Lett. 523177-181. [DOI] [PubMed] [Google Scholar]
- 7.DellaPenna, D., and B. J. Pogson. 2006. Vitamin synthesis in plants: tocopherols and carotenoids. Annu. Rev. Plant Biol. 57711-738. [DOI] [PubMed] [Google Scholar]
- 8.Dormann, P. 2007. Functional diversity of tocochromanols in plants. Planta 225269-276. [DOI] [PubMed] [Google Scholar]
- 9.Elhai, J., and C. P. Wolk. 1988. A versatile class of positive-selection vectors basted on the nonviability of palindrome-containing plasmids that allows the cloning into long polylinkers. Gene 68119-138. [DOI] [PubMed] [Google Scholar]
- 10.Elhai, J., and C. P. Wolk. 1988. Conjugal transfer of DNA to cyanobacteria. Methods Enzymol. 167747-754. [DOI] [PubMed] [Google Scholar]
- 11.Fryer, M. J. 1992. The antioxidant effects of thylakoid vitamin E (α-tocopherol). Plant Cell Environ. 15381-392. [Google Scholar]
- 12.Fukuzawa, K., A. Tokumura, S. Ouchi, and H. Tsukatani. 1982. Antioxidant activities of tocopherols on Fe2+-ascorbate-induced lipid peroxidation in lecithin liposomes. Lipids 17511-513. [DOI] [PubMed] [Google Scholar]
- 13.Gaber, A., M. Tamoi, T. Takeda, Y. Nakano, and S. Shigeoka. 2001. NADPH-dependent glutathione peroxidase-like proteins (Gpx-1, Gpx-2) reduce unsaturated fatty acid hydroperoxides in Synechocystis PCC 6803. FEBS Lett. 49932-36. [DOI] [PubMed] [Google Scholar]
- 14.Gao, H., Q. Tang, and X. Xu. 2007. Construction of copper-induced gene expression platform in Synechocystis sp. PCC 6803. Acta Hydrobiol. Sin. 31120-124. [Google Scholar]
- 15.Havaux, M., F. Eymery, S. Porfirova, P. Rey, and P. Dormann. 2005. Vitamin E protects against photoinhibition and photooxidative stress in Arabidopsis thaliana. Plant Cell 173451-3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huner, N. P. A., G. Öquist, and F. Sarhan. 1998. Energy balance and acclimation to light and cold. Trends Plant Sci. 6224-230. [Google Scholar]
- 17.Inaba, M., I. Suzuki, B. Szalontai, Y. Kanesaki, D. A. Los, H. Hayashi, and N. Murata. 2003. Gene-engineered rigidification of membrane lipids enhances the cold inducibility of gene expression in Synechocystis. J. Biol. Chem. 27812191-12198. [DOI] [PubMed] [Google Scholar]
- 18.Jiang, Z. Y., A. C. Woollard, and S. P. Wolff. 1996. Lipid hydroperoxide measurement by oxidation of Fe2+ in the presence of xylenol orange. Comparison with the TBA assay and an iodometric method. Lipids 26853-856. [DOI] [PubMed] [Google Scholar]
- 19.Kandror, O., A. Deleon, and A. L. Goldberg. 2002. Trehalose synthesis is induced upon exposure of Escherichia coli to cold and is essential for viability at low temperatures. Proc. Natl. Acad. Sci. USA 999727-9732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kunert, A., M. Hagemann, and N. Erdmann. 2000. Construction of promoter probe vectors for Synechocystis sp. PCC6803 using the light-emitting reporter systems Gfp and LuxAB. J. Microbiol. Methods 41185-194. [DOI] [PubMed] [Google Scholar]
- 21.Maeda, H., Y. Sakuragi, D. A. Bryant, and D. Dellapenna. 2005. Tocopherols protect Synechocystis sp. strain PCC 6803 from lipid peroxidation. Plant Physiol. 1381422-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maeda, H., W. Song, T. Sage, and D. Dellapenna. 2006. Tocopherols play a crucial role in low-temperature adaptation and phloem loading in Arabidopsis. Plant Cell 182710-2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murata, N., and H. Wada. 1995. Acyl-lipid desaturases and their importance in the tolerance and acclimation to cold of cyanobacteria. Biochem. J. 3081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prentki, P., A. Binda, and A. Epstein. 1991. Plasmid vectors for selecting IS1-promoted deletions in cloned DNA: sequence analysis of the omega interposon. Gene 10317-23. [DOI] [PubMed] [Google Scholar]
- 25.Preston, T., W. D. P. Steward, and C. S. Reynolds. 1980. Bloom-forming cyanobacterium Microcystis aeruginosa overwinters on sediment surface. Nature 288365-367. [Google Scholar]
- 26.Qi, Q., M. Hao, W. O. Ng, S. C. Slater, S. R. Baszis, J. D. Weiss, and H. E. Valentin. 2005. Application of the Synechococcus nirA promoter to establish an inducible expression system for engineering the Synechocystis tocopherol pathway. Appl. Environ. Microbiol. 715678-5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakamoto, T., V. B. Delgaizo, and D. A. Bryant. 1998. Growth on urea can trigger death and peroxidation of the cyanobacterium Synechococcus sp. strain PCC 7002. Appl. Environ. Microbiol. 642361-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakamoto, T., and N. Murata. 2002. Regulation of desaturation of fatty acids and its role in tolerance to cold and salt stress. Curr. Opin. Microbiol. 5208-210. [DOI] [PubMed] [Google Scholar]
- 29.Sakuragi, Y., H. Maeda, D. Dellapenna, and D. A. Bryant. 2006. α-Tocopherol plays a role in photosynthesis and macronutrient homeostasis of the cyanobacterium Synechocystis sp. PCC 6803 that is independent of its antioxidant function. Plant Physiol. 141508-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sattler, S. E., E. B. Cahoon, S. J. Coughlan, and D. DellaPenna. 2003. Characterization of tocopherol cyclases from higher plants and cyanobacteria: evolutionary implications for tocopherol synthesis and function. Plant Physiol. 1322184-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sattler, S. E., L. U. Gilliland, M. Magallanes-Lundback, M. Pollard, and D. DellaPenna. 2004. Vitamin E is essential for seed longevity, and for preventing lipid peroxidation during germination. Plant Cell 161419-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Savidge, B., J. D. Weiss, Y. H. Wong, M. W. Lassner, T. A. Mitsky, C. K. Shewmaker, D. Post-Beittenmiller, and H. E. Valentin. 2002. Isolation and characterization of homogentisate phytyltransferase genes from Synechocystis sp. PCC 6803 and Arabidopsis. Plant Physiol. 129321-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schledz, M., A. Seidler, P. Beyer, and G. Neuhaus. 2001. A novel phytyltransferase from Synechocystis sp. PCC 6803 involved in tocopherol biosynthesis. FEBS Lett. 49915-20. [DOI] [PubMed] [Google Scholar]
- 34.Shintani, D. K., Z. Cheng, and D. DellaPenna. 2002. The role of 2-methyl-6-phytylbenzoquinone methyltransferase in determining tocopherol composition in Synechocystis sp. PCC6803. FEBS Lett. 5111-5. [DOI] [PubMed] [Google Scholar]
- 35.Shintani, D., and D. DellaPenna. 1998. Elevating the vitamin E content of plants through metabolic engineering. Science 2822098-2100. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki, I., Y. Kanesaki, K. Mikami, M. Kanehisa, and N. Murata. 2001. Cold-regulated genes under control of the cold sensor Hik33 in Synechocystis. Mol. Microbiol. 40235-244. [DOI] [PubMed] [Google Scholar]
- 37.Takamura, N., M. Yasuno, and K. Sugahara. 1984. Overwintering of Microcystis aeruginosa Kutz. in a shallow lake. J. Plankton Res. 61019-1029. [Google Scholar]
- 38.Tsujimura, S., H. Tsukada, H. Nakahara, T. Nakajima, and M. Nishino. 2000. Seasonal variations of Microcystis populations in sediments of lake Biwa, Japan. Hydrobiology 434183-192. [Google Scholar]
- 39.Verspagen, J. M. H., E. O. F. M. Snelder, P. M. Visser, J. Huisman, L. R. Mur, and B. W. Ibelings. 2004. Recruitment of benthic Microcystis (Cyanophyceae) to the water column: internal buoyancy changes or resuspension? J. Phycol. 40260-270. [Google Scholar]
- 40.Visser, P. M., B. W. Ibelings, and L. R. Mur. 1995. Autumnal sedimentation of Microcystis spp. as result of an increase in carbohydrate ballast at reduced temperature. J. Plankton Res. 17919-933. [Google Scholar]
- 41.Wang, Y., and X. Xu. 2005. Regulation by hetC of genes required for heterocyst differentiation and cell division in Anabaena sp. J. Bacteriol. 1878489-8493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams, J. G. K. 1988. Construction of specific mutantions in photosystem II photosynthetic reaction center by engineering methods in Synechocystis 6803. Methods Enzymol. 167766-778. [Google Scholar]
- 43.Yin, C., W. Li, Y. Du, R. Kong, and X. Xu. 2007. Identification of a gene, ccr-1 (sll1242), required for chill-light tolerance and growth at 15°C in Synechocystis sp. PCC 6803. Microbiology 1531261-1267. [DOI] [PubMed] [Google Scholar]
- 44.Zhang, L., B. McSpadden, H. B. Pakrasi, and J. Whitmarsh. 1992. Copper-mediated regulation of cytochrome c553 and plastocyanin in the cyanobacterium Synechocystis 6803. J. Biol. Chem. 26719054-19059. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.